Thermal Analysis, Compressibility, and Decomposition of Synthetic Bastnäsite-(La) to Lanthanum Oxyfluoride
Abstract
1. Introduction
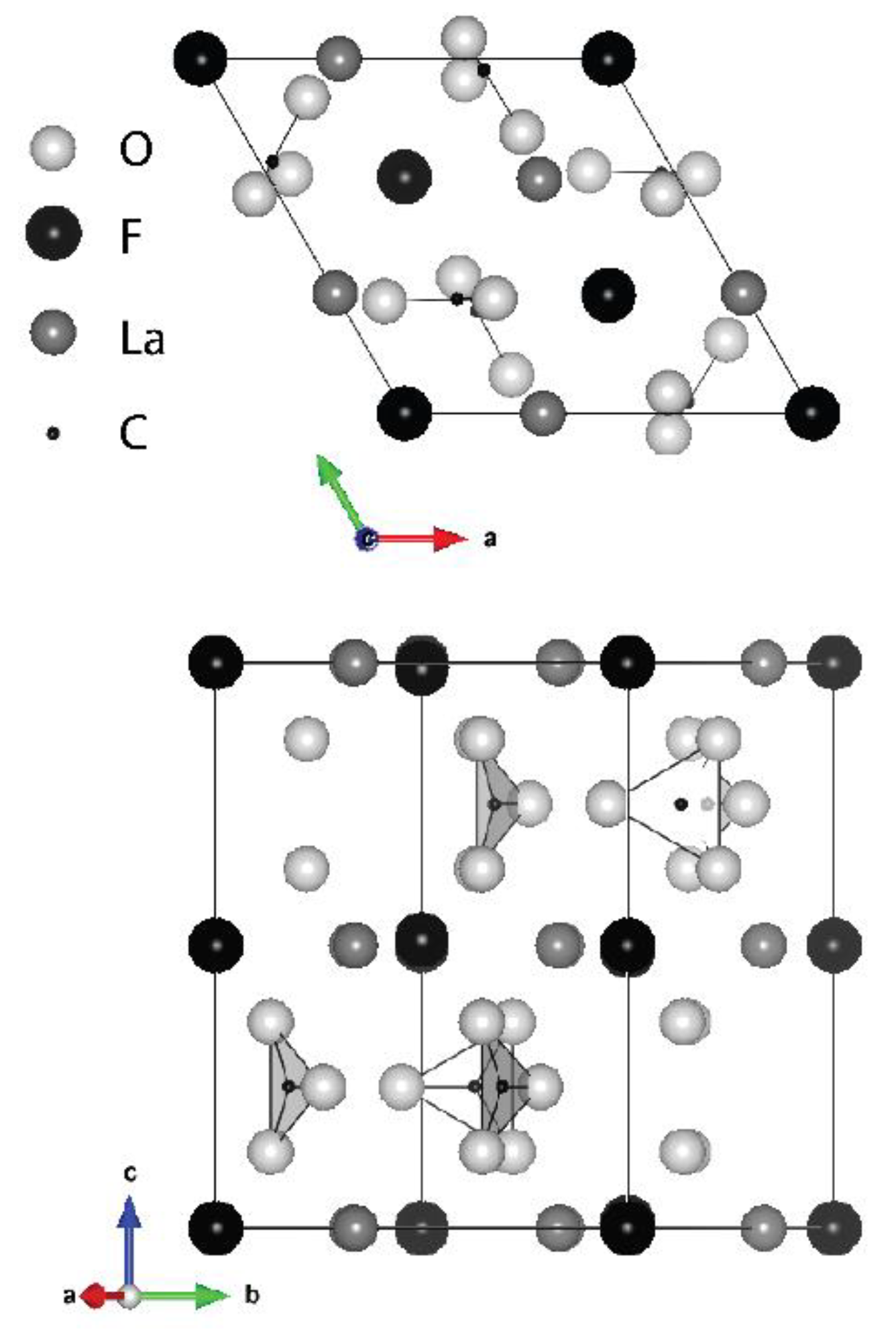
Structure and Decomposition of Bastnäsite-(La) to Lanthanum Oxyfluoride
2. Materials and Methods
2.1. Synthesis
2.2. Themogravimetric Analysis and Differential Scanning Calorimetry
2.3. High Temperature Powder X-ray Diffraction
2.4. Single Crystal X-ray Diffraction
3. Results
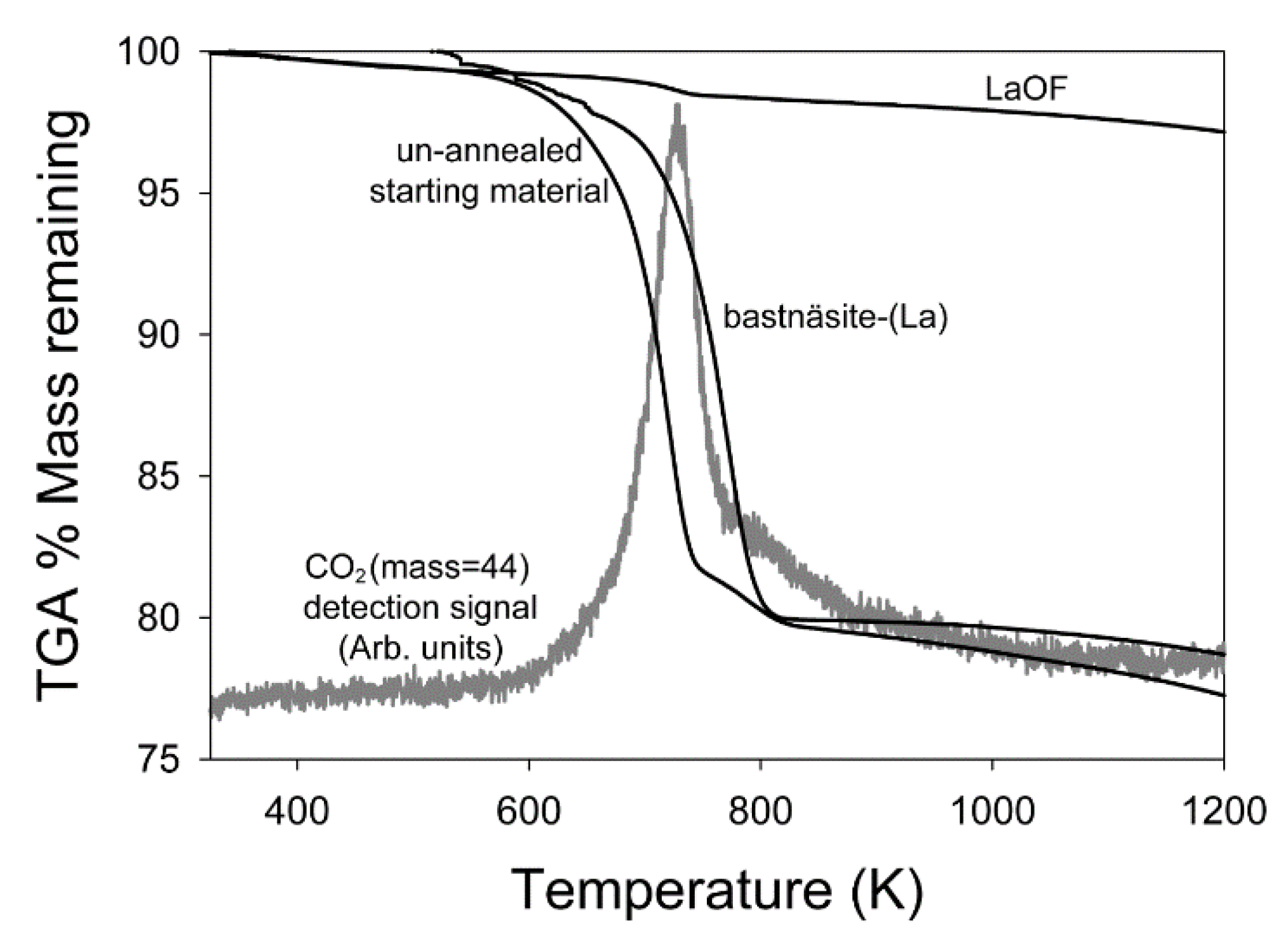
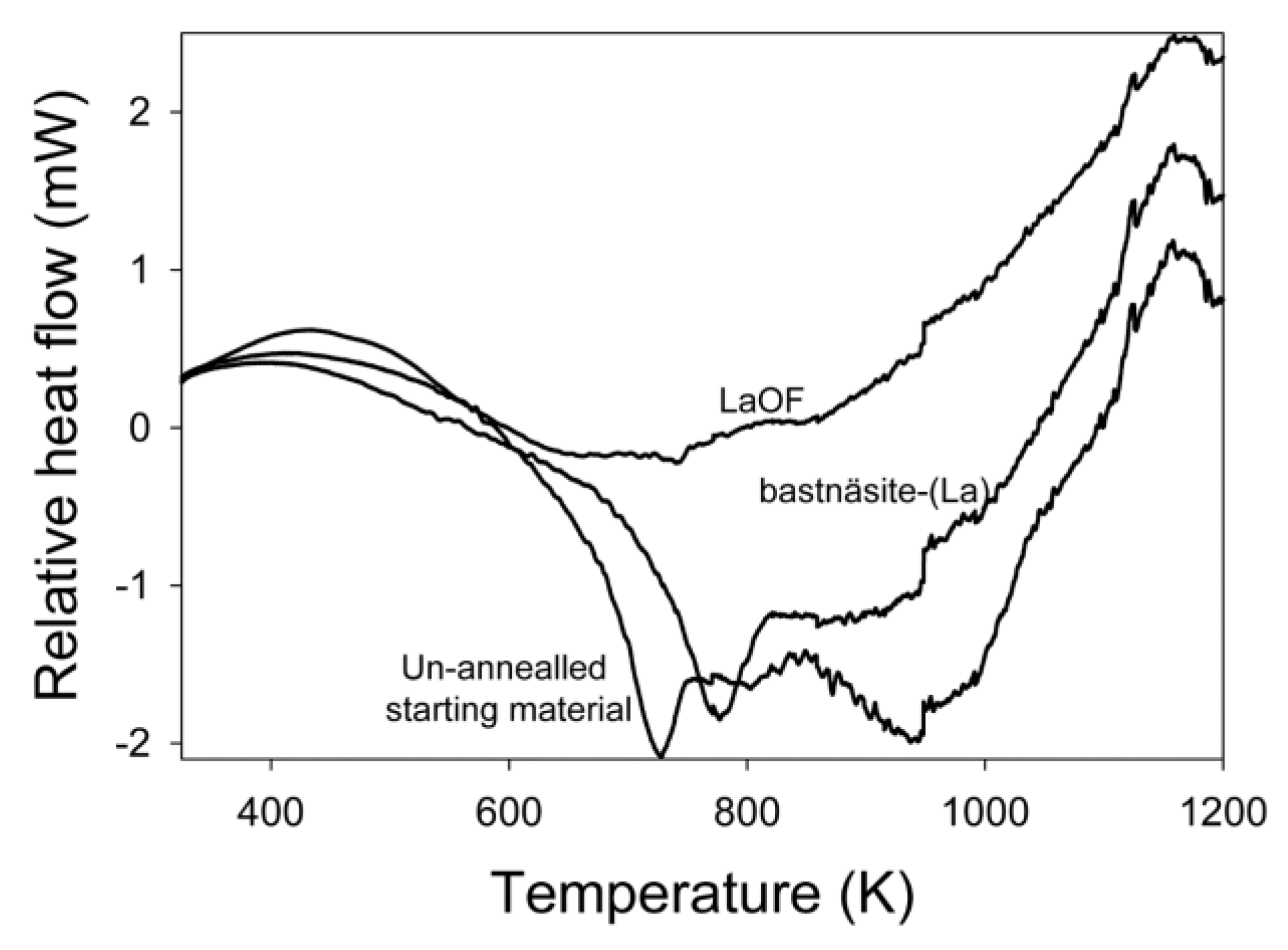
3.1. Thermogravimetric Analysis and Differential Scanning Calorimetry
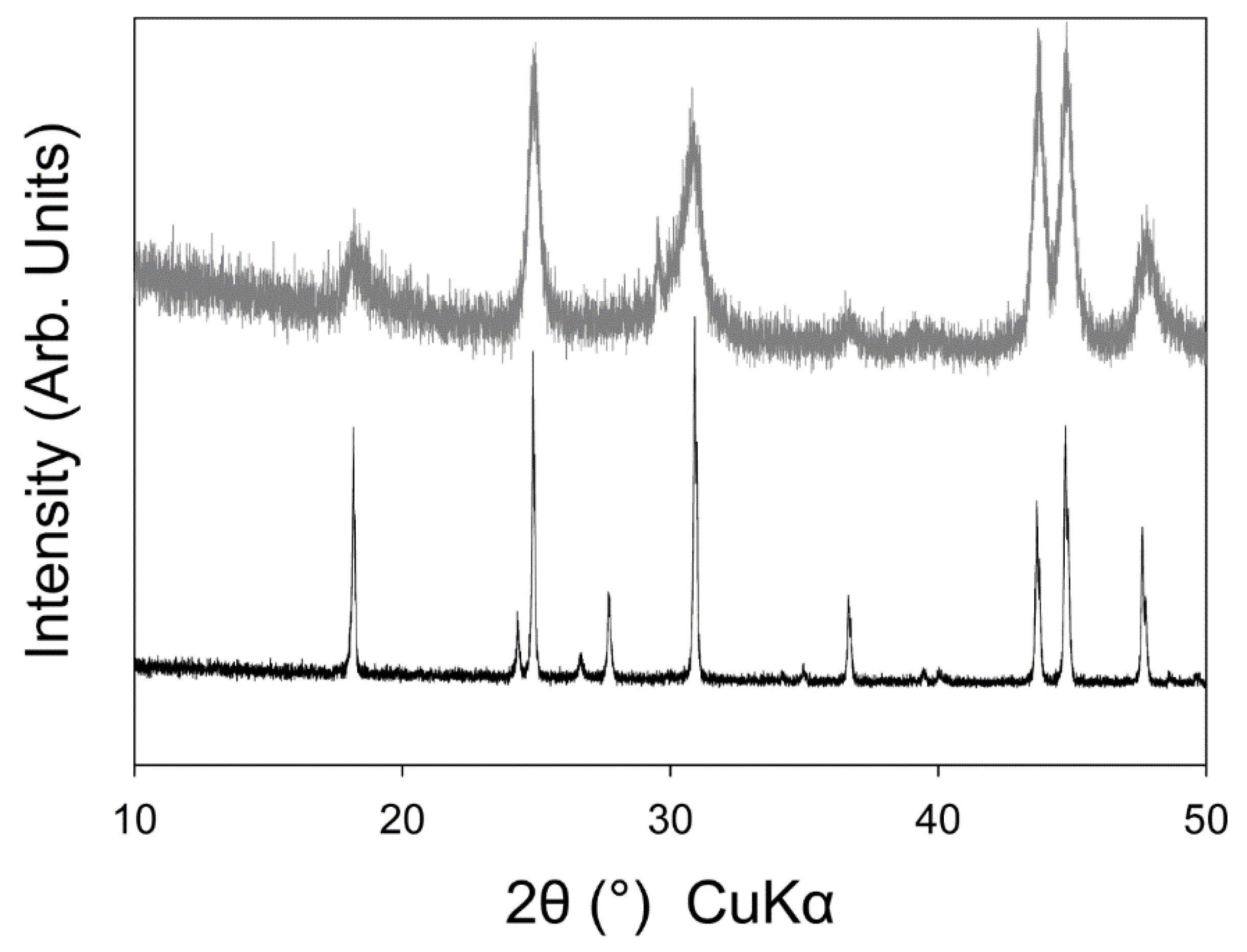
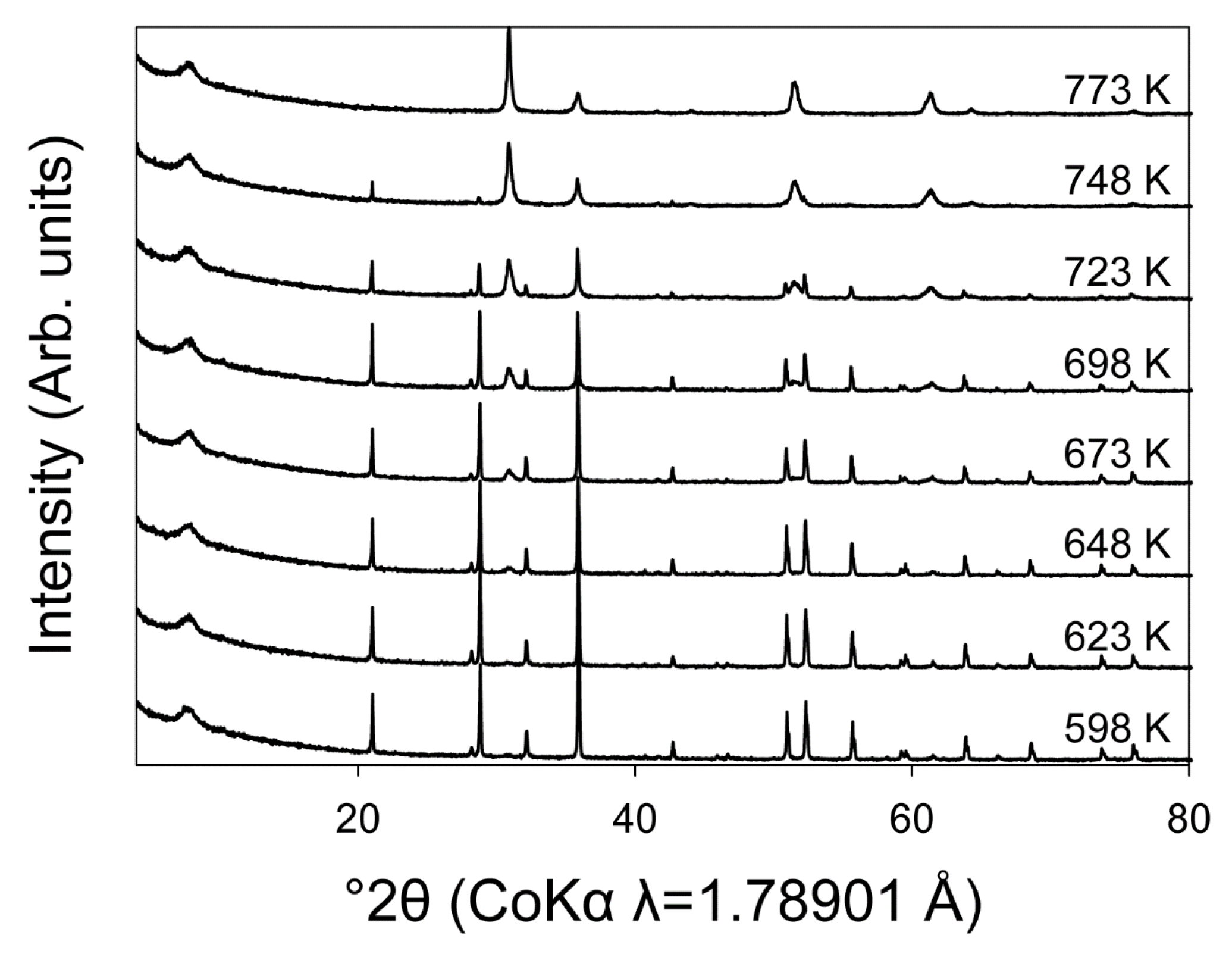
3.2. High Temperature Powder X-ray Diffraction
3.3. Single Crystal X-ray Diffraction
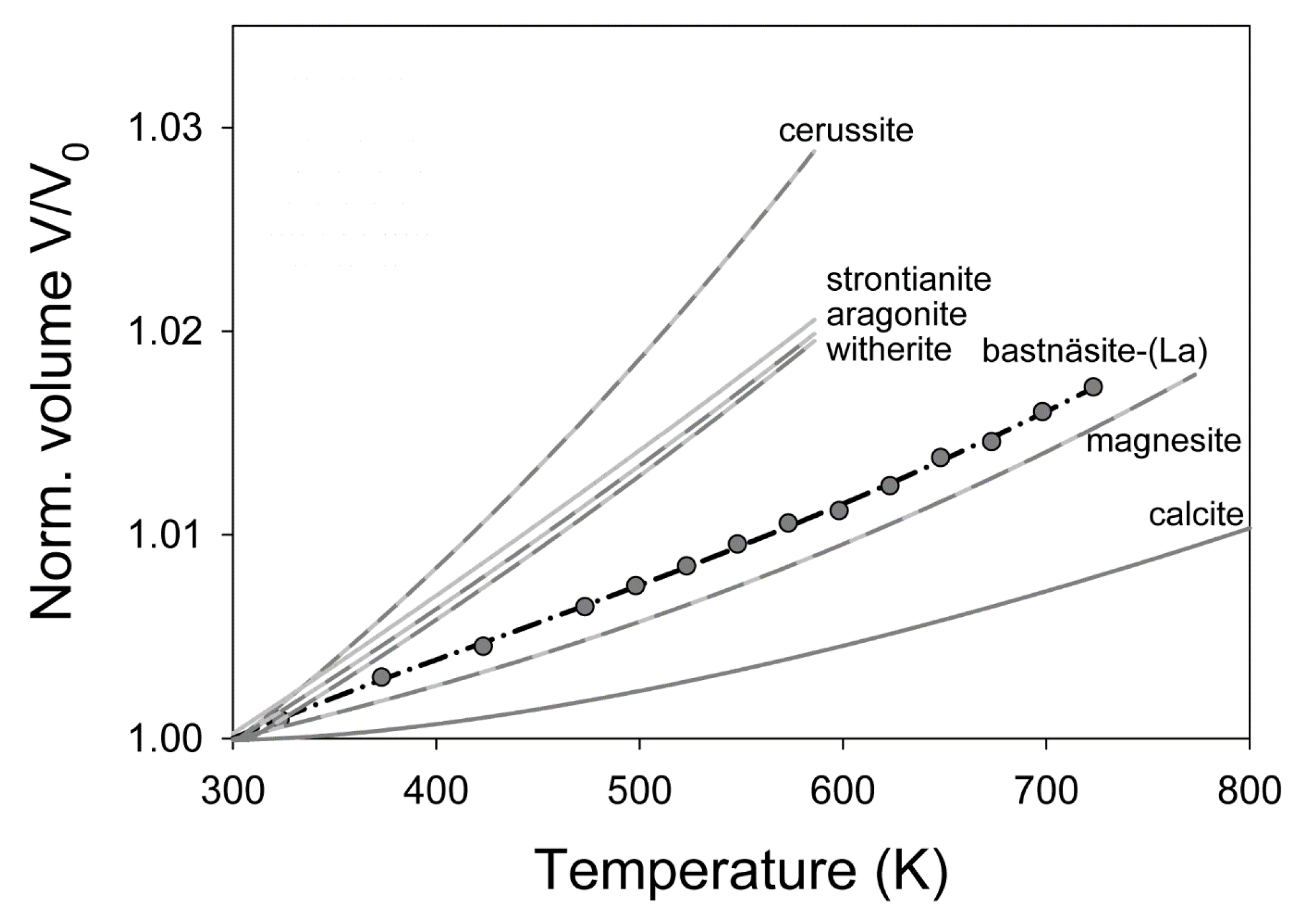
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Z. Global rare earth resources and scenarios of future rare earth industry. J. Rare Earths 2011, 29, 1–6. [Google Scholar] [CrossRef]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective. US Geol. Surv. Sci. Investig. 2010, 2010, 96. [Google Scholar]
- Workman, R.K.; Hart, S.R. Major and trace element composition of the depleted MORB mantle (DMM). Earth Planet. Sci. Lett. 2005, 231, 53–72. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M.; Mariano, A.N. The atomic arrangement of bastnäsite-(Ce), Ce(CO3)F, and structural elements of synchysite-(Ce), röntgenite-( Ce), and parisite-(Ce). Am. Mineral. 1993, 78, 415–418. [Google Scholar]
- Olson, J.C.; Shawe, D.R.; Pray, L.C.; Sharp, W.N.; Hewett, D.F. Rare-Earth Mineral Deposits of the Mountain Pass District San Bernardino County California. Geol. Surv. Prof. Pap. 1954, 261, 4–15. [Google Scholar]
- Williams-Jones, A.E.; Wood, S.A. A preliminary petrogenetic grid for REE fluorocarbonates and associated minerals. Geochim. Cosmochim. Acta 1992, 56, 725–738. [Google Scholar] [CrossRef]
- Hsu, L.C. Synthesis and Stability of Bastnaesites in a Part of the System (Ce,La)-F-H-C-O. Mineral. Petrol. 1992, 47, 87–101. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Wade, B.P.; Gilbert, S.; Kamenetsky, V.S. Rare earth element fluorocarbonate minerals from the olympic dam Cu-U-Au-Ag deposit, South Australia. Minerals 2017, 7, 202. [Google Scholar] [CrossRef]
- Oftedal, I. Zur Kristallstruktur von Bastnäsit, (Ce,La)FCO3. Zeitschrift fur Krist. Krist. Krist. Krist. 1931, 78, 462. [Google Scholar]
- Donnay, G.; Donnay, J.D.H. The Crystallography of Bastnaesite, Parisite, Roentgenite, and Synchisite. Am. Mineral. 1953, 38, 932–963. [Google Scholar]
- Castor, S.B. Rare Earth Deposits of North America. Resour. Geol. 2008, 58, 337–347. [Google Scholar] [CrossRef]
- Castor, S.B. The Mountain Pass Rare-Earth Carbonatite and Associated Ultrapotassic Rocks, California. Can. Mineral. 2008, 46, 779–806. [Google Scholar] [CrossRef]
- Shivaramaiah, R.; Anderko, A.; Riman, R.E.; Navrotsky, A. Thermodynamics of bastnaesite: A major rare earth ore mineral. Am. Mineral. 2016, 101, 1129–1134. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E. The thermodynamic properties of bastnäsite-(Ce) and parisite-(Ce). Chem. Geol. 2015, 392, 87–101. [Google Scholar] [CrossRef]
- Janka, O.; Schleid, T. Facile Synthesis of Bastnaesite-Type LaF[CO3] and Its Thermal Decomposition to LaOF for Bulk and Eu3+-Doped Samples. Eur. J. Inorg. Chem. 2009, 2009, 357–362. [Google Scholar] [CrossRef]
- Woo, D.C.; Lee, M.-H.; Jung, W.-S. Synthesis and characterization of rhombohedral- and tetragonal-lanthanum oxyfluoride powders. Ceram. Int. 2013, 39, 1533–1538. [Google Scholar] [CrossRef]
- Jacob, K.T.; Saji, V.S.; Waseda, Y. Lanthanum Oxyfluoride: Structure, Stability, and Ionic Conductivity. Int. J. Appl. Ceram. Technol. 2006, 3, 312–321. [Google Scholar] [CrossRef]
- Fergus, J.W.; Chen, H.-P. Structure and Conductivity of Tetragonal and Rhombohedral Lanthanum Oxyfluoride Compounds. J. Electrochem. Soc. 2000, 147, 4696–4704. [Google Scholar] [CrossRef]
- Shinn, D.B.; Eick, H.A. Phase Analyses of Lanthanide Oxide Fluorides. Inorg. Chem. 1969, 8, 232–235. [Google Scholar] [CrossRef]
- Zachariasen, W.H. Crystal Chemical Studies of the 5f-series of Elements. XIV. Oxyfluorides, XOF. Acta Crystallogr. 1951, 4, 231–236. [Google Scholar] [CrossRef]
- Pistorius, C.W.F.T. Effect of Pressure on the Rhombohedral/Cubic Transitions of Some Lanthanide Oxide Fluorides. J. Less-Common Met. 1973, 31, 119–124. [Google Scholar] [CrossRef]
- Klemm, V.W.; Klein, H.-A. Lanthanoxyfluorid [Lanthanum Oxyfluoride]. Zeitschrift fur Anorg. und Allg. Chemie 1941, 248, 167–171, in Germany. [Google Scholar]
- Mathews, M.D.; Tyagi, A.K.; Moorthy, P.N. Study of phase transition in REOF system by dilatometry (RE = La, Nd, Sm, Gd, Eu and Y). Thermochim. Acta 1997, 298, 165–167. [Google Scholar] [CrossRef]
- Achary, S.N.; Ambekar, B.R.; Mathews, M.D.; Tyagi, A.K.; Moorthy, P.N. Study of phase transition and volume thermal expansion in a rare-earth (RE) oxyfluoride system by high-temperature XRD (RE = La, Nd, Sm, Eu and Gd). Thermochim. Acta 1998, 320, 239–243. [Google Scholar] [CrossRef]
- Holtstam, D.; Grins, J.; Nysten, P. Håleniusite- (La) from the Bastnäs Deposit, Västmandland, Sweden: A new REE Oxyfluoride Mineral Species. Can. Mineral. 2004, 42, 1097–1103. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Tullis, T.E.; Tullis, J. Experimental Rock Deformation Techniques. Geophys. Monogr. 1986, 36, 297–324. [Google Scholar]
- Rowland, R.L., II. Phase Equilibria, Compressibility, and Thermal Analysis of Bastnaesite-(La); University of Nevada: Las Vegas, NV, USA, 2017. [Google Scholar]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW_COD. J. Appl. Crystallogr. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Redfern, S.A.T. Unit cell refinement from powder diffraction data: the use of regression diagnostics. Mineral. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]
- Angel, R.J.; Gonzalez-Platas, J.; Alvaro, M. EosFit7c and a Fortran module (library) for equation of state calculations. Zeitschrift fur Krist. 2014, 229, 405–419. [Google Scholar] [CrossRef]
- Kantor, I.; Prakapenka, V.; Kantor, A.; Dera, P.; Kurnosov, A.; Sinogeikin, S.; Dubrovinskaia, N.; Dubrovinsky, L. BX90: A new diamond anvil cell design for X-ray diffraction and optical measurements. Rev. Sci. Instrum. 2012, 83. [Google Scholar] [CrossRef] [PubMed]
- Hrubiak, R.; Sinogeikin, S.; Rod, E.; Shen, G. The laser micro-machining system for diamond anvil cell experiments and general precision machining applications at the High Pressure Collaborative Access Team. Rev. Sci. Instrum. 2015, 86. [Google Scholar] [CrossRef] [PubMed]
- Rivers, M.; Prakapenka, V.B.; Kubo, A.; Pullins, C.; Holl, C.M.; Jacobsen, S.D. The COMPRES/GSECARS gas-loading system for diamond anvil cells at the Advanced Photon Source. High Press. Res. 2008, 28, 273–292. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the Ruby Pressure Gauge to 800 kbar Under Quasi-Hydrostatic Conditions. J. Geophys. Res. 1986, 91, 4673. [Google Scholar] [CrossRef]
- Hirose, K.; Sata, N.; Komabayashi, T.; Ohishi, Y. Simultaneous volume measurements of Au and MgO to 140 GPa and thermal equation of state of Au based on the MgO pressure scale. Phys. Earth Planet. Inter. 2008, 167, 149–154. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Xu, H.; Lin, Z.; Daemen, L.L.; Zhao, Y.; Wang, L. Thermal equation of state of copper studied by high P-T synchrotron X-ray diffraction. Appl. Phys. Lett. 2009, 94, 4. [Google Scholar] [CrossRef]
- Hammersley, A.P. FIT2D: A multi-purpose data reduction, analysis and visualization program. J. Appl. Crystallogr. 2016, 49, 646–652. [Google Scholar] [CrossRef]
- Dera, P.; Zhuravlev, K.; Prakapenka, V.; Rivers, M.L.; Finkelstein, G.J.; Grubor-Urosevic, O.; Tschauner, O.; Clark, S.M.; Downs, R.T. High pressure single-crystal micro X-ray diffraction analysis with GSE_ADA/RSV software. High Press. Res. 2013, 33, 466–484. [Google Scholar] [CrossRef]
- Fei, Y. Thermal Expansion. In Mineral Physics and Crystallography: A Handbook of Physical Constants; Ahrens, T.J., Ed.; American Geophysical Union: Washington, DC, USA, 1995; pp. 29–44. ISBN 0-87590-852-7. [Google Scholar]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Ye, Y.; Smyth, J.R.; Boni, P. Crystal structure and thermal expansion of aragonite-group carbonates by single-crystal X-ray diffraction. Am. Mineral. 2012, 97, 707–712. [Google Scholar] [CrossRef]
- Markgraf, S.A.; Reeder, R.J. High-temperature structure refinements of calcite and magnesite. Am. Mineral. 1985, 70, 590–600. [Google Scholar]
- Ross, N.L.; Reeder, R.J. High-pressure structural study of dolomite and ankerite. Am. Mineral. 1992, 77, 412–421. [Google Scholar]
- Ross, N.L. The equation of state and high-pressure behavior of magnesite. Am. Mineral. 1997, 82, 682–688. [Google Scholar] [CrossRef]
- Zhang, J.; Reeder, R.J. Comparative compressibilities of calcite-structure carbonates: Deviations from empirical relations. Am. Mineral. 1999, 84, 861–870. [Google Scholar] [CrossRef]
- Vennari, C.E.; Williams, Q. High-pressure Raman and Nd3+ luminescence spectroscopy of bastnäsite-(REE)CO3F. Am. Mineral. 2019, 104, 1389–1401. [Google Scholar] [CrossRef]
- Anderson, O.L.; Nafe, J.E. The Bulk Modulus-Volume Relationship for Oxide Compounds and Related Geophysical Problems. J. Geophys. Res. 1965, 70, 3951–3963. [Google Scholar] [CrossRef]
- Knittle, E. Static Compression Measurements of Equation of State. In Mineral physics and Crystallography: A Handbook of Physical Constants; Ahrens, T.A., Ed.; American Geophysical Union: Washington, DC, USA, 1995; pp. 98–142. [Google Scholar]
- Merlini, M.; Perchiazzi, N.; Hanfland, M.; Bossak, A. Phase transition at high pressure in Cu2CO3(OH)2 related to the reduction of the Jahn–Teller effect. Acta Crystallogr. Sect. B Struct. Sci. 2012, B68, 266–274. [Google Scholar] [CrossRef]
- Xu, J.; Kuang, Y.; Zhang, B.; Liu, Y.; Fan, D.; Zhou, W.; Xie, H. High-pressure study of azurite Cu3(CO3)2(OH)2 by synchrotron radiation X-ray diffraction and Raman spectroscopy. Phys. Chem. Miner. 2015, 42, 805–816. [Google Scholar] [CrossRef]


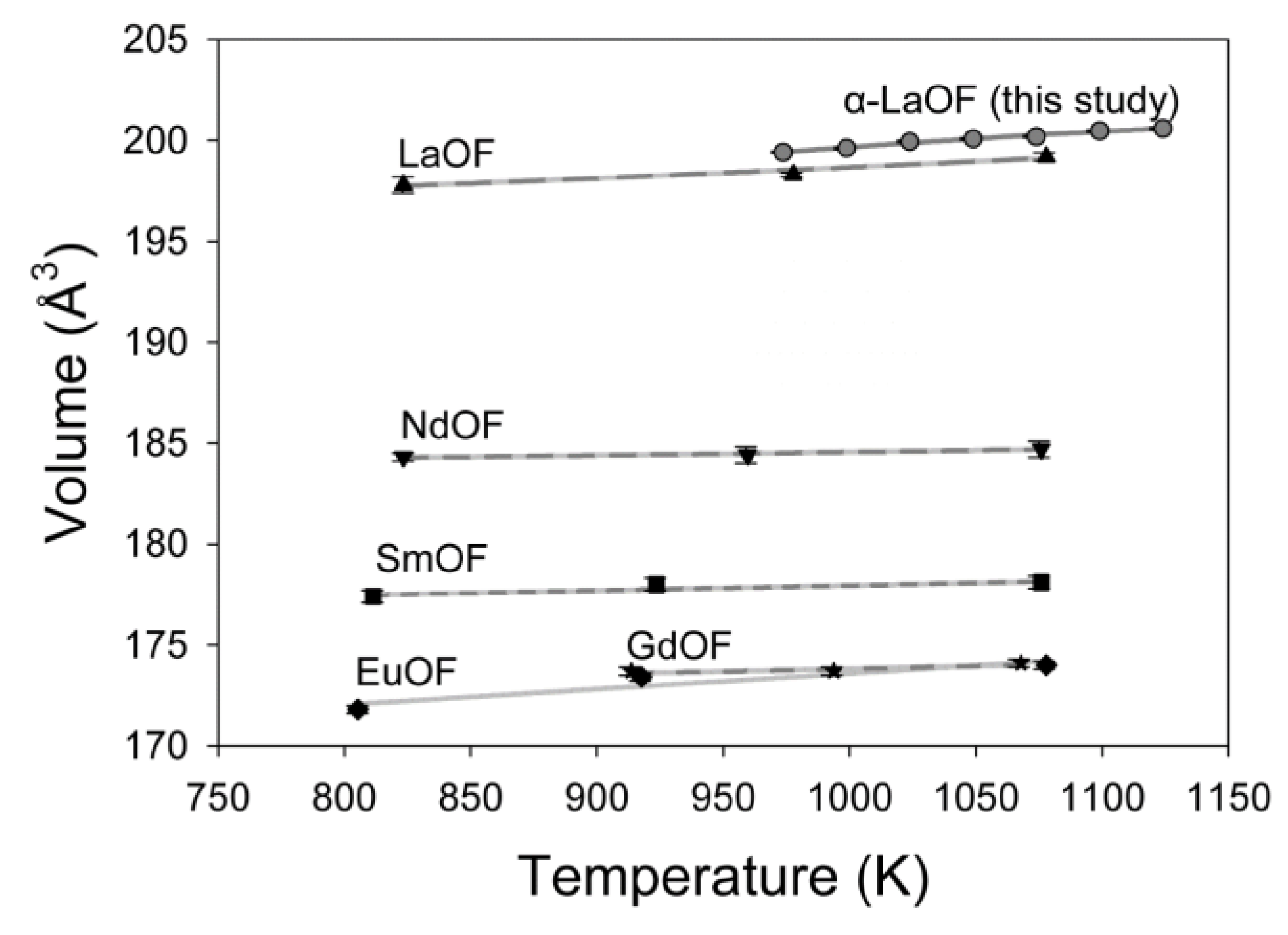
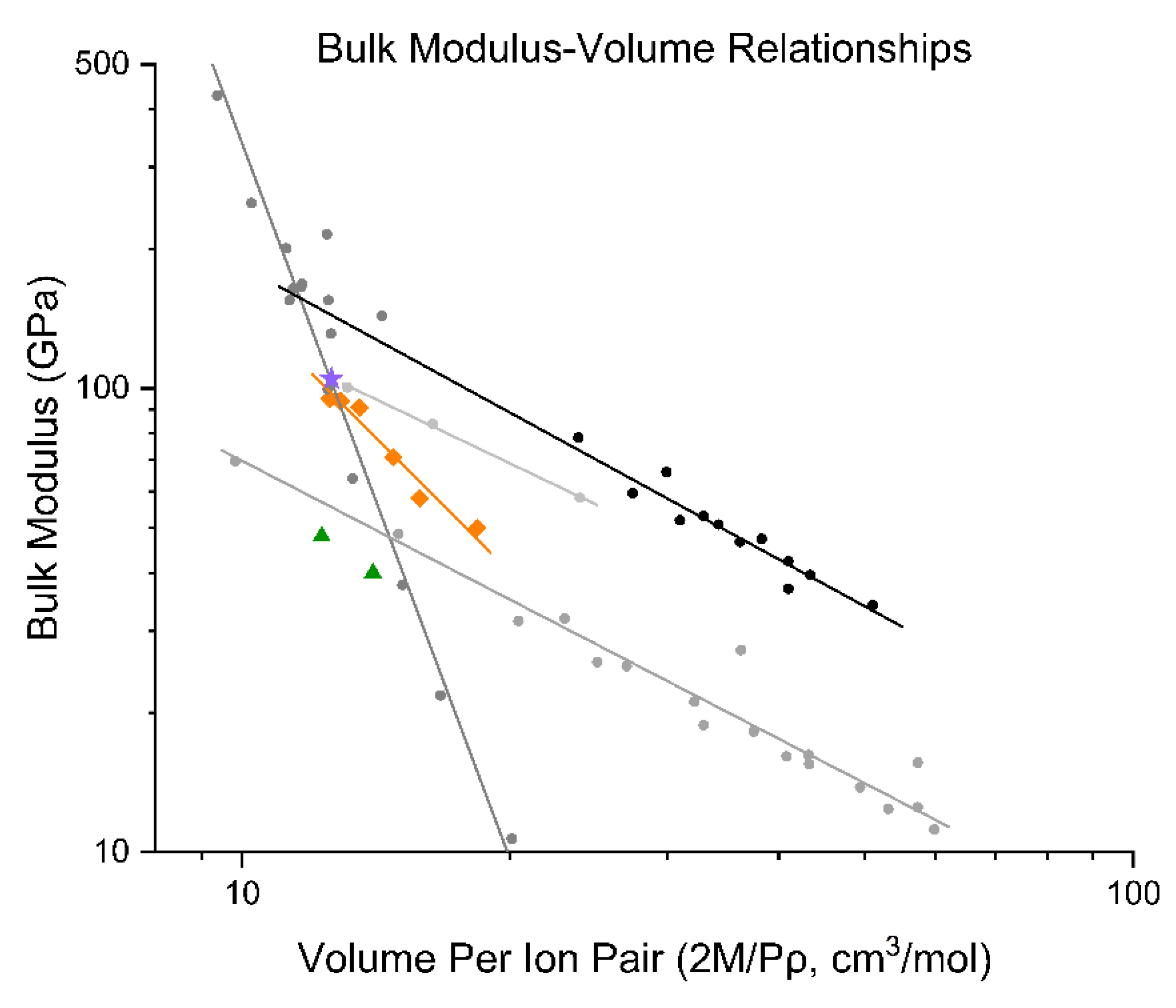
| Temperature (K) | a (Å) | Uncertainty | c (Å) | Uncertainty | V (Å3) | Uncertainty | a/c ratio |
|---|---|---|---|---|---|---|---|
| 298 | 7.1867 | 0.00085 | 9.8328 | 0.00083 | 439.82 | 0.185 | 0.7309 |
| 323 | 7.1897 | 0.00085 | 9.8347 | 0.00083 | 440.26 | 0.185 | 0.7311 |
| 373 | 7.1954 | 0.00085 | 9.8394 | 0.00083 | 441.18 | 0.185 | 0.7313 |
| 423 | 7.1992 | 0.00085 | 9.8438 | 0.00083 | 441.84 | 0.185 | 0.7313 |
| 473 | 7.2044 | 0.00085 | 9.8486 | 0.00083 | 442.69 | 0.186 | 0.7315 |
| 498 | 7.2074 | 0.00085 | 9.8504 | 0.00083 | 443.14 | 0.186 | 0.7317 |
| 523 | 7.2096 | 0.00085 | 9.8539 | 0.00083 | 443.57 | 0.186 | 0.7316 |
| 548 | 7.2125 | 0.00085 | 9.8564 | 0.00083 | 444.04 | 0.186 | 0.7318 |
| 573 | 7.2159 | 0.00085 | 9.8572 | 0.00083 | 444.50 | 0.186 | 0.7320 |
| 598 | 7.2172 | 0.00085 | 9.8598 | 0.00083 | 444.76 | 0.186 | 0.7320 |
| 623 | 7.2205 | 0.00086 | 9.8626 | 0.00083 | 445.30 | 0.186 | 0.7321 |
| 648 | 7.2238 | 0.00086 | 9.8669 | 0.00083 | 445.91 | 0.187 | 0.7321 |
| 673 | 7.2266 | 0.00086 | 9.8669 | 0.00083 | 446.25 | 0.187 | 0.7324 |
| 698 | 7.2296 | 0.00086 | 9.8730 | 0.00083 | 446.90 | 0.187 | 0.7323 |
| 723 | 7.2339 | 0.00086 | 9.8731 | 0.00083 | 447.43 | 0.187 | 0.7327 |
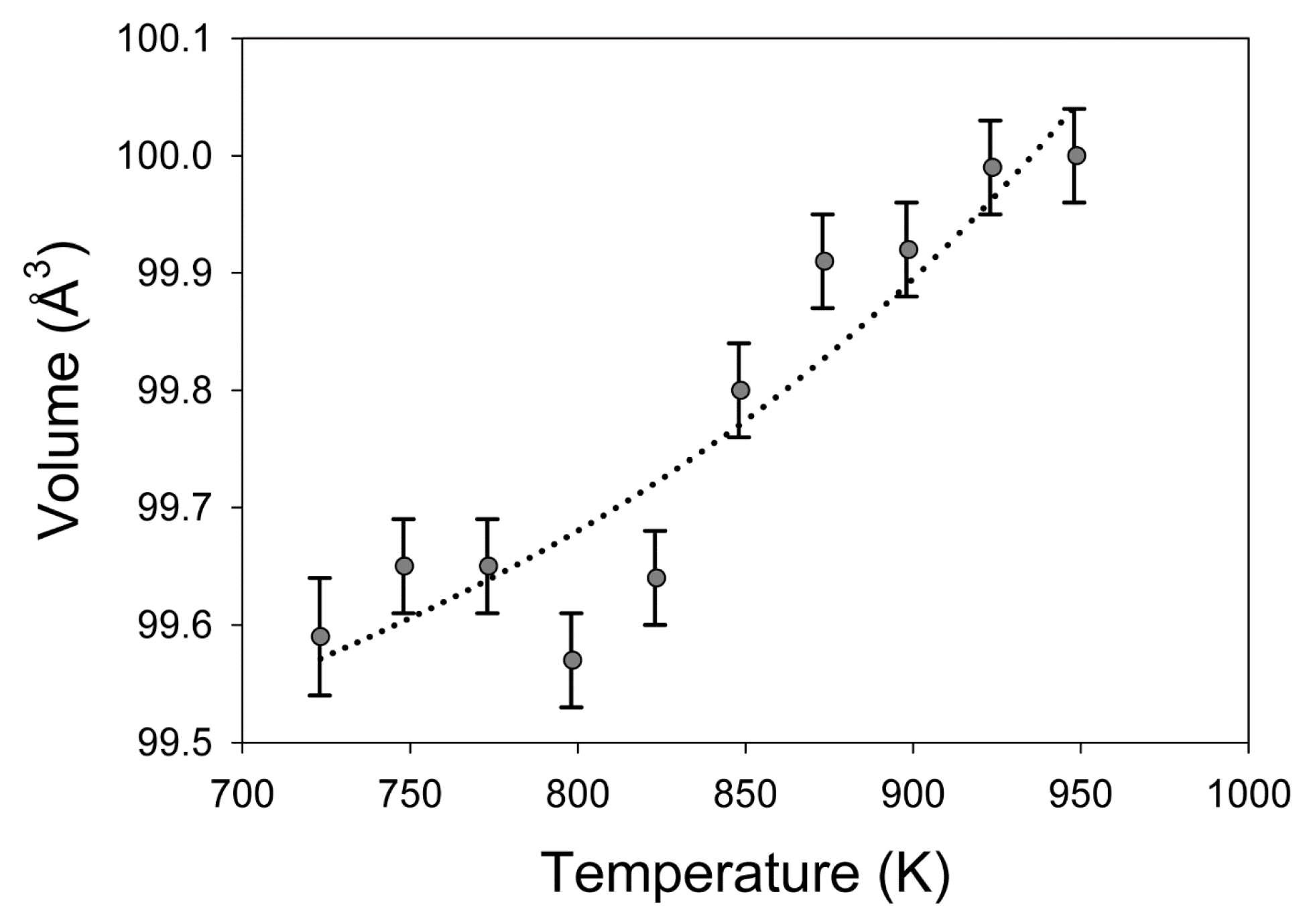
| Temperature (K) | a (Å) | Uncertainty | c (Å) | Uncertainty | V (Å3) | Uncertainty |
|---|---|---|---|---|---|---|
| 298 | 4.0798 | 0.00041 | 5.798 | 0.0020 | 96.51 | 0.118 |
| 723 | 4.1242 | 0.00045 | 5.855 | 0.0030 | 99.59 | 0.130 |
| 748 | 4.1192 | 0.00041 | 5.873 | 0.0020 | 99.65 | 0.119 |
| 773 | 4.1192 | 0.00041 | 5.873 | 0.0020 | 99.65 | 0.119 |
| 798 | 4.1213 | 0.00042 | 5.862 | 0.0020 | 99.57 | 0.119 |
| 823 | 4.1230 | 0.00042 | 5.862 | 0.0020 | 99.64 | 0.119 |
| 848 | 4.1246 | 0.00042 | 5.867 | 0.0020 | 99.80 | 0.119 |
| 873 | 4.1276 | 0.00042 | 5.864 | 0.0020 | 99.91 | 0.119 |
| 898 | 4.1293 | 0.00042 | 5.860 | 0.0020 | 99.92 | 0.119 |
| 923 | 4.1286 | 0.00050 | 5.866 | 0.0022 | 99.99 | 0.122 |
| 948 | 4.1311 | 0.00042 | 5.859 | 0.0020 | 100.00 | 0.119 |
| Temperature (K) | a (Å) | Uncertainty | V (Å3) | Uncertainty |
|---|---|---|---|---|
| 298 | 5.756 * | 0.003 * | 190.71 * | |
| 973 | 5.8424 | 0.00049 | 199.42 | 0.138 |
| 998 | 5.8443 | 0.00049 | 199.62 | 0.138 |
| 1023 | 5.8476 | 0.00050 | 199.95 | 0.138 |
| 1048 | 5.8489 | 0.00050 | 200.08 | 0.138 |
| 1073 | 5.8500 | 0.00050 | 200.20 | 0.138 |
| 1098 | 5.8526 | 0.00050 | 200.47 | 0.138 |
| 1123 | 5.8538 | 0.00050 | 200.60 | 0.138 |
| 1148 | 5.8531 | 0.00050 | 200.52 | 0.138 |
| 1173 | 5.8511 | 0.00050 | 200.32 | 0.138 |
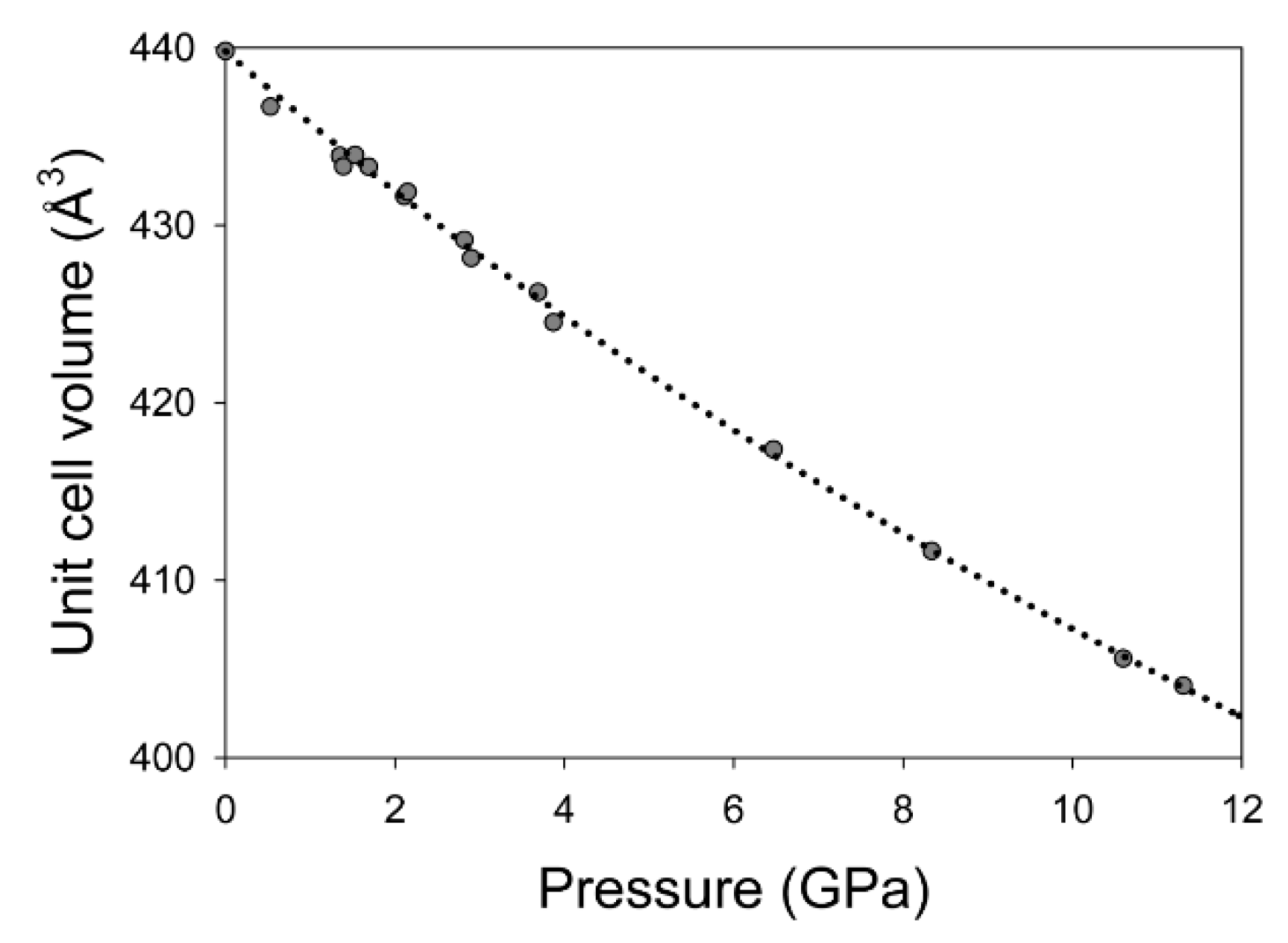
| Pressure (GPa) | a (Å) | Uncertainty | c (Å) | Uncertainty | V (Å3) | Uncertainty | a/c Ratio | Pressure Media | Pressure Standard |
|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 7.187 | 0.002 | 9.833 | 0.040 | 439.82 | 0.044 | 0.7309 | n/a | n/a |
| 0.53 | 7.169 | 0.003 | 9.811 | 0.003 | 436.68 | 0.009 | 0.7307 | Ne | Au |
| 1.35 | 7.148 | 0.003 | 9.806 | 0.003 | 433.91 | 0.008 | 0.7289 | Ne | Au |
| 1.39 | 7.159 | 0.001 | 9.763 | 0.003 | 433.32 | 0.006 | 0.7333 | He | Cu |
| 1.53 | 7.158 | 0.001 | 9.780 | 0.003 | 433.96 | 0.006 | 0.7319 | He | Cu |
| 1.69 | 7.150 | 0.002 | 9.787 | 0.004 | 433.30 | 0.008 | 0.7306 | He | Cu |
| 2.11 | 7.144 | 0.004 | 9.766 | 0.006 | 431.64 | 0.013 | 0.7315 | Ne | Au |
| 2.15 | 7.145 | 0.001 | 9.769 | 0.003 | 431.88 | 0.005 | 0.7314 | He | Cu |
| 2.82 | 7.129 | 0.001 | 9.751 | 0.003 | 429.18 | 0.005 | 0.7311 | He | Cu |
| 2.90 | 7.117 | 0.002 | 9.761 | 0.002 | 428.15 | 0.006 | 0.7291 | Ne | Au |
| 3.69 | 7.111 | 0.001 | 9.734 | 0.002 | 426.23 | 0.004 | 0.7305 | He | Cu |
| 3.87 | 7.095 | 0.003 | 9.738 | 0.003 | 424.53 | 0.009 | 0.7286 | Ne | Au |
| 6.47 | 7.051 | 0.003 | 9.694 | 0.003 | 417.38 | 0.009 | 0.7274 | Ne | Au |
| 8.34 | 7.014 | 0.003 | 9.663 | 0.003 | 411.65 | 0.009 | 0.7259 | Ne | Au |
| 10.60 | 6.973 | 0.003 | 9.632 | 0.003 | 405.59 | 0.009 | 0.7239 | Ne | Au |
| 11.31 | 6.974 | 0.004 | 9.593 | 0.003 | 404.06 | 0.011 | 0.7270 | Ne | Au |
| Pressure (GPa) | Temp (K) | a (Å) | Uncertainty | c (Å) | Uncertainty | V (Å3) | Uncertainty | a/c Ratio |
|---|---|---|---|---|---|---|---|---|
| 4.9 | 347 | 7.086 | 0.001 | 9.730 | 0.002 | 423.11 | 0.005 | 0.7283 |
| 5.1 | 324 | 7.084 | 0.002 | 9.721 | 0.002 | 422.47 | 0.006 | 0.7287 |
| 5.8 | 373 | 7.076 | 0.002 | 9.696 | 0.002 | 420.44 | 0.006 | 0.7298 |
| 6.5 | 423 | 7.070 | 0.002 | 9.699 | 0.003 | 419.85 | 0.007 | 0.7289 |
| 6.7 | 473 | 7.060 | 0.002 | 9.691 | 0.002 | 418.32 | 0.006 | 0.7285 |
| 6.7 | 523 | 7.063 | 0.002 | 9.697 | 0.003 | 418.93 | 0.007 | 0.7284 |
| 7.0 | 573 | 7.057 | 0.002 | 9.713 | 0.003 | 418.91 | 0.007 | 0.7266 |
| 7.3 | 623 | 7.065 | 0.001 | 9.694 | 0.002 | 419.01 | 0.005 | 0.7288 |
| 7.7 | 673 | 7.064 | 0.002 | 9.685 | 0.002 | 418.53 | 0.006 | 0.7294 |
| Material | Dimension | Temp. Range (K) | α298 × 10−5 | a0 × 10−5 | a1 × 10−7 | a2 |
|---|---|---|---|---|---|---|
| bastnäsite-(La) | a(a0 = 7.1867 Å) | 298–723 | 1.73 | −1.08 | 0.373 | 1.502 |
| c(c0 = 9.8328 Å) | 298–723 | 0.951 | 0.247 | 0.116 | 0.319 | |
| V(V0 = 439.82 Å3) | 298–723 | 4.32 | −1.68 | 0.834 | 3.126 | |
| γ-LaOF | a(a0 = 4.0798 Å) | 723–948 | 13.5 | −17.9 | 1.90 | 22.879 |
| c(c0 = 5.798 Å) | 723–948 | 0.179 | 0.1785 | −0.229 | 8.154 | |
| V(V0 = 96.51 Å3) | 723–948 | 29.5 | −24.1 | 2.42 | 41.147 | |
| α-LaOF | a(a0 = 5.756 Å) | 973–1123 | −0.844 | 8.46 | −0.625 | −6.606 |
| V(V0 = 190.71 Å3) | 973–1123 | −1.12 | 23.6 | −1.73 | −17.362 |
| Material | Temp. Range (K) | Vo (Å3) | α298 × 10−5 | ao × 10−5 | a1 × 10−7 | a2 |
|---|---|---|---|---|---|---|
| α-LaOF † | 973–1123 | 190.71 | −1.12 | 23.6 | −1.73 | −17.362 |
| α-LaOF * | 823–1077 | 196.1 | 0.855 | 0.0017 | 0.286 | 0.0001 |
| NdOF * | 823–1075 | 183.8 | 0.268 | 0.0007 | 0.0895 | 0.0001 |
| SmOF * | 811–1075 | 176.2 | 1.4 | 1.4 | 0.00028 | 0 |
| EuOF * | 815–1077 | 168.2 | 4.49 | 4.49 | 0.00052 | 0.0001 |
| GdOF * | 913–1067 | 172.6 | 0.461 | 0.0044 | 0.1530 | 0 |
| Temp (K) | V (Å3) | Measured P (GPa) | Calculated P (GPa) | Difference | Error |
|---|---|---|---|---|---|
| 347 | 423.11 | 4.90 | 5.03 | 0.13 | 0.03 |
| 324 | 422.47 | 5.09 | 5.11 | 0.02 | 0.00 |
| 373 | 420.44 | 5.78 | 6.02 | 0.24 | 0.04 |
| 423 | 419.85 | 6.46 | 6.46 | 0.00 | 0.00 |
| 473 | 418.32 | 6.74 | 7.23 | 0.49 | 0.07 |
| 523 | 418.93 | 6.71 | 7.28 | 0.57 | 0.08 |
| 573 | 418.91 | 6.97 | 7.57 | 0.60 | 0.09 |
| 623 | 419.01 | 7.33 | 7.84 | 0.51 | 0.07 |
| 673 | 418.53 | 7.69 | 8.33 | 0.64 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowland, R.L., II; Lavina, B.; Vander Kaaden, K.E.; Danielson, L.R.; Burnley, P.C. Thermal Analysis, Compressibility, and Decomposition of Synthetic Bastnäsite-(La) to Lanthanum Oxyfluoride. Minerals 2020, 10, 212. https://doi.org/10.3390/min10030212
Rowland RL II, Lavina B, Vander Kaaden KE, Danielson LR, Burnley PC. Thermal Analysis, Compressibility, and Decomposition of Synthetic Bastnäsite-(La) to Lanthanum Oxyfluoride. Minerals. 2020; 10(3):212. https://doi.org/10.3390/min10030212
Chicago/Turabian StyleRowland, Richard L., II, Barbara Lavina, Kathleen E. Vander Kaaden, Lisa R. Danielson, and Pamela C. Burnley. 2020. "Thermal Analysis, Compressibility, and Decomposition of Synthetic Bastnäsite-(La) to Lanthanum Oxyfluoride" Minerals 10, no. 3: 212. https://doi.org/10.3390/min10030212
APA StyleRowland, R. L., II, Lavina, B., Vander Kaaden, K. E., Danielson, L. R., & Burnley, P. C. (2020). Thermal Analysis, Compressibility, and Decomposition of Synthetic Bastnäsite-(La) to Lanthanum Oxyfluoride. Minerals, 10(3), 212. https://doi.org/10.3390/min10030212






