New Insights into the Mineralogy and Geochemistry of Sb Ores from Greece
Abstract
1. Introduction
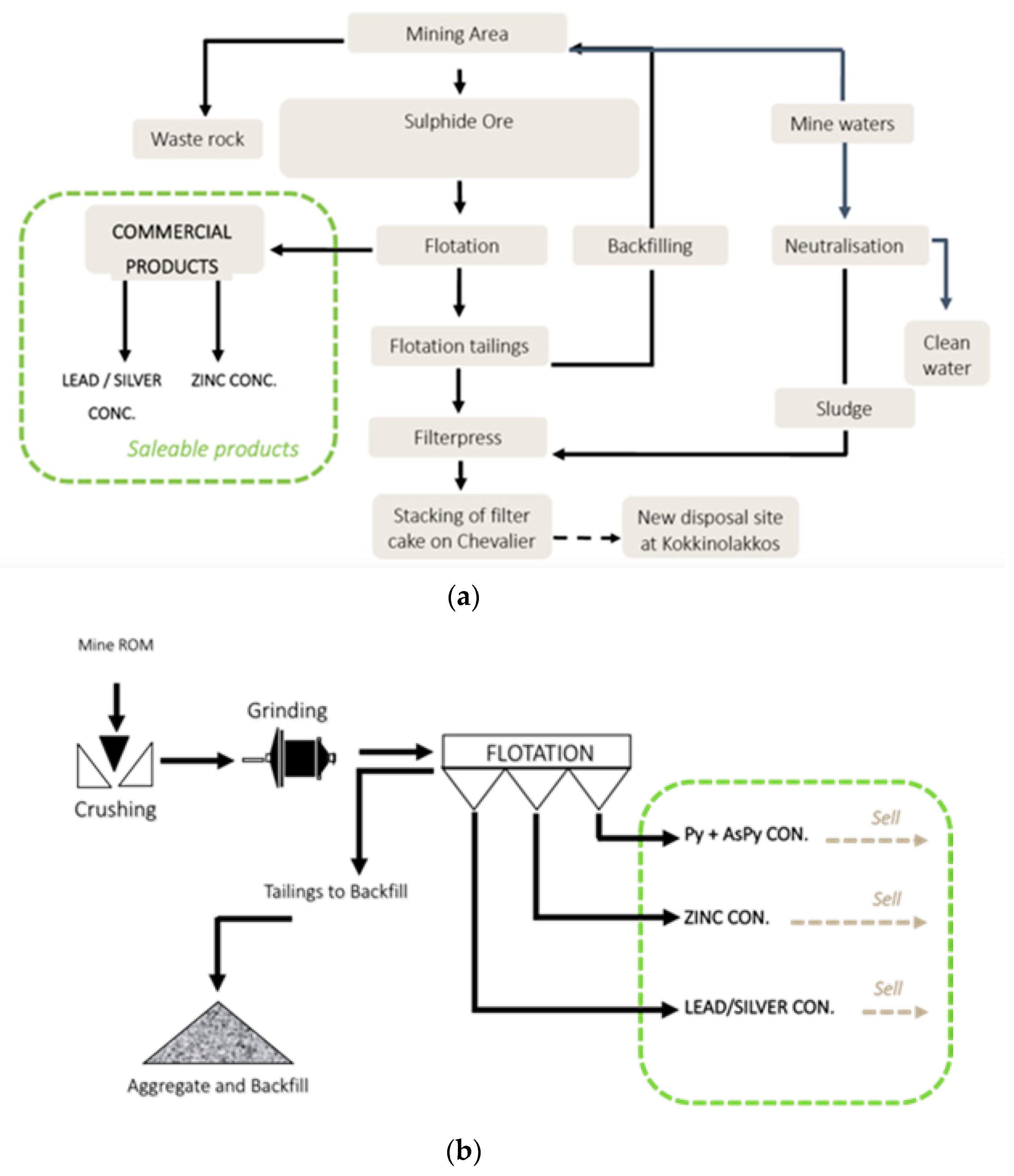
2. Materials and Methods
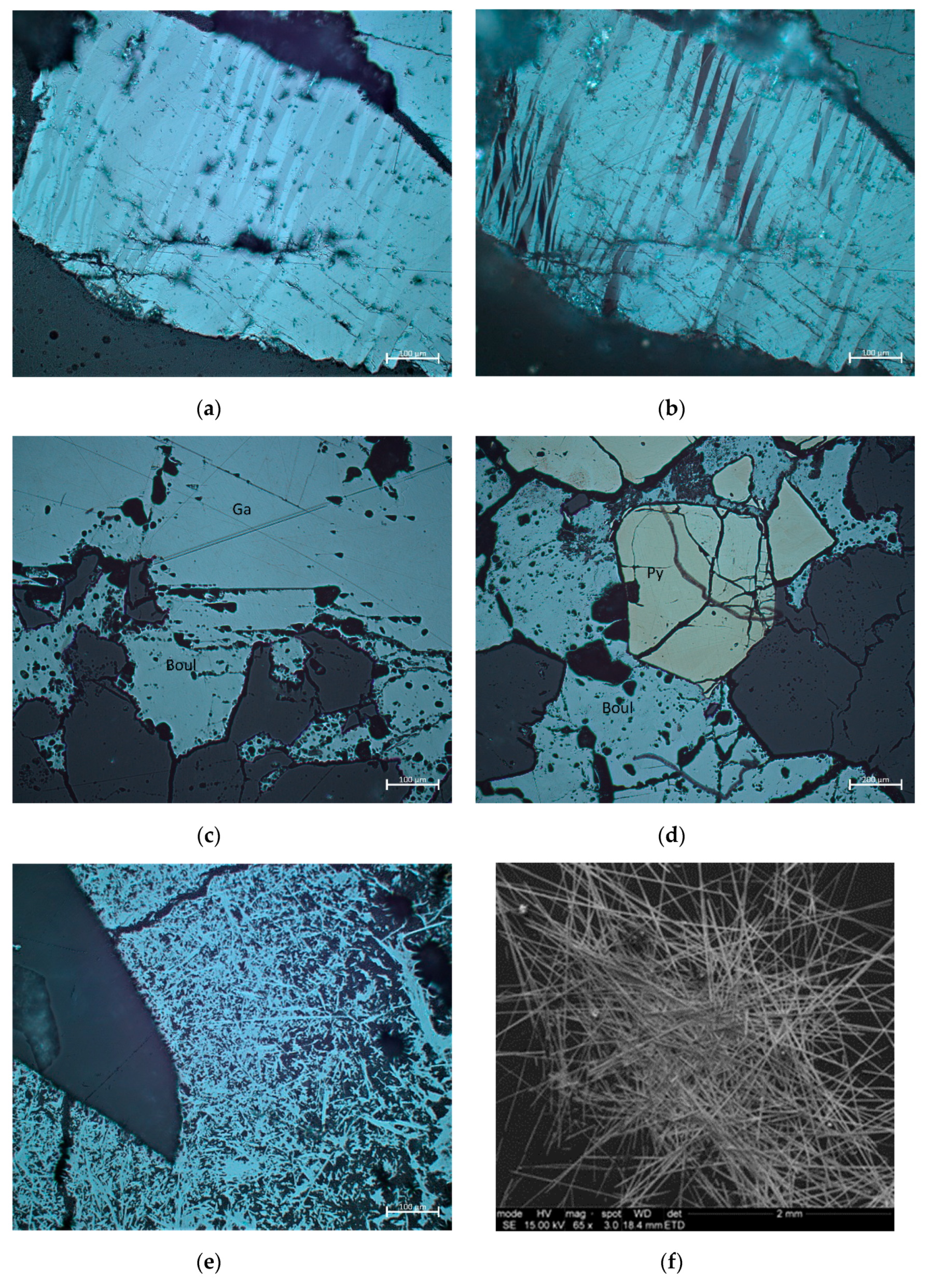
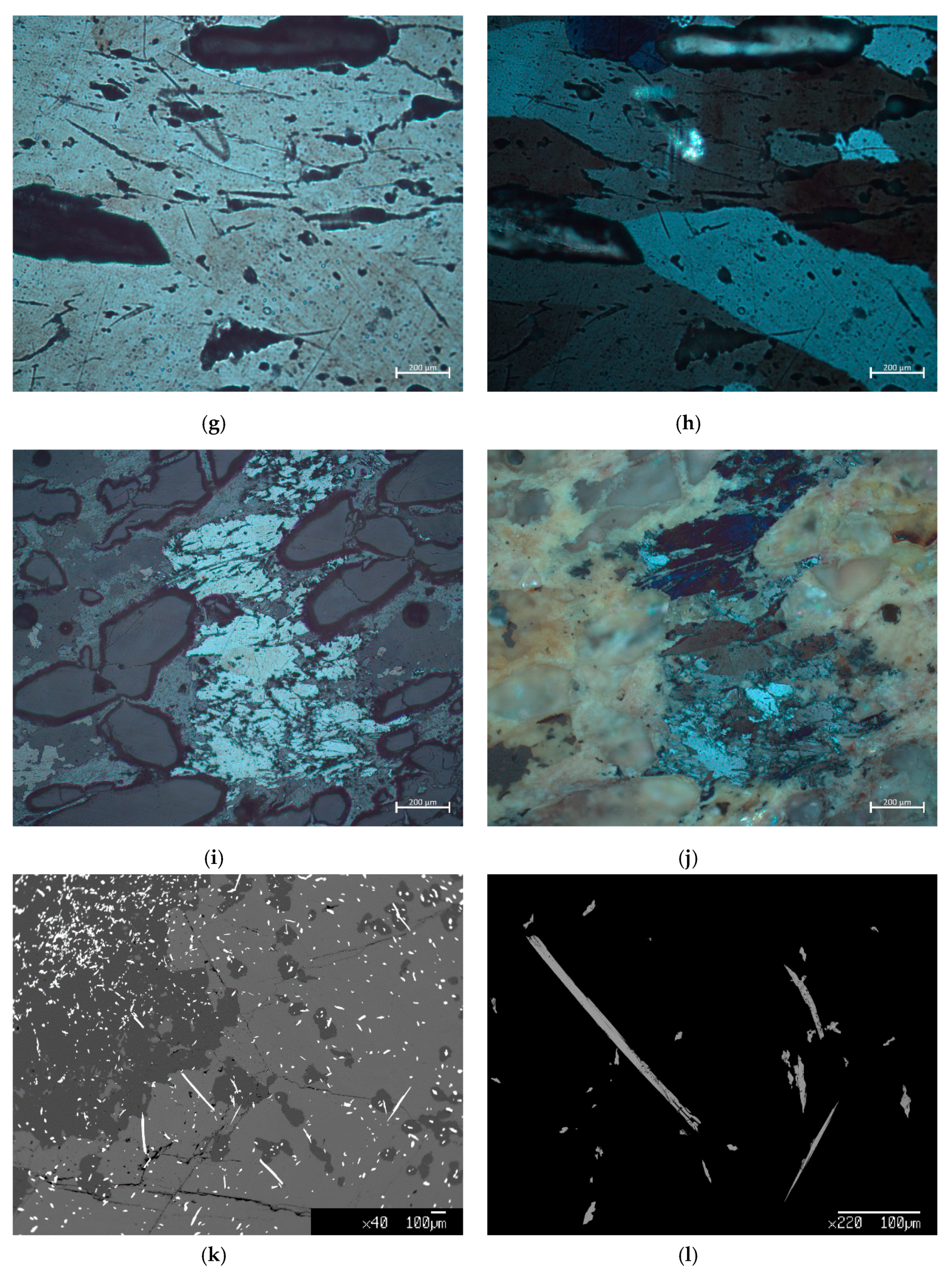
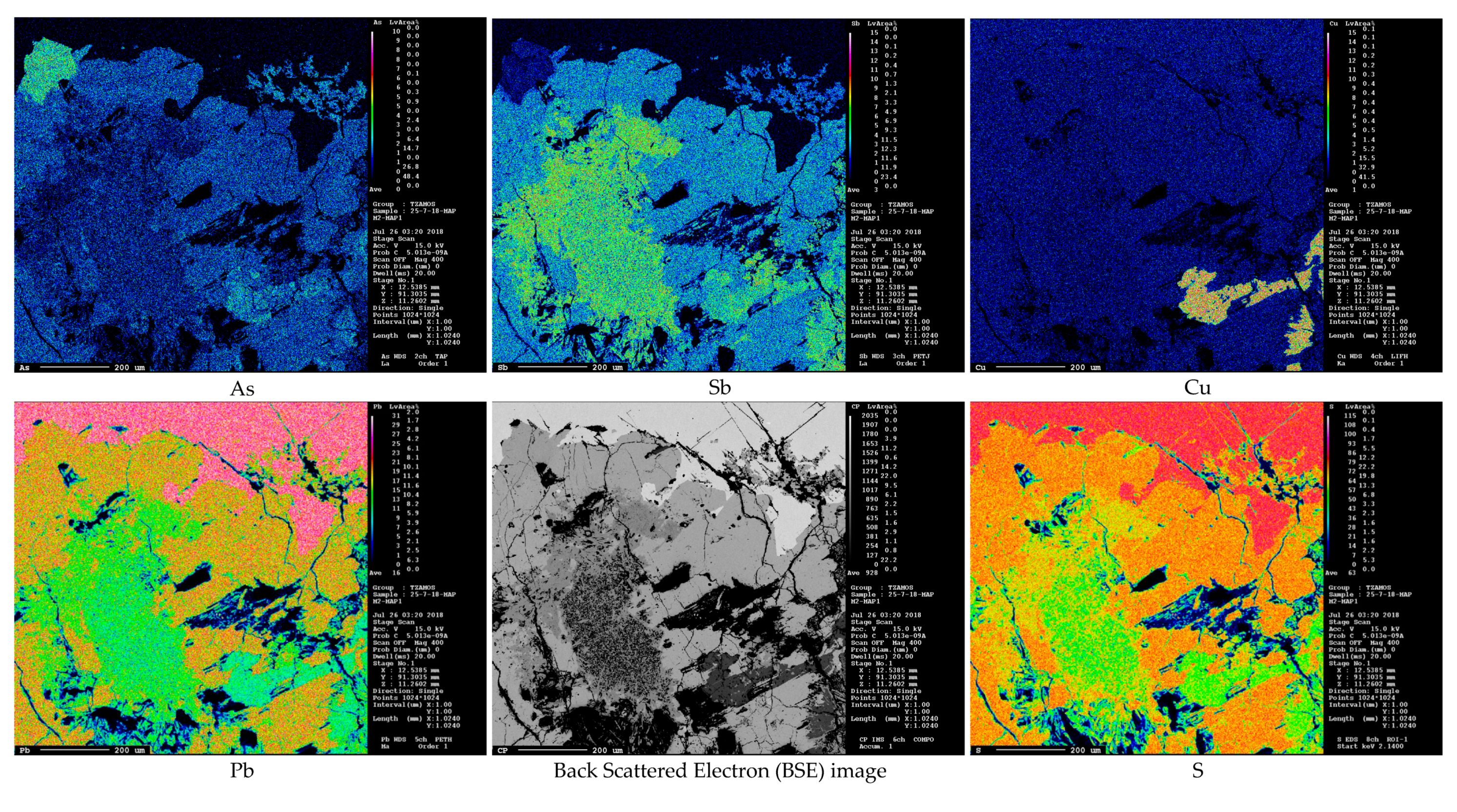
3. Results
- Stibnite from Olympiada locality: Sb2.02As0.01S3.00
- Stibnite from Stratoni/Mantem Lakkos locality: Sb2.06As0.01S3.00
- Stibnite from Chios locality: Sb2.03As0.01S3.00
- Boulangerite from Olympiada locality: Pb5.02Zn0.02Cu0.01Fe0.01Ni0.01W0.01Se0.01Sb4.09As0.30S11.00
- Boulangerite from Kilkis area: Pb5.16Zn0.01Cu0.01Ni0.01W0.01Hg0.01Mn0.01Sb4.03As0.09S11.00
- Bournonite from Olympiada locality: Pb1.00Cu1.00Sb0.68As0.36S3.00
- Bertherite from Chios island: Fe1.00Sb2.14As0.01S4.00
- The boulangerite sample from Kassandra Mines (Olympiada locality) shows unusual high concentrations in Bi (1610 ppm), Ag (>200 ppm), and Tl (92 ppm), and is particularly enriched in Cd (22 ppm) and Sn (70 ppm).
- The stibnite sample from Kassandra Mines (Stratoni/Mantem Lakkos locality) has an elevated concentration of Se (10 ppm) and Ag (27 ppm).
- The stibnite sample from Kassandra Mines (Olympiada locality) is enriched in As (157 ppm), Sn (83 ppm), Ag (70 ppm), and Tl (15 ppm).
- The stibnite sample from the Kilkis area (Lachanas/Rizana locality) contains a significant amount of Hg (10.08 ppm).
4. Discussion
5. Conclusions
- Greek Sb-ores are identified as stibnite, boulangerite, bournonite, bertherite, and valentinite. Bournonite is associated with seligmannite, and bertherite and valentinite occur together with primary stibnite.
- Significant amounts of trace elements can be found in Sb minerals Bi, Ag, Tl, Cd, and Sn in boulangerite from Olympiada mine; Ag and Se in stibnite from Olympiada mine; As, Sn, Ag, and Tl in stibnite from Olympiada mine; and Hg in stibinitesample from Lachanas/Rizana mine.
- The presence of trace elements in elevated concentrations in Greek Sb minerals is mainly attributed to the co-presence of mineral phases rich in these elements.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- British Geological Survey, Bureau de Recherches Géologiques et Minières, Deloitte Sustainability, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs (European Commission), TNO. Study on the Review of the List of Critical Raw Materials; Publications Office of the European Union: Brussels, Belgium, 2017; p. 93.
- Grund, S.C.; Hanusch, K.; Breuning, H.J.; Wolf, H.U. Antimony and antimony compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, The Netherlands, 2006; Available online: http://criticalrawmaterials.org/critical-raw-materials (accessed on 21 March 2019).
- Klocho, K. Antimony. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2019; pp. 22–23.
- Tsirambides, A.; Filippidis, A. Sb- Bi-Bearing Metallogeny of the Serbomacedonian-Rhodope Metallogenetic Belt (SRMB). In Proceedings of the Abstracts of 15th International Congress of the Geological Society of Greece, Athens, Greece, 22–24 May 2019. [Google Scholar]
- Paraskevopoulos, G. Genesis of the W- and Sb-bearing ores of Lachanas area in the central Macedonia. Ann. Geol. Pays. Hell. 1958, 9, 227–241. [Google Scholar]
- Liatsikas, N.; Solomos, I.; Kogevinas, S.; Andreakos, G. The Mineral Wealth of Greece; U.N.R.R.A.: Athens, Greece, 1947. [Google Scholar]
- Vassilatos, C.; Barlas, K.; Stamatakis, M.; Tsivilis, S. Wolframite—Stibnite mineral assemblages from Rizana Lachanas, Macedonia, Greece and their possible use as flux agent in the manufacturing of clinker. Bull. Geol. Soc. Greece 2001, 34, 827–834. [Google Scholar] [CrossRef]
- Tzamos, E.; Papadopoulos, A.; Grieco, G.; Stoulos, S.; Bussolesi, M.; Daftsis, E.; Vagli, E.; Dimitriadis, D.; Godelitsas, A. Investigation of trace and critical elements (including actinides) in flotation sulphide concentrates of Kassandra mines (Chalkidiki, Greece). Geosciences 2019, 9, 164. [Google Scholar] [CrossRef]
- Chatzidiakos, E.; Fanouraki, M.; Kelepertsis, A.; Argyraki, A.; Alexakis, D. Speciation and mobility of arsenic and antimony in groundwater at Melivoia, East Thessaly and Keramos area NW Chios, Greece. In Proceedings of the 8th International Hydrogeological Congress of Greece, Athens, Greece, 8–10 October 2008; Volume 1, pp. 219–228. [Google Scholar]
- Fanouraki, M. Environmental Pollution by Arsenic and Antimony in the Soil and Groundwater of NW Chios Island, Greece. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2011. [Google Scholar]
- Cidu, R.; Biddau, R.; Dore, E.; Vacca, A.; Marini, L. Antimony in the soil–water–plant system at the Su Suergiu abandoned mine (Sardinia, Italy): Strategies to mitigate contamination. Sci. Tot. Envir. 2014, 497, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, S.; Takahashi, Y.; Araki, Y.; Tanimizu, M. Comparison of antimony and arsenic behavior in an Ichinokawa River water—Sediment system. Chem. Geol. 2012, 334, 1–8. [Google Scholar] [CrossRef]
- Casiot, C.; Ujevic, M.; Munoz, M.; Seidel, J.L.; Elbaz-Poulichet, F. Antimony and arsenic mobility in a creek draining an antimony mine abandoned 85 years ago (Upper Orb Basin, France). Appl. Geochem. 2007, 22, 788–798. [Google Scholar] [CrossRef]
- Fillela, M.; Philippo, S.; Belzile, N.; Chen, Y.; Quentel, F. Natural attenuation processes applying to antimony: A study in the abandoned antimony mine in Goesdorf, Luxembourg. Sci. Total. Envir. 2009, 407, 6205–6216. [Google Scholar] [CrossRef] [PubMed]
- Ondrejková, I.; Ženišová, Z.; Flaková, R.; Krčmáŕ, D.; Sracek, O. The distribution of antimony and arsenic in waters of the Dúbrava abandoned mine site, Slovak Republic. Mine Water Envir. 2013, 32, 207–221. [Google Scholar] [CrossRef]
- Ritchie, V.J.; Ilgen, A.G.; Mueller, S.H.; Trainor, T.P.; Goldfarb, R.J. Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chem. Geol. 2013, 335, 172–188. [Google Scholar] [CrossRef]
- Minz, F.-E.; Bolin, N.-J.; Lamberg, P.; Bachmann, K.; Gutzmer, J.; Wanhainen, C. Distribution of Sb minerals in the Cu and Zn flotation of Rockliden massive sulphide ore in north-central Sweden. Miner. Eng. 2015, 82, 125–135. [Google Scholar] [CrossRef]
- Pažout, R.; Sejkora, J.; Šrein, V. Ag–Pb–Sb sulfosalts and Se-rich mineralization of anthony of padua mine near poličany—Model example of the mineralization of silver lodes in the historic Kutná Hora Ag–Pb ore district, Czech Republic. Minerals 2019, 9, 430. [Google Scholar] [CrossRef]
- Mumme, W. The crystal structure of Pb5.05 (Sb3.75Bi0.28)S10.72Se0.28 boulangerite of near ideal composition. Neu. Jahr. Miner. Mon. 1989, 11, 498–512. [Google Scholar]
- Sejkora, J.; Ozdín, D.; Vitáloš, J.; Ďuďa, R. Schafarzikite from the type locality Pernek (Male Karpaty Mountains, Slovak Republic) revisited. Europ. J. Miner. 2007, 19, 419–427. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, H.; Qin, C.; Du, S.; Zhu, C.; Fan, A.; Zhang, J. The geological significance of Pb–Bi- and Pb–Sb-sulphosalts in the Damajianshan tungsten polymetallic deposit, Yunnan Province, China. Ore Geol. Rev. 2015, 71, 203–214. [Google Scholar] [CrossRef]
- Sheng, X.; Bi, X.; Hu, R.; Tang, Y.; Lan, Q.; Xiao, J.; Tao, Y.; Huang, M.; Peng, J.; Xu, L. The mineralization process of the Lanuoma Pb–Zn–Sb deposit in the Sanjiang Tethys region: Constraints from in situ sulfur isotopes and trace element compositions. Ore Geol. Rev. 2019, 111, 1029–1041. [Google Scholar] [CrossRef]
- Sabeva, R.; Mladenova, V.; Mogessie, A. Ore petrology, hydrothermal alteration, fluid inclusions, and sulfur stable isotopes of the Milin Kamak intermediate sulfidation epithermal Au-Ag deposit in Western Srednogorie, Bulgaria. Ore Geol. Rev. 2017, 88, 400–415. [Google Scholar]
- Frias, J.M. Sulphide and sulphosalt mineralogy and paragenesis from the Sierra Alamgrera Veins, Beta Cordillera (SE Spain). Estud. Geol. 1991, 47, 271–279. [Google Scholar]
- Li, W.; Cook, J.N.; Ciobani, L.; Cristiana, L.C.; Xie, G.; Wade, P.B.; Gilbert, E.S. Trace element distributions in (Cu)–Pb–Sb sulfosalts from the Gutaishan Au-Sb deposit, South China: Implications for formation of high fineness native gold. Am. Mineral. 2019, 104, 425–437. [Google Scholar] [CrossRef]



| wt. % | Stibnite (Olympiada) Average, n = 31 | Stibnite (Stratoni/M.Lakkos) Average, n = 15 | Stibnite (Chios) Average, n = 9 |
| Sb | 71.833 | 71.784 | 71.694 |
| S | 28.049 | 27.543 | 27.928 |
| As | 0.261 | 0.215 | 0.183 |
| W | 0.080 | 0.074 | 0.030 |
| Pb | 0.054 | 0.050 | 0.059 |
| Zn | 0.053 | 0.076 | 0.045 |
| Se | 0.025 | 0.040 | 0.019 |
| Hg | 0.021 | 0.038 | 0.036 |
| Ag | 0.020 | 0.009 | 0.014 |
| Ni | 0.017 | 0.017 | 0.015 |
| Fe | 0.015 | 0.015 | 0.012 |
| Mn | 0.015 | 0.010 | 0.005 |
| Cu | 0.011 | 0.023 | 0.037 |
| Mg | 0.009 | 0.010 | 0.010 |
| Co | 0.009 | 0.016 | 0.021 |
| Bi | 0.000 | 0.000 | 0.000 |
| Ca | 0.000 | 0.000 | 0.000 |
| Total | 100.472 | 99.919 | 100.107 |
| Atom | |||
| S | 3.000 | 3.000 | 3.000 |
| Sb | 2.024 | 2.059 | 2.028 |
| As | 0.012 | 0.010 | 0.008 |
| Zn | 0.003 | 0.004 | 0.002 |
| W | 0.001 | 0.001 | 0.001 |
| Mg | 0.001 | 0.001 | 0.001 |
| Se | 0.001 | 0.002 | 0.001 |
| Ni | 0.001 | 0.001 | 0.001 |
| Fe | 0.001 | 0.001 | 0.001 |
| Mn | 0.001 | 0.001 | 0.000 |
| Pb | 0.001 | 0.001 | 0.001 |
| Ag | 0.001 | 0.000 | 0.000 |
| Cu | 0.001 | 0.001 | 0.002 |
| Co | 0.000 | 0.001 | 0.001 |
| Hg | 0.000 | 0.001 | 0.001 |
| Bi | 0.000 | 0.000 | 0.000 |
| Ca | 0.000 | 0.000 | 0.000 |
| Total | 5.048 | 5.085 | 5.049 |
| wt. % | Boulangerite (Olympiada) Average, n = 13 | Boulangerite (Kilkis) Average, n = 10 | |
| Pb | 54.468 | 55.932 | |
| Sb | 26.090 | 25.701 | |
| S | 18.465 | 18.474 | |
| As | 1.161 | 0.338 | |
| Zn | 0.057 | 0.027 | |
| W | 0.053 | 0.093 | |
| Hg | 0.033 | 0.094 | |
| Bi | 0.026 | 0.000 | |
| Cu | 0.024 | 0.033 | |
| Fe | 0.020 | 0.011 | |
| Se | 0.020 | 0.010 | |
| Ni | 0.017 | 0.026 | |
| Co | 0.011 | 0.006 | |
| Ag | 0.007 | 0.000 | |
| Mn | 0.003 | 0.038 | |
| Mg | 0.000 | 0.000 | |
| Ca | 0.000 | 0.000 | |
| Total | 100.454 | 100.784 | |
| Atom | |||
| S | 11.000 | 11.000 | |
| Pb | 5.022 | 5.155 | |
| Sb | 4.094 | 4.031 | |
| As | 0.295 | 0.086 | |
| Zn | 0.017 | 0.008 | |
| Cu | 0.007 | 0.010 | |
| Fe | 0.007 | 0.004 | |
| Ni | 0.006 | 0.008 | |
| W | 0.005 | 0.010 | |
| Se | 0.005 | 0.003 | |
| Co | 0.004 | 0.002 | |
| Hg | 0.003 | 0.009 | |
| Bi | 0.002 | 0.000 | |
| Ag | 0.001 | 0.000 | |
| Mn | 0.001 | 0.013 | |
| Mg | 0.000 | 0.000 | |
| Ca | 0.000 | 0.000 | |
| Total | 20.470 | 20.338 | |
| wt. % | Bournonite-Seligmannite (Olympiada) Average, n = 5 | wt. % | Bertherite (Chios) Average, n = 6 |
| Pb | 43.132 | Sb | 57.982 |
| S | 20.134 | S | 28.615 |
| Sb | 17.244 | Fe | 12.415 |
| Cu | 13.242 | As | 0.237 |
| As | 5.620 | Cu | 0.135 |
| Se | 0.053 | Pb | 0.072 |
| Zn | 0.049 | W | 0.065 |
| Hg | 0.042 | Mn | 0.051 |
| Ni | 0.034 | Zn | 0.048 |
| Fe | 0.018 | Hg | 0.041 |
| W | 0.018 | Se | 0.037 |
| Mn | 0.014 | Mg | 0.019 |
| Co | 0.010 | Co | 0.013 |
| Mg | 0.000 | Ni | 0.011 |
| Bi | 0.000 | Ag | 0.003 |
| Ag | 0.000 | Bi | 0.000 |
| Ca | 0.000 | Ca | 0.000 |
| Total | 99.610 | Total | 99.745 |
| Atom | |||
| S | 3.000 | S | 4.000 |
| Cu | 0.996 | Sb | 2.135 |
| Pb | 0.995 | Fe | 0.997 |
| Sb | 0.677 | As | 0.014 |
| As | 0.358 | Cu | 0.009 |
| Zn | 0.004 | Mn | 0.004 |
| Se | 0.003 | Mg | 0.004 |
| Ni | 0.003 | Zn | 0.003 |
| Fe | 0.002 | Se | 0.002 |
| Mn | 0.001 | W | 0.002 |
| Hg | 0.001 | Pb | 0.002 |
| Co | 0.001 | Co | 0.001 |
| W | 0.000 | Hg | 0.001 |
| Mg | 0.000 | Ni | 0.001 |
| Bi | 0.000 | Ag | 0.000 |
| Ag | 0.000 | Bi | 0.000 |
| Ca | 0.000 | Ca | 0.000 |
| Total | 6.040 | Total | 7.175 |
| Locality | Kilkis (Lachanas/Rizana) | Kassandra Mines (Olympiada) | Kassandra Mines (Olympiada) | Kassandra Mines (Stratoni/M.Lakkos) |
|---|---|---|---|---|
| Main Phase | Stibnite (Sb2S3) | Stibnite (Sb2S3) | Boulangerite (Pb5Sb4S11) | Stibnite (Sb2S3) |
| Fe | 100 | 100 | 300 | 3200 |
| Ba | 89 | 158 | 34 | 115 |
| Zn | 33 | 2099 | 64 | 3 |
| Cu | 19 | 242 | 190 | 43 |
| Hg | 10.08 | 0.01 | 0.22 | 0.37 |
| Tl | 8.8 | 15.1 | 92.0 | 0.6 |
| Pb | 7 | >10,000 | >10,000 | 1 |
| Bi | 6.7 | 4.2 | 1609.7 | 0.7 |
| Mn | 3 | 3074 | 428 | 63 |
| Ag | 1.4 | 70 | >200 | 26.8 |
| *ΣREE + Y + Sc | <2 | <6 | <3 | <2 |
| Ni | 1.2 | 8.5 | 0.1 | 0.2 |
| V | 1.0 | 3.0 | 1.0 | 1.0 |
| As | 1 | 157 | 105 | 0 |
| Cd | 0.7 | 8.2 | 21.9 | 0.9 |
| Se | 0.30 | 0.70 | 0.80 | 10.00 |
| Sn | 0.2 | 83.7 | 69.7 | 15.7 |
| U + Th | <0.2 | <1 | <0.7 | <0.2 |
| W | 0.10 | 0.10 | 0.10 | 0.10 |
| Ta | 0.10 | 0.10 | 0.10 | 0.10 |
| Ga | 0.07 | 1.28 | 0.27 | 0.20 |
| Mo | 0.05 | 0.29 | 0.12 | 0.05 |
| Te | 0.05 | 0.17 | 2.74 | 0.05 |
| Nb | 0.04 | 0.04 | 0.04 | 0.04 |
| In | <0.01 | 0.02 | 0.16 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzamos, E.; Gamaletsos, P.N.; Grieco, G.; Bussolesi, M.; Xenidis, A.; Zouboulis, A.; Dimitriadis, D.; Pontikes, Y.; Godelitsas, A. New Insights into the Mineralogy and Geochemistry of Sb Ores from Greece. Minerals 2020, 10, 236. https://doi.org/10.3390/min10030236
Tzamos E, Gamaletsos PN, Grieco G, Bussolesi M, Xenidis A, Zouboulis A, Dimitriadis D, Pontikes Y, Godelitsas A. New Insights into the Mineralogy and Geochemistry of Sb Ores from Greece. Minerals. 2020; 10(3):236. https://doi.org/10.3390/min10030236
Chicago/Turabian StyleTzamos, Evangelos, Platon N. Gamaletsos, Giovanni Grieco, Micol Bussolesi, Anthimos Xenidis, Anastasios Zouboulis, Dimitrios Dimitriadis, Yiannis Pontikes, and Athanasios Godelitsas. 2020. "New Insights into the Mineralogy and Geochemistry of Sb Ores from Greece" Minerals 10, no. 3: 236. https://doi.org/10.3390/min10030236
APA StyleTzamos, E., Gamaletsos, P. N., Grieco, G., Bussolesi, M., Xenidis, A., Zouboulis, A., Dimitriadis, D., Pontikes, Y., & Godelitsas, A. (2020). New Insights into the Mineralogy and Geochemistry of Sb Ores from Greece. Minerals, 10(3), 236. https://doi.org/10.3390/min10030236











