Micro-Raman Spectroscopy Assessment of Chemical Compounds of Mantle Clinopyroxenes
Abstract
1. Introduction
2. Materials and Methods
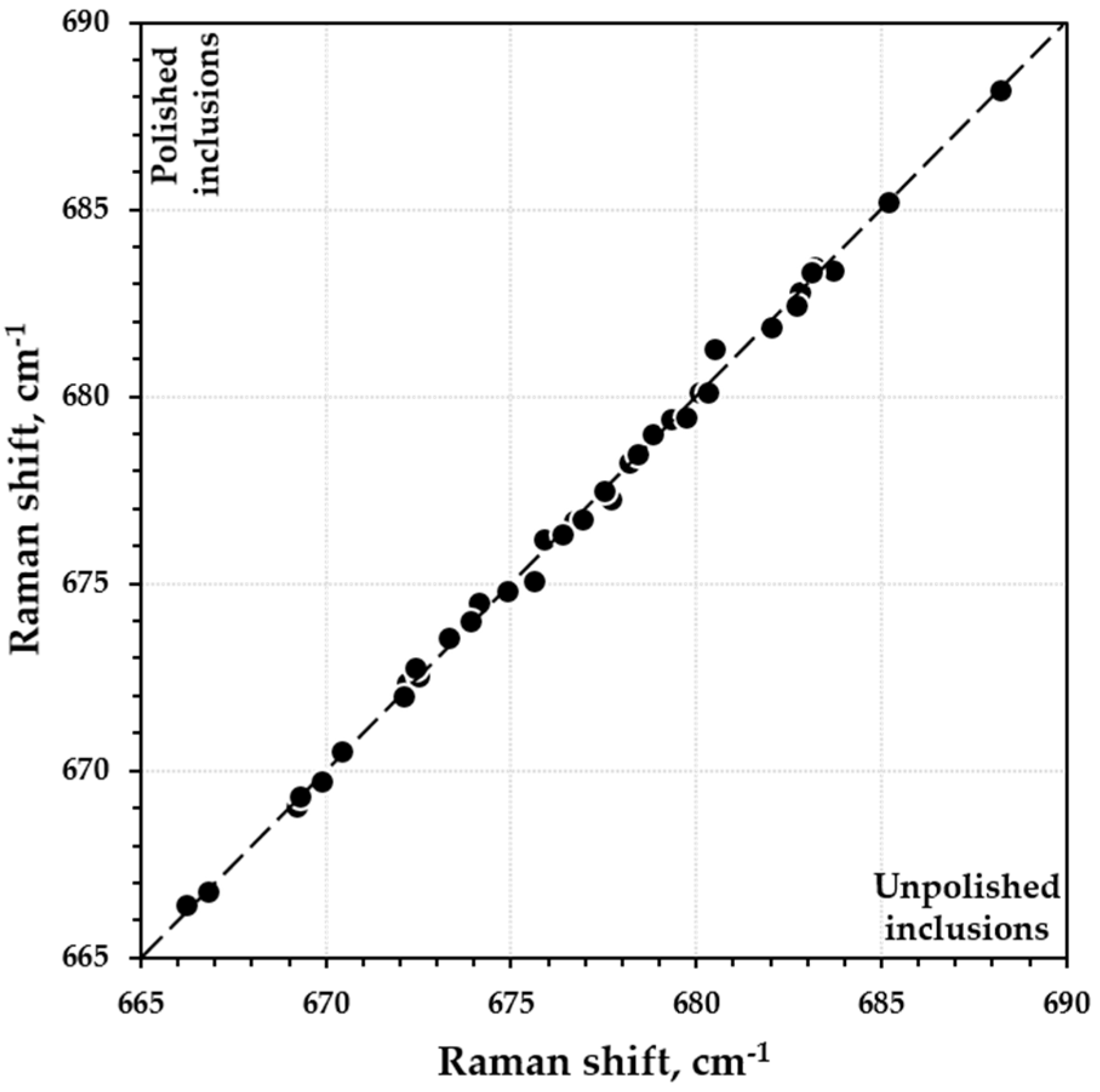
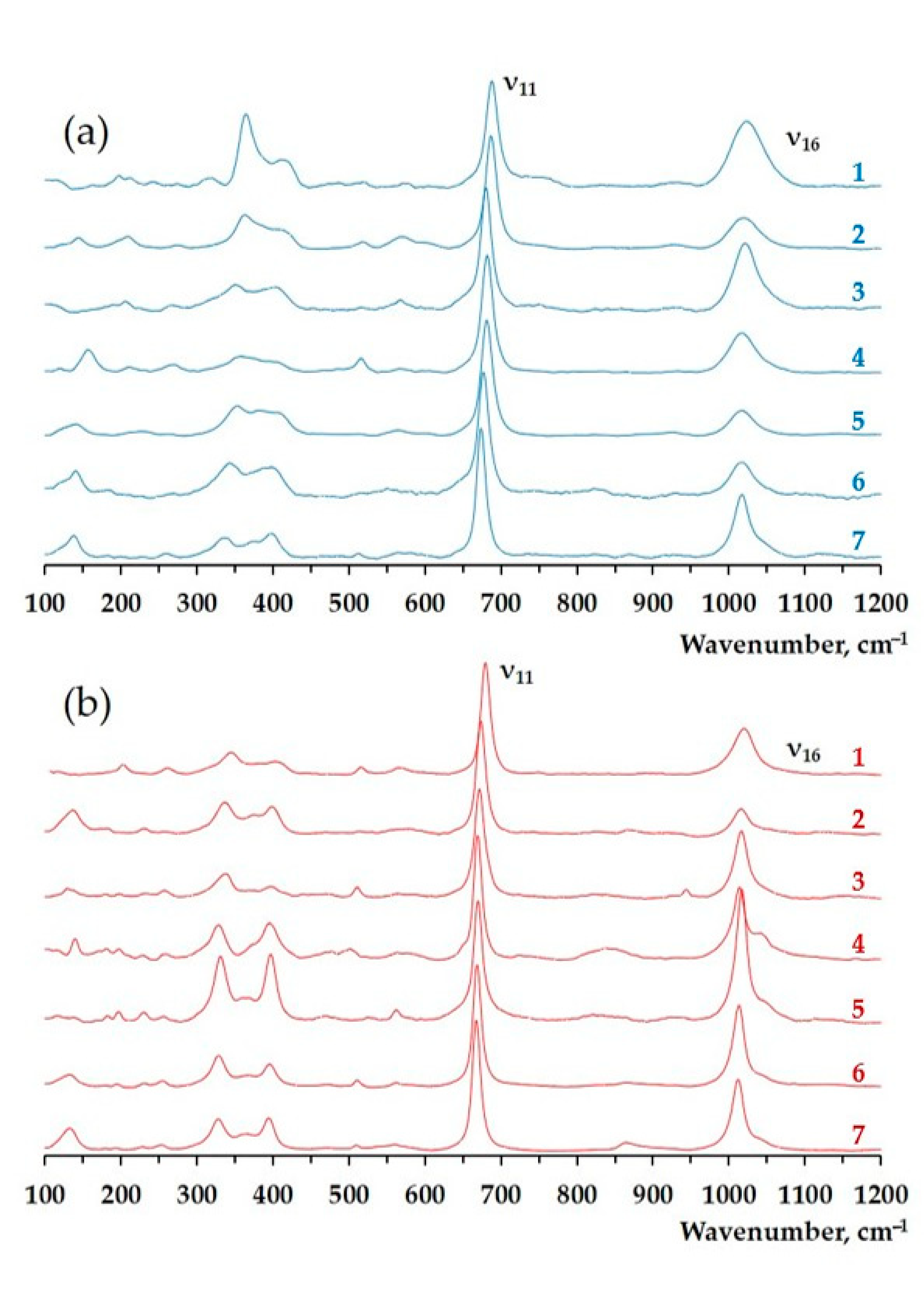
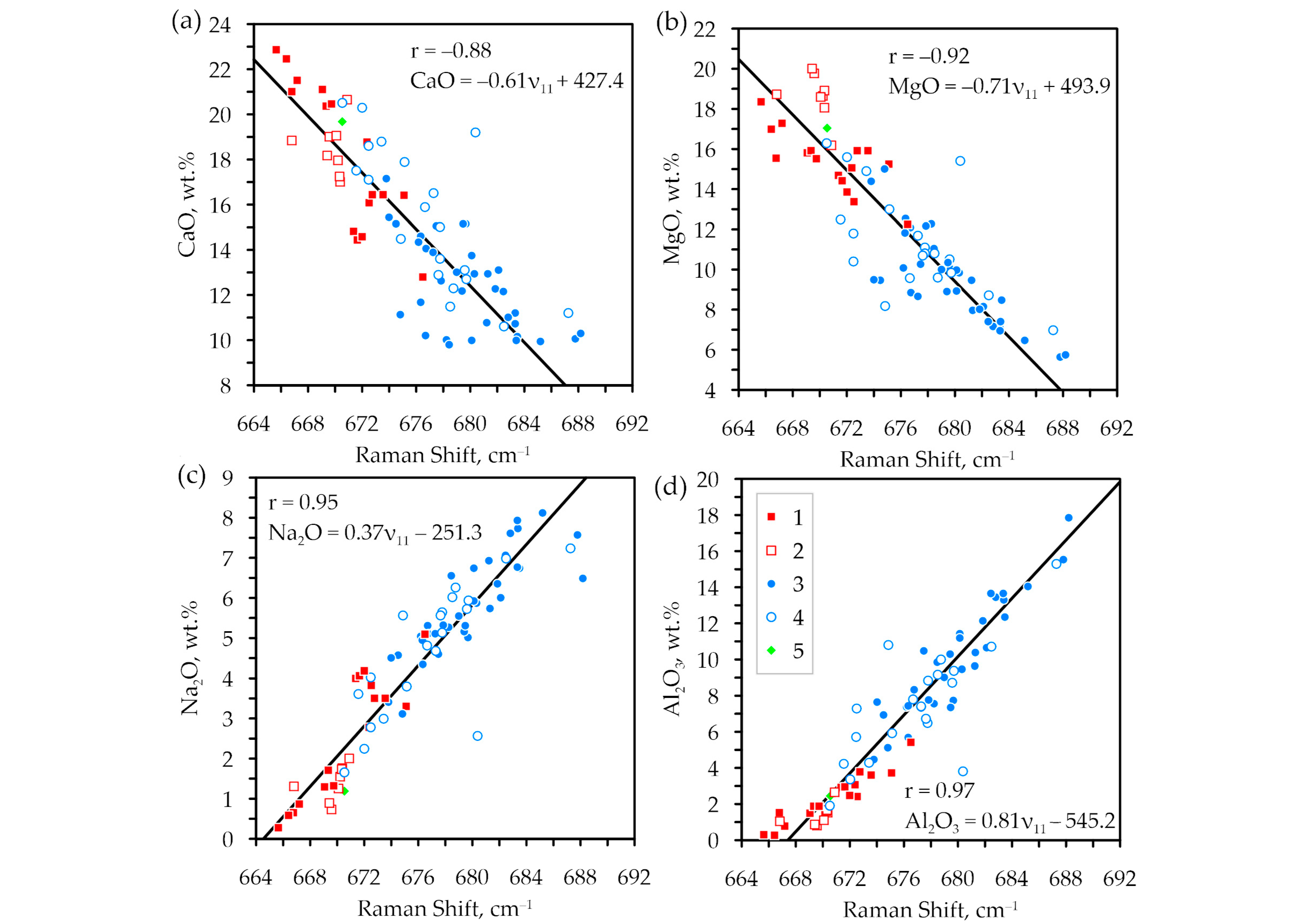
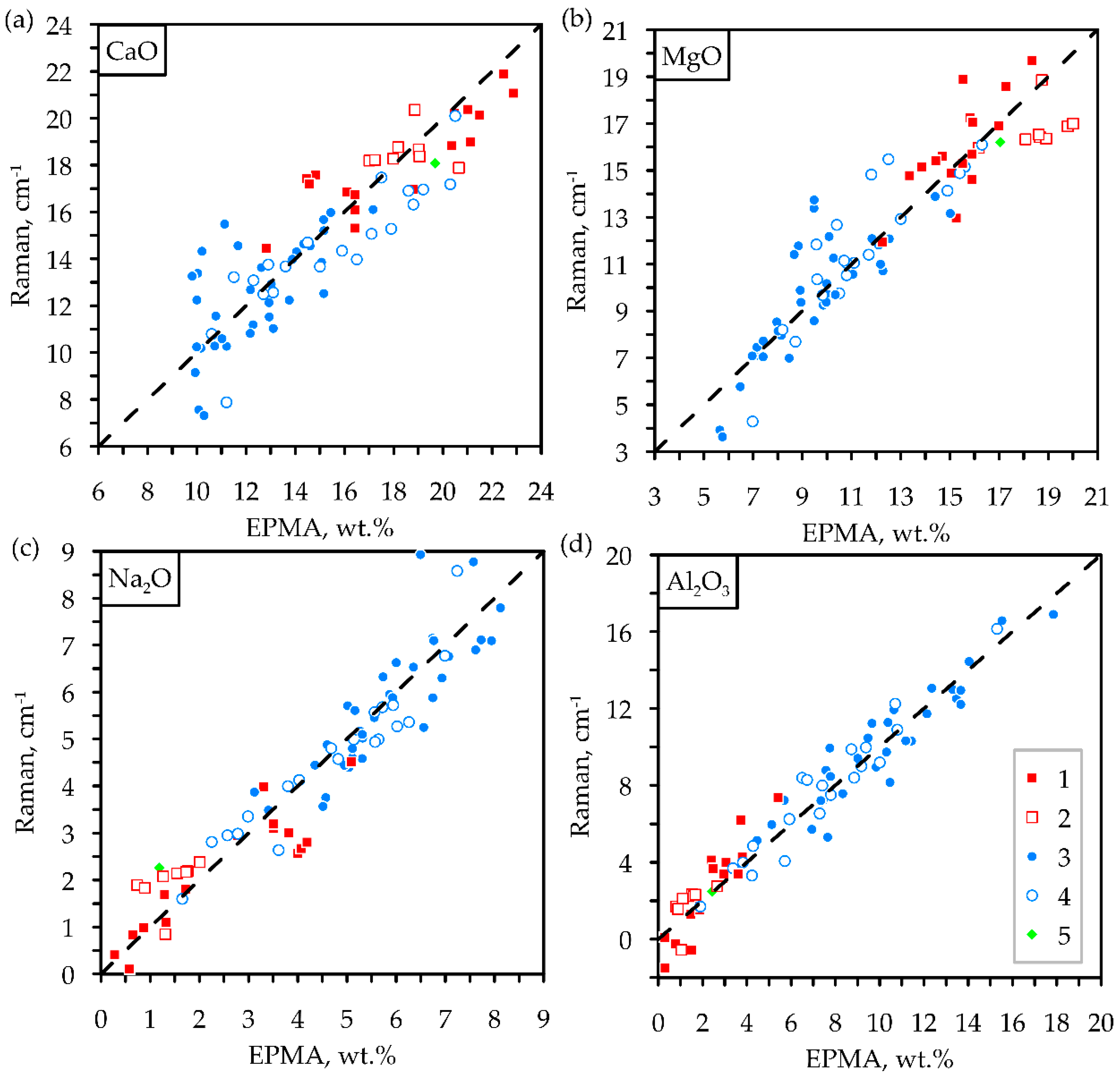
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Sobolev, N.V. Deep Inclusions in Kimberlites and the Problem of the Upper Mantle Composition; Sobolev, V.S., Ed.; Nauka: Moscow, Russia, 1974. [Google Scholar]
- Stachel, T.; Harris, J.W. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Taylor, L.A.; Neal, C.R. Eclogites with oceanic crustal and mantle signatures from the Bellsbank kimberlite, South Africa, Part I: Mineralogy, petrography, and whole rock chemistry. J. Geol. 1989, 97, 551–567. [Google Scholar] [CrossRef]
- Liu, L.G.; Mernagh, T.P.; Jaques, A.L. A mineralogical Raman spectroscopy study on eclogitic garnet inclusions in diamonds from Argyle. Contrib. Mineral. Petrol. 1990, 105, 156–161. [Google Scholar] [CrossRef]
- Izraeli, E.S.; Harris, J.W.; Navon, O. Raman barometry of diamond formation. Earth Planet. Sci. Lett. 1990, 173, 351–360. [Google Scholar] [CrossRef]
- McMillan, P. Theory and practice–lattice vibrations and spectroscopy of mantle phases. In Treatise on Geophysics; Price, D.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 2, pp. 153–196. [Google Scholar]
- Kagi, H.; Odake, S.; Fukura, S.; Zedgenizov, D.A. Raman spectroscopic estimation of depth of diamond origin: Technical developments and the application. Russ. Geol. Geophys. 2009, 50, 1183–1187. [Google Scholar] [CrossRef]
- Nestola, F.; Alvaro, M.; Casati, M.N.; Wilhelm, H.; Kleppe, A.K.; Jephcoat, A.P.; Domeneghetti, M.C.; Harris, J.W. Source assemblage types for cratonic diamonds from X-ray synchrotron diffraction. Lithos 2016, 265, 334–338. [Google Scholar] [CrossRef]
- Kalugina, A.D.; Zedgenizov, D.A. Raman discrimination of garnet inclusions in Siberian diamonds. J. Raman Spectrosc. 2020, 51, 1438–1444. [Google Scholar] [CrossRef]
- Chopelas, A.; Serghiou, G. Spectroscopic evidence for pressure-induced phase transitions in diopside. Phys. Chem. Miner. 2002, 29, 403–408. [Google Scholar] [CrossRef]
- Lin, C.C. Pressure-induced polymorphism in enstatite (MgSiO3) at room temperature: Clinoenstatite and orthoenstatite. J. Phys. Chem. Solids 2004, 65, 913–921. [Google Scholar] [CrossRef]
- Kolesnichenko, M.V.; Zedgenizov, D.A.; Litasov, K.D.; Safonova, I.Y.; Ragozin, A.L. Heterogeneous distribution of water in the mantle beneath the central Siberian Craton: Implications from the Udachnaya Kimberlite Pipe. Gondwana Res. 2017, 47, 249–266. [Google Scholar] [CrossRef]
- Kolesnichenko, M.V.; Zedgenizov, D.A.; Ragozin, A.L.; Litasov, K.D.; Shatsky, V.S. The role of eclogites in the redistribution of water in the subcontinental mantle of the Siberian craton: Results of determination of the water content in minerals from the Udachnaya pipe eclogites. Russ. Geol. Geophys. 2018, 59, 763–779. [Google Scholar] [CrossRef]
- Lavrent’ev, Y.G.; Karmanov, N.S.; Usova, L.V. Electron probe microanalysis of minerals: Microanalyzer or scanning electron microscope? Russ. Geol. Geophys. 2015, 56, 1154–1161. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Zedgenizov, D.A.; Ragozin, A.L.; Kalinina, V.V. Diamondiferous subcontinental lithospheric mantle of the northeastern Siberian Craton: Evidence from mineral inclusions in alluvial diamonds. Gondwana Res. 2015, 28, 106–120. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Kalinina, V.V.; Malkovets, V.G.; Pomazansky, B.S. Mineral inclusions in diamonds from Nyurbinskaya kimberlite pipe (Yakutia). In Geology and Mineral Resources of the North-East of Russia, Proceedings of Russian Scientific-Practical Conference “Geology and Mineral Resources of the North-East of Russia”, Yakutsk, Russia, 31 March–2 April 2015; Biller, A.Y., Ed.; M.K. Ammosov North-Eastern Federal University: Yakutsk, Russia, 2015; pp. 173–176. [Google Scholar]
- Gubanov, N.; Zedgenizov, D.; Sharygin, I.; Ragozin, A. Origin and evolution of high-Mg carbonatitic and low-Mg carbonatitic to silicic high-density fluids in coated diamonds from Udachnaya kimberlite pipe. Minerals 2019, 9, 734. [Google Scholar] [CrossRef]
- Smith, D.C. The RAMANITA1© method for non-destructive and in situ semi-quantitative chemical analysis of mineral solid-solutions by multidimensional calibration of Raman wavenumber shifts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 61, 2299–2314. [Google Scholar] [CrossRef]
- Wang, A.; Jolliff, B.L.; Haskin, L.A.; Kuebler, K.E.; Viskupic, K.M. Characterization and comparison of structural and compositional features of planetary quadrilateral pyroxenes by Raman spectroscopy. Am. Mineral. 2001, 86, 760–806. [Google Scholar] [CrossRef]
- Mernagh, T.P.; Hoatson, D.M. Raman Spectroscopic Study of Pyroxene Structures from the Layered Intrusion, Munni Munni Western Australia. J. Raman Spectrosc. 1997, 28, 647–658. [Google Scholar] [CrossRef]
- Chopelas, A. Estimates of mantle relevant Clapeyron slopes in the MgSiO3 system from high-pressure spectroscopic data. Am. Mineral. 1999, 84, 233–244. [Google Scholar] [CrossRef]
- Huang, E.; Chen, C.H.; Huang, T.; Lin, E.H.; Xu, J.A. Raman spectroscopic characteristics of Mg-Fe-Ca pyroxenes. Am. Mineral. 2000, 85, 473–479. [Google Scholar] [CrossRef]
- Prencipe, M.; Maschio, L.; Kirtman, B.; Salustro, S.; Erba, A.; Dovesi, R. Raman spectrum of NaAlSi2O6 jadeite. A quantum mechanical simulation. J. Raman Spectrosc. 2014, 45, 703–709. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Pyroxene Group. In An Introduction to the Rock-Forming Minerals, 2nd ed.; Longman Scientific & Technical: London, UK, 1992. [Google Scholar]
- Safonov, O.G.; Litvin, Y.A.; Perchuk, L.L. Synthesis of omphacites and isomorphic features of clinopyroxenes in the system CaMgSi2O6–NaAlSi2O6–KAlSi2O6. Petrology. 2004, 12, 84–97. [Google Scholar]
- Papike, J.J.; Karner, J.M.; Shearer, C.K. Comparative planetary mineralogy: Valence state partitioning of Cr, Fe, Ti, and V among crystallographic sites in olivine, pyroxene, and spinel from planetary basalts. Am. Mineral. 2005, 90, 277–290. [Google Scholar] [CrossRef]
- Compomenosi, N.; Mazzucchelli, M.L.; Mihailova, B.; Scambelluri, M.; Angel, R.J.; Nestola, F.; Reali, A.; Alvaro, M. How geometry and anisotropy affect residual strain in host-inclusion systems: Coupling experimental and numerical approaches. Am. Mineral. 2018, 103, 2032–2035. [Google Scholar] [CrossRef]



| SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MgO | CaO | Na2O | ν11 | |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 1 | ||||||||
| TiO2 | −0.06 | 1 | |||||||
| Al2O3 | 0.27 | 0.48 | 1 | ||||||
| Cr2O3 | −0.11 | −0.32 | −0.51 | 1 | |||||
| FeO | −0.40 | 0.36 | 0.33 | −0.49 | 1 | ||||
| MgO | −0.09 | −0.51 | −0.95 | 0.42 | −0.45 | 1 | |||
| CaO | −0.23 | −0.47 | −0.86 | 0.35 | −0.52 | 0.80 | 1 | ||
| Na2O | 0.19 | 0.50 | 0.94 | −0.37 | 0.42 | −0.94 | −0.92 | 1 | |
| ν11 | 0.27 | 0.42 | 0.97 | −0.48 | 0.36 | −0.92 | −0.88 | 0.95 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalugina, A.D.; Zedgenizov, D.A. Micro-Raman Spectroscopy Assessment of Chemical Compounds of Mantle Clinopyroxenes. Minerals 2020, 10, 1084. https://doi.org/10.3390/min10121084
Kalugina AD, Zedgenizov DA. Micro-Raman Spectroscopy Assessment of Chemical Compounds of Mantle Clinopyroxenes. Minerals. 2020; 10(12):1084. https://doi.org/10.3390/min10121084
Chicago/Turabian StyleKalugina, Anastasiya D., and Dmitry A. Zedgenizov. 2020. "Micro-Raman Spectroscopy Assessment of Chemical Compounds of Mantle Clinopyroxenes" Minerals 10, no. 12: 1084. https://doi.org/10.3390/min10121084
APA StyleKalugina, A. D., & Zedgenizov, D. A. (2020). Micro-Raman Spectroscopy Assessment of Chemical Compounds of Mantle Clinopyroxenes. Minerals, 10(12), 1084. https://doi.org/10.3390/min10121084





