Insight into the Influence of Surface Roughness on the Wettability of Apatite and Dolomite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polishing Treatment
2.3. Surface Roughness Measurements
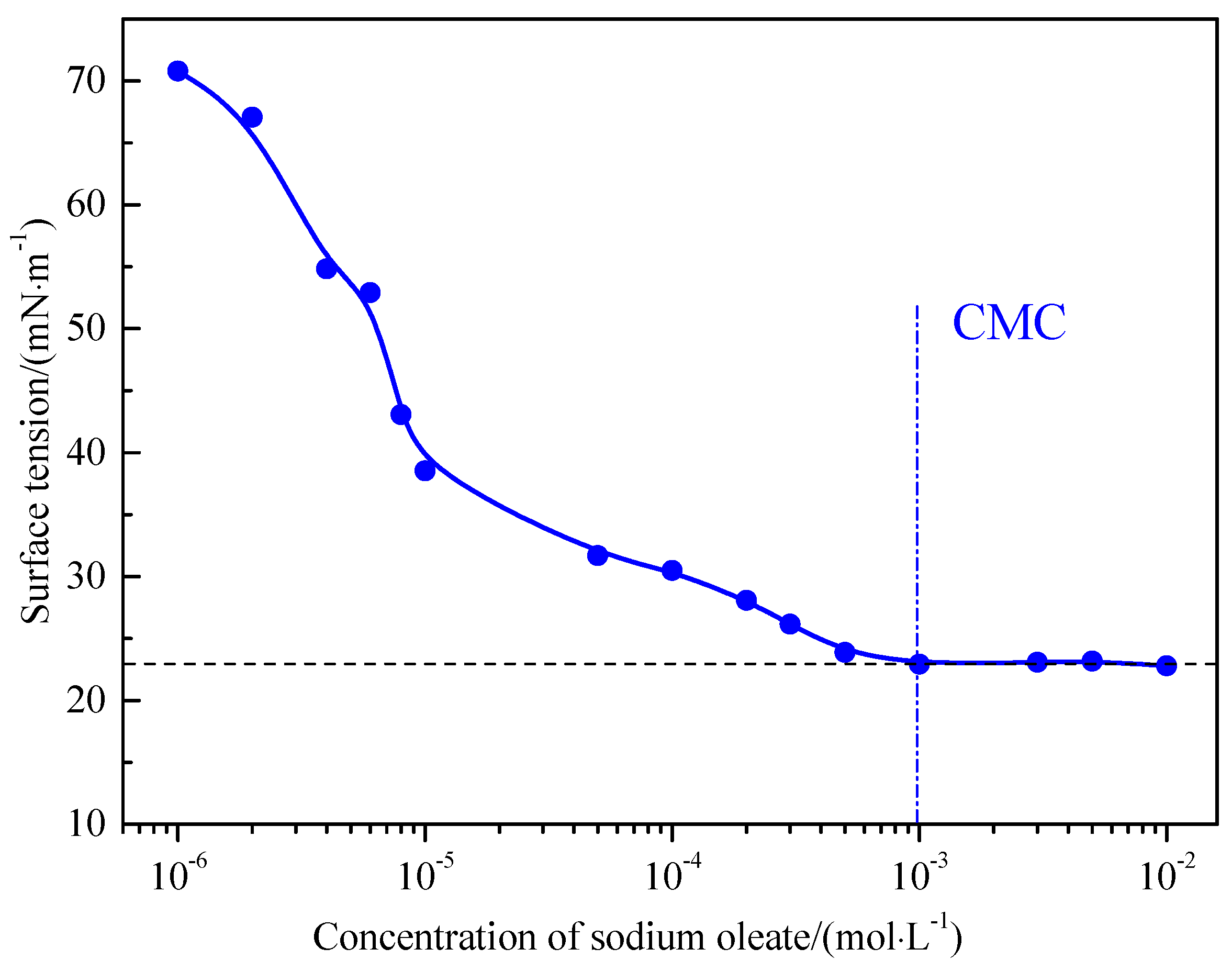
2.4. Surface Tension Measurements
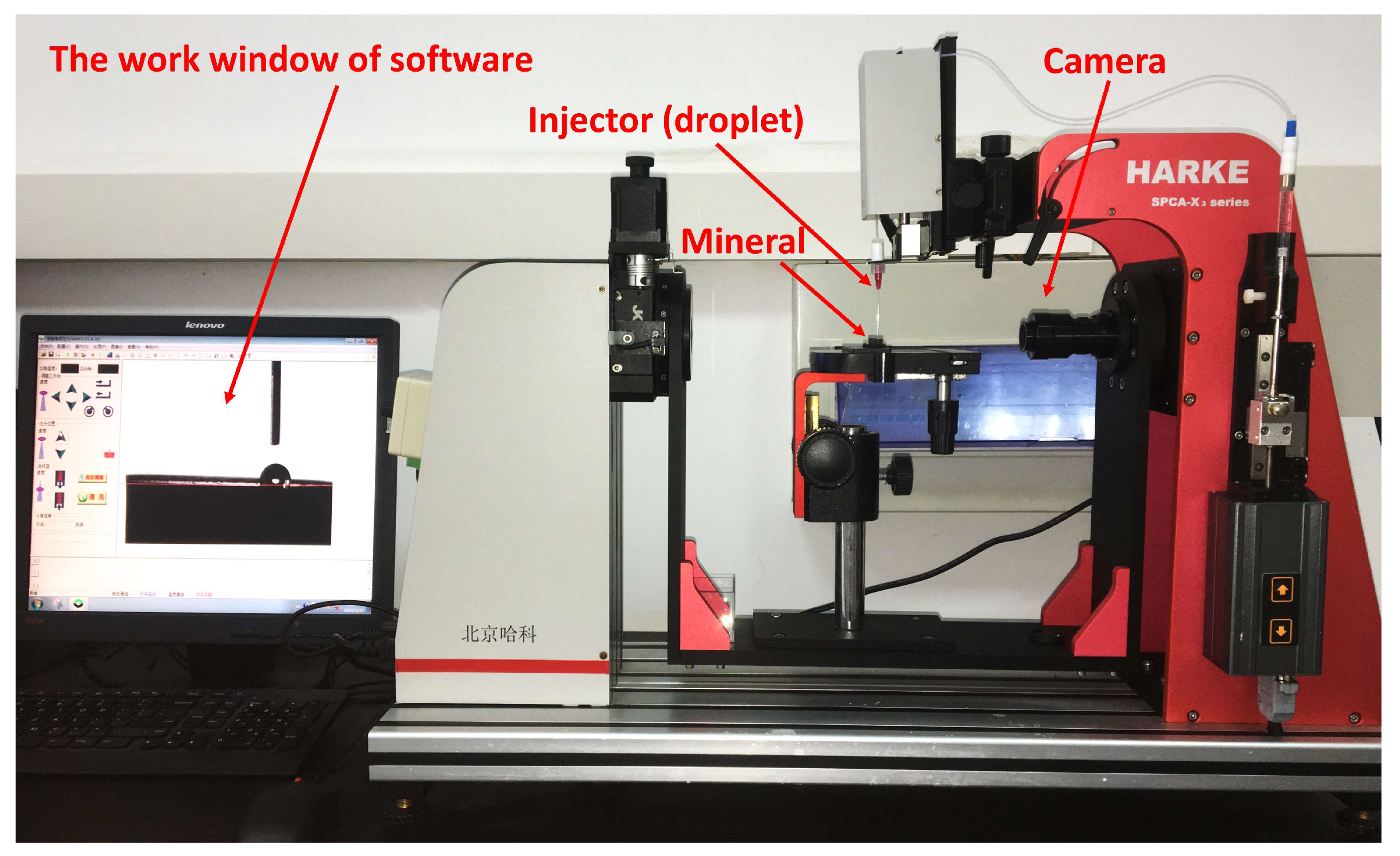
2.5. Contact Angle Measurements
2.6. Surface Energy Measurements
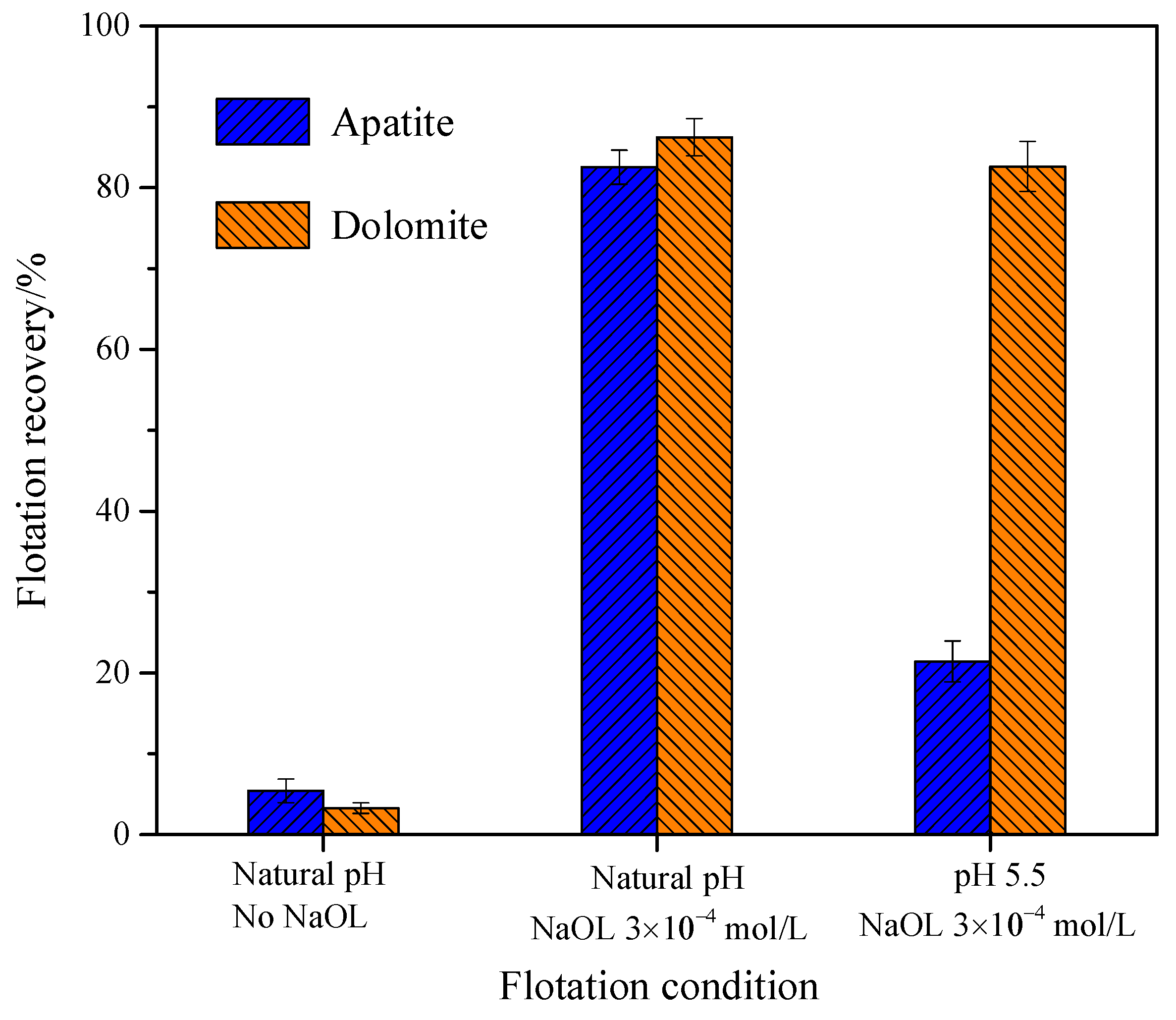
2.7. Micro-Flotation of Single Mineral
3. Results
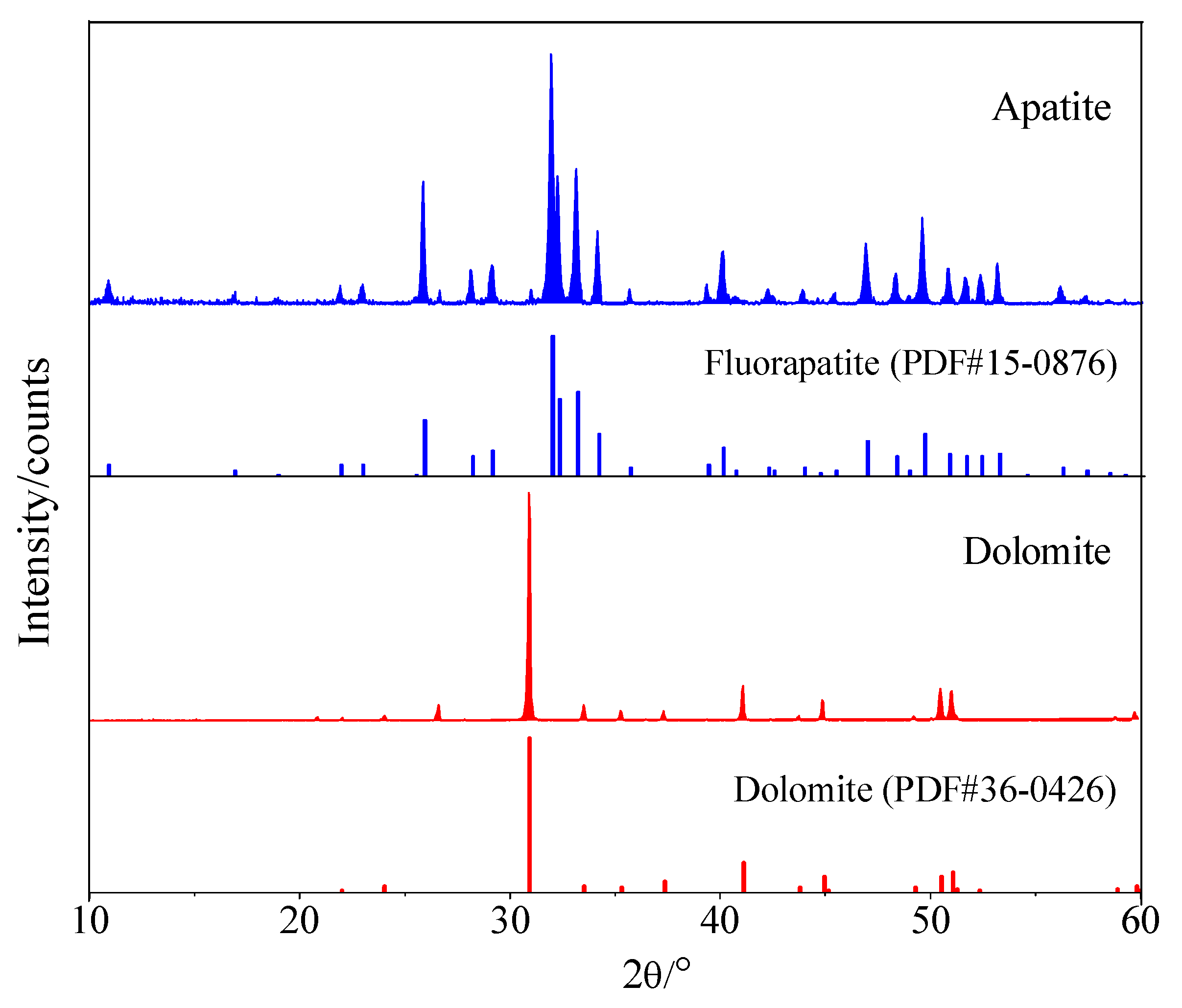
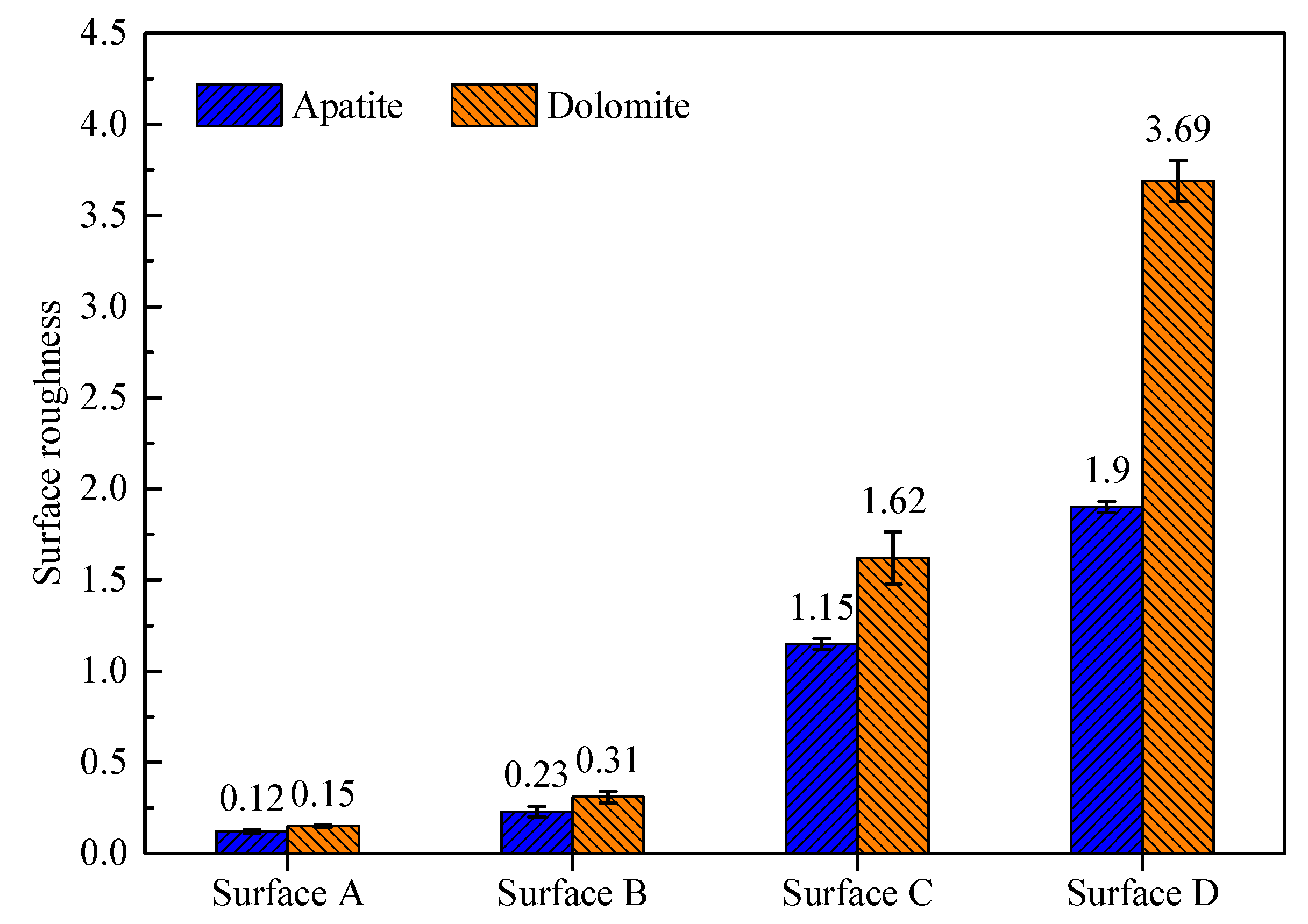
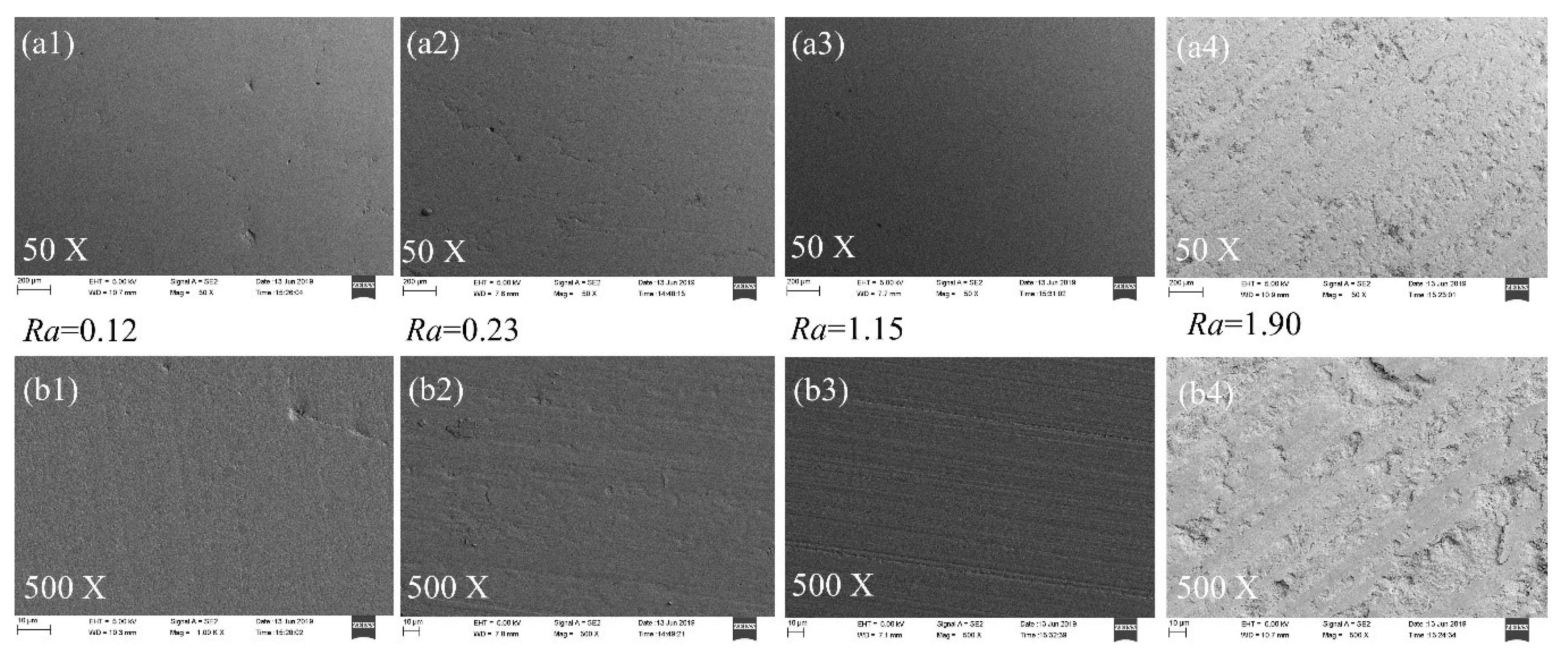
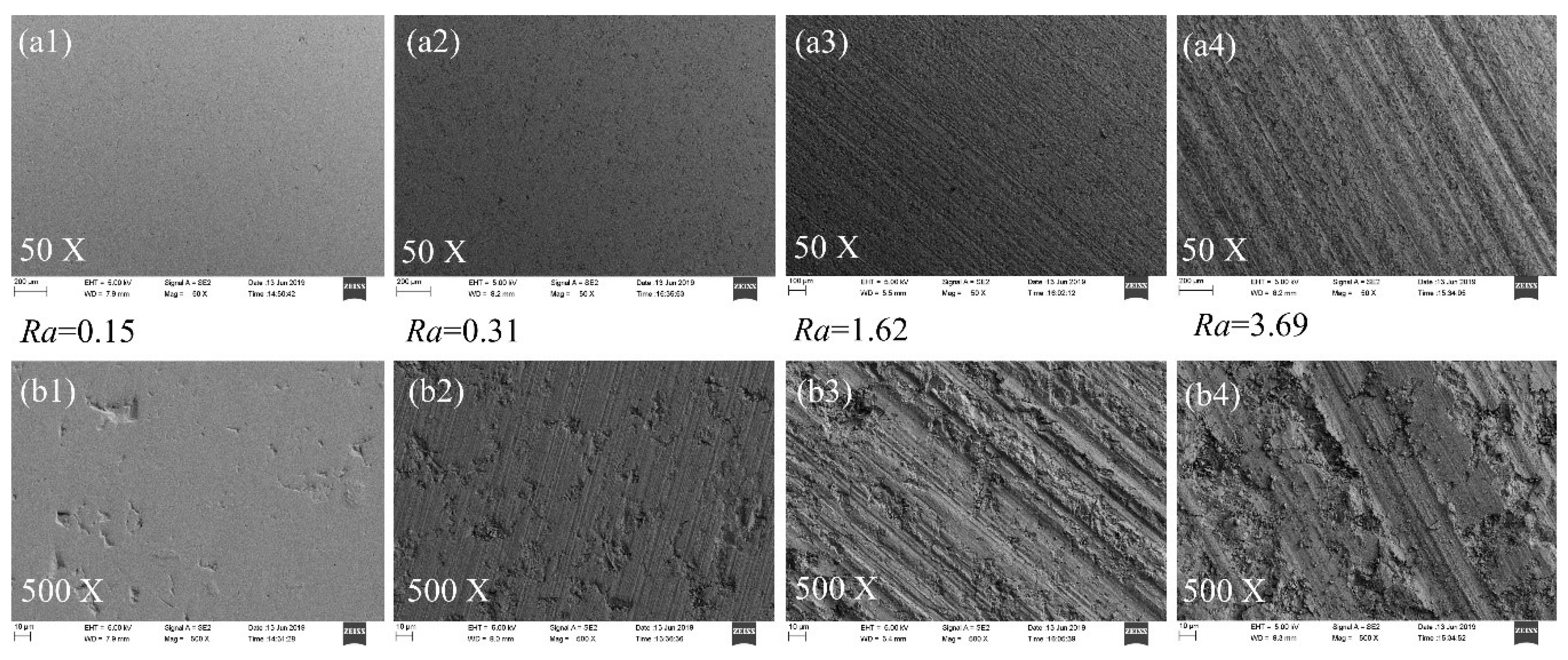
3.1. Surface Roughness and Morphology Characteristics
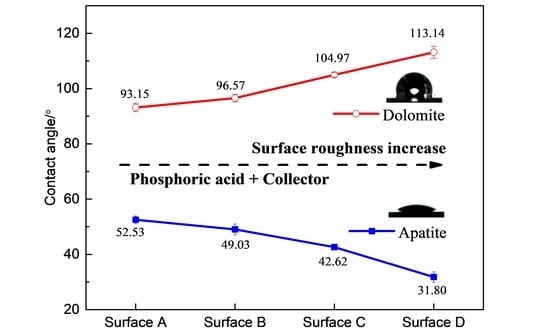
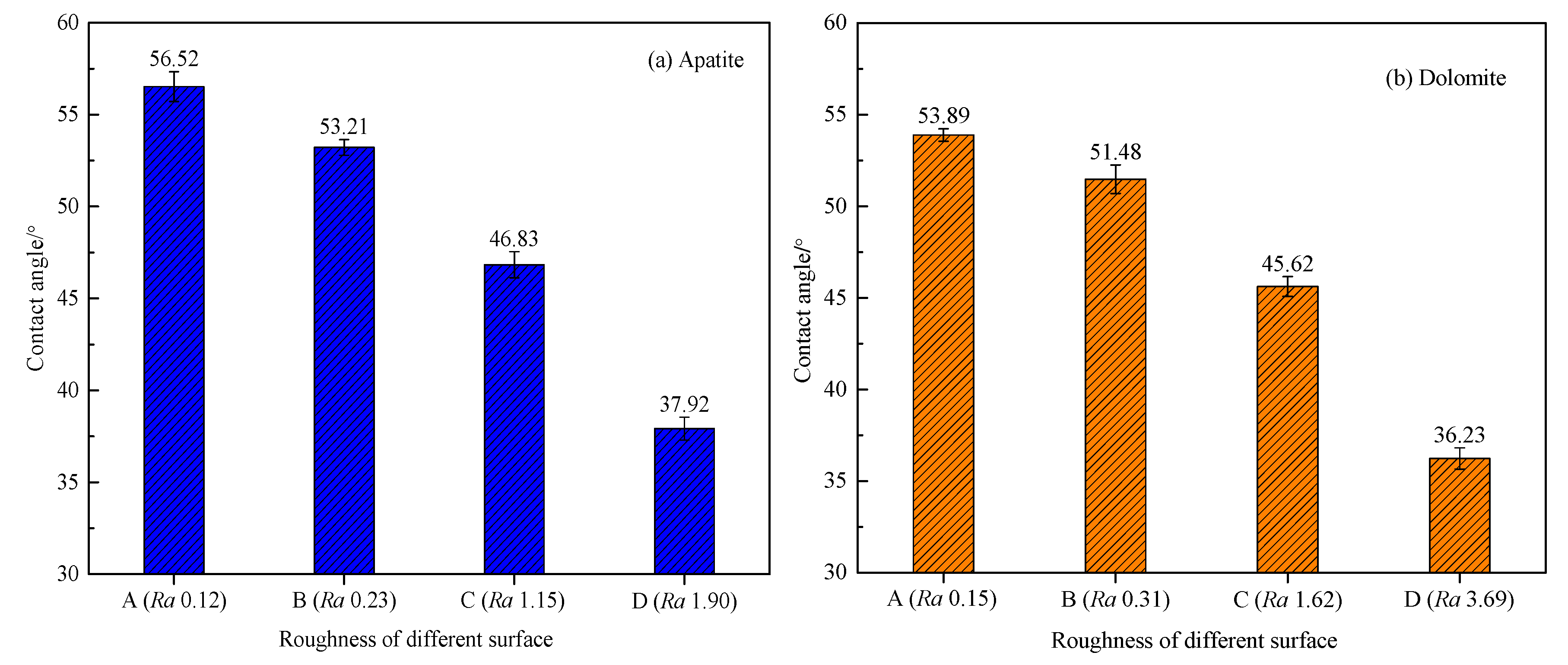
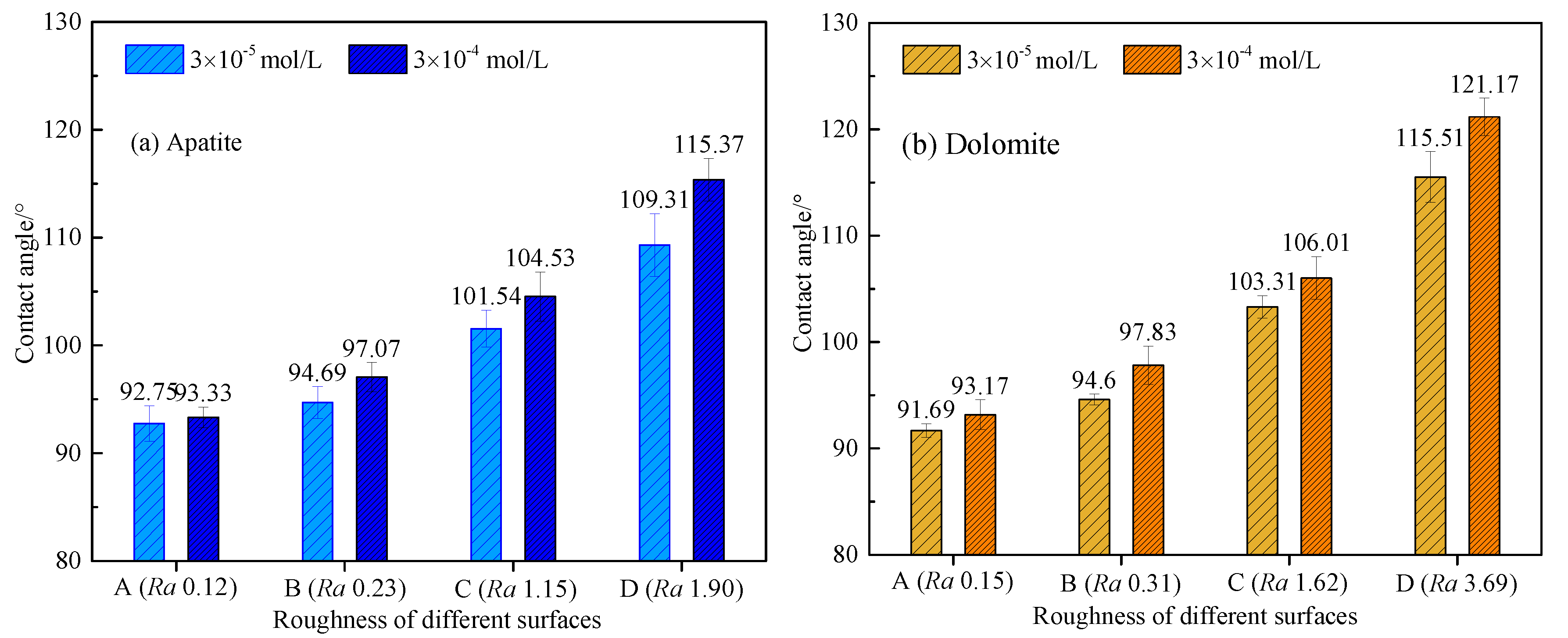
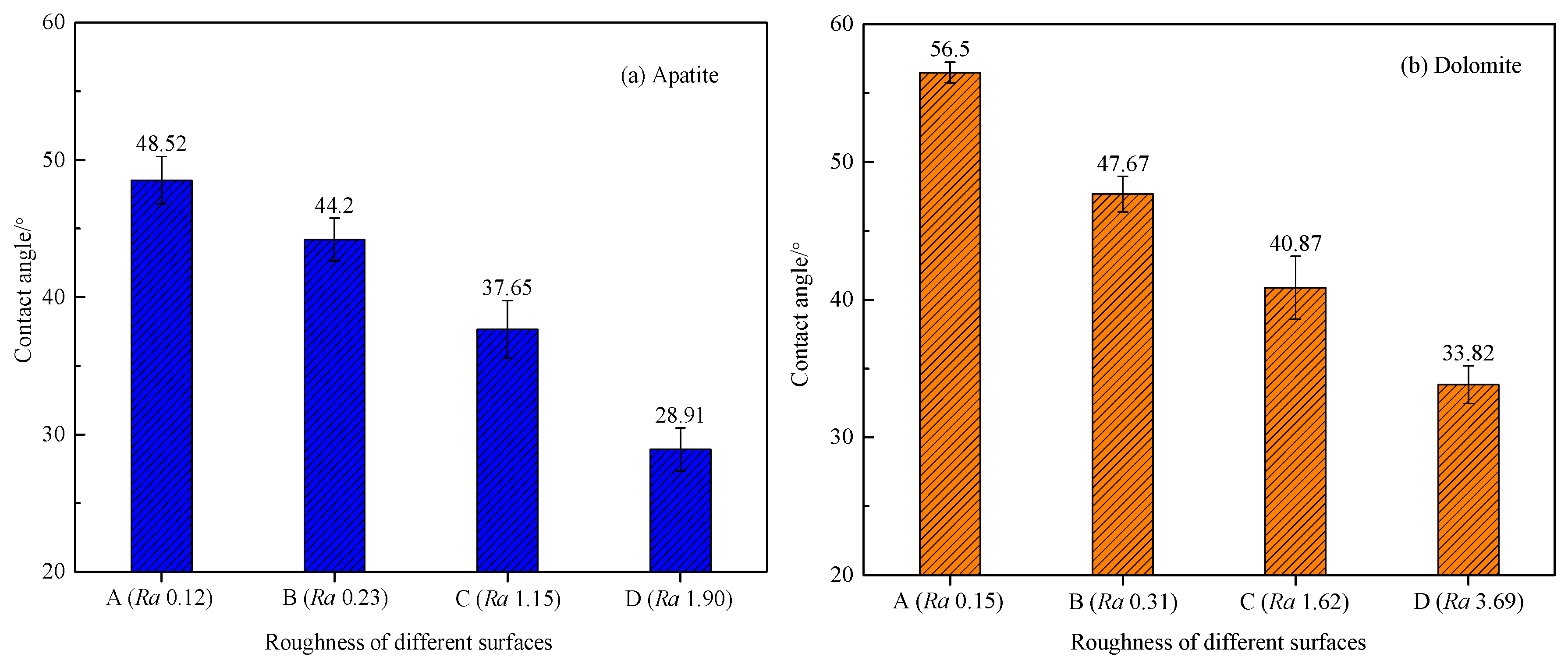
3.2. Effect of Surface Roughness on Contact Angle of Apatite and Dolomite Surfaces
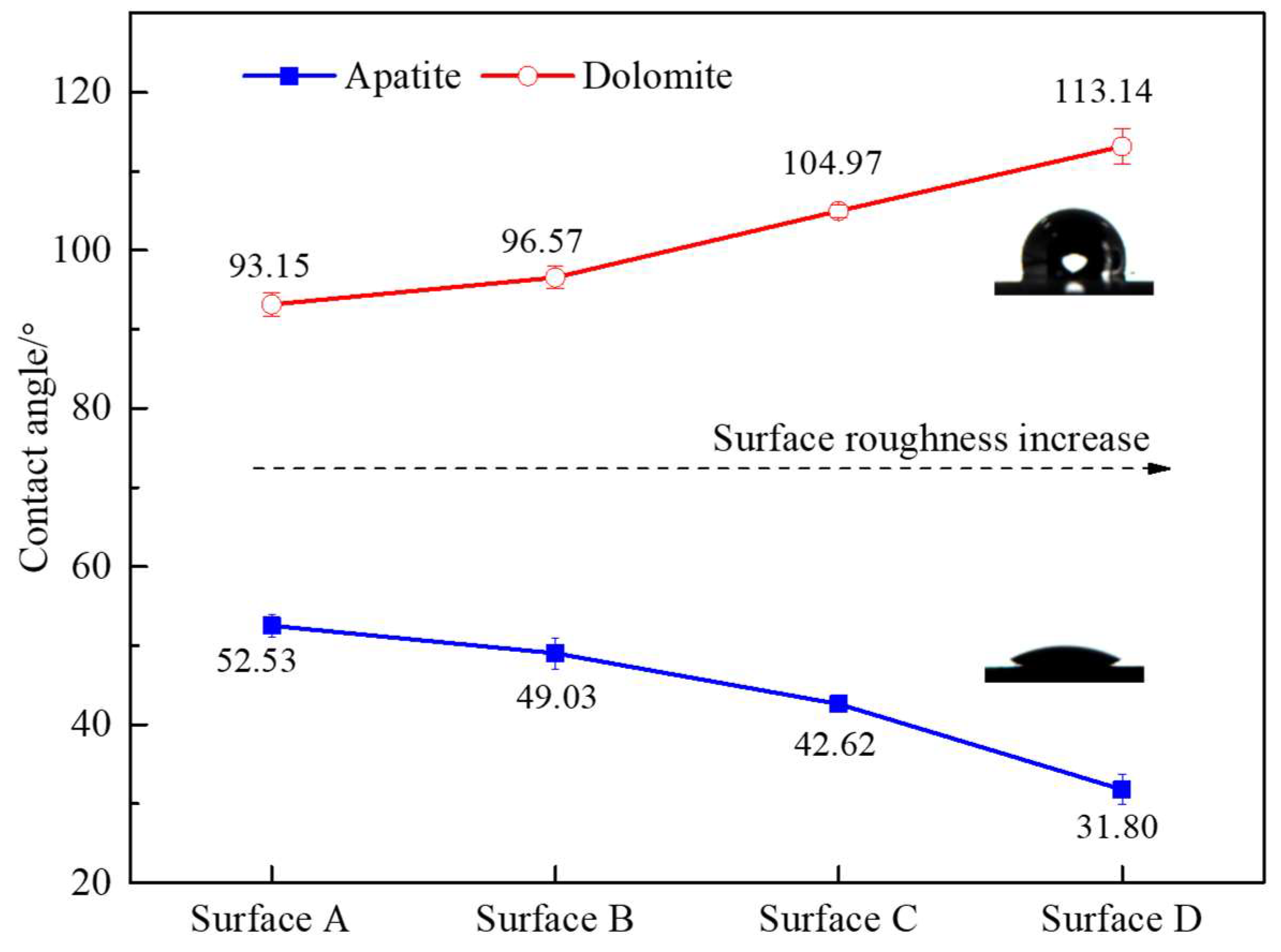
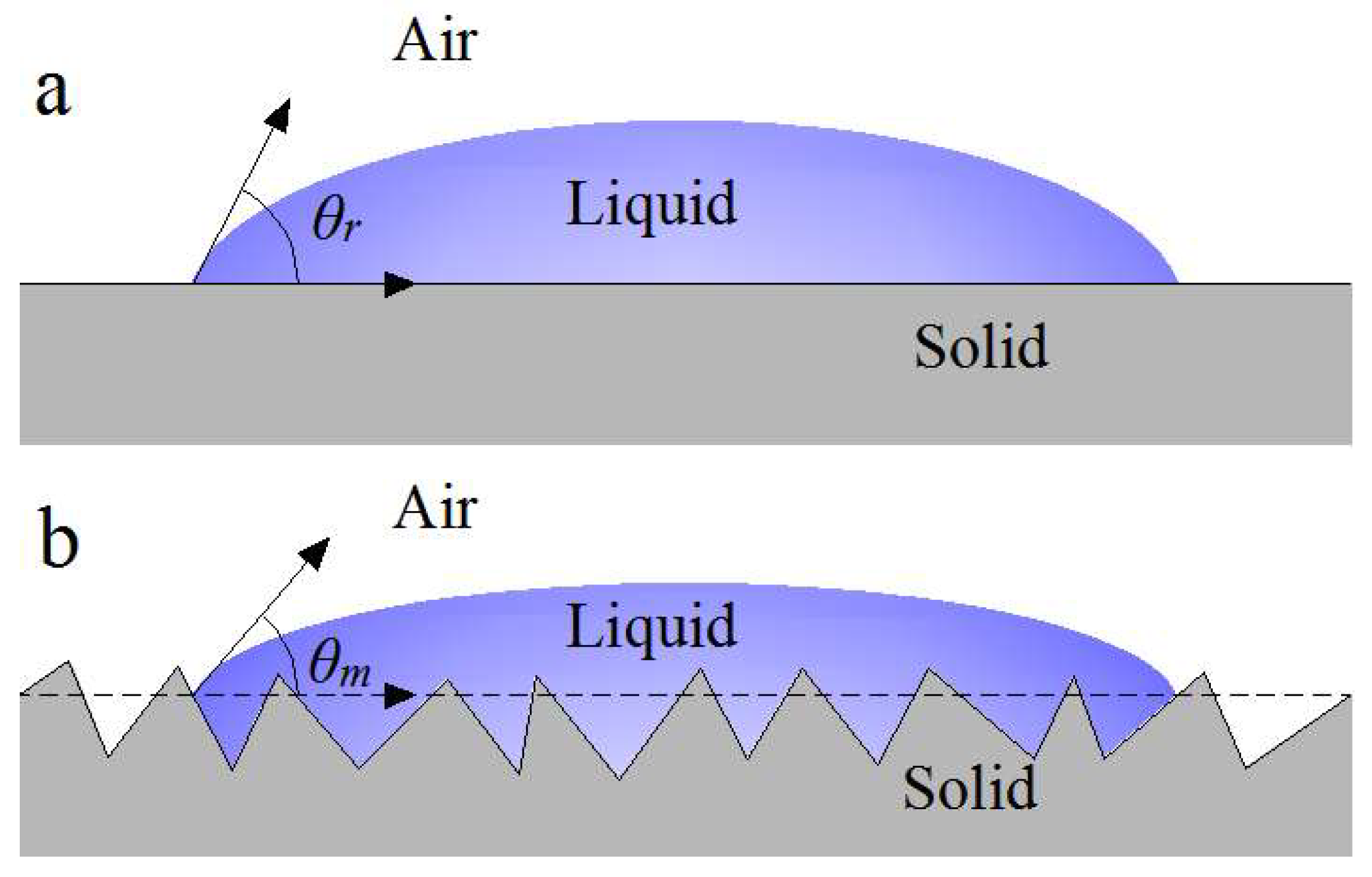
3.3. Surface Wetting Model
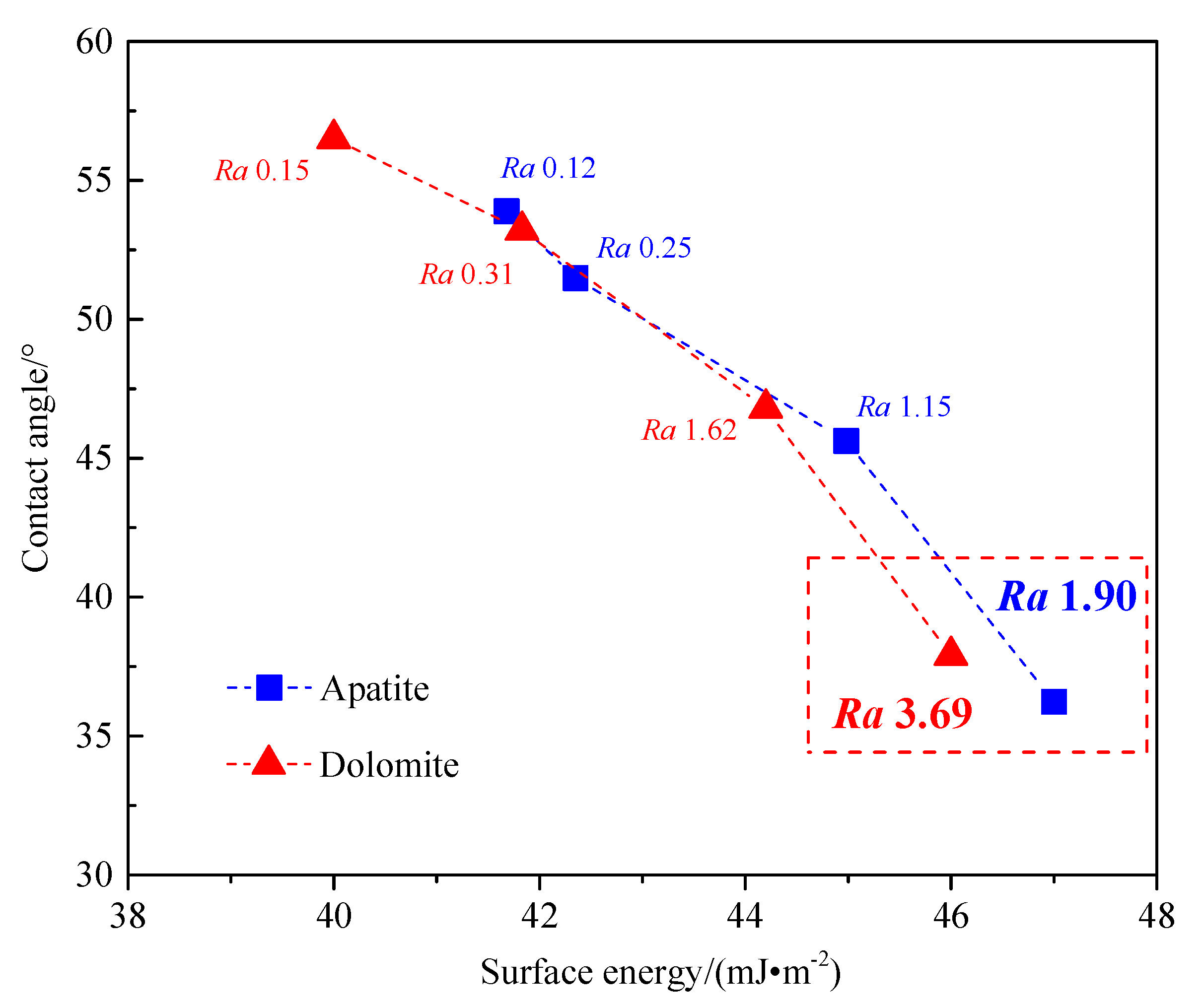
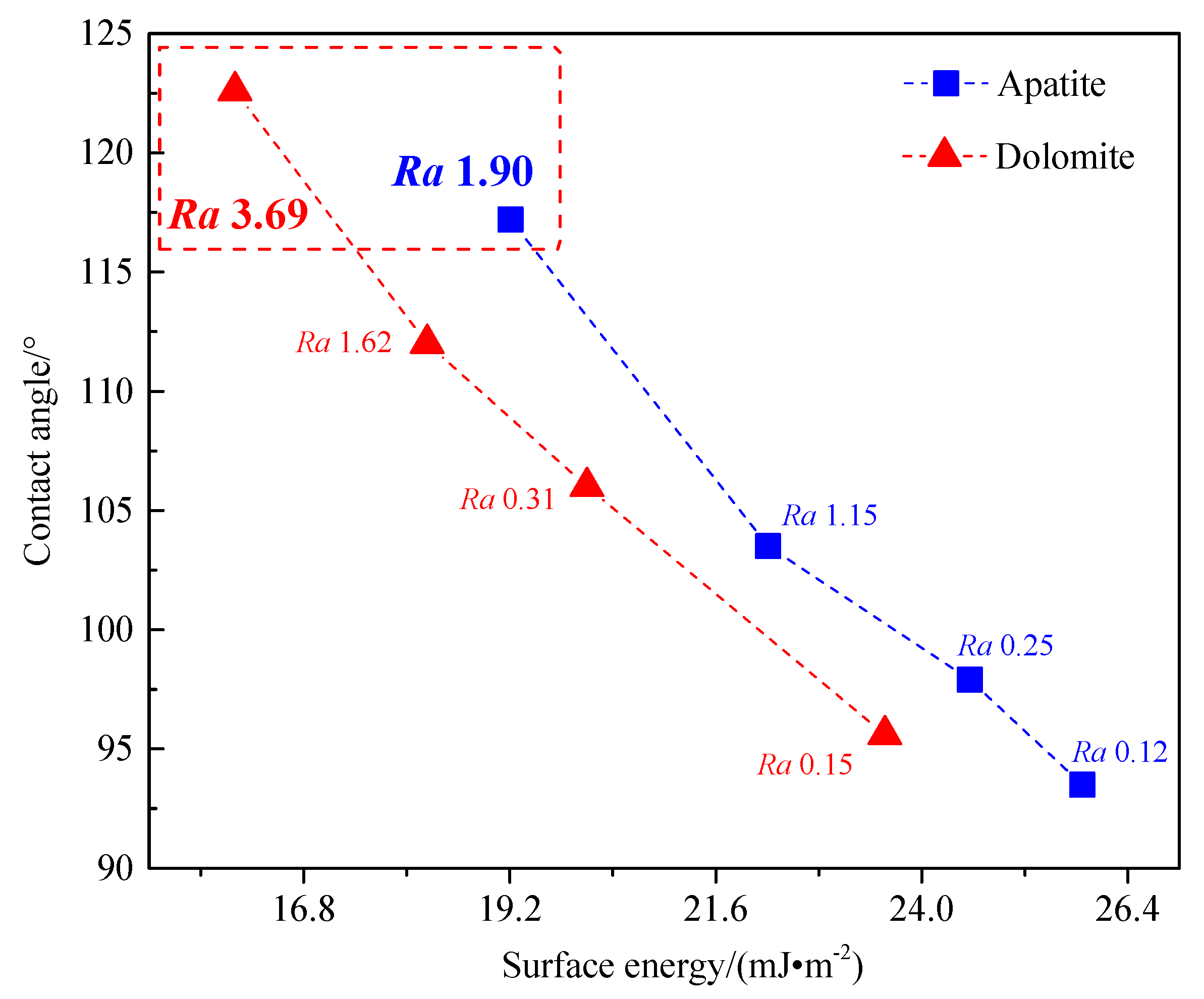
3.4. The Relationship between Surface Roughness, Surface Energy, and Wettability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Ge, Y.Y.; Guo, X.; Guo, W. The depression effect and mechanism of NSFC on dolomite in the flotation of phosphate ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Luo, H.; Cheng, R.; Liu, F. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Rao, S.R. Surface Chemistry of Froth Flotation, 2nd ed.; Springer Science and Business Media: New York, NY, USA, 2004. [Google Scholar]
- Ulusoy, U.; Hiçyılmaz, C.; Yekeler, M. Role of shape properties of calcite and barite particles on apparent hydrophobicity. Chem. Eng. Process. 2004, 43, 1047–1053. [Google Scholar] [CrossRef]
- Ulusoy, U.; Yekeler, M. Floatability of barite particles with different shape and roughness. Indian J. Chem. Techn. 2007, 14, 616–625. [Google Scholar]
- Ulusoy, U.; Yekeler, M.; Hiçyılmaz, C. Determination of the shape, morphological and wettability properties of quartz and their correlations. Miner. Eng. 2003, 16, 951–964. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Caliskan, H.; Guven, O.; Karakas, F.; Cinar, M.; Celik, M.S. Effect of roughness and shape factor on flotation characteristics of glass beads. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 88–99. [Google Scholar] [CrossRef]
- Xia, W.C. Role of particle shape in the floatability of mineral particle: An overview of recent advances. Powder Technol. 2017, 317, 104–116. [Google Scholar] [CrossRef]
- Xia, W.C.; Ma, G.X.; Bu, X.N.; Peng, Y.L. Effect of particle shape on bubble-particle attachment angle and flotation behavior of glass beads and fragments. Powder Technol. 2018, 338, 168–172. [Google Scholar] [CrossRef]
- Yekeler, M.; Ulusoy, U.; Hiçyılmaz, C. Effect of particle shape and roughness of talc mineral ground by different mills on the wettability and floatability. Powder Technol. 2004, 140, 68–78. [Google Scholar] [CrossRef]
- Wonyen, D.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S. A review of flotation feparation of Mg carbonates (dolomite and magnesite). Minerals 2018, 8, 354. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Adsorption and contact angle of single and binary mixtures of surfactants on apatite. Miner. Eng. 2003, 16, 839–848. [Google Scholar] [CrossRef]
- Santos, E.P.; Dutra, A.J.B.; Oliveira, J.F. The effect of jojoba oil on the surface properties of calcite and apatite aiming at their selective flotation. Int. J. Miner. Process. 2015, 143, 34–38. [Google Scholar] [CrossRef]
- Xia, W.C.; Yang, J.G. Changes in surface properties of anthracite coal before and after inside/outside weathering processes. Appl. Surf. Sci. 2014, 313, 320–324. [Google Scholar] [CrossRef]
- Xia, W.C.; Yang, J.G.; Liang, C. Investigation of changes in surface properties of bituminous coal during natural weathering processes by XPS and SEM. Appl. Surf. Sci. 2014, 293, 293–298. [Google Scholar] [CrossRef]
- Quast, K. Literature review on the interaction of oleate with non-sulphide minerals using zeta potential. Miner. Eng. 2016, 94, 10–20. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Chen, X.M.; Peng, Y.J. Understanding and improving the flotation of coals with different degrees of surface oxidation. Powder Technol. 2017, 321, 190–196. [Google Scholar] [CrossRef]
- Rahimi, M.; Aslani, M.R.; Rezai, B. Influence of surface roughness on flotation kinetics of quartz. J. Cent South Univ 2012, 19, 1206–1211. [Google Scholar] [CrossRef]
- Guven, O.; Ozdemir, O.; Karaagaclioglu, I.E.; Çelik, M.S. Surface morphologies and floatability of sand-blasted quartz particles. Miner. Eng. 2015, 70, 1–7. [Google Scholar] [CrossRef]
- Yekeler, M.; Ulusoy, U. Characterisation of surface roughness and wettability of salt-type minerals: Calcite and barite. Miner. Process. Extr. Metall. 2004, 113, 145–152. [Google Scholar] [CrossRef]
- Li, Z.L.; Rao, F.; Corona-Arroyo, M.A.; Bedolla-Jacuinde, A.; Song, S.X. Comminution effect on surface roughness and flotation behavior of malachite particles. Miner. Eng. 2019, 132, 1–7. [Google Scholar] [CrossRef]
- Karakas, F.; Hassas, B.V. Effect of surface roughness on interaction of particles in flotation. Physicochem. Probl. Miner. Process. 2016, 52, 18–34. [Google Scholar]
- Krasowska, M.; Malysa, K. Kinetics of bubble collision and attachment to hydrophobic solids: I. Effect of surface roughness. Int. J. Miner. Process. 2007, 81, 205–216. [Google Scholar] [CrossRef]
- Xing, Y.W.; Zhang, Y.F.; Liu, M.; Xu, M.D.; Guo, F.Y.; Han, H.S.; Gao, Z.Y.; Cao, Y.J.; Gui, X.H. Improving the floatability of coal with varying surface roughness through hypobaric treatment. Powder Technol. 2019, 345, 643–648. [Google Scholar] [CrossRef]
- Ulusoy, U.; Yekeler, M. Correlation of the surface roughness of some industrial minerals with their wettability parameters. Chem. Eng. Process. 2005, 44, 555–563. [Google Scholar] [CrossRef]
- Hicyilmaz, C.; Ulusoy, U.; Bilgen, S.; Yekeler, M. Flotation responses to the morphological properties of particles measured with three-dimensional approach. Int. J. Miner. Process. 2005, 75, 229–236. [Google Scholar] [CrossRef]
- Akdogan, G.; Hicyilmaz, C.; Ulusoy, U.; Bilgen, M.S. Response of rough and acute surfaces of pyrite with 3-D approach to the flotation. J. Min. Sci. 2006, 42, 393–402. [Google Scholar]
- Ahmed, M.M. Effect of comminution on particle shape and surface roughness and their relation to flotation process. Int. J. Miner. Process. 2010, 94, 180–191. [Google Scholar] [CrossRef]
- Xia, W.C.; Ni, C.; Xie, G.Y. The influence of surface roughness on wettability of natural/gold-coated ultra-low ash coal particles. Powder Technol. 2016, 288, 286–290. [Google Scholar] [CrossRef]
- Dippenaar, A. The destabilization of froth by solids. I. The mechanism of film rupture. Int. J. Miner. Process. 1982, 9, 1–14. [Google Scholar] [CrossRef]
- Dippenaar, A. The destabilization of froth by solids. II. The rate-determining step. Int. J. Miner. Process. 1982, 9, 15–22. [Google Scholar] [CrossRef]
- Chen, Y.R.; Xia, W.C.; Xie, G.Y. Contact angle and induction time of air bubble on flat coal surface of different roughness. Fuel 2018, 222, 35–41. [Google Scholar] [CrossRef]
- Hoang, D.H.; Hassanzadeh, A.; Peuker, U.A.; Rudolph, M. Impact of flotation hydrodynamics on the optimization of fine-grained carbonaceous sedimentary apatite ore beneficiation. Powder Technol. 2019, 345, 223–233. [Google Scholar] [CrossRef]
- Ruan, Y.Y.; He, D.S.; Chi, R.A. Review on beneficiation techniques and reagents used for phosphate ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.H.; Mao, S.; Li, L.J.; Ke, B.L.; Zhang, Q. Effects of structure of fatty acid collectors on the adsorption of fluorapatite (001) surface: A first-principles calculations. Appl. Surf. Sci. 2018, 444, 699–709. [Google Scholar] [CrossRef]
- Ye, J.J.; Zhang, Q.; Li, X.B.; Wang, X.C.; Ke, B.L.; Li, X.H.; Shen, Z.H. Effect of the morphology of adsorbed oleate on the wettability of a collophane surface. Appl. Surf. Sci. 2018, 444, 87–96. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, Q.; Hou, B.; Ye, J.J.; Mao, S.; Li, X.H. Flotation separation of quartz from collophane using an amine collector and its adsorption mechanisms. Powder Technol. 2017, 318, 224–229. [Google Scholar] [CrossRef]
- Gu, G.H.; Wang, H.; Suo, J.; Qiu, G.Z.; Hao, Y. Interfacial interaction of bio-leaching of pyrite mineral. J. Cent. South Univ. Technol. 2008, 15, 49–53. [Google Scholar] [CrossRef]
- Wang, H.; Gu, G.H.; Qiu, G.Z. Evaluation of surface free energy of polymers by contact angle goniometry. J. Cent. South Univ. 2006, 37, 943–948. [Google Scholar]
- Hirva, P.; Tikka, H. Ab initio study on the interaction of anionic collectors with calcite and dolomite surfaces. Langmuir 2002, 18, 5002–5006. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Fan, R.Y.; John, R.; Sun, W.; Hu, Y.H. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Jiang, T.; Liu, Q. Flotation of coarse and fine pyrochlore using octyl hydroxamic acid and sodium oleate. Miner. Eng. 2019, 132, 191–201. [Google Scholar] [CrossRef]
- Barros, L.A.F.; Ferreira, E.E.; Peres, A.E.C. Floatability of apatites and gangue minerals of an igneous phosphate ore. Miner. Eng. 2008, 21, 994–999. [Google Scholar] [CrossRef]
- Paiva, P.R.P.; Monte, M.B.M.; Sim O, R.A.; Gaspar, J.C. In situ AFM study of potassium oleate adsorption and calcium precipitate formation on an apatite surface. Miner. Eng. 2011, 24, 387–395. [Google Scholar] [CrossRef]
- Li, C.W.; Gao, Z.Y. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Rong, G.Q.; Xia, Y.C.; Zhang, Y.F.; Guo, F.Y.; Wang, D.Y.; Zhang, R.; Xing, Y.W.; Gui, X.H. Effect of Comminution methods on low-rank coal bubble-particle attachment/detachment: Implications for flotation. Minerals 2019, 9, 452. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxte, S. Wettability of porous surfaces. Trans. Faraday Soc. 1994, 40, 546–551. [Google Scholar] [CrossRef]
- Miller, J.D.; Veeramasuneni, S.; Drelich, J.; Yalamanchili, M.R.; Yamauchi, G. Effect of roughness as determined by microscopy on the wetting properties films. Polym. Eng. Sci. 1996, 36, 1849–1855. [Google Scholar] [CrossRef]


| Examined Liquid | ||||
|---|---|---|---|---|
| Second Purified Water | 72.8 | 21.8 | 25.5 | 25.5 |
| Diiodomethane | 50.8 | 50.8 | 0 | 0 |
| Formamide | 58.0 | 39.0 | 2.3 | 39.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Q. Insight into the Influence of Surface Roughness on the Wettability of Apatite and Dolomite. Minerals 2020, 10, 114. https://doi.org/10.3390/min10020114
Wang X, Zhang Q. Insight into the Influence of Surface Roughness on the Wettability of Apatite and Dolomite. Minerals. 2020; 10(2):114. https://doi.org/10.3390/min10020114
Chicago/Turabian StyleWang, Xianchen, and Qin Zhang. 2020. "Insight into the Influence of Surface Roughness on the Wettability of Apatite and Dolomite" Minerals 10, no. 2: 114. https://doi.org/10.3390/min10020114
APA StyleWang, X., & Zhang, Q. (2020). Insight into the Influence of Surface Roughness on the Wettability of Apatite and Dolomite. Minerals, 10(2), 114. https://doi.org/10.3390/min10020114