Abstract
The new eudialyte-group mineral, odikhinchaite, was discovered in a peralkaline pegmatite vein hosted by melteigite at the Odikhincha ultrabasic alkaline–carbonatite intrusion, Taimyr Peninsula, Krasnoyarsk Krai, Russia. Associated minerals are orthoclase, albite, aegirine, cancrinite, ancylite-(Ce), catapleiite, and wadeite. Odikhinchaite occurs as dense rosette-like aggregates up to 11 mm across, consisting of split lamellar individuals. The mineral is translucent to transparent, deep purple, with vitreous luster. Odikhinchaite is brittle, with uneven fracture; distinct cleavage on (001) is observed. Hardness determined by the micro-indentation method is equal to 430 kgf/mm2; the Mohs hardness is 5. D(meas.) is 2.97(1) g·cm−3, D(calc.) is 3.04 g·cm–3. Odikhinchaite is optically uniaxial (–), ω = 1.638(2), ε = 1.630(2). The IR spectrum shows the presence of the IVMn2+O4 polyhedra, H2O molecules and CO32– anions. The chemical composition is (electron microprobe, H2O determined by the modified Penfield method, CO2 determined by selection sorption of gaseous annealing products; wt%): Na2O 9.25, K2O 0.59, CaO 12.77, MnO 5.49, FeO 0.75, MgO 0.24, La2O3 0.38, Ce2O3 0.39, Nd2O3 0.15, Al2O3 0.07, SiO2 44.80, ZrO2 11.13, TiO2 0.07, Nb2O5 4.17, Cl 0.69, CO2 0.90, H2O 2.22, –O = Cl –0.16, total 99.72. The crystal structure was solved using single-crystal X-ray diffraction data. Odikhinchaite is trigonal, space group R3m; the unit-cell parameters are: a = 14.2837(2) Å, c = 30.0697(3) Å, V = 5313.04(12) Å3. The new mineral is isostructural with other 12-layered members of the eudialyte group with the space group R3m. Its crystal chemical formula is (Z = 3): {N1(Na2.58Ca0.42)N2[Na2.37Ca0.51(H2O)0.12]N3(Sr2.00K0.45Na0.35REE0.20)N4Na3N5[(H2O)1.8Na1.2]}{ZZr3M1Ca6M2(Mn2.49Fe2+0.51)[M3Nb(OH)1.82O1.18](M4SiOH)[Si3O9]2[Si9O27]2X1[(CO3)0.53Cl0.47]X2[(H2O)0.6(O,F)0.4]XM4(CO3)0.15. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 11.42 (64) (101), 4.309 (41) (205), 3.405 (53) (131), 3.208 (45) (208, 036), 3.167 (44) (217), 2.978 (100) (315), 2.858 (86) (404).
1. Introduction
Eudialyte was first discovered in Greenland about 200 years ago [1] and was considered as a mineral species with unstable chemical composition. Later it was supposed that eudialyte is an endmember of the “eudialyte–eucolite” solid solution, however, later, based on a chemical and X-ray structural study, it was concluded that the term “eucolite” was applied to different eudialyte-related mineral species. At present, thirty valid eudialyte-group minerals (EGMs) are distinguished [2,3,4,5,6,7,8].
During recent decades, EGMs attracted attention of researchers due to their remarkable set of chemical and crystal-chemical features, unique structural variability and complexity. These minerals are excellent models, demonstrating interesting complex mechanisms of homovalent, heterovalent, and, especially, blocky isomorphism involving groups of atoms that have different valence states and coordination [7,8]. These minerals are considered as geochemical markers which play an important role in the understanding of the distribution of different metals in peralkaline rocks and melts. Due to high concentrations of so-called strategic metals, EGMs are a potential source of some rare elements—first Zr, Hf and REE.
The general formula of EGM can be written as [N1N2N3N4N5]3M16M23M3M4Z3(Si9O27)2(Si3O9)2Ø4–6X1X2, where Z = Zr, Ti, Nb; M1 = Ca, Mn2+, Fe2+, Na, REE; M2 = Fe2+, Fe3+, Mn2+, Zr, Na; M3–4 = Si, Nb, W; N1–5 = Na, Ca, Mn, Sr, K, REE, H2O, H3O; Ø = OH; X = Cl, H2O, CO3. The eudialyte-type crystal structure is based on a heteropolyhedral pseudo-framework built up by the rings of tetrahedra (Si3O9 and Si9O27) and of octahedra (M16O24) connected via M2O4–7 polyhedra and ZO6 octahedra containing additional M3 and M4 sites, which are situated at the centers of two nonequivalent Si9O27 rings and can be filled by additional TØ4-tetrahedra (predominantly occupied by silicon) or (MØ6)-octahedra (occupied by different cations having octahedral coordination) [1,2]. Five extra-framework sites, N1–5, are usually occupied by Na, but the N3 and N4 sites may contain significant and even dominant amounts of other cations (K+, Ca2+, Mn2+, Sr2+, REE3+). In so-called “hydroeudialytes”, the N sites contain H2O molecules in significant amounts [1]. The X1 and X2 sites located on the three-fold axis are occupied by extra-framework anions (Cl–, F–, OH–, S2–, SO42–, and CO32–) and water molecules.
In this paper we describe a new eudialyte-group mineral, odikhinchaite, from the Odikhincha ultrabasic alkaline–carbonatite intrusion belonging to the Maimecha–Kotuy alkaline province. It is situated in the Kotuy river basin, at the southern part of the Taimyr Peninsula, Krasnoyarsk Krai, Polar Siberia, Russia. The specific crystal-chemical feature of this mineral, distinguishing it from other members of the eudialyte group, is the combination of N3Sr, N5(H2O), M2Mn2+, M3Nb, and X1(CO32–) as species-defining components.
Odikhinchaite is named after the discovery locality. The mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA no. 2020-064). The type specimens are deposited in the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia with the registration numbers 5587/1 and 5588/1.
2. Geological Setting
The Odikhincha ultrabasic alkaline–carbonatite complex is the second in size intrusion of the Maimecha–Kotuy alkaline province. The Odikhincha intrusion is situated at the watershed of the Medvezhya and Kotuy rivers, in the peripheral part of the Anabarskiy Pre-Cambrian shield and is hosted by Cambrian dolomites [9]. The area of the modern erosional truncation of the intrusion is 56 km2.
Odikhincha is a multistage ring intrusive complex formed as a result of consecutive intrusion of olivinites, jacupirangites, melilite-bearing rocks, melteigites, and ijolites. The latest vein rocks are alkaline syenites, nepheline syenites, melanephelinites, and carbonatites. The veins are up to 3 m thick and no more than ~100 m long. Olivinites mainly occur as xenoliths typically to several hundred meters hosted by ijolites, jacupirangites and melteigites in the northern and eastern parts of the Odikhincha intrusion. Melilite-bearing rocks (okaites and turjaites) form two large bodies up to 9 km long in the NE part of the intrusion. Ijolites occur in the central, western and southern parts of the intrusive complex. The central part of the intrusion is predominantly composed of coarse-grained ijolites whereas medium- and fine-grained ijolites and melteigites occur in the peripheral parts of Odikhincha. Veins of alkaline syenites and nepheline syenites up to 2 m thick are the latest formations. They are confined to the peripheral parts of the complex and crosscut all other intrusive rocks. Different rocks of the intrusion host multiple lenticular bodies (up to 30 m long) and veins (up to 1 m thick) of calcite carbonatites. Pegmatoid andradite- and melilite-bearing veins with variable contents of pyroxene and nepheline are widespread too.
Outcrops of calcite carbonatite and two pegmatite veins related to nepheline syenites were found among fine-grained melteigites in the valley of the second right tributary of the Ebe-Yuryakh stream, in the southeastern part of the intrusion, 0.5–1 km from the contact with the host dolomites.
The largest pegmatite vein, 3.5 m thick, was exposed on the stream slope by four ditches. Two zones are observed in this peralkaline pegmatite body: a fine-grained zone composed of nepheline–feldspar–aegirine aggregate, with poikilite-developed crystals of lamprophyllite and sheaf-like rinkite-(Ce) segregations, and a coarse-grained zone composed with tabular feldspar crystals up to a few centimeters in size and subordinate xenomorphic rinkite-(Ce) (lovchorrite) and lamprophyllite individuals. EGM in this pegmatite are represented only by taseqite. It forms large (up to 1 × 0.5 cm2) light brown flattened crystals in the central part of pegmatite and is associated with lovchorrite, aegirine, orthoclase, and titanite. The ranges of main components in this mineral are: Na12.3–12.4K0.5Ca6–6.4Sr1.5–1.7Fe1.9–2.4Mn0.5–0.7Nb0.5–0.6Ti0.2–0.5Zr2.5–2.7Si25.3–25.5O73(O,H2O,Cl)5 [10].
Downslope, in the stream bed, 70 m from the large pegmatite vein, another vein of peralkaline pegmatite was found among fine-grained melteigites. This thin (up to 2 cm thick) pegmatite vein was composed of coarse-crystalline orthoclase, albite, aegirine, cancrinite, and subordinate odikhinchaite.
3. Materials and Methods
Odikhinchaite occurs as dense rosette-like aggregates up to 11 mm across, consisting of split lamellar individuals (Figure 1). Along the periphery, odikhinchaite spherulites overgrow and are partially replaced by a fine-grained catapleiite aggregate, typically together with ancylite-(Ce) (Figure 2). Other associated minerals are orthoclase, albite, aegirine, cancrinite, and wadeite.
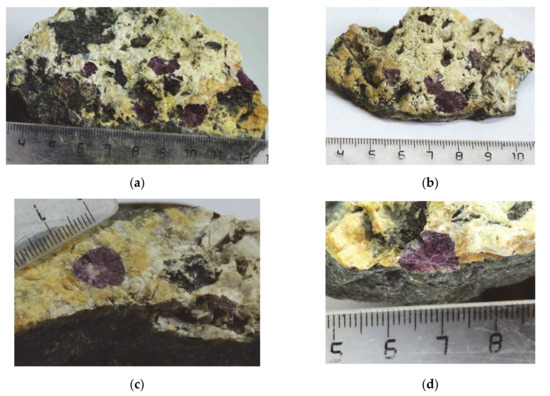
Figure 1.
Odikhinchaite aggregates (purple rosettes) in pegmatite with white alkali feldspars, yellow cancrinite and dark green to black aegirine.
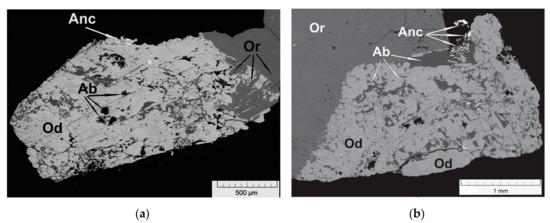
Figure 2.
Fragments of odikhinchaite (Od) individuals with albite (Ab) inclusions in association with ancylite-(Ce) (Anc) and orthoclase (Or). BSE images of polished sections.
Five chemical analyses were carried out using a Tescan VEGA-II XMU INCA Energy 450, (EDS mode, 20 kV, 190 pA; TESCAN, Brno, Czech Republic). The H2O was determined by means of the modified Penfield method. Selective sorption of CO2 was carried out on askarite sorbent (an asbestiform matter saturated by NaOH) from gaseous products obtained by heating of the mineral at 1080 °C in oxygen flow at 1 atm.
In order to obtain IR absorption spectra of odikhinchaite and taseqite, powdered samples were mixed with anhydrous KBr, pelletized, and analyzed using an ALPHA FTIR spectrometer (Bruker Optics, Karlsruhe, Germany)) at a resolution of 4 cm–1. Sixteen scans were collected. The IR spectrum of an analogous pellet of pure KBr was used as a reference.
Powder X-ray diffraction data were collected using a Rigaku R-AXIS Rapid II (Rigaku Corporation, Tokyo, Japan) diffractometer (image plate), CoKα, 40 kV, 15 mA, rotating anode with the microfocus optics, Debye–Scherrer geometry, d = 127.4 mm, exposure 15 min. The raw powder XRD data were collected using program suite designed by Britvin et al. [11]. Calculated intensities were obtained by means of STOE WinXPOW v. 2.08 program suite based on the atomic coordinates and unit-cell parameters.
Single-crystal X-ray diffraction studies of odikhinchaite were carried out at room temperature with an Xcalibur Oxford Diffraction (Oxford Diffraction Ltd.: Abingdon, Oxfordshire, UK) diffractometer equipped with a CCD detector using the ω scanning mode. Raw data were integrated by using the CrysAlis (Oxford Diffraction Ltd.: Abingdon, Oxfordshire, UK) program [12] and then scaled, merged. A total of 27,254 reflections within the sphere limited by θ = 32.66° were measured. Based on the single crystal X-ray analysis, the following unit-cell parameters have been obtained by the least-squares refinement of all reflections: a = 14.2837(2) Å, c = 30.0697(3) Å, V = 5313.04(12) Å3. The analysis of systematic absences of reflections shows R-centering, common for eudialyte-group minerals. The space group R3m was chosen. Experimental details are given in Table 1.

Table 1.
Crystal data, data collection information and structure refinement details for odikhinchaite.
4. Results
4.1. Physical Properties
Odikhinchaite is translucent to transparent, deep purple, with vitreous luster. The streak is white. The new mineral is brittle, with uneven fracture; distinct cleavage on (001) is observed. Hardness determined by the micro-indentation method (the Vickers hardness number = VHN, load 20 g) is equal to 430 kgf/mm2 (range 394–491, n = 6). The Mohs hardness is 5. Density measured by flotation in heavy liquids (mixtures of methylene iodide and benzene) is 2.97(1) g·cm–3. Density calculated using the empirical formula and unit cell volume refined from single crystal XRD data is 3.04 g·cm–3.
4.2. Infrared Spectroscopy
Absorption bands in the IR spectrum of odikhinchaite (curve a in Figure 2) and their assignments are (cm–1; s—strong band, w—weak band, sh—shoulder): 3435, 3220sh (O–H stretching vibrations), 1635w (H–O–H bending vibrations), 1507w, 1451, 1407w (asymmetric stretching vibrations of the CO32– groups), 1060sh, 1016s, 975s, 923s (Si–O stretching vibrations), 740, (mixed vibrations of tetrahedral rings—“ring band”), 697w, 662 (mixed vibrations of tetrahedral rings combined with Nb–O stretching vibrations), 545sh (IVFe2+–O stretching vibrations), 526 (IVMn2+–O stretching vibrations), 479s, 453s (lattice mode involving predominantly bending vibrations of tetrahedral rings), 390, 364 (lattice modes involving Ca–O stretching vibrations). The assignment of IR bands was made based on the analysis of IR spectra of several tens structurally investigated eudialyte-group minerals, in accordance with [1].
The IR spectrum of odikhinchaite differs from that of its M2Fe2+-dominant and CO2-deficient analogue taseqite with the crystal-chemical formula [Na10.5K0.5(Sr1.71Na1.29)][Ca5.85REE0.15][Zr2.96Hf0.03](Fe2+2.43Mn0.45)Si(Nb0.64Ti0.27)(Si9O27)2(Si3O9)2(OH,O,Cl)5 from the Odikhincha massif (curve b on Figure 3) in higher intensities of the bands of O–H, C–O, Nb–O and IVMn2+–O stretching vibrations and a lower intensity of the band of IVFe2+–O stretching vibrations, which is in accordance with crystal-chemical features of these minerals.
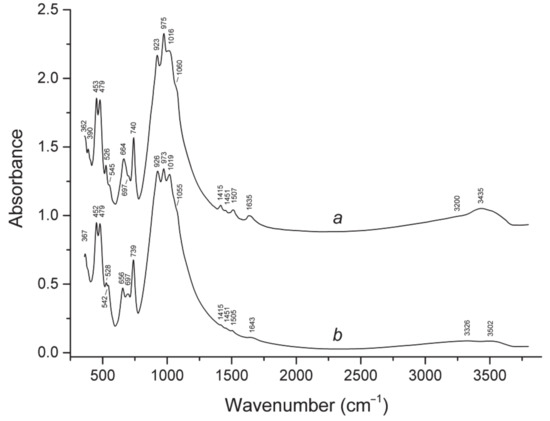
Figure 3.
Powder infrared absorption spectra of (a) odikhinchaite and (b) taseqite, both from the Odikhincha alkaline complex.
4.3. Optical Properties
Odikhinchaite is optically uniaxial (–), ω = 1.638(2), ε = 1.630(2) (λ = 589 nm). Under the microscope, the mineral is strongly pleochroic: O = bright crimson, E = light yellow. The absorption scheme is: O > E.
4.4. Chemical Composition
Chemical data for odikhinchaite are given in Table 2. Contents of other elements with atomic numbers > 8 are below detection limits.

Table 2.
Chemical data for odikhinchaite.
The formula coefficients based on 29 high force-strength framework cations, i.e., Si + Al + Nb + Zr + Ti atoms per formula unit (apfu, for Z = 3), in accordance with structural data are H8.22Na9.97K0.42Ca7.59Sr1.87Ce0.08La0.08Nd0.03Mn2.58Fe0.35Mg0.20Ti0.03Zr3.01Nb1.05Si24.87Al0.05Cl0.65C0.68O81.71.
The end-member formula is Na9Sr3[(H2O)2Na]Ca6Mn3Zr3NbSi(Si24O73)(OH)3(CO3)·H2O.
The compatibility index 1—(Kp/Kc) is equal to –0.005 (superior) with density calculated using the crystal-chemical formula and −0.024 (excellent) with measured density.
4.5. X-Ray Diffraction Data and Crystal Structure
Powder X-ray diffraction data are presented in Table 3. Diffraction peaks are readily indexed in the space group R3m. The unit-cell parameters calculated from the powder data are: a = 14.2179(1), c = 30.3492(3) Å, V = 5313.1(1) Å3.

Table 3.
Powder X-ray diffraction data (d in Å) of odikhinchaite.
The structure determination and refinement of odikhinchaite were carried out based on 2312 independent reflections with I > 2σ(I) using the program JANA2006 (Institute of Physics, Praha, Czech Republic) [13]. Extra-framework sites including split and partially occupied ones have been localized from a series of difference electron-density maps calculations. Atomic scattering factors for neutral atoms together with anomalous dispersion corrections were taken from International Tables for Crystallography [14]. Illustrations were produced with the JANA2006 program package in combination with the program DIAMOND, Version 3 (Crystal Impact GbR, Bonn, Germany) [15].
Table 4 lists the fractional atomic coordinates, site multiplicities, atomic displacement parameters and site occupancies. Selected interatomic distances are given in Table 5.

Table 4.
Atom coordinates (x, y, z), atomic displacement parameters (U, Å2), site multiplicities (Mult), site occupancies and bond-valence sums (BVS) in the structure of odikhinchaite.

Table 5.
Selected interatomic distances (Å) in the structure of odikhinchaite.
Odikhinchaite is isostructural with other 12-layered members of the eudialyte group with the space group R3m, which is most common for eudialyte-group minerals (see Figure 4 and Figure 5). Based on the refined site-scattering factors, the crystal chemical formula of odikhinchaite can be written as follows (Z = 3): {N1(Na2.58Ca0.42)N2[Na2.37Ca0.51(H2O)0.12]N3(Sr2.00K0.45Na0.35REE0.20)N4Na3N5[(H2O)1.8Na1.2]}{ZZr3M1Ca6M2(Mn2.49Fe2+0.51)[M3Nb(OH)1.82O1.18](M4SiOH)[Si3O9]2[Si9O27]2X1[(CO3)0.53Cl0.47]X2[(H2O)0.6(O,F)0.4]XM4(CO3)0.15 where braces and brackets enclose contents of the key sites.
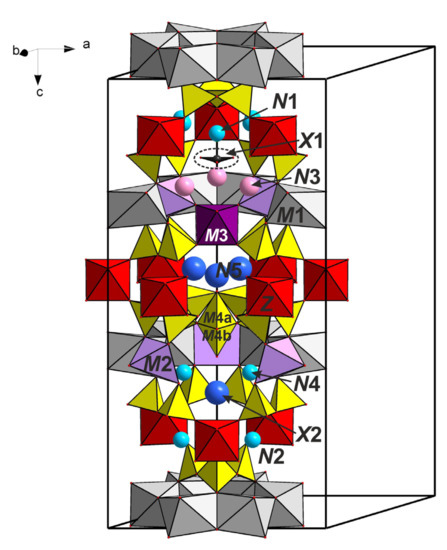
Figure 4.
The general view of the crystal structure of odikhinchaite (the unit cell is outlined).
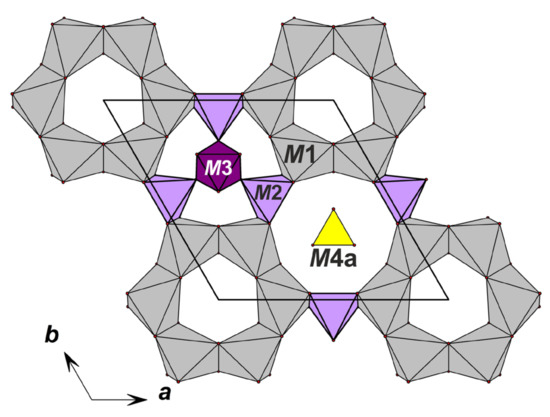
Figure 5.
Heteropolyhedral layer involving rings of CaO6 octahedra in the structure of odikhinchaite.
In the crystal structure of odikhinchaite, manganese (2.49 apfu) predominates over iron(II) (0.51 apfu) at the M2-site, which is located in the center of the М2φ5-square pyramids (φ = O2–, OH–) with the mean distance <M2–φ> = 2.109 Å. The octahedral M3 site (the mean distance <M3–φ> = 1.986 Å) located on the threefold axis at the center of one nine-membered Si9O27-tetrahedral ring is occupied by niobium (1 apfu) while the M4 site located at the center of the symmetrically non-equivalent nine-membered tetrahedral ring is split into two M4a- and M4b-sites (М4а–М4b = 1.076 Å) occupied by silicon (0.82 and 0.18 apfu, respectively) in tetrahedral environment (the mean distances are: <M4a–φ> = 1.622 Å; <M4b–φ> = 1.579 Å). The M3φ6 octahedron links with the M2φ6 square pyramid via the common OHM3-vertex forming a heteropolyhedral [NbMn3(OH,O)3] cluster (Figure 6).
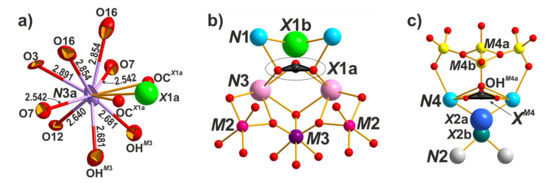
Figure 6.
Local coordinational environments of the N3a site (a), the X1 site (b), and the X2 site (c).
The distribution of large cations over the N1–5 sites in odikhinchaite is similar to that in taseqite [16] and its Cl-deficient variety [10]. The N1 site is split into two sites with the distance N1a–N1b = 0.945 Å. The N1a is occupied by sodium (2.58 apfu), while N1b is occupied by calcium (0.42 apfu). The N2 site has a complex composition with the predominance of sodium (2.37 apfu) and admixture of calcium (0.51 apfu) and water molecules (0.12 molecules pfu). The N4 site is fully occupied by sodium. The N5 site is predominantly occupied by water molecules (1.8 apfu) and sodium (1.2 apfu). The most distinguished feature of odikhinchaite and taseqite [10,16] is the predominance of strontium at the N3 site. In the structure of odikhinchaite, the N3 is split into two sites with the distance N3a–N3b = 0.891 Å. The N3a site has a complex chemical composition with the predominance of strontium (2.00 apfu) and admixture of potassium (0.45 apfu) and rare-earth elements (0.20 apfu) and has a variable coordination number (8 or 10 in the cases when the X1a site contains Cl– or CO32–, respectively) (Figure 6a). The N3b site is occupied by sodium (0.35 apfu).
Odikhinchaite is characterized by the considerable amount of CO32– anions (0.68 anions pfu in total) which are located at the at the X sites, on the threefold axis (Figure 6b,c). It was found that CO32– group predominates over chlorine at the X1 site, which is split into two sites with the distance X1a–X1b = 1.461 Å. The X1a is occupied by CO32– (0.53 anions pfu) with admixture of chlorine (0.35 apfu), while the X1b site is occupied only by chlorine (0.12 apfu). The X2 site is also split into two sites with the distance X2a–X2b = 1.028 Å. The X2a site is occupied by water molecules (0.6 apfu) and the X2b site is occupied by the mix of hydroxyl group and fluorine atoms (0.4 apfu). Additional amount of the CO32– groups (0.15 apfu) were also localized at the XM4 site between the X2a and OHM4b sites with the distances XM4–X2a = 1.762 Å and XM4–OHM4a = 0.662 Å.
5. Discussion
Odikhinchaite Na9Sr3[(H2O)2Na]Ca6Mn3Zr3NbSi(Si24O72)O(OH)3(CO3)·H2O belongs to the eudialyte group and adopts the eudialyte structure type. It is closely related to taseqite whose end-member formula is Na12Sr3Ca6Fe2+3Zr3NbSi(Si24O72)(O,OH,H2O)4Cl2 [18]. Unlike taseqite, which is Fe2+-dominant at the M2 site, in odikhinchaite, this site is predominantly populated by Mn2+. In addition, in odikhinchaite, the X2 site is predominantly occupied by the СO32– groups, whereas in taseqite, this site is Cl-dominant, and the N5 site of odikhinchaite is predominantly populated by H2O.
Odikhinchaite can also be the [N3Sr, N5(H2O)] analogue of carbokentbrooksite (Na,⎕,REE)15Ca6Mn3Zr3NbSi(Si24O72)O(OH)3(CO3)·H2O [19] and the [M3Nb, N5(H2O), X1(CO3)] analogue of manganokhomyakovite Na12Sr3Ca6Mn3Zr3WSi(Si24O72)(O,OH,H2O)4(OH,Cl)2 [20].
Comparative data for odikhinchaite, other M3Nb- and M2Mn2+-dominant eudialyte-group minerals structurally related to odikhinchaite and taseqite are given in Table 6.

Table 6.
Comparative data for odikhinchaite and some related eudialyte-group minerals *.
Author Contributions
N.V.C., Y.D.G. and S.M.A. wrote the paper. Y.D.G. collected the type material. D.A.K. obtained single-crystal X-ray diffraction data. S.M.A. solved and refined the crystal structure. S.N.B. and I.V.P. obtained and treated powder X-ray diffraction data. D.A.V. conceived and designed chemical data. N.V.C. obtained IR spectra. L.A.P. obtained optical data and measured hardness. S.A.V. measured density. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed in accordance with the state task, state registration no. ААА-А19-119092390076-7 (chemical data and crystal-chemical analysis) and was partly funded by the Russian Foundation for Basic Research, grant no. 18-29-12007-mk (IR spectroscopy, powder X-ray diffraction, and physical properties) and Russian Science Foundation, grant no. 20-77-10065 (solving and refinement of the crystal structure; crystal chemical analysis and structure description). S.N.B. acknowledges Saint-Petersburg State University for financial support, grant no. 3.42.741.2017 (in part of powder X-ray diffraction measurements).
Acknowledgments
The authors thank the X-ray Diffraction Centre of Saint-Petersburg State University for instrumental and computational resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sjöqvist, A.S.L. The tale of greenlandite: Commemorating the two-hundredth anniversary of eudialyte (1819–2019). Minerals 2019, 9, 497–510. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Aksenov, S.M. Minerals of Eudialyte Group: Crystal Chemistry, Properties, Genesis; University of Nizhniy Novgorod: Nizhniy Novgorod, Russia, 2012; p. 230. ISBN 9785913262073. (In Russian) [Google Scholar]
- Johnsen, O.; Grice, J.D.; Gault, R.A. The crystal chemistry of the eudialyte group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Pekov, I.V. The nomenclature of eudialyte group minerals. Can. Mineral. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Khomyakov, A.P. Crystal chemistry of modular eudialytes. Crystallogr. Rep. 2003, 48, S78–S90. [Google Scholar]
- Rastsvetaeva, R.K.; Chukanov, N.V. Classification of eudialyte-group minerals. Geol. Ore Depos. 2012, 54, 487–497. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Pekov, I.V.; Schäfer, C.; Van, K.V. New data on the isomorphism in eudialyte-group minerals. 1. Crystal chemistry of eudialyte-group members with Na incorporated into the framework as a marker of hyperagpaitic conditions. Minerals 2020, 10, 587. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. New data on the isomorphism in eudialyte-group minerals. 2. Crystal-chemical mechanisms of blocky isomorphism at the key sites (a review). Minerals 2020, 10, 720. [Google Scholar] [CrossRef]
- Egorov, L.S. Iiolite-Carbonatite Pluton (For Example Maimeche-Kotuy Province Polar Siberia); Nedra: Moscow, Russia, 1991; p. 260. (In Russian) [Google Scholar]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Zaitsev, В.А.; Aksenov, S.M.; Viktorova, K.A. Crystal structure of Cl-deficiente analog of tasekite from the Odihincha massif. Crystallogr. Rep. 2018, 63, 392–400. [Google Scholar] [CrossRef]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zap. Ross. Mineral. Obs. (Proc. Russ. Mineral. Soc.) 2017, 146, 104–107. (In Russian) [Google Scholar]
- Oxford Diffraction. CrysAlisPro; Oxford Diffraction Ltd.: Abingdon, UK, 2009. [Google Scholar]
- Petřiček, V.; Dušek, M.; Palatinus, L. Structure Determination Software Programs; Institute of Physics: Praha, Czech Republic, 2006. [Google Scholar]
- Prince, E. (Ed.) International Tables for Crystallography, Volume C: Mathematical, Physical and Chemical Tables, 3rd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Brandenburg, K.; Putz, H. DIAMOND Version 3; Crystal Impact GbR: Bonn, Germany, 2005. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. Sect. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Petersen, O.V.; Johnsen, O.; Gault, R.A.; Niederayr, G.; Grice, A.D. Taseqite, a new member of the eudialyte group from the Ilímaussaq alkaline complex, South Greenland. Neues Jahrb. Mineral. Mon. 2004, 2, 83–96. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Dusmatov, V.D.; Ferraris, G.; Gula, A.; Ivaldi, G.; Nechelyustov, G.N. Zirsilite-(Ce), (Na,⎕)12(Ce,Na)3Ca6Mn3Zr3Nb(Si25O73)(OH)3(CO3)·H2O, and carbokentbrooksite, (Na,⎕)12(Na,Ce)3Ca6Mn3Zr3Nb(Si25O73)(OH)3(CO3)·H2O, two new eudialyte-group minerals from the Dara-i-Pioz alkaline massif, Tajikistan. Zap. Vserossiyskogo Mineral. Obs. (Proc. Russ. Mineral. Soc.) 2003, 132, 40–51. (In Russian) [Google Scholar]
- Johnsen, O.; Gault, R.A.; Grice, J.D.; Ercit, T.S. Khomyakovite and manganokhomyakovite, two new members of the eudialyte group, from Mont Saint-Hilaire, Quebec. Can. Mineral. 1999, 37, 893–899. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).