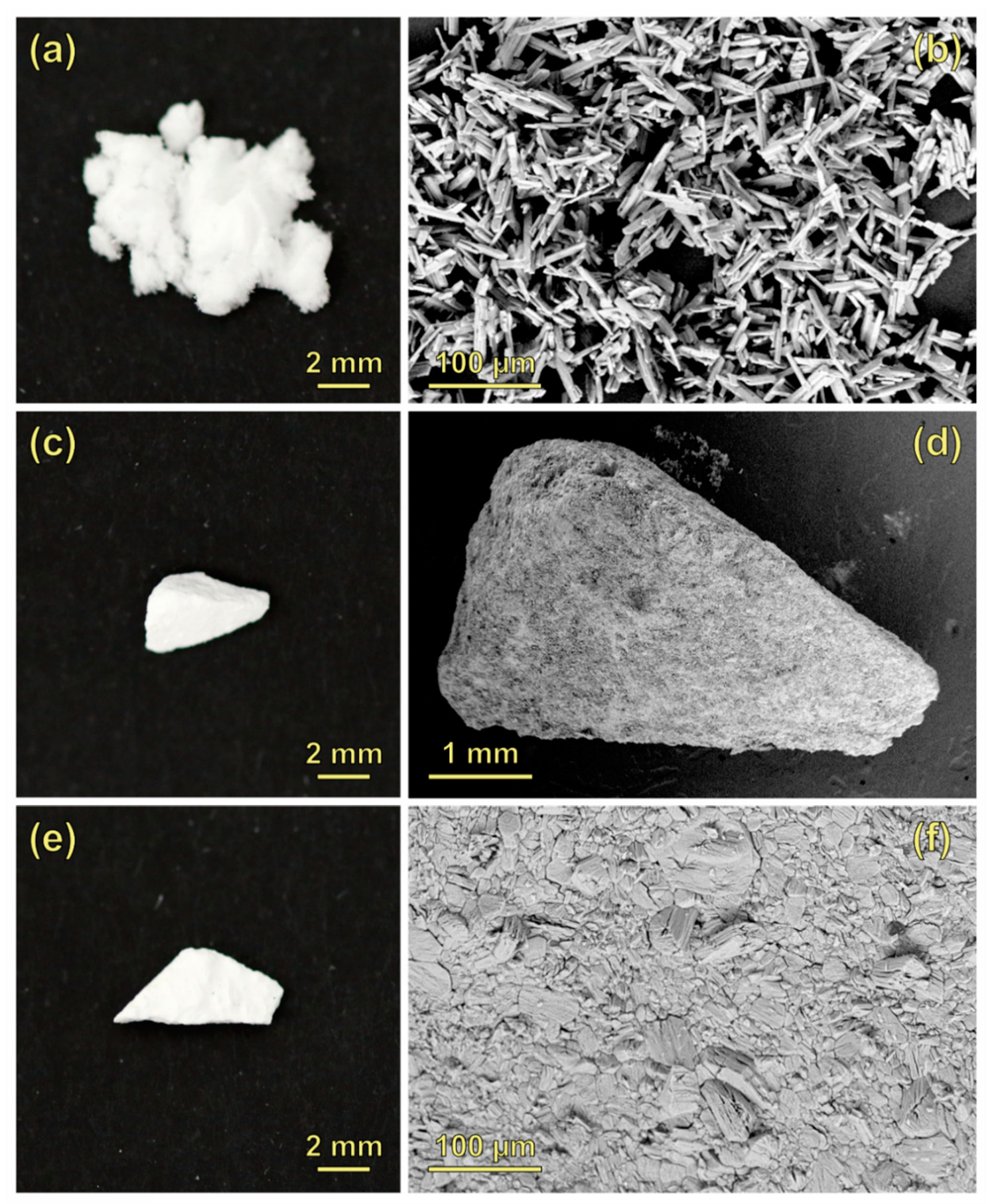
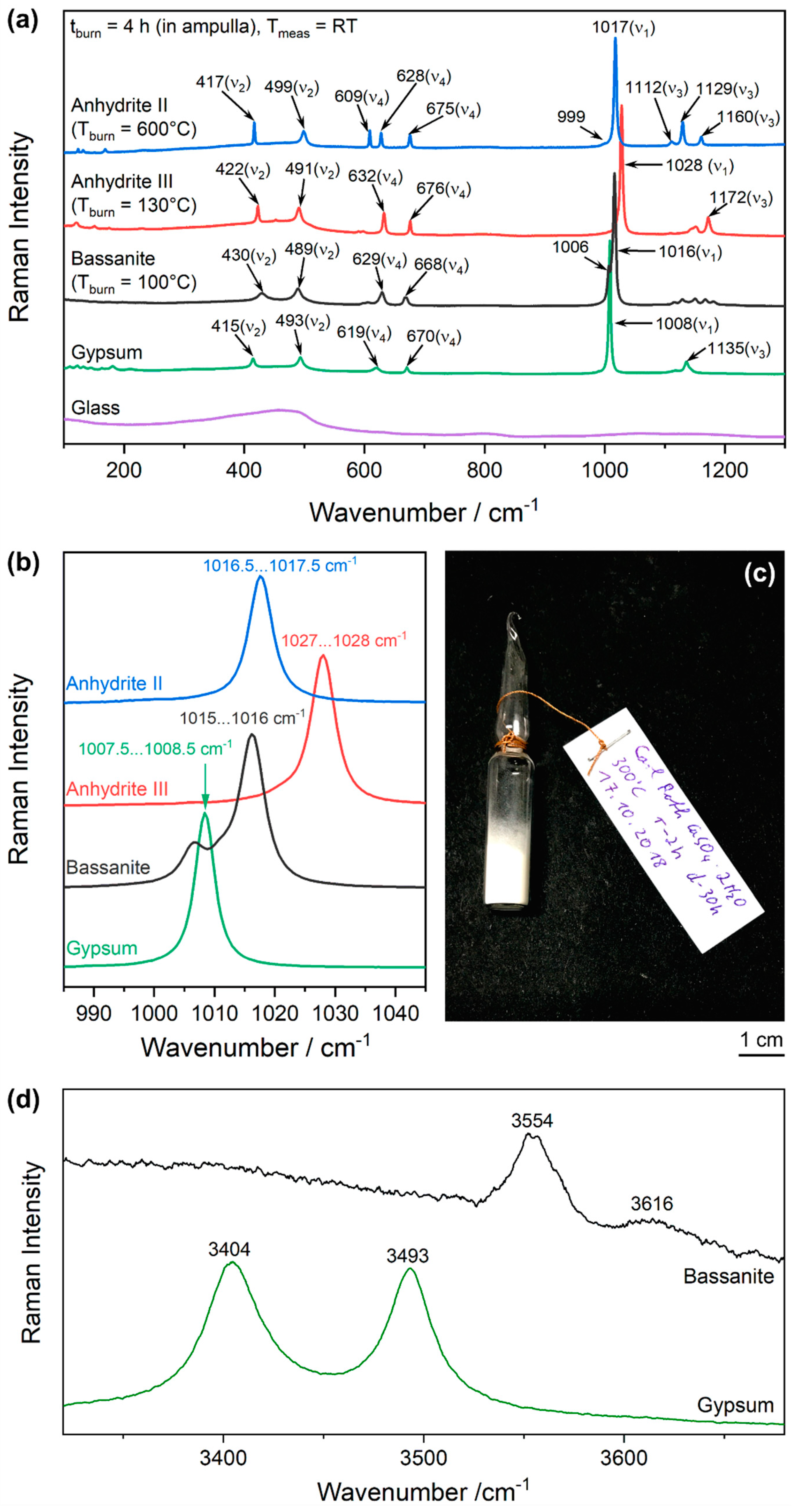
3.1. Raman Spectra of the CaSO4–H2O System
Since anhydrite I is described to be stable only at temperatures above 1180 °C, only the other four main phases of the CaSO
4–H
2O system are accessible for Raman spectroscopy at room temperature. The spectra of these phases are summarised in
Figure 2. They were synthesised from gypsum at the burning temperatures (T
burn) given there. At room temperature, the conversion of anhydrite III into bassanite under the influence of air humidity takes only minutes (see
Section 3.4 below), which makes the acquisition of Raman spectra of anhydrite III impossible. This issue was overcome by burning gypsum in open glass ampullae in a laboratory furnace and sealing them by melting the glass before cooling of the reaction products to room temperature (see
Figure 2c, and the ampulla method described in
Section 2.2). Since Raman measurements were conducted through the glass, we show a glass spectrum for comparison in
Figure 2a.
A peak list is given in
Table 1. This is not intended to be a complete collection of all (theoretically) possible Raman-active modes, but a guide including the most practically relevant and typically the strongest Raman bands. For example, the primitive unit cell of anhydrite II (CaSO
4) contains two formula units, i.e.,
N = 12 atoms. Thus, such crystal can conduct 3
N = 36 vibrations. Three vibrations are acoustic and 3(
N − 1) = 33 are optical modes of which 18 are Raman active [
25]. We typically observe a maximum of 15 modes in strong Raman spectra, and, depending on the actual sample material and measurement conditions, this number can easily shrink because of the challenging detection of the weak low-wavenumber modes. Detection of weak modes can be hampered by spectrally broad fluorescence emission, which, in some cases, is observed after calcination at certain temperatures [
21]. Since such emission is not necessarily observed in the gypsum used as a starting material and often vanishes after calcination at higher temperatures, it is most likely due to crystal lattice defects (colour centres or F centres, respectively) appearing and healing depending on calcination [
22,
23]. This effect is detailed below in
Section 3.7 (see Figure 20c). Furthermore, some (even the strong) modes can be suppressed by crystal-orientation effects [
25].
As shown in
Table 1, the Raman bands of the phases of the CaSO
4–H
2O system can be grouped into crystal-lattice phonons, modes assigned to the sulphate ion, and vibrations of crystal water. Crystal-lattice vibrations or phonons moving through the whole crystal, respectively, which are also termed external modes, are motions of Ca
2+ against SO
42− ions. They appear at <300 cm
−1 and are typically weak and easily overwhelmed by fluorescence emission from the sample, which was most challenging in measurements of all bassanite samples included in this study.
The sulphate ion in a solvated state consists of
N = 5 atoms arranged in tetrahedral (T
d) symmetry. Thus, it has 3
N = 15 degrees of freedom of which three are translations of the whole SO
42− ion and three are rotations. The remaining 3
N − 6 = 9 modes are vibrations. In T
d symmetry, they are distributed as follows: one (non-degenerated) symmetric stretch vibration ν
1, two degenerated symmetric bending vibrations ν
2, three degenerated antisymmetric stretch vibrations ν
3, and three degenerated anti-symmetric bending vibrations ν
4 (notation according to Reference [
26]). Due to degeneration, in aqueous solution, only four Raman bands of sulphate ions can be observed. When integrated into crystal lattices, the tetrahedra are distorted (e.g., to C
2h symmetry in gypsum [
27] and D
2h in anhydrite II [
25]), and the crystal field leads to changes of energy levels and (partial) breaking of degeneracies. A more simplistic explanation is that orientations in space of the same vibration cannot be distinguished in case of freely moveable ions in solution but can play a role for ions implemented into a crystal lattice. Therefore,
Table 1 shows the four frequencies of free sulphate ions in solution compared to the split-up modes observed in the crystalline compounds. The ν
n nomenclature is also applied to the latter, internal modes of gypsum, bassanite, anhydrite III, and anhydrite II.
In practice, the ν
1 mode, both ν
2 bands, and two or three ν
3 and ν
4 modes, respectively, are typically observed. Some modes can be missing either due to crystal orientation effects or merging of neighbouring bands. The symmetric stretch vibration ν
1 of the sulphate ion is the most prominent mode in all spectra and indicative of the phase within the CaSO
4–H
2O system, as shown in
Figure 2b, which also includes typical ranges of observed wavenumbers, considering variations within one cm
−1 from sample to sample due to variations of stress and strain.
The Raman spectra of the crystal-water-containing phases gypsum and bassanite are completed by the OH stretch modes of water of which the lower wavenumber is assigned to the symmetric and the higher wavenumber to the antisymmetric stretch vibration (
Figure 2d).
In the 1970s, Brian J. Berenblut et al. published comprehensive studies on the Raman spectra of gypsum and anhydrite II, including theoretical predictions and experimental proof (involving different crystal orientations) of all bands of these compounds [
25,
27]. Later research focused on excerpts of the spectra, mainly around the most prominent bands enabling phase identification [
28,
29,
30,
31]. Especially, complete spectra of bassanite and anhydrite III including low-wavenumber lattice phonons have been missing so far [
32,
33]. Therefore,
Table 1 gives the most complete list of wavenumbers of these phases, even though we do not lay any claim to completeness.
An interesting observation is the 1006-cm
−1 band overlapping with the most prominent mode of bassanite at 1016 cm
−1, which, at first look, seems to be a shifted ν
1 band of gypsum. In fact, Prasad [
29] observed a typical temperature-induced downshift of the gypsum ν
1 mode during heating, but also saw that, after conversion into bassanite and cooling to room temperature, a seeming gypsum band remained at 1006 cm
−1, i.e., shifted from the normal room-temperature wavenumber of 1008 cm
−1. Based on this observation, the band at 1006 cm
−1 was assigned to gypsum in a “disordered form” [
28] or a “different structural environment” [
29], which seems reasonable. We confirm this experimental observation of a steady downshift in
Section 3.3 below. Having the full spectrum of bassanite available, we come to a different hypothesis. If this mode would be due to gypsum, shifted bands would be expected in other parts of the spectrum as well. For example, additional sulphate bending and water stretching modes at the according (or slightly shifted) wavenumbers of gypsum would be expected to appear in the bassanite spectrum, which is not the case. Contrarily, we observe the same number of water and sulphate bending modes as in the other spectra of the CaSO
4–H
2O system, while a higher number of sulphate stretching bands appears. There is a doubling of the ν
1 and an almost doubling of the ν
3 modes, as we observe five bands in the range of the latter. In case of a different (e.g., a gypsum-like) type of sulphate ions in the structure, we would expect such doubling for all bands. Our hypothesis is that this is rather due to a splitting of modes because of coupled vibrations than due to sulphate ions in different structural environments.
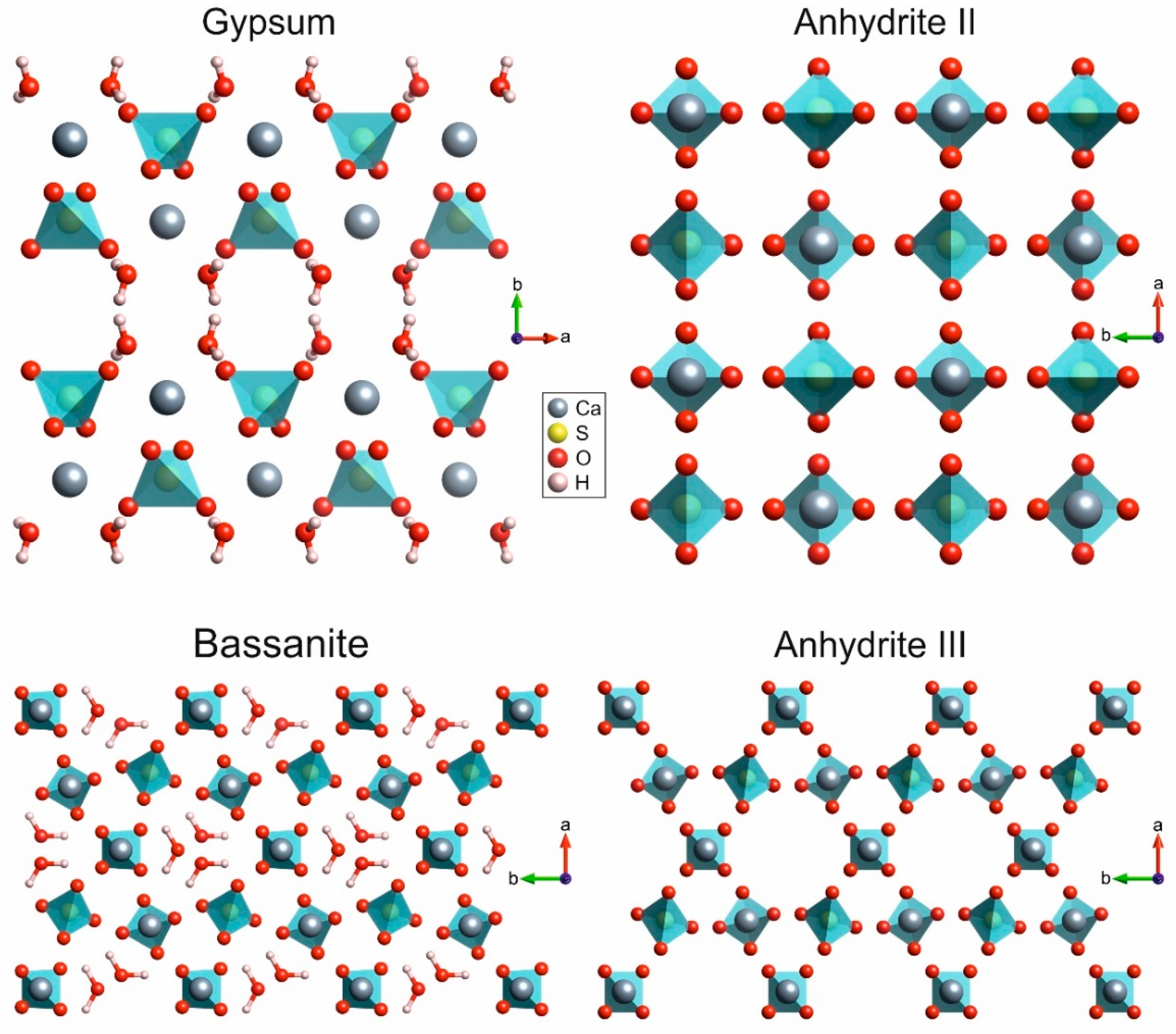
The CaSO
4–H
2O system includes the case of two phases with almost the same structure, mainly distinct by the absence (anhydrite III) or presence of crystal water (bassanite). Thus, the differences in their spectra is mostly due to the implementation of water molecules into channels in their structures (see bassanite and anhydrite III in
Figure 1). The general downshift of wavenumbers in the conversion of anhydrite III to bassanite can be explained by the strong influence of hydrogen bonding, which leads to weakening of bonds within the sulphate ions, and thus, to lower vibrational frequencies. The observed band splitting is most likely induced by the integration of water molecules into the structure because it is not observed in the spectrum of anhydrite III. According to
Figure 1, each water channel is surrounded by six Ca
2+–SO
42− chains. Stretch vibrations of the sulphate ions interfere with the water molecules, and differences in force constants can be hypothesised for the symmetric and antisymmetric vibrations of two opposite sulphate ions (each of both performing a symmetric ν
1 stretch motion), while much less influence on vibrational frequencies is expected in case of the bending vibrational motions. Further experimental and theoretical work is needed to fully understand this peak splitting, but, at this point, we can conclude that the 1006-cm
−1 band has to be treated as an intrinsic bassanite mode and not be assigned to gypsum or another type of sulphate. For that reason,
Table 1 lists 1016 cm
−1 as the most prominent band of bassanite with the shoulder at 1006 cm
−1 in parentheses. We show below, in
Section 3.3,
Section 3.4, and
Section 3.5, that the shoulder is present in all spectra of bassanite, independent of the route of its synthesis.
In strong anhydrite II spectra, a shoulder of the ν
1 (A
g) band at 999 cm
−1–1000 cm
−1 can be observed (compare Reference [
21]). This might be a weak contribution from the infrared-active A
u mode, which is similarly described for gypsum [
27]. The same assignment may be hypothesised for the weak bands present at 599 cm
−1/606 cm
−1 and 590 cm
−1/599 cm
−1 in spectra of bassanite and anhydrite III, respectively, whose origins remain unclear.
3.3. Gypsum–Bassanite–Anhydrite III Conversion Reactions
Dehydration of gypsum at elevated temperatures below 200 °C yields bassanite and anhydrite III. Since these phases are difficult to discriminate in XRD [
31,
36,
37,
38], some previous studies on the dehydration reaction of gypsum rely on Raman spectroscopy and make use of the clearly different Raman shifts of the ν
1 bands, as shown in
Figure 2b [
28,
29,
30,
31,
39,
40,
41]. Nevertheless, some of the studies yield contradictive results, which range from the formation of gypsum, bassanite, and anhydrite III at increasing burning temperatures [
31,
39] to the direct formation of anhydrite III out of gypsum, which is followed by conversion into bassanite upon cooling in ambient air with air humidity acting as a reactant [
29,
30,
34]. In the present, more detailed study involving both ex situ and in situ experiments, we investigated further factors such as the burning time and the comminution of the raw material and performed mechanistic investigations to better understand these conversion reactions and to find reasons for such seemingly contradictive observations.
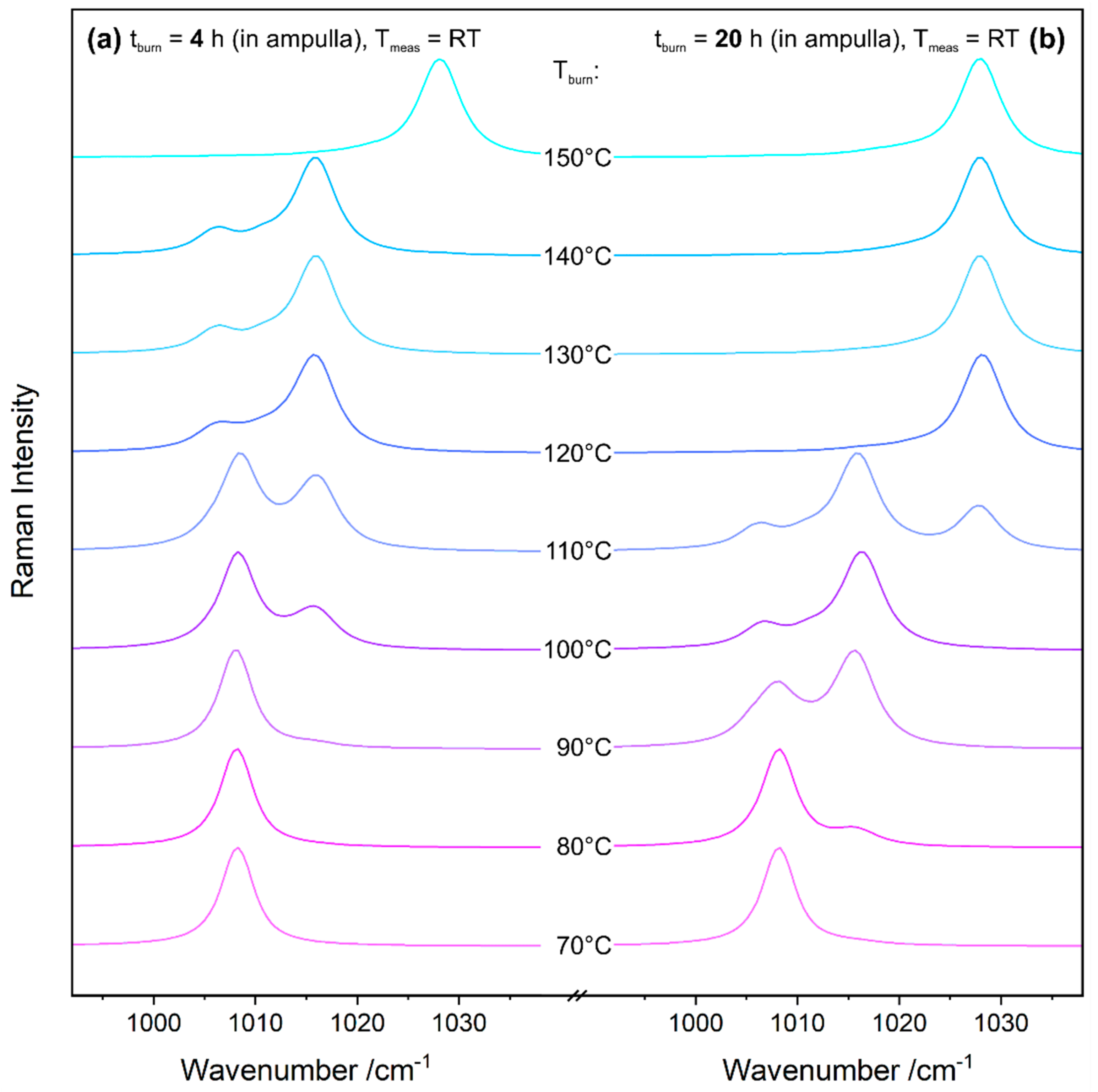
Figure 5 reveals the results of gypsum burning experiments in a laboratory furnace conducted with different holding times t
burn of either 4 h or 20 h, according to the ampulla method described in
Section 2.2. The spectral range shown in
Figure 5 contains the most prominent ν
1 modes of gypsum, bassanite, and anhydrite III at approximately 1008 cm
−1, 1016 cm
−1, and 1028 cm
−1, respectively. The data easily enables the monitoring of the conversion of gypsum into bassanite and anhydrite III. For both burning durations, these transitions occur in this order.
Of special interest in
Figure 5 are the differences of the results depending on holding time t
burn. While a clear bassanite signal is seen from 100 °C upwards (and a weak shoulder from 90 °C) at 4 h burning time, bassanite is already present at 80 °C if gypsum is calcinated for 20 h. The differences are even more distinct regarding the formation of anhydrite III, which starts (due to a seemingly immediate depletion of bassanite) at 150 °C with t
burn = 4 h, while already present in a mixture with bassanite at 110 °C with t
burn = 20 h. Calcination time has a significant influence on the product composition. Thus, in situ measurements for studying reaction kinetics in detail were performed.
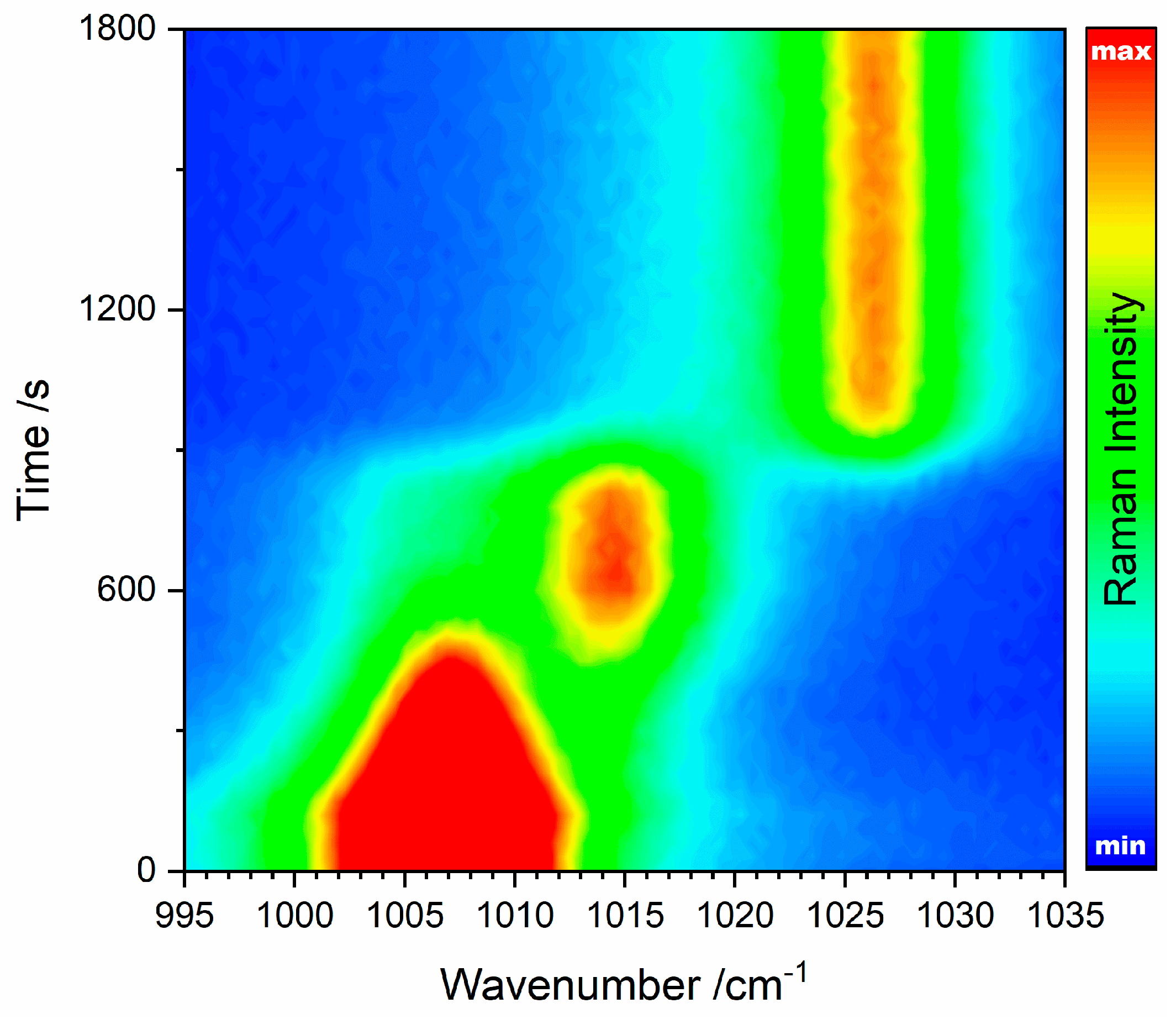
Figure 6 shows all in situ Raman spectra acquired during calcination of 10 mg gypsum powder (see
Section 2.3 for experimental details) with the wavenumber axis and time scale as two dimensions and the Raman intensity as third dimension represented by colours. The conversion of gypsum via bassanite into anhydrite III can be qualitatively monitored. Quantitative data can be extracted from the spectra by (multiple) peak fitting.
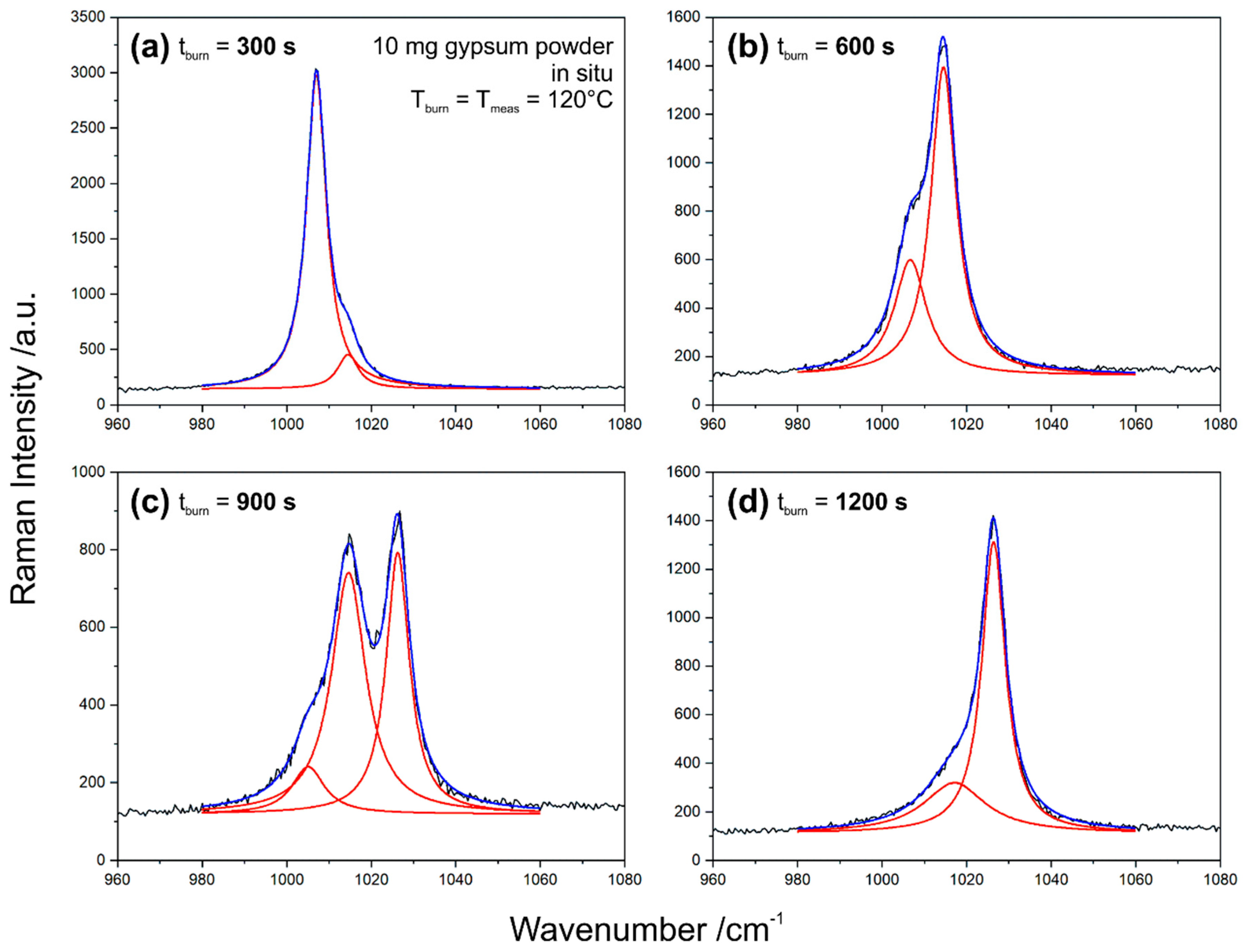
Figure 7 exemplifies the data analysis based on selected spectra from this set. Depending on burning time, the range including the ν
1 modes can be described by one, or a linear combination of two or three Lorentzian profiles.
From the fit functions, properties such as the peak areas and positions can be derived. In this case, the focus is on areas with the aim of turning the qualitative data in
Figure 6 into semi-quantitative information by enabling insights into reaction kinetics. In order to yield semi-quantitative results, the individual band areas assigned to the three phases were converted into relative Raman intensities, expressed as a percentage, according to the equation below.
where I
rel(
i) is the relative Raman intensity of phase
i, A(
i) is the peak area assigned to phase
i, and Σ
i(A(
i)) is the sum of all peak areas. We would like to point out that this is not an exact quantification in mole percent, as we do not consider the (presumably different) Raman activities of the involved phases. Independent from the amount of a substance, Raman intensities can also change due to the crystal orientation [
42] and other effects. In fact, Prasad [
29] describes a continuous decrease of the gypsum ν
1 band intensity due to structural disorder preceding dehydration. We also observed a weakening of the gypsum peak during the first phase of such experiments with only the gypsum ν
1 present in the spectra. Because of the reproducibility of the effect, it can surely not only be explained by the rearrangement of the powder inside the measurement cell (thus, partly moving out of the laser focus) because of air convection. The semi-quantitative data given in
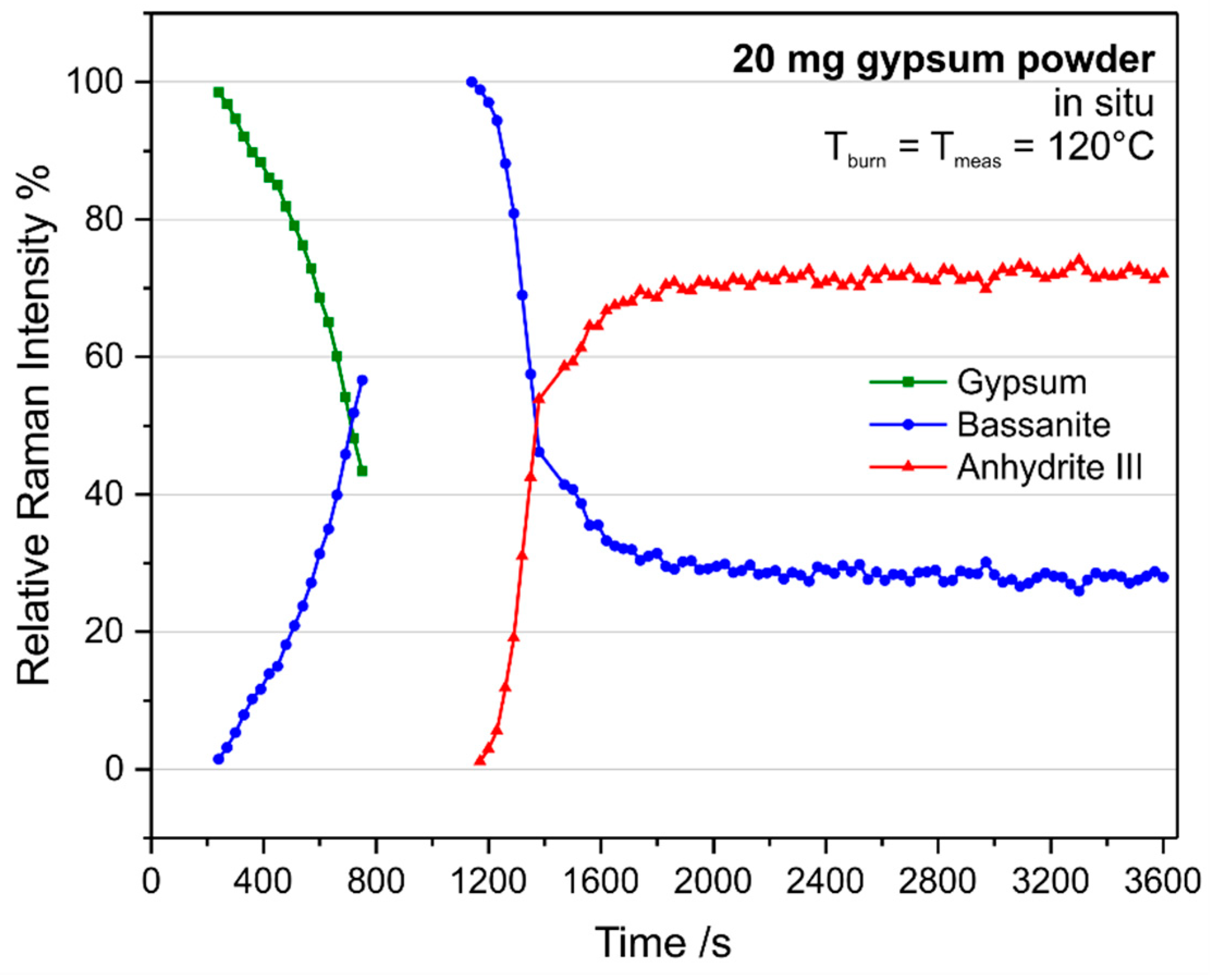
Figure 8a starts when two ν
1 modes, assigned to gypsum and basanite, can be detected simultaneously and, thus, Equation (1) yields percentages other than the trivial values of 0 or 100%.
During calcination of 10 mg gypsum, 240 s after reaching the T
burn of 120 °C, the bassanite peak becomes strong enough for the described spectra analysis (compare
Figure 7a). The amount of gypsum decreases while bassanite is formed, which both reach approximately equal Raman intensities after 510 s. Anhydrite III formation starts at 780 s, and the bassanite concentration decreases. Both peaks reach equal intensities between 900 s and 930 s, which is followed by an asymptotic approach to the chemical equilibrium at these conditions. Note that bassanite is not fully consumed during this reaction. Instead, it reaches its equilibrium concentration at the given temperature. For a rough comparison of reaction rates, we have a look at the (linear) slopes of curves at the intersection points of equal relative Raman intensities. The approximated rate of bassanite formation is 0.22% s
−1, while anhydrite III is formed within 0.41% s
−1. These numbers are consistent with a look at the graphs: The conversion of gypsum into bassanite is slower than the reaction of bassanite into anhydrite III. An explanation might be that only little structural rearrangement is needed in the second reaction, while the water molecules simply leave bassanite through channels (see
Figure 1).
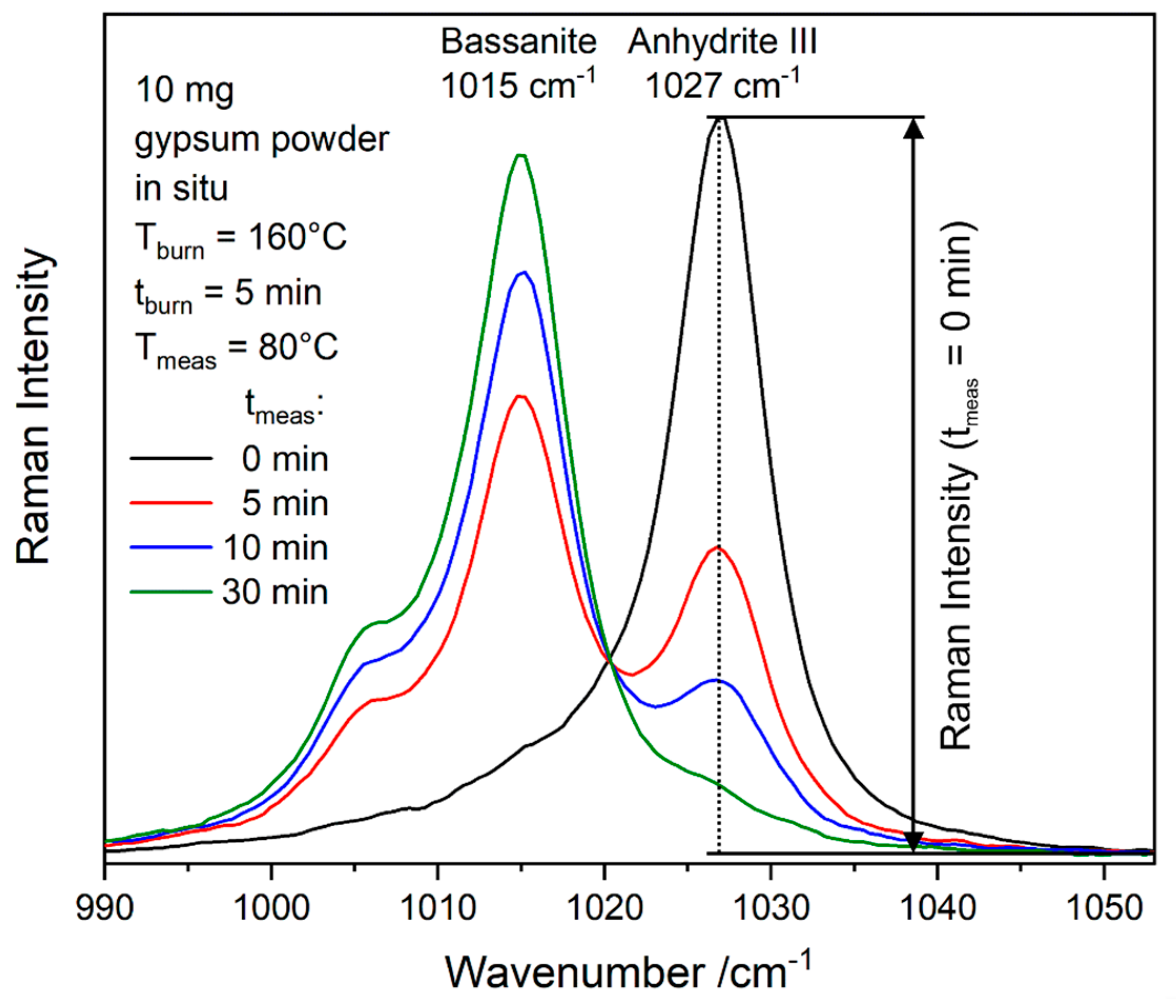
As shown in
Figure 2, the most prominent bassanite band has a shoulder toward lower wavenumbers. Note that, due to the elevated measurement temperature of 120 °C, all bands appear downshifted with respect to the room temperature data shown in
Figure 2. Thus, the shoulder appears at approximately 1005 cm
−1 instead of 1006 cm
−1. The downshift of the gypsum ν
1 mode during dehydration as a function of temperature and its downshifted position at 1006 cm
−1 even after cooling the resulting bassanite to room temperature were previously observed [
29]. We see a gradual downshift also at a constant temperature of T
burn = 120 °C during gypsum–bassanite conversion (see blue-green dotted line in
Figure 8b), which ends in the position of the bassanite shoulder at 1005 cm
−1. According to the interpretation given in
Section 3.1, this is the result of two parallel effects: The decrease of gypsum band intensity and simultaneous increase of the strongly overlapping bassanite shoulder leads to a seemingly shifting signal. The relative Raman intensity in the range of the gypsum ν
1 mode (1008 cm
−1 at room temperature) and the bassanite shoulder (1006 cm
−1 at room temperature) is disregarded in the data analysis from the point when the bassanite ν
1 becomes stronger than the gypsum ν
1 mode. From this point, bassanite and gypsum cannot be unambiguously distinguished due to strongly overlapping bands, and quantifications start again at the beginning of anhydrite III formation. It is unclear whether the upshift of the bassanite peak at low concentrations in the presence of anhydrite III is a real effect (which, due to the instability of anhydrite III, is difficult to study at room temperature) or at least partly related to the mathematical deconvolution of this weak and strongly overlapping band from the anhydrite III band (see
Figure 7d).
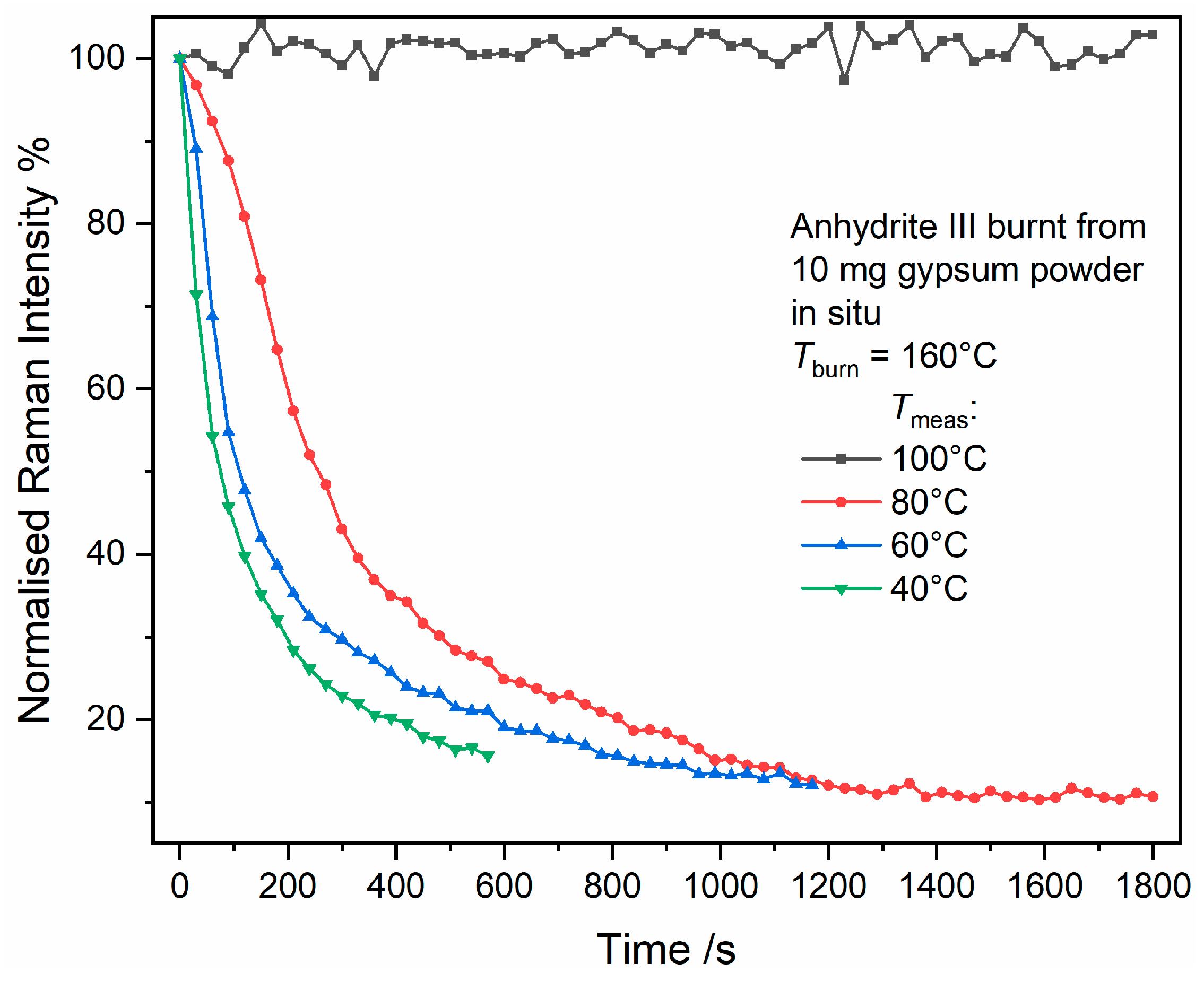
If the experiment is repeated with 20 mg instead of 10 mg gypsum powder as starting material, the measurements result in a different picture (see
Figure 9). Note that the time scale in
Figure 9 covers 1 h instead of 0.5 h in
Figure 8. Similar to the first experiment, bassanite can be detected from 240 s after reaching the setpoint temperature of 120 °C, but it takes until 720 s to achieve the same gypsum and bassanite band intensities, which is a difference of 210 s compared to the 10-mg experiment. In the following transition of bassanite to anhydrite III, equal band intensities are reached at approximately 1380 s (780 s during 10-mg experiment). All times approximately double if twice the amount of starting material is used.
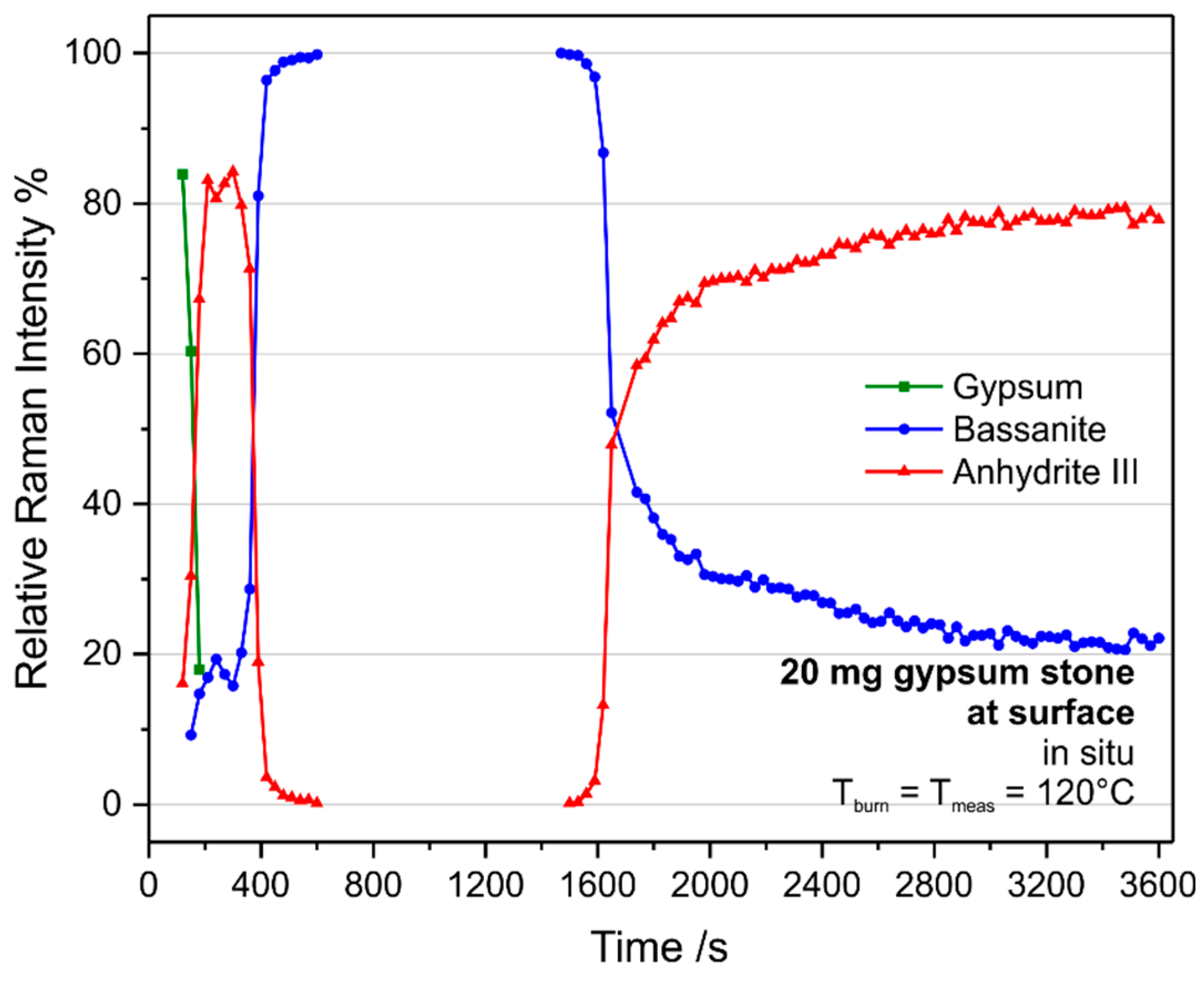
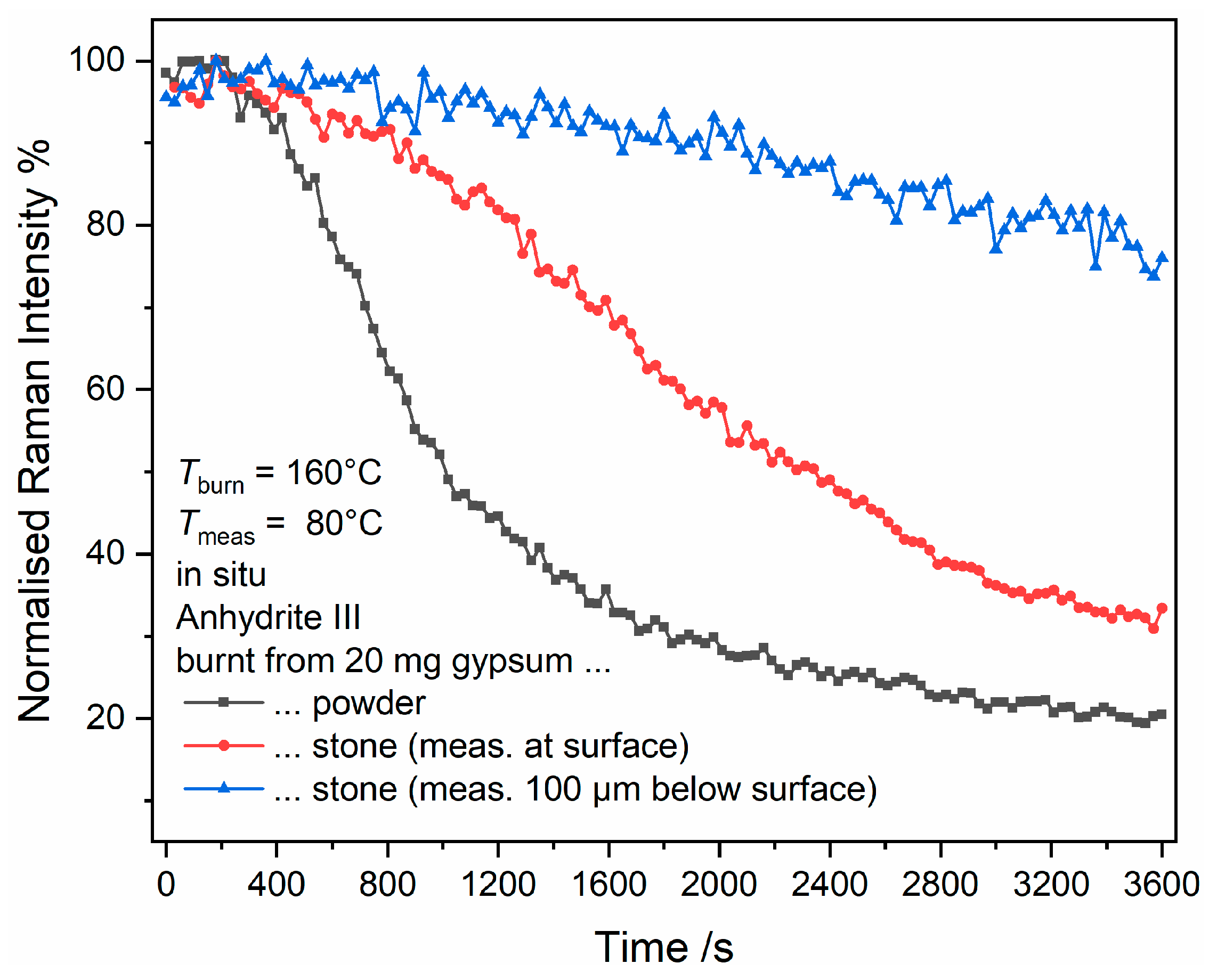
The measurements during calcination of gypsum stones, which had a mass of 20 mg and typically around 10 mm
3 volume, revealed very different trends of the reactions as compared to the powder studies (see
Figure 10 and
Figure 11). Note that the increased noise in the sub-surface measurement shown in
Figure 11 is due to the reduced peak heights achieved in the raw data of these measurements. In both cases, anhydrite III is formed prior to the detection of bassanite. The restricted time resolution of the experiment does not allow us to monitor the intermediate formation of bassanite, if present. While bassanite is formed later, anhydrite III is depleted and reappears at a later stage when, very similar to the powder experiments, the bassanite concentration drops. While the initial anhydrite III can be detected from 120 s to 600 s at the surface of the gypsum stone, it appears only between 210 s and 450 s at 100 µm below the surface. Another difference is the point of equal bassanite and anhydrite III Raman intensities during the final anhydrite III formation, which is at approximately 1650 s at the surface and 1500 s 100 µm below the surface.
These observations reveal that, even though these are chemical transformation reactions, there are significant parallels to physical drying of solid materials, which consists of a drying phase of the surface layer with a constant rate corresponding to the evaporation rate of free water, which is followed by a phase of falling rate (or several distinguishable phases of varying rates), which is determined by the mass transport of water from inside the material toward its surface, which is the only part of the material where water can leave the system [
43,
44]. A rate-determining influence of the water transport also explains the discrepancies between the conversions of 10 mg and 20 mg gypsum powder, and the conversion of anhydrite III to anhydrite II discussed in
Section 3.6 below provides further evidence for this assumption. Thus, the local oven atmosphere changes if the transport of water vapour out of the reaction volume is hampered by a more voluminous kiln run. The comminution of the kiln run also plays a role for the same reasons.
Furthermore, local measurements at the surface or sub-surface provide mechanistic insights. Clearly, we have to differentiate between surface and bulk “drying”. At the surface, heat can attack as well as water vapour can leave the gypsum stone very rapidly, which leads to an almost immediate formation of anhydrite III (whether or not via bassanite cannot be proven due to limited time resolution of this experiment). At the same time, the “drying” process also starts within the bulk, but can only take place if water is transported to the surface from where it can leave the reaction volume. Thus, at some point, a water front reaches the surface, which converts anhydrite III back into bassanite, and bassanite is also formed within the bulk. In accordance with these similarities with a drying process, conversion of the initially formed anhydrite III into bassanite happens first within the sub-surface and last at the outer surface. Similarly, the reaction front of bassanite and anhydrite III moves from the inside out after completion of the gypsum–bassanite transformation. In that sense, the final transformation of bassanite into anhydrite III occurs last at the surface while the sub-surface of the 20-mg stone behaves similarly to 20 mg gypsum powder, which is also a consequence of a reaction front moving from the bulk toward the surface.
3.4. Spectroscopic Differentiation of the α and β Forms of Bassanite
Bassanite is known to exist in two forms, termed α and β, which are the results of different routes of synthesis (i.e., wet or dry method) and have differing properties in terms of reactivity with water as well as physical-mechanical qualities of the hydration product [
40,
45]. Their discriminative microscopic properties (crystal habit and size) most likely arise from variations in their internal structure, which are expected to be spectroscopically measurable. Nevertheless, there are only a few analytical studies on this subject available so far. A study on X-ray diffractometry (XRD) shows the widening of three overlapping signals when comparing the diffractograms of the crystallographic (
hkl) = (212) planes of α-bassanite and β-bassanite. Stacking order faults within the same crystal structure are mentioned as a reason for the peak widening, which is quantified based on the stacking order index calculated from the areas of a central and two satellite peaks [
46]. In contrast, another XRD study provides indications for differences in the crystal structure between the two forms [
47]. Research aiming for an analytical approach capable of quantifying the α-character and β-character of bassanite mixtures went on, and thermoanalysis had to be ruled out as a candidate [
40].
Within the present study, a Raman-microspectroscopy-based approach for detailed quantitative analyses of the differences between the α and β forms of bassanite was found, which enables repeated measurements on either the same spot or a range of different sample spots for testing the significance of differences and probing heterogeneity within a sample material.
Figure 12 shows typical spectra of two samples of industrial products, which mainly consist of either α-bassanite or β-bassanite, which reveal no clear changes in band positions or relative band intensities. A close look at the ν
1 mode of bassanite at 1016 cm
−1 and the overlapping shoulder at 1006 cm
−1 discussed above yields a stronger overlap between these modes in case of the β form. This is in close agreement with the previous observations made regarding the broadening of XRD signals [
46]. Peak broadening in Raman spectroscopy during retention of an approximately Lorentzian peak shape can be explained by a decrease of crystallinity and a higher number of crystal lattice defects [
48,
49,
50,
51]. The same explanation applies to gradual changes of Raman band widths found recently within the stability range of anhydrite II [
21,
22,
23], which are described in more detail in
Section 3.7 below. Standard deviations and mean values from repeated measurements enable judgment of the significance of the differences in band FWHMs (as graphically depicted by arrows in the inset of
Figure 12).
One advantage of Raman microspectroscopy over XRD is its intrinsic spatial resolution. Automated mapping measurements can easily provide insight into the (microscopic) heterogeneity of a material.
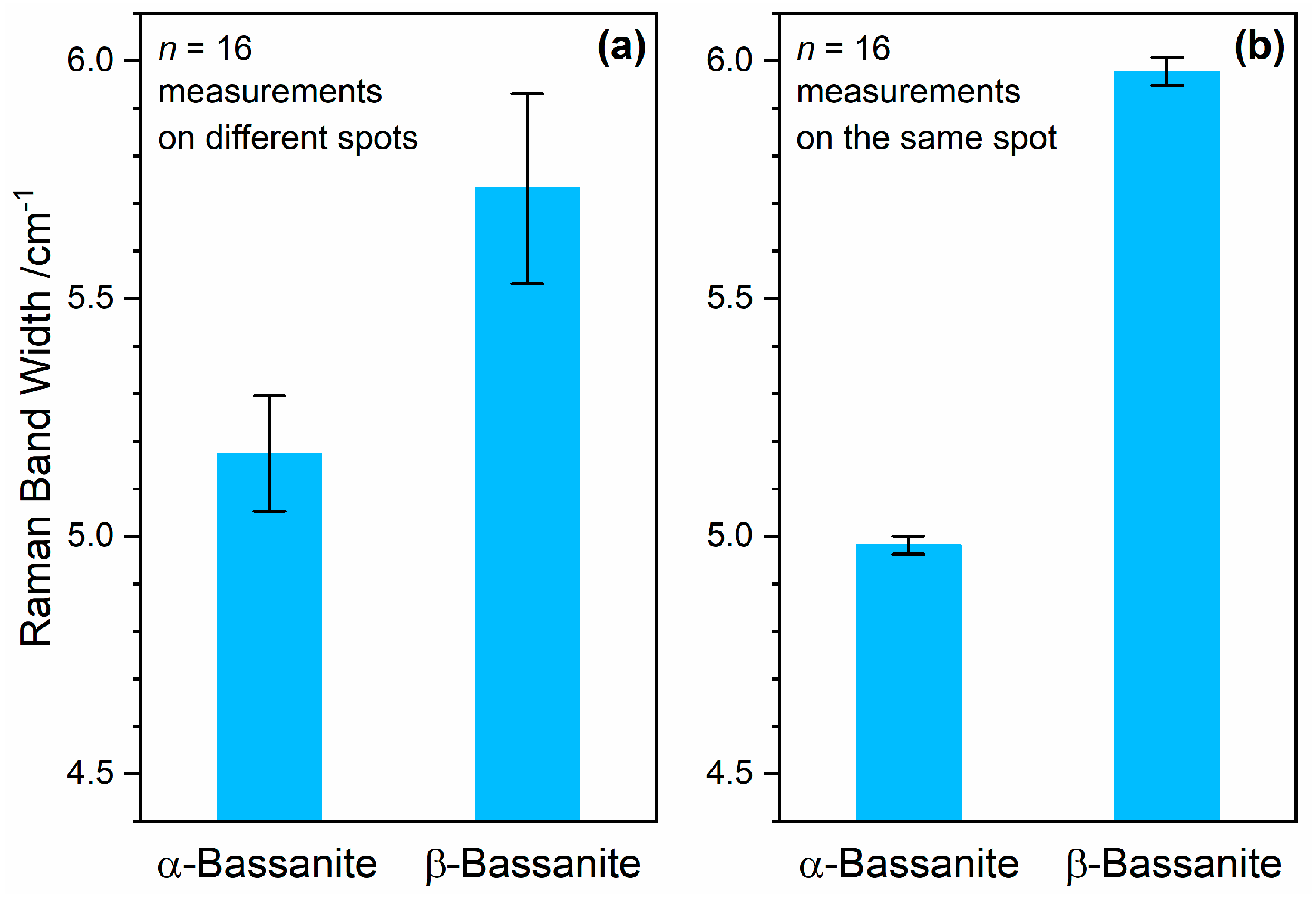
Figure 13a shows mean values and standard deviations of Raman measurements on 16 different spots of α-bassanite and β-bassanite powder samples. Even though heterogeneities within the sample materials are included, the error bars do not overlap, which indicates a significant difference. A t-test proves this assumption, as detailed in
Appendix B.
In order to assess the measurement uncertainty of the method, repeated measurements of the same sample spot were performed, and the mean values and standard deviations are plotted in
Figure 13b. Even though, by coincidence, bassanite grains with specifically high (α-bassanite) and low crystallinities (β-bassanite) were measured, very similar values of both FWHMs are included in the data sets represented by
Figure 13a. The standard deviations strongly decrease compared to the measurements performed on different sample spots. The latter include both measurement uncertainties and sample heterogeneities, and amount to 2.4% (α-bassanite) and 3.5% (β-bassanite), respectively (
n = 16). The measurement uncertainty represented by relative standard deviations of
n = 16 measurements repeated on the same sample spot is on the order of only 0.25% (0.2% in the case of α-bassanite and 0.3% for β-bassanite). This demonstrates that Raman band width measurements can be employed to discriminate different sub-types of bassanite and are also suitable to draw conclusions about heterogeneities of mixtures.
3.7. Structural Changes within the Stability Range of Anhydrite II
As pointed out in the Introduction, three sub-phases of anhydrite II are proposed due to different hydration reactivities: sparingly soluble AII (AII-s), insoluble AII (AII-u), and estrich gypsum (AII-E) [
19,
20]. There has been no spectroscopic study in the literature on the possible structural reasons for these differences in behaviour until our previous publication in 2017 [
21], which revealed decreases of Raman band width, specific surface area (according to the Brunauer–Emmett–Teller method) and strain, and an increase in crystallite size (as determined by XRD) in the burning temperature range of 500 °C to 900 °C. The following studies included T
burn = 400 °C and 1000–1100 °C, and the sharpening of Raman bands along the temperature axis was attributed to an increase in crystallinity of anhydrite II [
22,
23]. All mentioned property changes agree with the known decrease in reactivity.
So far, all studies used 5 g gypsum as starting material, which was burnt in open crucibles [
21,
22,
23]. Even though it was placed in a desiccator during cooling and for storage, at least during Raman measurements at ambient air, the significant amounts of anhydrite III present in samples with T
burn < 400 °C (see
Figure 17) readily convert into bassanite (compare
Figure 15). As a consequence, the clearly separated bands seen in
Figure 17 merge into one peak consisting of the ν
1 modes of bassanite at 1015–1016 cm
−1 and anhydrite II at 1016.5–1017.5 cm
−1 (see
Figure 2b). Due to these strongly overlapping peaks, deconvolution into two Lorentzian profiles for exact band width determination does not yield meaningful or reproducible results in most cases, which is the reason why the range below 400 °C is not accessible by such an experiment. The red curve in
Figure 18 shows Raman band widths of the anhydrite II ν
1 mode determined after burning in crucibles, which reproduces the sigmoidal trend of previous studies [
21,
22,
23].
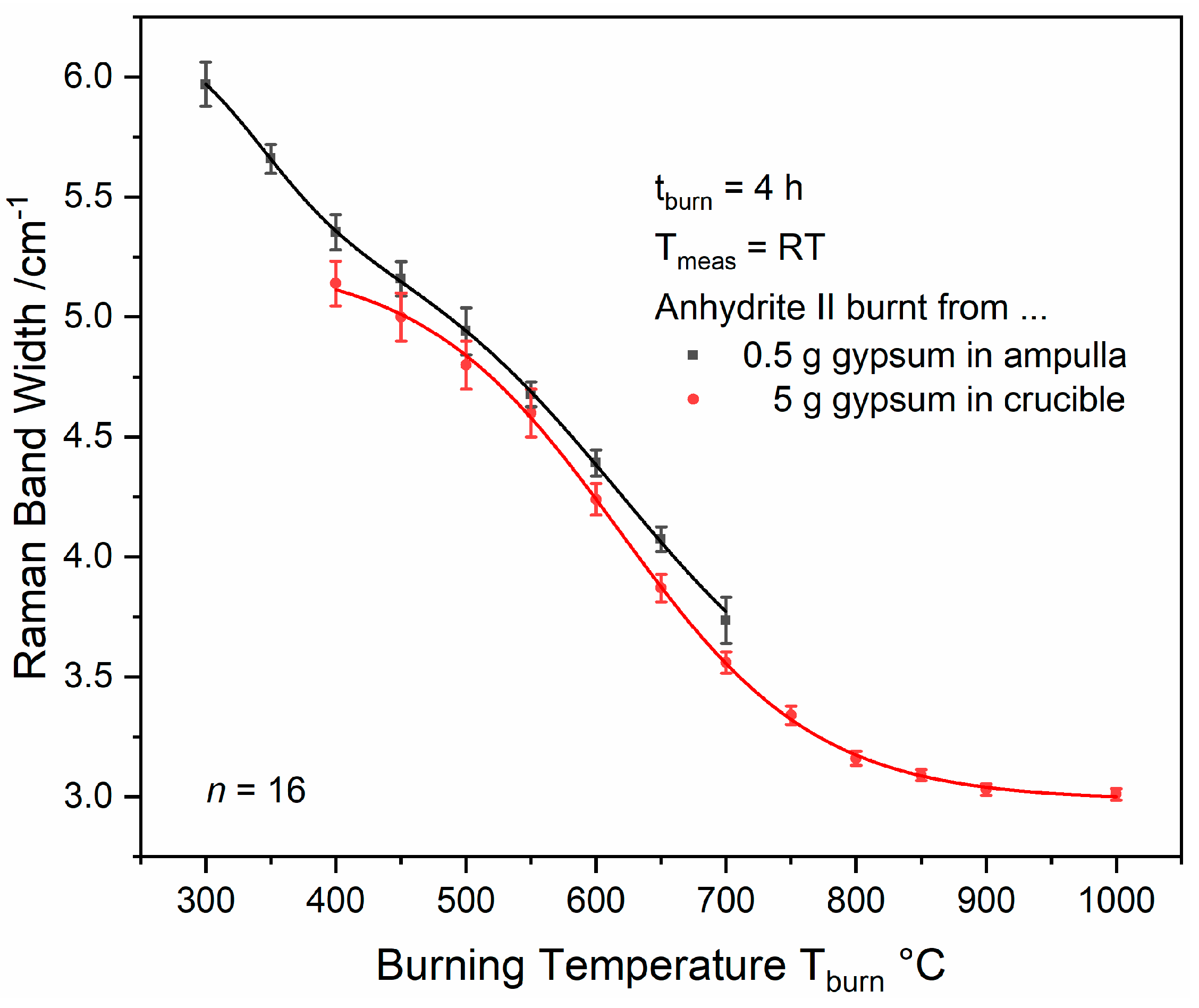
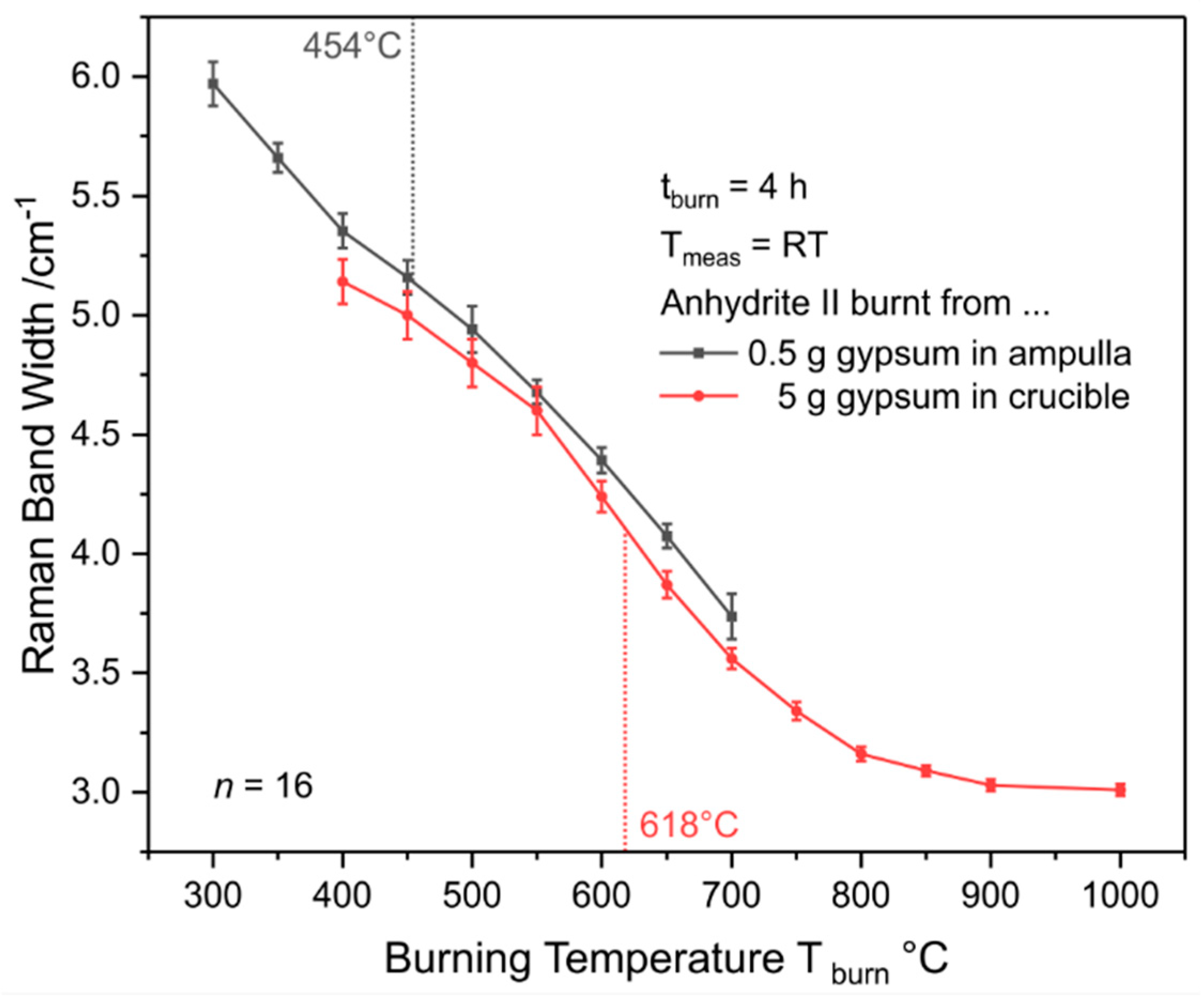
In order to overcome the issue of overlapping bassanite and anhydrite II bands, gypsum powder was calcinated by employing the ampulla method described in
Section 2.2. That way, spectra as shown in
Figure 17 were obtained. Band widths (FWHMs) were determined as described in
Section 2.6. Ampulla experiments can only be performed up to T
burn = 700 °C due to melting of the glass during the holding time at higher burning temperatures while experiments with crucibles can be used to cover the range of AII-E. There is a slight discrepancy between both data sets, which is not significant at a few temperatures when taking the standard deviations into account. An average difference of approximately 0.15 cm
−1 between ampulla and crucible FWHMs was observed, which we presumably attribute to the different conditions during gypsum burning. While water can easily leave a crucible via its surface of a diameter of 3.5 cm, the opening of a glass ampulla is only 0.5 cm in width. Thus, due to mass transport limitation, gypsum–bassanite–anhydrite III conversion is expected to take longer in the ampullae, so that the starting point of the anhydrite III–anhydrite II conversion is later in that case, which results in a slightly (from 450 °C to 550 °C not significantly) less crystalline product.
Both curves can be mathematically approximated by sigmoidal or double-sigmoidal functions, respectively, as detailed in
Appendix C. Curve fitting reveals a plateau at 454 °C within the ampulla data and an inflexion point at the steepest negative slope of the sigmoid at 618 °C within the crucible curve. Both temperatures highlighted in
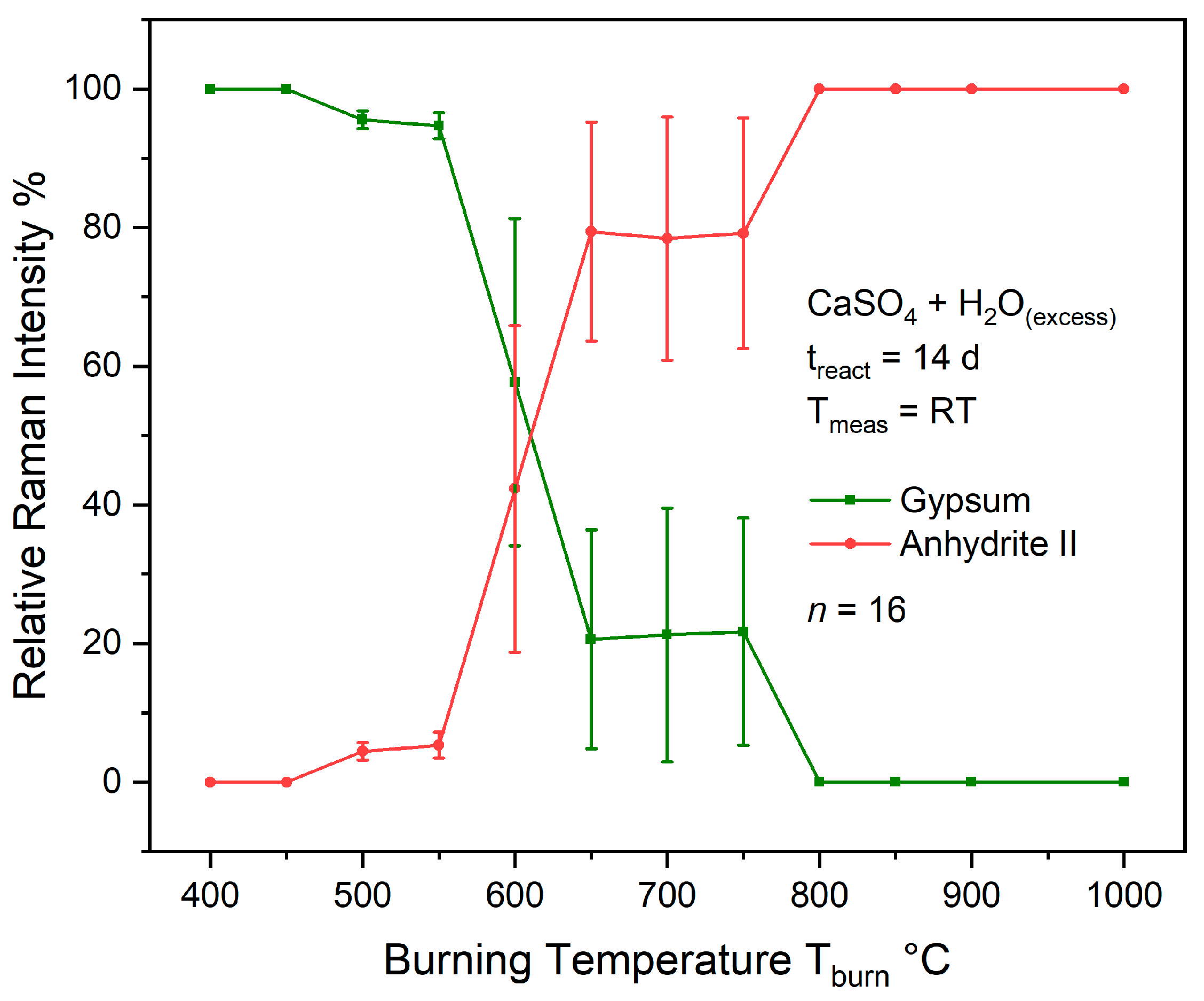
Figure 18 are near the postulated AII-s–AII-u and AII-u–AII-E transition points, and, thus, seem to coincide with changes in the material’s reactivity. Therefore, a simple reactivity test was performed as described in
Section 2.4. Relative Raman intensities calculated according to Equation (1) and plotted in
Figure 19 are measures for the relative amounts of anhydrite II and gypsum in the product mixtures obtained after exposing the different anhydrites to an excess of water for 14 days. Note that the large standard deviations in some experiments are most likely because the reactions were conducted without shaking or stirring of the mixtures.
Full conversion into gypsum was achieved for anhydrites burnt at <500 °C, while small amounts of anhydrite were still present when using anhydrite II calcinated at 500–550 °C. Only partial hydration with relative Raman intensities of anhydrite II between approximately 40% and 80% in the product mixture was observed at T
burn ranging from 600 °C to 750 °C, while the material did not react with water within 14 days when burnt at >800 °C, which is in accordance with previous studies [
52,
53,
54,
55]. This simple reactivity test can be discussed based on the three postulated sub-phases AII-s, AII-u, and AII-E. A comparison of the reactivity with the FWHM curve shows that the plateau coincides with the AII-s–AII-u conversion, while the inflexion point at the steepest slope falls within the range of partial conversion, i.e., of strongest changes in reactivity (at 618 °C, by coincidence almost identical relative Raman intensities of anhydrite II and gypsum are predicted by this data). At the transition to AII-E, the FWHM curve reaches the final plateau phase exhibiting only small changes in crystallinity and approximates the values of natural anhydrite crystals [
21,
23].
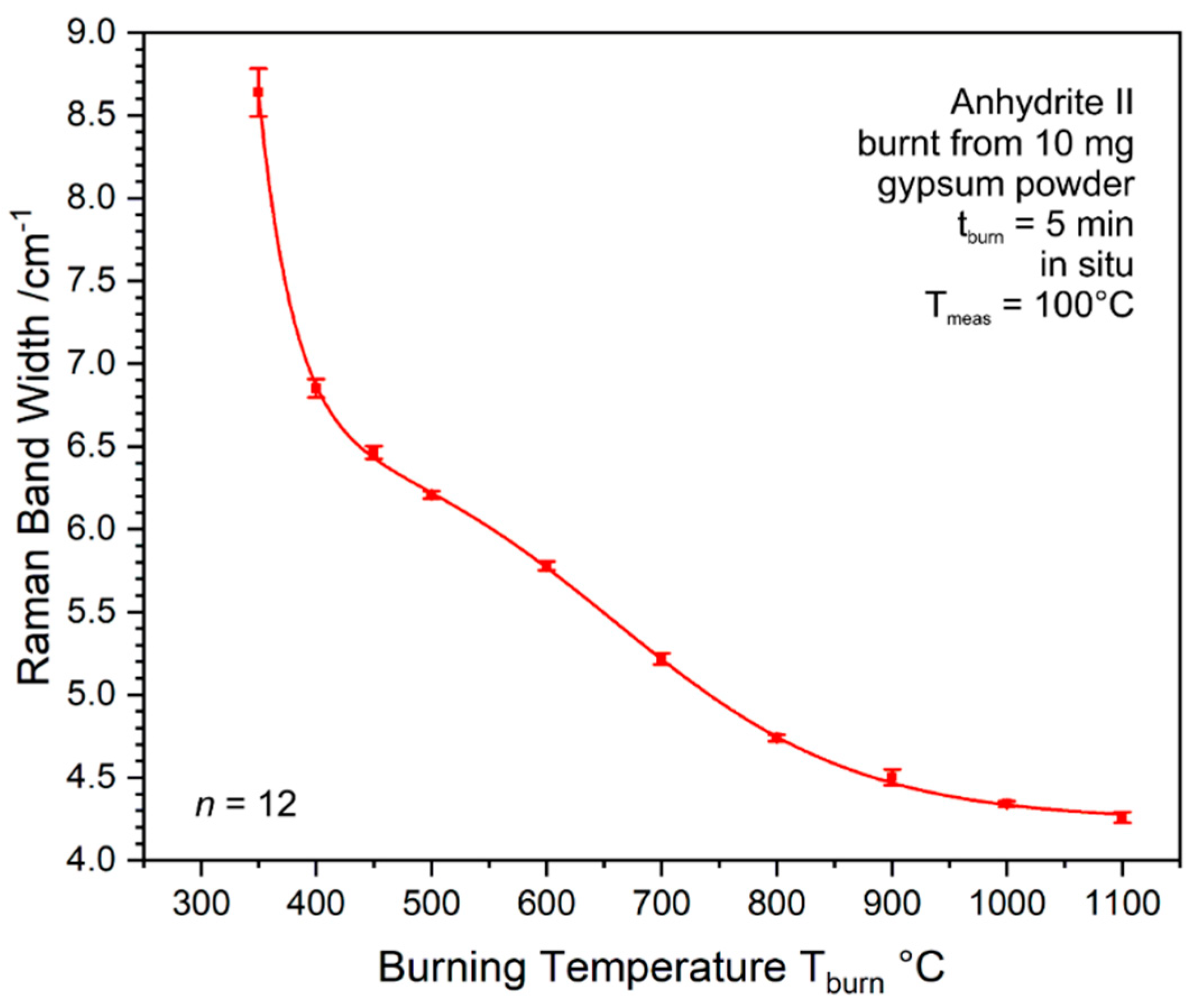
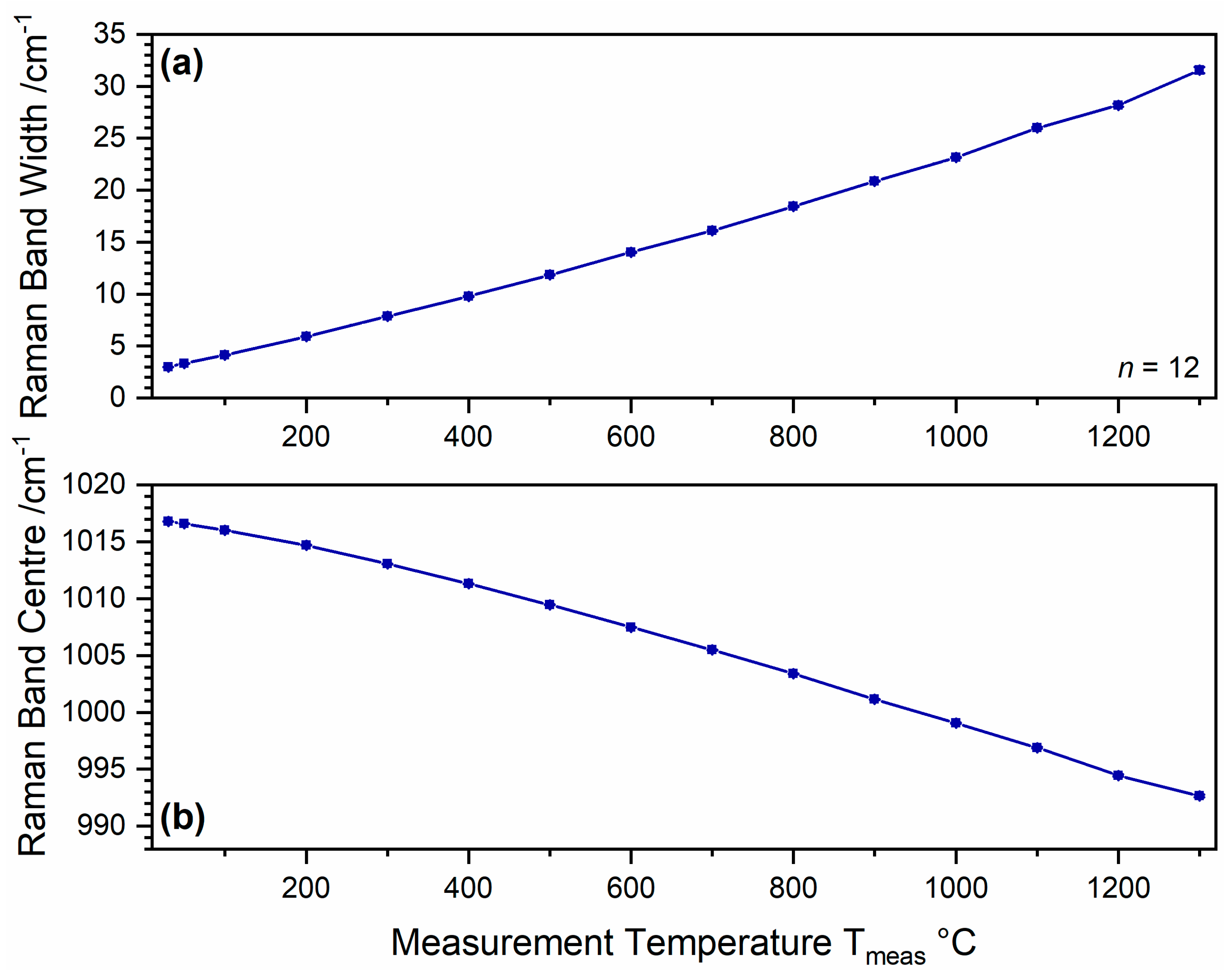
Due to the mentioned experimental limitations, a continuous measurement of the FWHM development throughout the whole stability range of anhydrite II can only be achieved by in situ analyses (see
Section 2.3 for experimental details). In order to protect anhydrite III present in the product at T
burn ≤ 500 °C (see
Figure 17) from air humidity, we made use of the finding shown in
Figure 15 by heating gypsum to different burning temperatures and subsequent cooling down to the measurement temperature of T
meas = 100 °C at which anhydrite III is stabilised. Within this experiment,
n = 12 measurements were performed at the same sample spot for each temperature step. Therefore, the resulting standard deviations are smaller than in ex situ measurements (compare
Figure 18), where they also reflect sample heterogeneities.
Figure 20a shows the relative Raman intensities assigned to anhydrite II and anhydrite III, which are in good agreement with a previous XRD investigation [
56] and confirm the observation of anhydrite III at T
burn ≤ 500 °C (see
Figure 17).
Because the anhydrite II ν
1 mode was too weak to be analysed at T
burn ≤ 300 °C, the FWHM curve in
Figure 20b starts at 350 °C. The curve confirms the double-sigmoidal trend predicted based on the ampulla and crucible data shown in
Figure 18. As described in
Appendix C, curve fitting yields a plateau at 494 °C and an inflexion point at 659 °C. Both characteristic temperatures are shifted up by approximately 40 K, which might be due to a slightly too low burning time or generally due to the different conditions of 5 g, 0.5 g, and 10 mg starting material as well as 4 h vs. 5 min holding time period but is most likely within the overall uncertainty of these experiments. Generally, we can conclude that ex situ and in situ experiments agree well and result in a plateau phase at around 460–500 °C and an inflexion point reflecting the strongest change of properties at approximately 620–660 °C, while the system reaches a final plateau at around 800 °C.
The evaluation of autofluorescence of the material can provide a deeper insight. Fluorescence was observed in the form of a raised baseline of the Raman spectra. Previous studies have shown an emission maximum of anhydrite II at approximately 695 nm in the red spectral range [
21] and varying intensities depending on the temperature applied during its synthesis, i.e., increase of the emission at low burning temperatures and vanishing of the fluorescence at around T
burn = 900 °C [
21,
22,
23]. Quantitative measurements are difficult to perform in ex situ experiments because of high variabilities within the samples. Within in situ experiments, measurements can be performed at the same sample spot while only varying the calcination temperature, which leads to quantitatively comparable results.
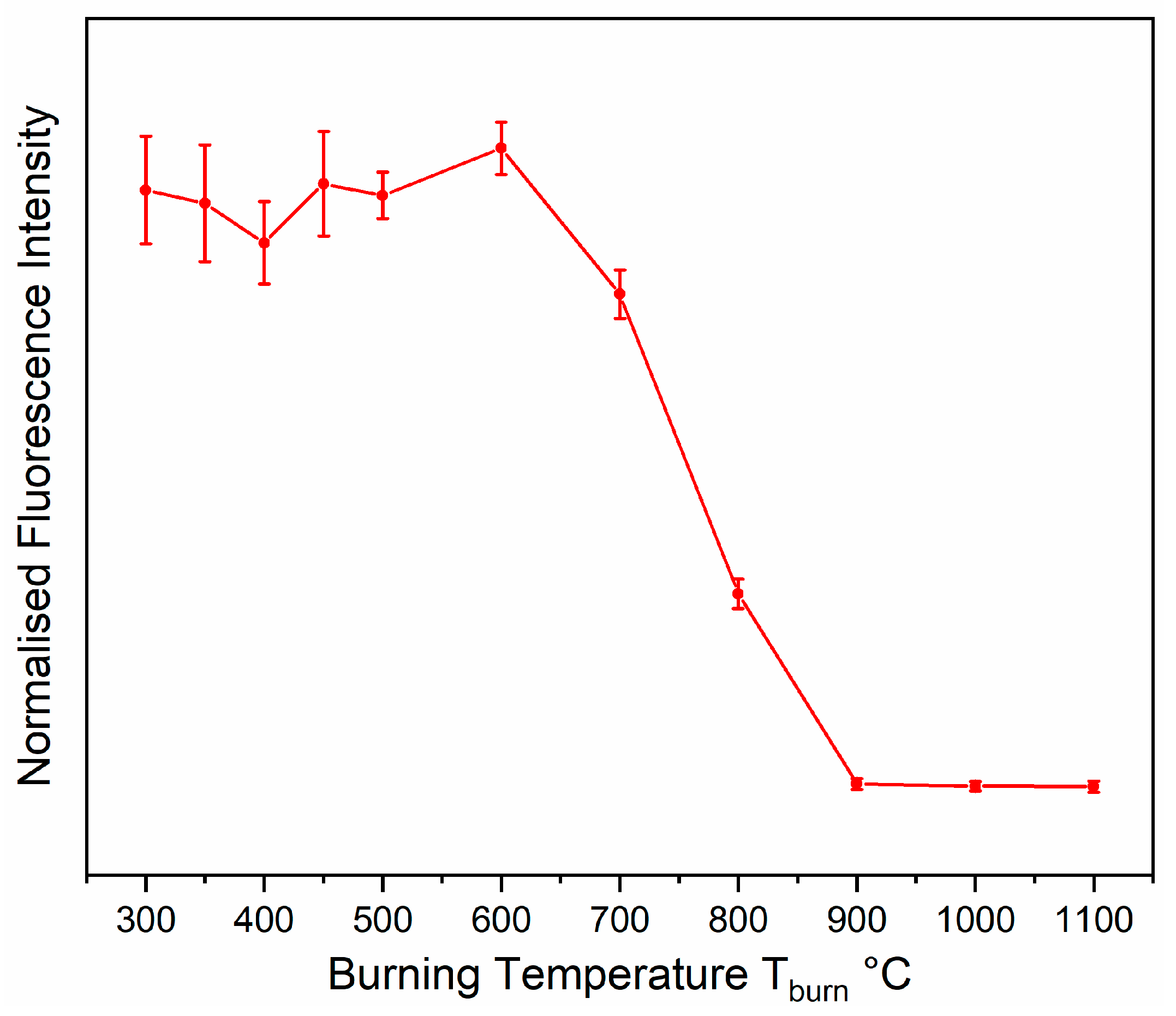
Figure 20c reveals an increase of fluorescence emission measured at 570 nm (i.e., 1253 cm
−1 Stokes-shifted from the laser emission wavelength 532 nm within the actual green spectral range, which is still on the rising edge of the spectrally broad red emission) at burning temperatures from 300°C to 600 °C, which is followed by a decrease and its disappearance at T
burn = 900 °C.
Fluorescence emission in inorganic materials can have several reasons including organic contaminants, integration of foreign ions (d-block or f-block elements) into crystal lattices, or colour centre defects (F-centres) in crystals. Organic contaminants can be ruled out because the gypsum powder used as starting material was free from fluorescence emission [
23]. In case of the integration of foreign ions into the anhydrite II lattice, the decrease of fluorescence at high temperatures is difficult to explain since a constant or steadily increasing emission (due to further integration of ions) would be expected. Yet, this behaviour agrees with the generation and healing of fluorescing crystal lattice defects. Thus, fluorescence intensity can be employed as an indicator for internal crystal lattice defects, whereas the observed changes in Raman band width under simultaneous conservation of the approximately Lorentzian peak shape are attributed to changes of both inner defects and crystallite surfaces [
48,
49,
50,
51]. Therefore, evaluation of fluorescence and Raman band widths in the same spectra (
Figure 20b,c) enables us to separate these two effects.
The increase of emission within T
burn = 300–500 °C can be explained by the proportionality between fluorescence intensity and concentration. The curves representing the relative Raman (
Figure 20a) and fluorescence intensities (
Figure 20c) not only follow the same trend, the ratio of fluorescence and relative Raman intensity yields almost constant values within this range (see
Appendix D). This means that the phase transition from anhydrite III to anhydrite II generates a defective material with an almost constant defect concentration. Lattice defects can only heal if diffusion within the material is enabled by temperature. Therefore, the defect concentration slightly further increases toward 600 °C, which is followed by a healing phase within approximately T
burn = 600–900 °C.
Coming back to the trend of the Raman band widths representing changes in both crystallite surfaces and crystal lattice defects, the plateau at approximately 500 °C can be understood as a coincidence of two effects: (1) while in the low burning temperature range, individual anhydrite II crystallites are formed within anhydrite III, which grow during the progress of the reaction, full conversion is reached at approximately 500 °C and crystallites can further reduce their specific surface areas only by sintering, and (2) the highest concentration of crystal lattice defects is reached. The loss of surface area slows down over further increasing burning temperatures, following an exponential decay [
21], while the ongoing increase in crystallinity is dominated by the healing of inner defects. Thus, the Raman band width and fluorescence curves follow a very similar trend within T
burn = 600–800 °C, with the strongest overall change in crystallinity at the inflexion point at approximately 620–660 °C and the steepest trend in fluorescence depletion at around 770 °C. At higher burning temperatures, both effects reach a plateau phase toward highest crystallinity and weakest reactivity.
Summarising these findings, we can conclude that the reactivity of anhydrite produced at T
burn ≥ 300 °C is influenced by (1) the presence of anhydrite III and its reaction product bassanite (according to
Section 3.5) at T
burn ≤ 500 °C, (2) the healing of crystal-lattice defects within anhydrite II at T
burn > 600 °C (reflected by changing autofluorescence), and (3) the increase of overall crystallinity by growth and sintering of crystallites as well as healing of inner defects, which reveals the strongest changes at around 620–660 °C and leads to a final plateau at T
burn ≥ 800 °C (shown by sharpening of Raman bands). A comparison with the postulated sub-phases AII-s, AII-u, and AII-E reveals sparingly soluble anhydrite II as a mixture of low-crystalline AII and bassanite, while AII-u is pure anhydrite II of medium and strongly changing reactivity with the strongest variations at approximately 650 °C ± 50 K (conservatively estimated by taking the measurement uncertainty according to Reference [
23] into account), and AII-E is anhydrite II of the lowest reactivity due to the highest crystallinity. This sub-phase might gain reactivity from calcium oxide, which is not necessarily formed by CaSO
4 decomposition [
19], but might be derived from calcite (and dolomite) present in both gypsum obtained from flue-gas desulphurisation and many natural gypsum stones.
When only looking at the data from reactivity and spectroscopy studies, it seems contradictive to sub-divide AII into three sub-phases, with the middle, AII-u, being characterised by strongly changing crystallinity and reactivity. There are several indications from both literature and our own studies pointing toward a two-fold separation at the mentioned transition temperature of 650 °C ± 50 K. The simple reactivity test shown in
Figure 19 reveals a transition from a reactive anhydrite II form into a much less reactive form at approximately this temperature. Paul Rohland mentions the temperature range of 525–600 °C to be ideal for synthesis of reactive anhydrite II, which coincides with the reactive and pure (bassanite-free) anhydrite II of the present study [
57]. Our previous studies of several examples of high-fired medieval gypsum mortars yielded anhydrite II grains assigned to burning temperatures ranging from approximately 650 °C up to 900 °C (uncertainty of measurement: ± 50 K), [
21,
22,
23,
24] confirming previous light microscopic observations reported in References [
58,
59,
60]. Even though heterogeneous heat distribution in medieval kilns is expected, including lower burning temperatures as well, anhydrite grains generated at the latter were completely converted into gypsum over the centuries, and only the much less reactive grains survived. In this case, the characteristic temperature of 650 °C ± 50 K seems to mark the lower limit. The arguments for this hypothesis are completed by an infrared-spectroscopic study by John Bensted and Satya P. Varma [
61] who relate changes within the ν
3 bands of anhydrite to two different modifications: β’-CaSO
4 (or anhydrite II’, respectively, when translated into the nomenclature employed in the present study) in the burning temperature range of ≤600 °C (determined with a temperature resolution of 100 K) and β-CaSO
4 (or anhydrite II, respectively) in the range of ≥700 °C. Additionally, these authors describe different stabilities and reactivities of these two forms and further explain that the modifications are not different crystal structures, but, according to X-ray diffractometry, are related to different stacking arrangements within the same orthorhombic crystal lattice.
Our previous study proofs the indifferent crystal structures within the stability range of anhydrite II as reflected by exactly the same Raman spectra in terms of wavenumbers of bands [
23], and our investigations of anhydrite II so far (including the present) elucidate the known differences in reactivity as being due to an increase in overall crystallinity (loss of a specific surface area and healing of crystal lattice defects) as a function of an increasing burning temperature [
21,
22,
23,
24]. Since we do not want these to be confused with polymorphs and we would like to point out the structural reason for this classification, we propose the terminology “disordered anhydrite II” and “crystalline anhydrite II” for the different crystallinities obtained by heat treatment either below or above 650 °C ± 50 K.
Further studies are needed to investigate the processes in the high-temperature range including generation of anhydrite I and thermal dissociation of calcium sulphate [
8,
62,
63,
64]. So far, our in situ experiments did not show clear changes of Raman spectra pointing to a high-temperature phase transition (see
Figure 3). This might be related to the practical difficulties that arise from black-body radiation emitted by anhydrite and the crucible material around the conversion temperature, but also Raman-inactivity of anhydrite I cannot be ruled out, as XRD studies point toward a sodium-chloride crystal structure, which typically leads to solely Raman-inactive 1st order vibrational modes [
65,
66]. Since calcium oxide is Raman-inactive for the same reason [
67], analytical techniques other than Raman spectroscopy are better suited to study calcium sulphate decomposition.