Separation of Products from Mineral Sequestration of CO2 with Primary and Secondary Raw Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Examination of Possible Input Material
2.2. Pretreatment
2.3. Lab-Scale Carbonation
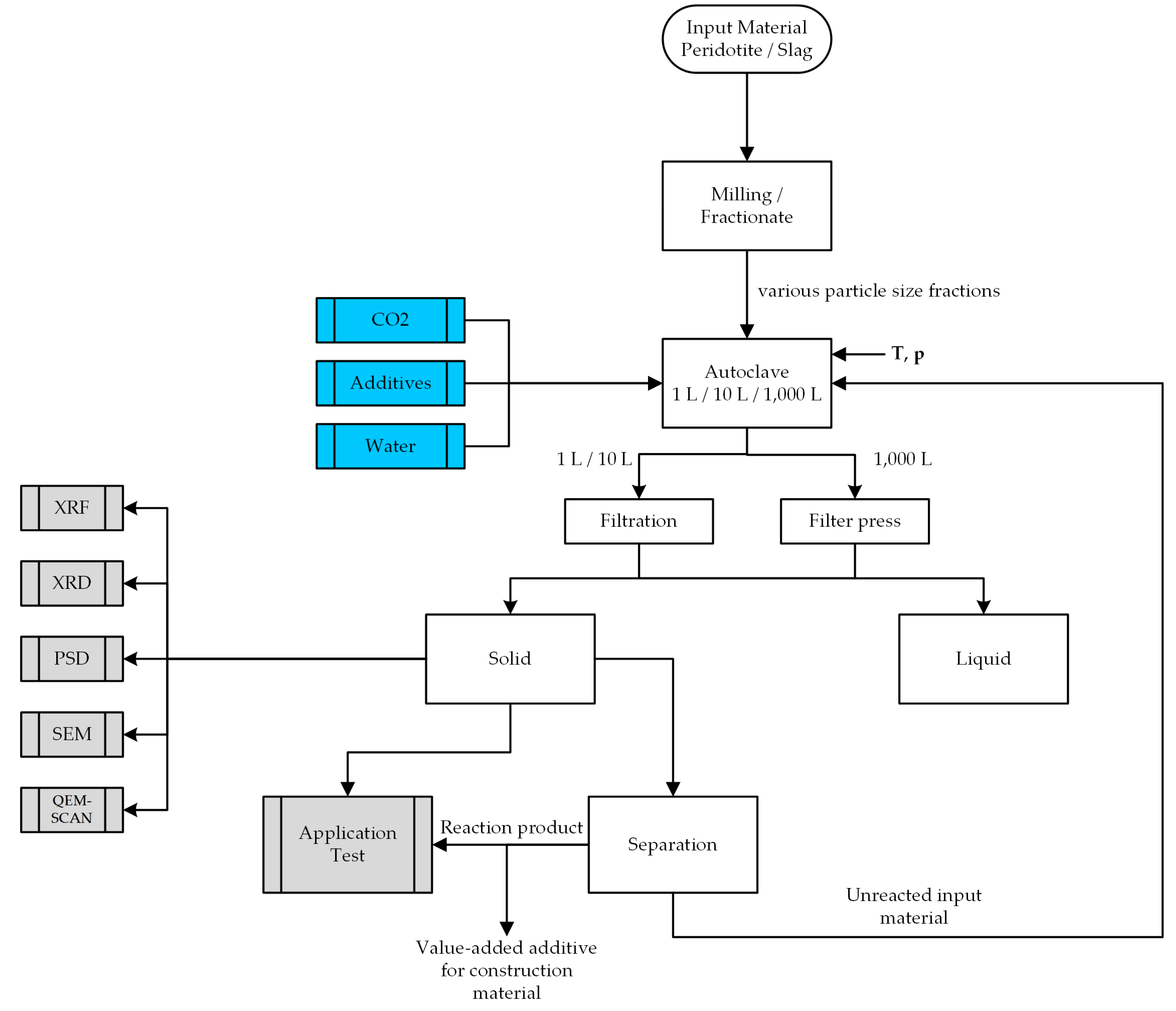
2.4. Experimental Conditions and Procedures
2.5. Separation of Carbonation Products
3. Results
3.1. Results of Flocculation
3.2. Results of Flotation
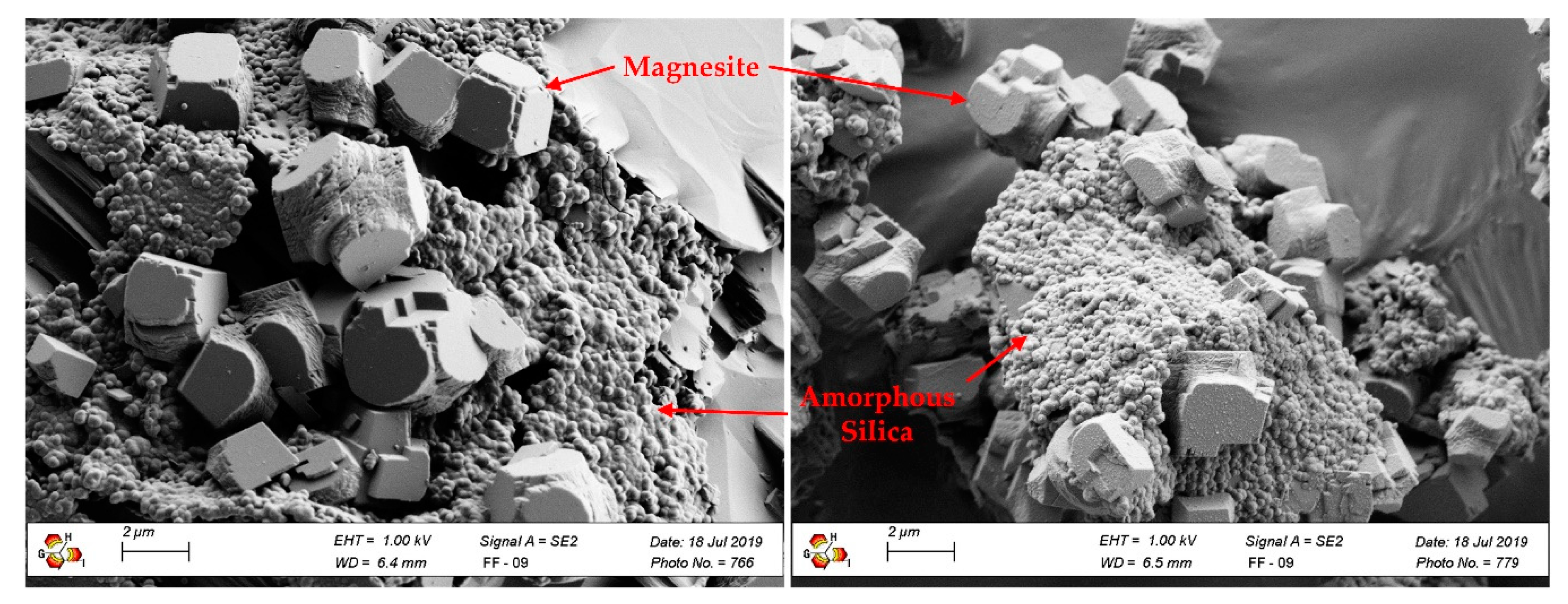
3.3. Results of Deagglomeration and Classification
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rebecca Lindsey. Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 5 November 2020).
- Blunden, J.; Arndt, D.S. State of the Climate in 2018. Bull. Amer. Meteor. Soc. 2019, 100, Si–S306. [Google Scholar] [CrossRef]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 297. [Google Scholar] [CrossRef]
- Huijgen, W.J.J. Carbon Dioxide Sequestration by Mineral Carbonation; Energy research Centre of the Netherlands (ECN): Petten, The Netherlands, 2007; ISBN 90-8504-573-8. [Google Scholar]
- Pan, S.-Y.; Chiang, A.; Chang, E.-E.; Lin, Y.-P.; Kim, H.; Chiang, P.-C. An Innovative Approach to Integrated Carbon Mineralization and Waste Utilization: A Review. Aerosol Air Qual. Res. 2015, 15, 1072–1091. [Google Scholar] [CrossRef]
- Ostovari, H.; Sternberg, A.; Bardow, A. Rock ‘n’ use of CO2: Carbon footprint of carbon capture and utilization by mineralization. Sustain. Energy Fuels 2020, 4, 4482–4496. [Google Scholar] [CrossRef]
- Kremer, D.; Etzold, S.; Boldt, J.; Blaum, P.; Hahn, K.M.; Wotruba, H.; Telle, R. Geological Mapping and Characterization of Possible Primary Input Materials for the Mineral Sequestration of Carbon Dioxide in Europe. Minerals 2019, 9, 485. [Google Scholar] [CrossRef]
- Bremen, A.M.; Ploch, T.; Mhamdi, A.; Mitsos, A. A mechanistic model of direct forsterite carbonation. Chem. Eng. J. 2021, 404, 126480. [Google Scholar] [CrossRef]
- Baris, K.; Ozarslan, A.; Sahin, N. The Assesment for CO2 Sequestration Potential by Magnesium silicate Minerals in Turkey: Cases of Orhaneli-Bursa and Divrigi-Sivas Regions. Energy Explor. Exploit. 2008, 26, 293–309. [Google Scholar] [CrossRef]
- Daval, D. Carbon dioxide sequestration through silicate degradation and carbon mineralisation: Promises and uncertainties. NPJ Mater. Degrad. 2018, 2, 1677. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 Emissions from Cement Production, 1928–2018; Earth System Science Data; Copernicus: Göttingen, Germany, 2019; Volume 11, pp. 1675–1710. [Google Scholar] [CrossRef]
- Gibbs, M.J.; Soyka, P.; Conneely, D. CO2 Emissions from Cement Production. Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change (ipcc): Geneva, Switzerland, 2000; Available online: https://www.ipcc-nggip.iges.or.jp/public/gp/bgp/3_1_Cement_Production.pdf (accessed on 10 November 2020).
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef]
- Rothon, R.; Paynter, C. Calcium Carbonate Fillers. In Fillers for Polymer Applications; Rothon, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 149–160. ISBN 978-3-319-28116-2. [Google Scholar]
- Khan, I.; Bhat, A.H. CHAPTER 16. Micro and Nano Calcium Carbonate Filled Natural Rubber Composites and Nanocomposites. In Natural Rubber Materials; Thomas, S., Han Chan, C., Pothen, L., Joy, J., Maria, H., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 467–487. ISBN 978-1-84973-631-2. [Google Scholar]
- Min, Y.; Jun, Y.-S. Wollastonite carbonation in water-bearing supercritical CO2: Effects of water saturation conditions, temperature, and pressure. Chem. Geol. 2018, 483, 239–246. [Google Scholar] [CrossRef]
- Huijgen, W.J.; Witkamp, G.-J.; Comans, R.N. Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chem. Eng. Sci. 2006, 61, 4242–4251. [Google Scholar] [CrossRef]
- Ryu, K.W.; Jo, H.; Choi, S.H.; Chae, S.C.; Jang, Y.-N. Changes in mineral assemblages during serpentine carbonation. Appl. Clay Sci. 2016, 134, 62–67. [Google Scholar] [CrossRef]
- Blencoe, J.G.; Anovitz, L.M.; Beard, J.S.; Palmer, D.A. Carbonation of Serpentine for Long-Term CO2 Sequestration; Final Report; FY 2003 ORNL Laboratory Directed Research and Development Annual Report Project Number: 3210-2024; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2003. [Google Scholar]
- Okrusch, M.; Matthes, S. Mineralogie; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-34659-0. [Google Scholar]
- O’Driscoll, M. Olivine-Going where the grass is greener. Ind. Miner. 2004, 438, 40–49. [Google Scholar]
- Boyd, R.; Gautneb, H. Mineral Resources in Norway: Potential and Strategic Importance, 2016 Update; Geological Survey of Norway: Trondheim, Norway, 2016; Available online: https://www.researchgate.net/publication/309807416 (accessed on 23 January 2020).
- Stopic, S.; Dertmann, C.; Modolo, G.; Kegler, P.; Neumeier, S.; Kremer, D.; Wotruba, H.; Etzold, S.; Telle, R.; Rosani, D.; et al. Synthesis of Magnesium Carbonate via Carbonation under High Pressure in an Autoclave. Metals 2018, 8, 993. [Google Scholar] [CrossRef]
- Stopic, S.; Dertmann, C.; Koiwa, I.; Kremer, D.; Wotruba, H.; Etzold, S.; Telle, R.; Knops, P.; Friedrich, B. Synthesis of Nanosilica via Olivine Mineral Carbonation under High Pressure in an Autoclave. Metals 2019, 9, 708. [Google Scholar] [CrossRef]
- Béarat, H.; McKelvy, M.J.; Chizmeshya, A.V.G.; Gormley, D.; Nunez, R.; Carpenter, R.W.; Squires, K.; Wolf, G.H. Carbon sequestration via aqueous olivine mineral carbonation: Role of passivating layer formation. Environ. Sci. Technol. 2006, 40, 4802–4808. [Google Scholar] [CrossRef]
- Eikeland, E.; Blichfeld, A.B.; Tyrsted, C.; Jensen, A.; Iversen, B.B. Optimized carbonation of magnesium silicate mineral for CO2 storage. ACS Appl. Mater. Interfaces 2015, 7, 5258–5264. [Google Scholar] [CrossRef]
- Lazaro, A.; Brouwers, H.; Quercia, G.; Geus, J.W. The properties of amorphous nano-silica synthesized by the dissolution of olivine. Chem. Eng. J. 2012, 211–212, 112–121. [Google Scholar] [CrossRef]
- Raza, N.; Raza, W.; Madeddu, S.; Agbe, H.; Kumar, R.V.; Kim, K.-H. Synthesis and characterization of amorphous precipitated silica from alkaline dissolution of olivine. RSC Adv. 2018, 8, 32651–32658. [Google Scholar] [CrossRef]
- Matus, C.; Stopic, S.; Etzold, S.; Kremer, D.; Wotruba, H.; Dertmann, C.; Telle, R.; Friedrich, B.; Knops, P. Mechanism of Nickel, Magnesium, and Iron Recovery from Olivine Bearing Ore during Leaching with Hydrochloric Acid Including a Carbonation Pre-Treatment. Metals 2020, 10, 811. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Dahlin, C.L.; Collins, W.K. Carbon Dioxide Sequestration by Direct Mineral Carbonation: Process Mineralogy of Feed and Products: 2001. In Proceedings of the 2001 SME Annual Meeting & Exhibit, Denver, CO, USA, 26–28 February 2001; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2001. [Google Scholar]
- Ding, K.; Laskowski, J.S. Application of a modified water glass in a cationic flotation of calcite and dolomite. Can. Metall. Q. 2013, 45, 199–206. [Google Scholar] [CrossRef]
- Yao, J.; Hou, Y.; Wang, Y.L.; Zhong, W.X.; Yin, W.Z. Research on the Floatability of Magnesite and its Gangue Minerals with Sodium Oleate and Lauryl Amine as Collectors. AMR 2013, 798–799, 328–332. [Google Scholar] [CrossRef]
- Katzmarzyk, J.L.; Silin, I.; Hahn, K.M.; Wotruba, H.; Gerold, C.; Stapelmann, M. Investigation on flotation behavior of a copper sulfide ore after grinding by Loesche vertical roller mill: Project: Flotation of sulphides after dry comminution. In Proceedings of the 58th Annual Conference of Metallurgists (COM 2019), Vancouver, BC, Canada, 18–21 August 2019; The Metallurgy and Materials Society (MetSoc): Westmount, QC, Canada, 2019. [Google Scholar]
- Achimovičová, M.; Vonderstein, C.; Friedrich, B. Titanium Dioxide. Mechanically Activated Rutile and Ilmenite as the Starting Materials for Process of Titanium Alloys Production; InTechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Schosseler, J.; Trentmann, A.; Friedrich, B.; Hahn, K.; Wotruba, H. Kinetic Investigation of Silver Recycling by Leaching from Mechanical Pre-Treated Oxygen-Depolarized Cathodes Containing PTFE and Nickel. Metals 2019, 9, 187. [Google Scholar] [CrossRef]
- Silin, I.; Huben, J.; Wotruba, H.; Ognyanova, A. Study on the Characterisation and Processing of Iron Ore after Grinding by HPGR. In Proceedings of the 29th International Mineral Processing Congress (IMPC 2018), Moscow, Russia, 17–21 September 2018; “Ore and Metals” Publishing house: Moscow, Russia; pp. 17–21. [Google Scholar]


| Component [wt.%] | Olivine | Ferrochrome Slag |
|---|---|---|
| Norway | Germany | |
| SiO2 | 48.6 | 32.7 |
| Al2O3 | 0.5 | 6.3 |
| Fe2O3 | 7.8 | 0.2 |
| TiO2 | 0.0 | 0.2 |
| CaO | 0.2 | 43.9 |
| MgO | 41.1 | 11.6 |
| K2O | 0.1 | 0.1 |
| Na2O | 0.0 | 0.0 |
| NiO | 1.2 | 0.0 |
| Cr2O3 | 0.4 | 4.7 |
| Experimental Parameters | |
|---|---|
| Temperature: | 175 °C |
| Pressure: | p0 = 17.5 bar, pmax = 30 bar (limitation of 1000 L autoclave) |
| Stirring Speed: | 600 rpm |
| Retention Time: | 4 h |
| Solid/Liquid Ratio: | 1:8 |
| Additives: | 0.64 M sodium bicarbonate 0.05 M oxalic acid 0.01 M ascorbic acid |
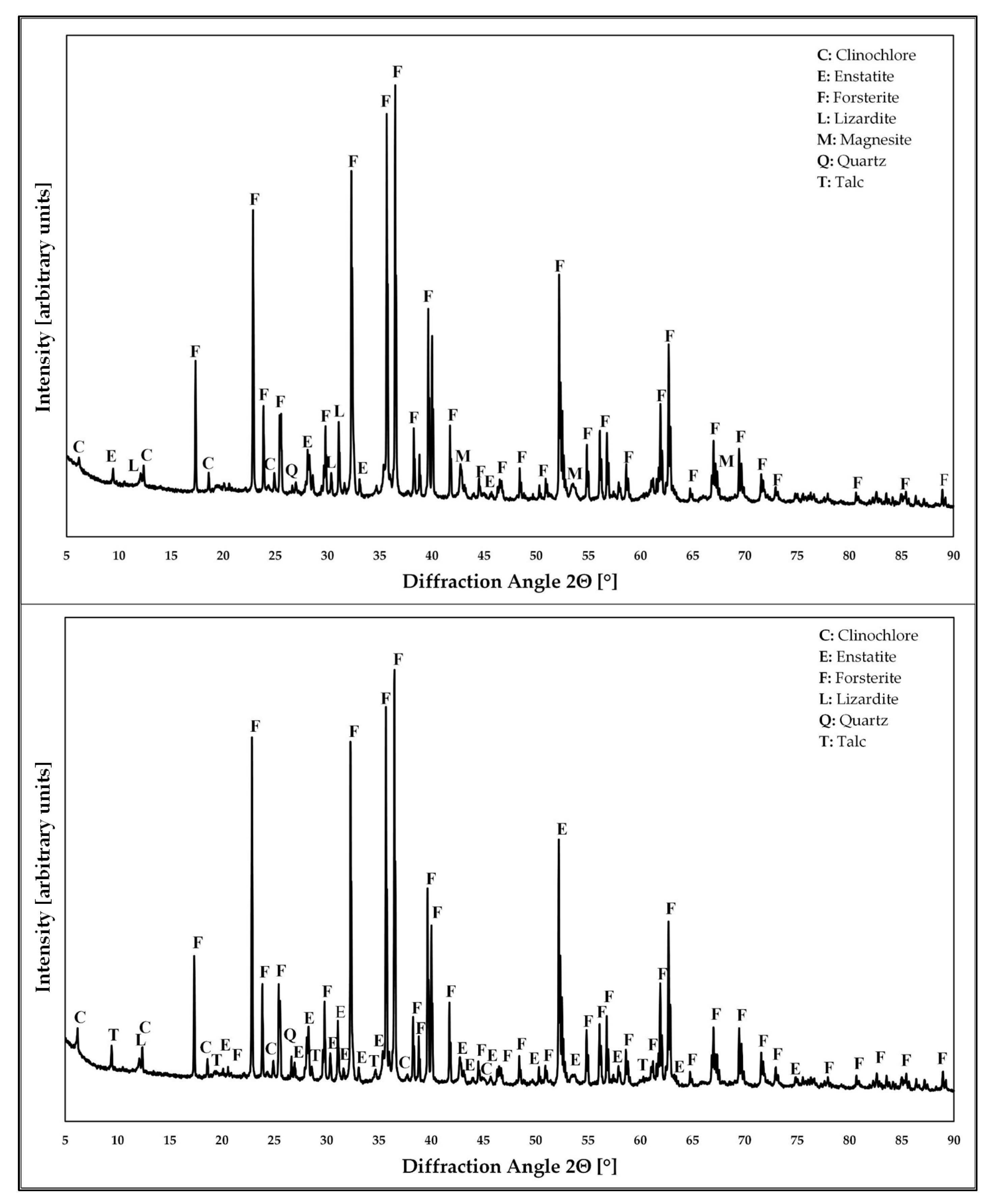
| Mineral Phase Fraction [%] | Olivine (Steinsvik) | ||
|---|---|---|---|
| Input Material | Carbonation Product | Flotation Product | |
| Forsterite | 75–80 | 50–55 | 45–50 |
| Enstatite | 10–15 | 10–15 | 5–10 |
| Lizardite | ≤5 | ≤5 | ≤5 |
| Clinochlore | ≤5 | ≤5 | ≤5 |
| Talc | ≤5 | ≤5 | ≤5 |
| Magnesite | - | 20–25 | 30–35 |
| Amorphous Silica | - | 5–10 | indeterminable |
| Mineral Phase Fraction [%] | Carbonation Product 0–20 µm | 0–5 µm | 5–11 µm | 11–15 µm |
|---|---|---|---|---|
| Mass fraction | 100 * | ~45 | ~40 | ~12 |
| Forsterite | 50–55 | 45–50 | 65–70 | 70–75 |
| Magnesite | 20–25 | 30–35 | 10–15 | 5–10 |
| Enstatite | 10–15 | 5–10 | 10–15 | 10–15 |
| Clinochlore | 5–10 | ≤5 | ≤5 | ≤5 |
| Talc | ≤5 | ≤5 | ≤5 | ≤5 |
| Lizardite | ≤5 | ≤5 | ≤5 | ≤5 |
| Quartz | ≤5 | ≤5 | ≤5 | ≤5 |
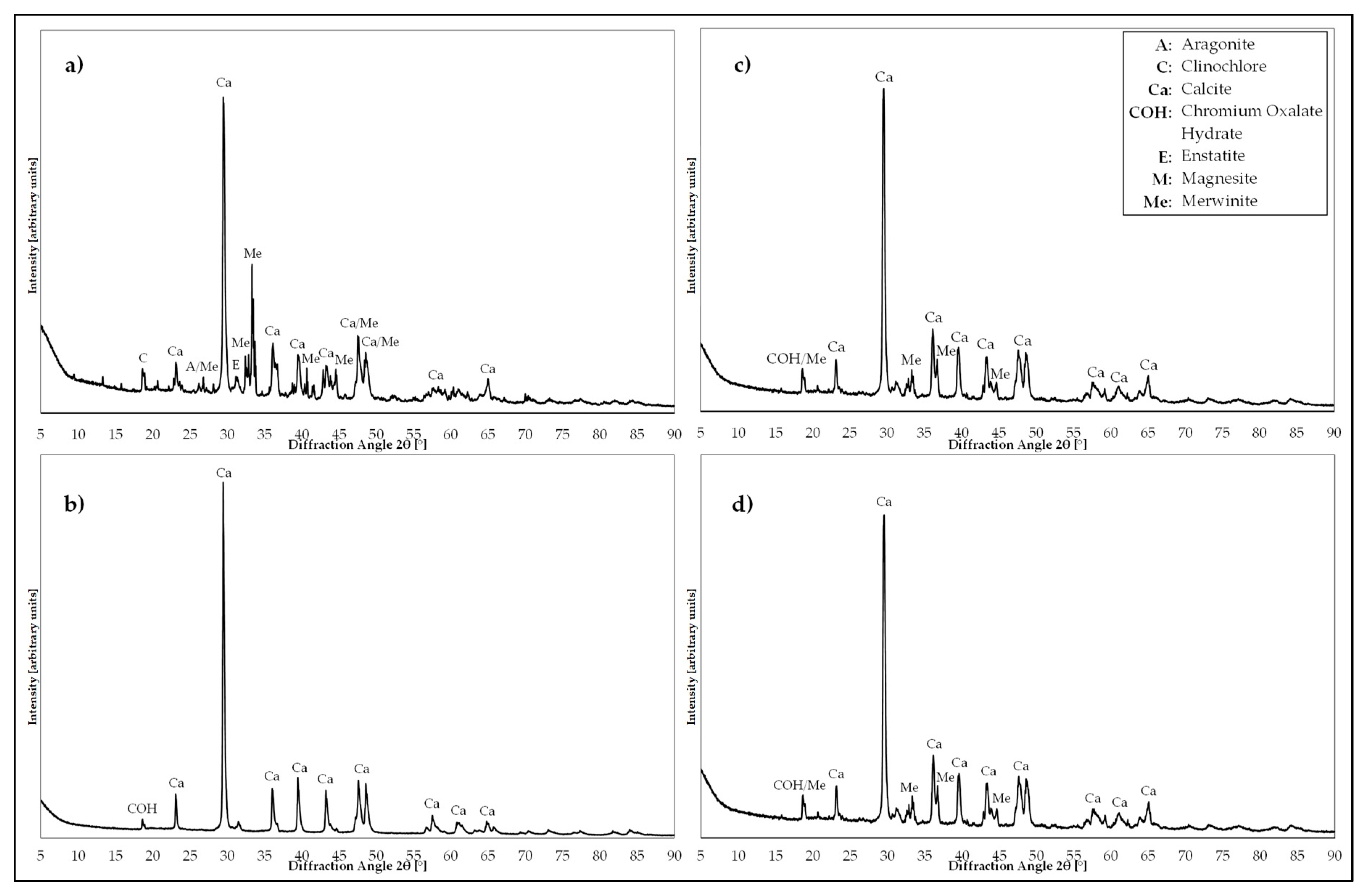
| Mineral Phase Fraction [%] | Carbonation Product 0–63 µm | 0–5 µm | 5–11 µm | 11–15 µm | 15–20 µm |
|---|---|---|---|---|---|
| Mass Fraction | 100 * | ~10 | ~25 | ~10 | ~8 |
| Calcite | 50–55 | 100 ** | 100 ** | 95–100 | 75–80 |
| Merwinite | 15–20 | 5–10 | 20–25 | ||
| Magnesite | 5–10 | below detection limit (bdl) | bdl | bdl | bdl |
| Aragonite | ≤5 | ||||
| Clinochlore | 10–15 | ||||
| Forsterite | 5–10 | ||||
| Enstatite | ≤5 | ||||
| Quartz | ≤5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremer, D.; Wotruba, H. Separation of Products from Mineral Sequestration of CO2 with Primary and Secondary Raw Materials. Minerals 2020, 10, 1098. https://doi.org/10.3390/min10121098
Kremer D, Wotruba H. Separation of Products from Mineral Sequestration of CO2 with Primary and Secondary Raw Materials. Minerals. 2020; 10(12):1098. https://doi.org/10.3390/min10121098
Chicago/Turabian StyleKremer, Dario, and Hermann Wotruba. 2020. "Separation of Products from Mineral Sequestration of CO2 with Primary and Secondary Raw Materials" Minerals 10, no. 12: 1098. https://doi.org/10.3390/min10121098
APA StyleKremer, D., & Wotruba, H. (2020). Separation of Products from Mineral Sequestration of CO2 with Primary and Secondary Raw Materials. Minerals, 10(12), 1098. https://doi.org/10.3390/min10121098





