Graphite-Based Geothermometry on Almahata Sitta Ureilitic Meteorites
Abstract
1. Introduction
2. Materials and Methods
3. Results
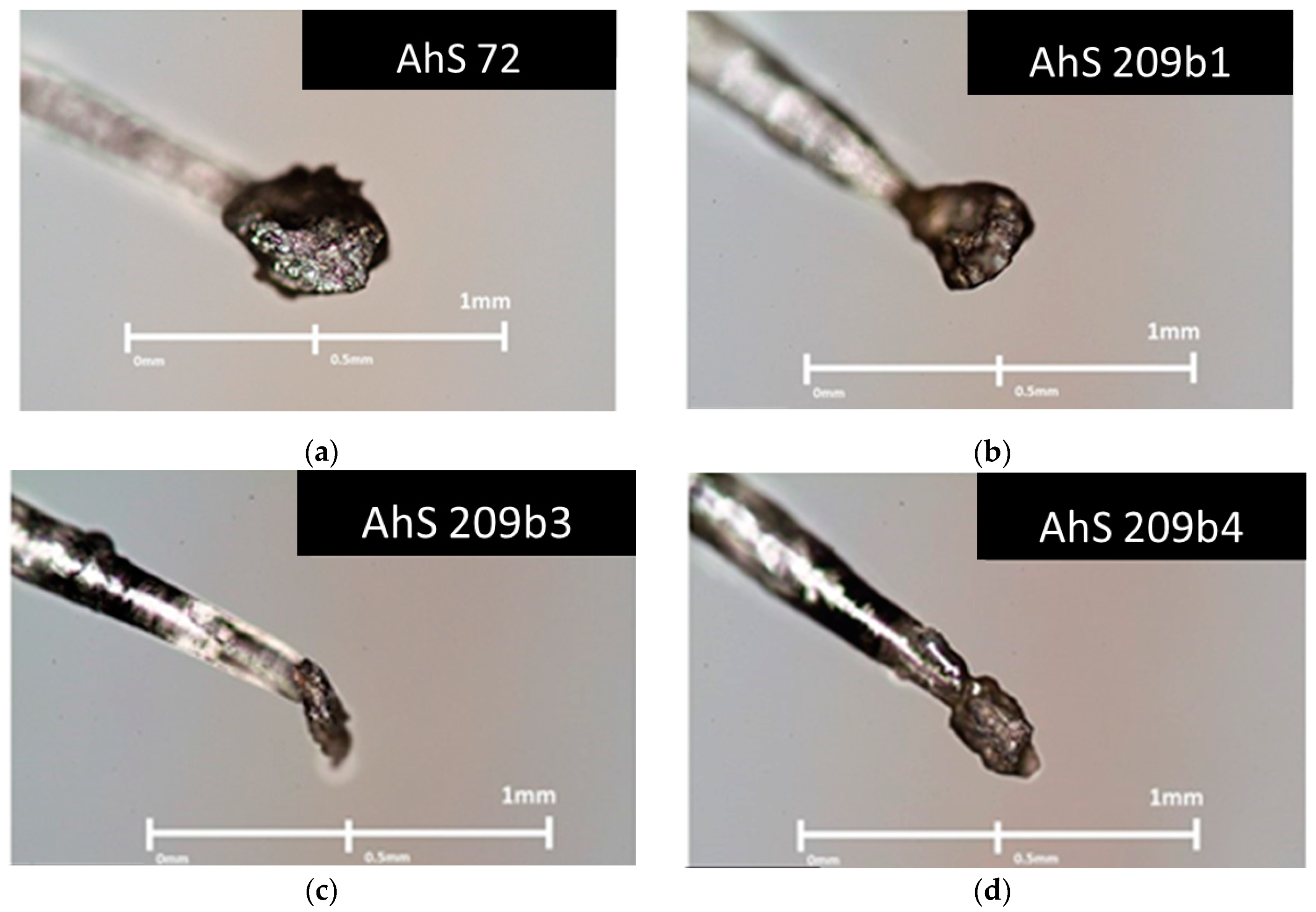
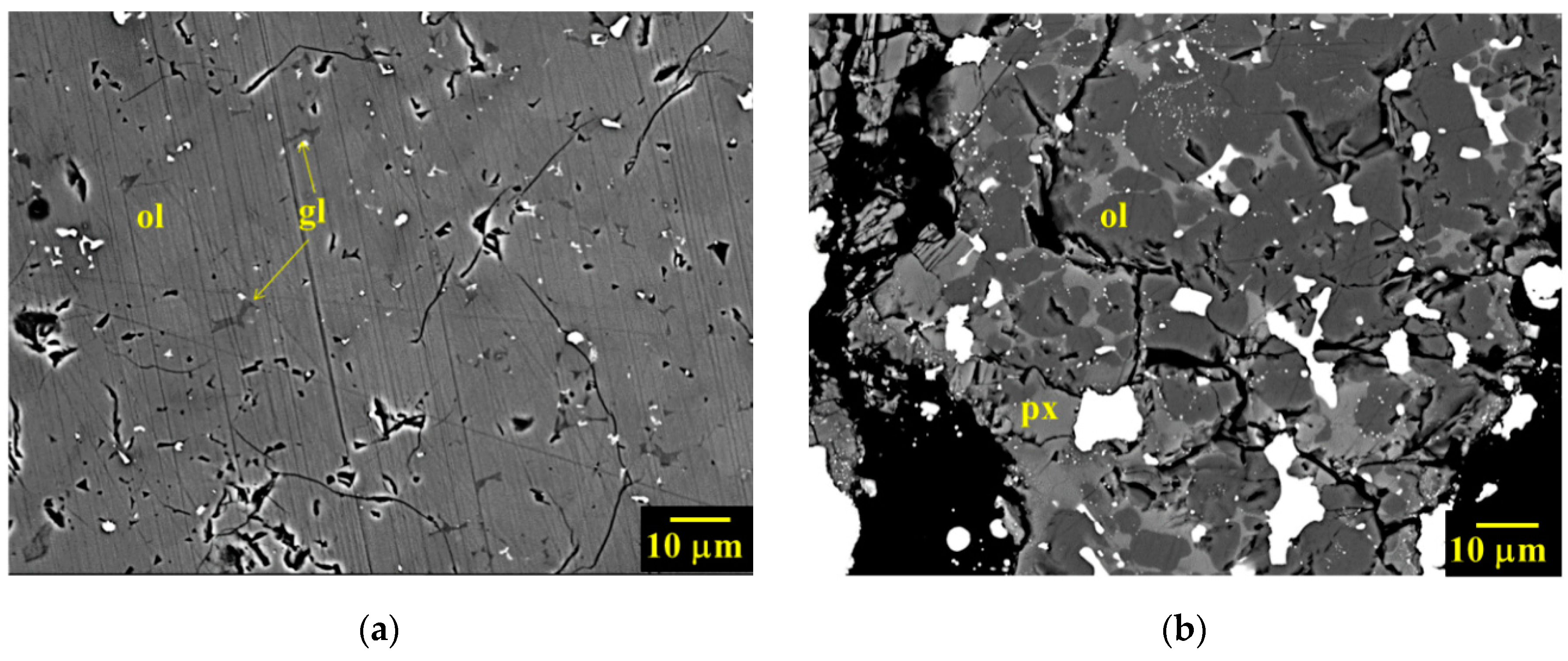
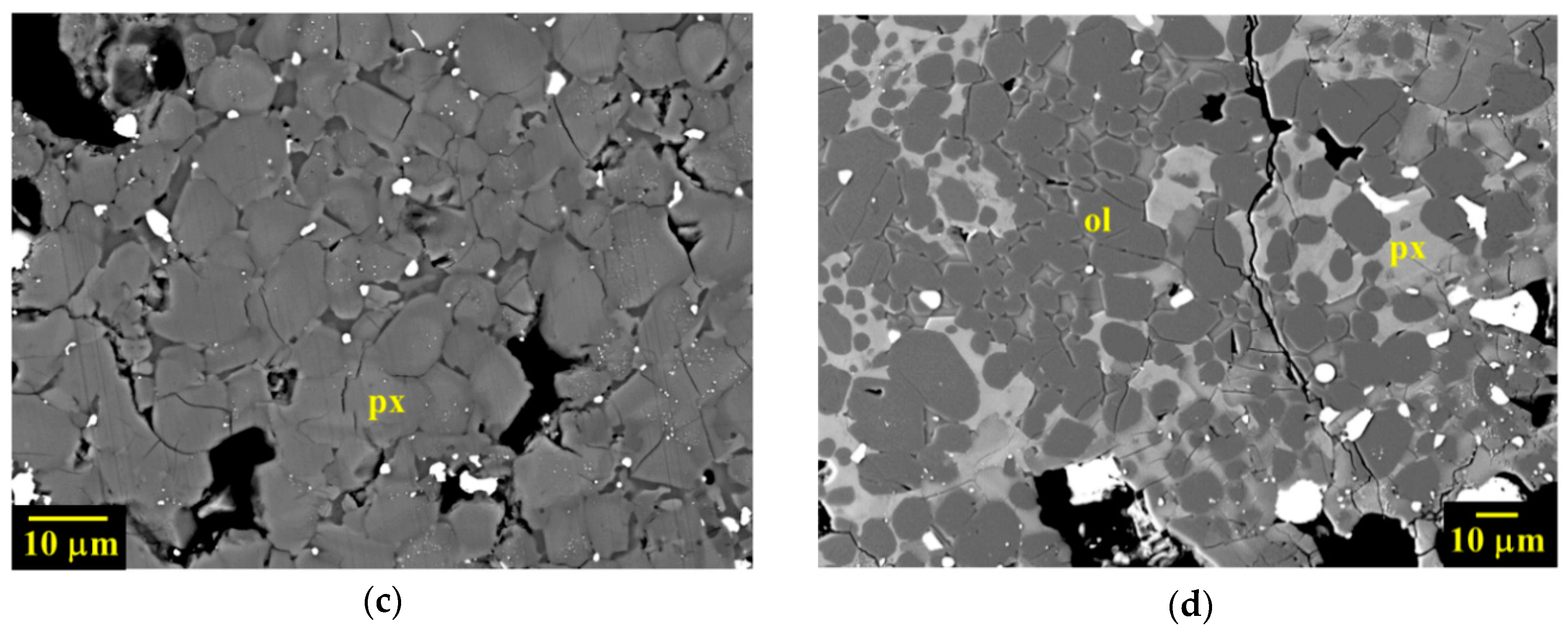
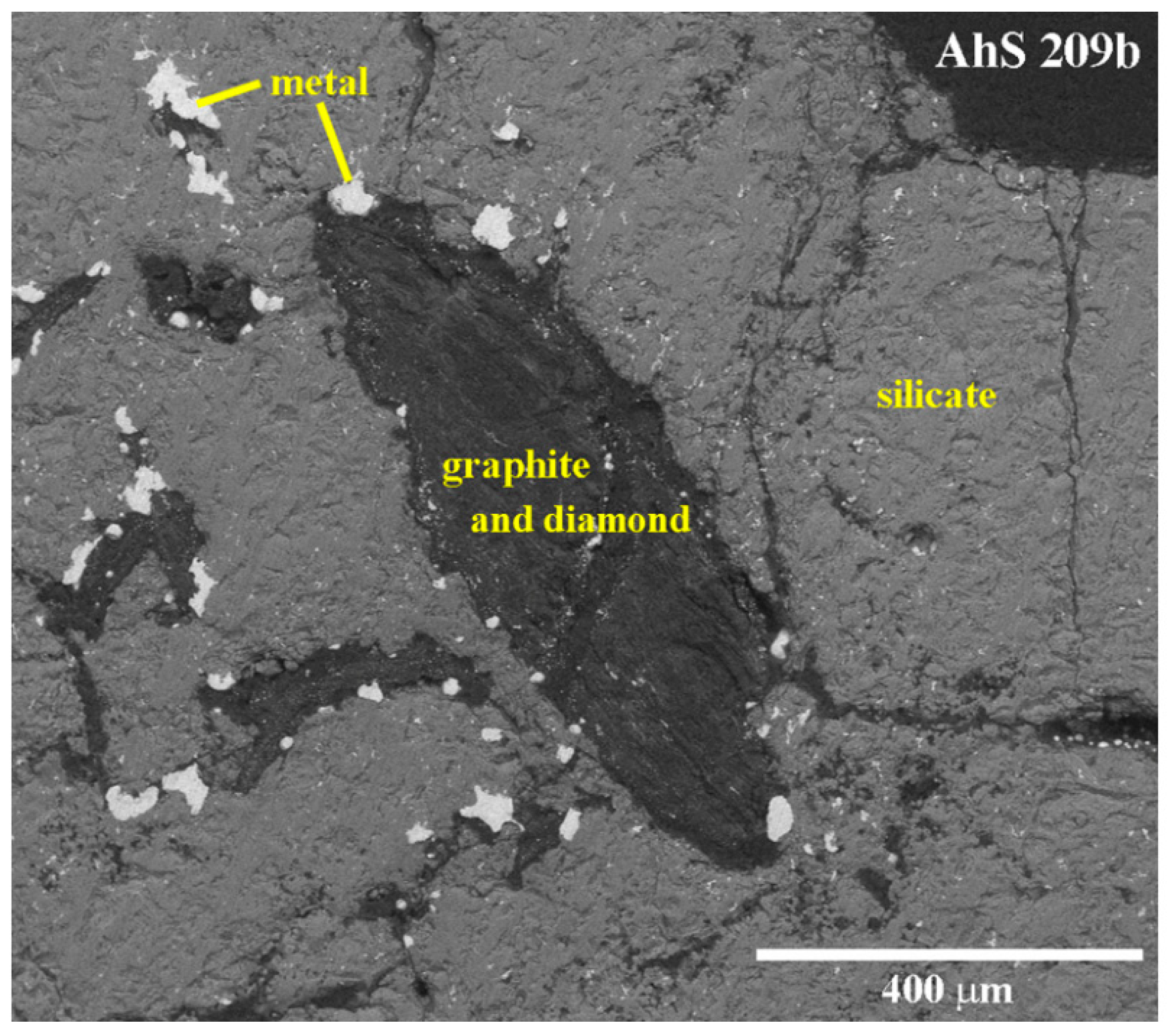
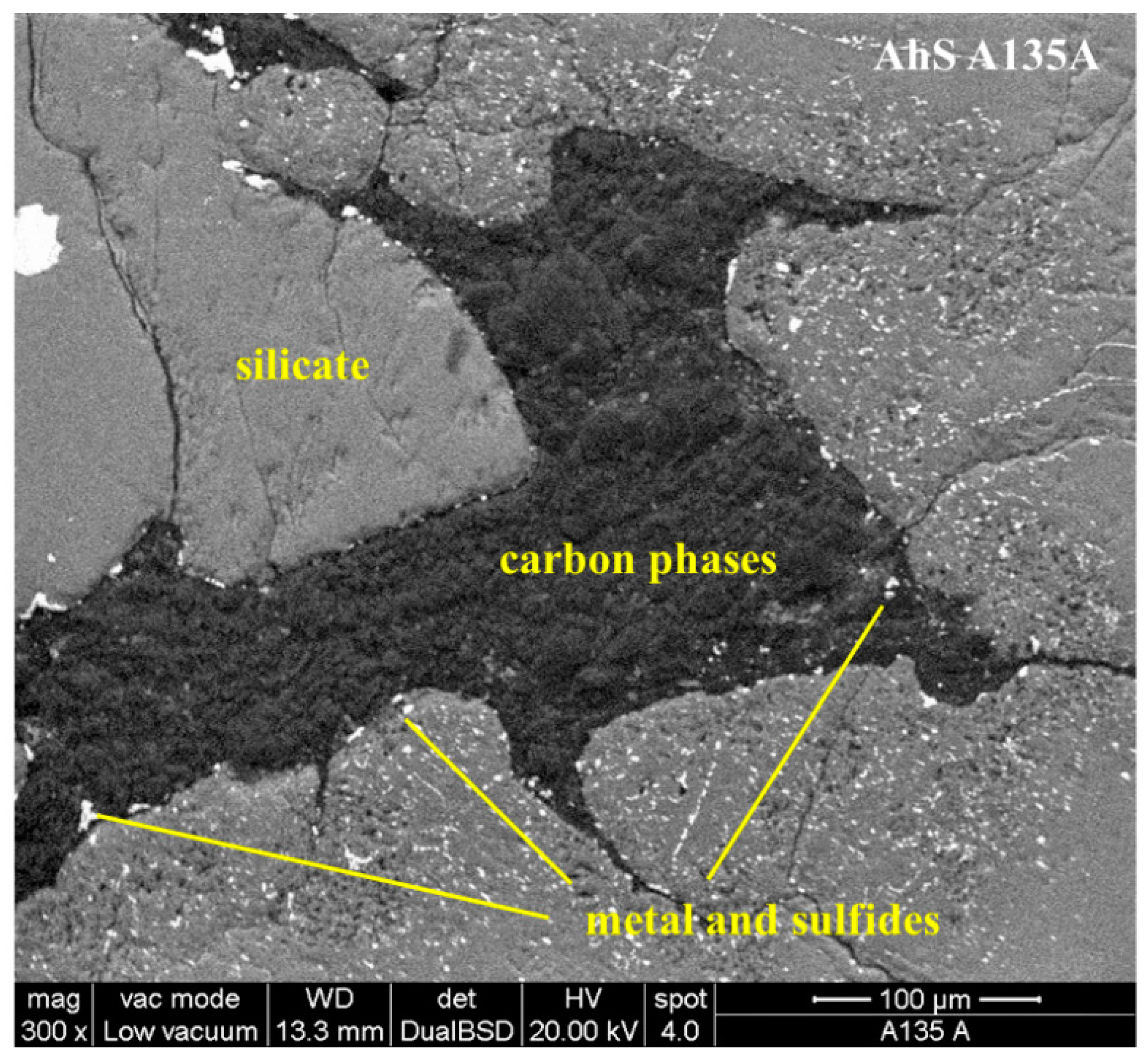
3.1. Petrographic Analysis: Characterization of AhS Graphite Phases
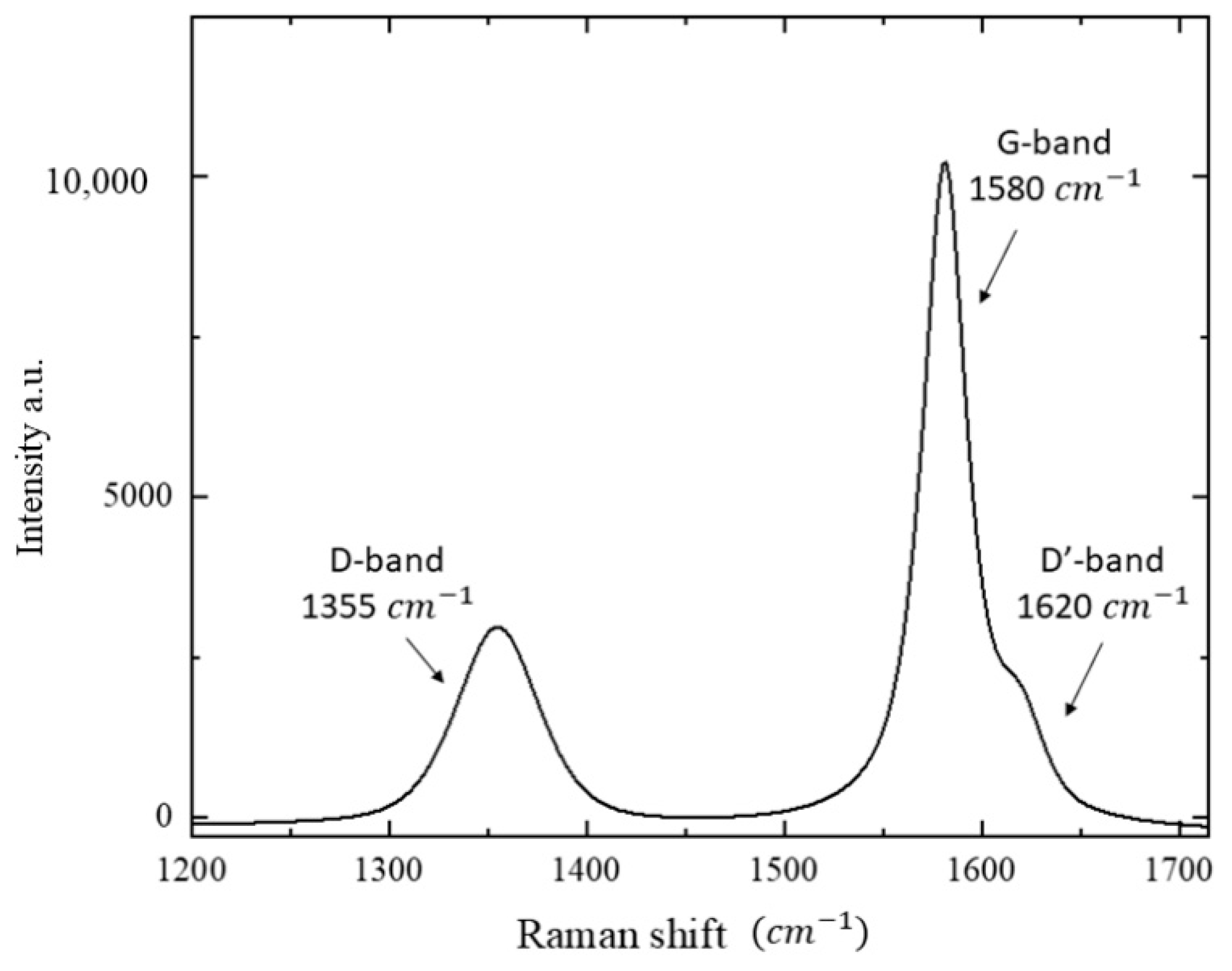
3.2. Micro-Raman Analysis: Characterization of AhS Graphite Phases
3.3. Geothermometry Application to Graphite in AhS Ureilite
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jenniskens, P.; Shaddad, M.H.; Numan, D.; Elsir, S.; Kudoda, A.M.; Zolensky, M.E.; Le, L.; Robinson, G.A.; Friedrich, J.M.; Rumble, D.; et al. The impact and recovery of asteroid 2008 TC3. Nature 2009, 458, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Shaddad, M.H.; Jenniskens, P.; Numan, D.; Kudoda, A.M.; Elsir, S.; Riyah, I.F.; Ali, A.E.; Alameen, M.; Alameen, N.M.; Eid, O.; et al. The recovery of asteroid 2008 TC3. Meteorit. Planet. Sci. 2010, 45, 1618–1637. [Google Scholar] [CrossRef]
- Horstmann, M.; Bischoff, A. The Almahata Sitta polymict breccia and the late accretion of asteroid 2008 TC3. Geochemistry 2014, 74, 149–183. [Google Scholar] [CrossRef]
- Goodrich, C.A.; Hartmann, W.K.; O’Brien, D.P.; Weidenschilling, S.J.; Wilson, L.; Michel, P.; Jutzi, M. Origin and history of ureilitic material in the solar system: The view from asteroid 2008 TC3 and the Almahata Sitta meteorite. Meteorit. Planet. Sci. 2015, 50, 782–809. [Google Scholar] [CrossRef]
- Downes, H.; Mittlefehldt, D.W.; Kita, N.T.; Valley, J.W. Evidence from polymict ureilite meteorites for a disrupted and re-accreted single ureilite parent asteroid gardened by several distinct impactors. Geochim. Cosmochim. Acta 2008, 72, 4825–4844. [Google Scholar] [CrossRef]
- Goodrich, A.C.; Scott, E.R.D.; Fioretti, A.M. Ureilitic breccias: Clues to the petrologic structure and impact disruption of the ureilite parent asteroid. Geochemistry 2004, 64, 283–327. [Google Scholar] [CrossRef]
- Herrin, J.S.; Zolensky, M.E.; Ito, M.; Le, L.; Mittlefehldt, D.W.; Jenniskens, P.; Ross, A.J.; Shaddad, M.H. Thermal and fragmentation history of ureilitic asteroids: Insights from the Almahata Sitta fall. Meteorit. Planet. Sci. 2010, 45, 1789–1803. [Google Scholar] [CrossRef]
- Michel, P.; Jutzi, M.; Richardson, D.C.; Goodrich, C.A.; Hartmann, W.K.; Brien, D.P.O. Selective sampling during catastrophic disruption: Mapping the location of reaccumulated fragments in the original parent body. Planet. Space Sci. 2015, 107, 24–28. [Google Scholar] [CrossRef]
- Mikouchi, T.; Zolensky, M.E.; Ohnishi, I.; Suzuki, T.; Takeda, H.; Jenniskens, P.; Shaddad, M.H. Electron microscopy of pyroxene in the Almahata Sitta ureilite. Meteorit. Planet. Sci. 2010, 1820, 1812–1820. [Google Scholar] [CrossRef]
- Nakamuta, Y.; Kitajima, F.; Shimada, K. In situ observation, X–ray diffraction and Raman analyses of carbon minerals in ureilites: Origin and formation mechanisms of diamond in ureilites. J. Petrol. Sci. 2016, 111, 252–269. [Google Scholar] [CrossRef]
- Berkley, J.L.; Taylor, G.J.; Keil, K.; Harlow, G.E.; Prinz, M. The nature and origin of ureilites. Geochim. Cosmochim. Acta 1980, 44, 1579–1597. [Google Scholar] [CrossRef]
- Berkley, J.L.; Jones, J.H. Primary igneous carbon in ureilites: Petrological implications. J. Geophys. Res. 1982, 87, A353–A364. [Google Scholar] [CrossRef]
- Treiman, A.H.; Berkley, L. Igneous petrology of the new ureilites Nova 001 and Nullarbor 010. Meteorit. Planet. Sci. 1994, 848, 843–848. [Google Scholar] [CrossRef]
- Miyahara, M.; Ohtani, E.; El Goresy, A.; Lin, Y.; Feng, L.; Zhang, J.; Gillet, P.; Nagase, T.; Muto, J.; Nishijima, M. Unique large diamonds in a ureilite from Almahata Sitta 2008 TC3 asteroid. Geochim. Cosmochim. Acta 2015, 163, 14–26. [Google Scholar] [CrossRef]
- Nabiei, F.; Badro, J.; Dennenwaldt, T.; Oveisi, E.; Cantoni, M.; Hèbert, C.; El Goresy, A.; Barrat, J.; Gillet, P. A large planetary body inferred from diamond inclusions in an ureilite meteorite. Nat. Commun. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Goodrich, C.A.; Nestola, F.; Jakubek, R.; Erickson, T.; Fries, M.; Fioretti, A.M.; Ross, D.K.; Brenker, F.E. The origin of diamonds in ureilites. In Proceedings of the 51st Lunar and Planetary Science Conference, Houston, TX, USA, 16–20 March 2020. [Google Scholar]
- Nestola, F.; Goodrich, C.A.; Morana, M.; Barbaro, A.; Christ, O.; Brenker, F.E.; Domeneghetti, M.D.; Dalconi, M.C.; Alvaro, M.; Fioretti, A.M.; et al. Impact shock origin of diamonds in ureilite meteorites. Proc. Natl. Acad. Sci. USA 2020. [Google Scholar] [CrossRef]
- Cody, G.D.; Alexander, C.M.O.D.; Yabuta, H.; Kilcoyne, A.L.D.; Araki, T.; Ade, H.; Dera, P.; Fogel, M.; Militzer, B.; Mysen, B.O. Organic thermometry for chondritic parent bodies. Earth Planet. Sci. Lett. 2008, 272, 446–455. [Google Scholar] [CrossRef]
- Ross, A.J.; Steele, A.; Fries, M.D.; Kater, L.; Downes, H.; Jones, A.P.; Smith, C.L.; Jenniskens, P.; Zolensky, M.E.; Shaddad, M.H. MicroRaman spectroscopy of diamond and graphite in Almahata Sitta and comparison with other ureilites. Meteorit. Planet. Sci. 2011, 46, 364–378. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Roberson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ammar, M.R.; Galy, N.; Rouzaud, J.N.; Toulhoat, N.; Vaudey, C.E.; Simon, P.; Moncoffre, N. Characterizing various types of defects in nuclear graphite using Raman scattering: Heat treatment, ion irradiation and polishing. Carbon 2015, 95, 364–373. [Google Scholar] [CrossRef]
- Warren, P.H.; Rubin, A.E. Pyroxene -selective impact smelting in ureilites. Geochim. Cosmochim. Acta 2010, 74, 5109–5133. [Google Scholar] [CrossRef]
- Matthews, M.J.; Pimenta, M.A. Origin of dispersive effects of the Raman D band in carbon materials. Phys. Rev. B 1999, 59, 6585–6588. [Google Scholar] [CrossRef]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Busemann, H.; Alexander, C.M.O.; Nittler, L.R. Characterization of insoluble organic matter in primitive meteorites by microRaman spectroscopy. Meteorit. Planet. Sci. 2007, 42, 1387–1416. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffe, B.; Chopin, C.; Rouzaud, J.N. Raman spectra of carbonaceous material in metasediments: A new geothermometer. J. Metamorphic. Geol. 2012, 20, 859–871. [Google Scholar] [CrossRef]
- Goodrich, C.A.; Wilson, L.; Van Orman, J.A.; Michel, P. Comment on “Parent body depth-pressure-temperature relationships and the style of the ureilite anatexis” by P. H. Warren (MAPS 47:209-227). Meteorit. Planet. Sci. 2013, 48, 1096–1106. [Google Scholar] [CrossRef]
- Warren, P.H. Parent body depth-pressure-temperature relationships and the style of the ureilite anatexis. Meteorit. Planet. Sci. 2012, 47, 209–227. [Google Scholar] [CrossRef]
- Goodrich, C.A.; Van Orman, J.A.; Wilson, L. Fractional melting and smelting of the ureilite parent body. Geochim. Cosmochim. Acta 2007, 71, 2876–2895. [Google Scholar] [CrossRef]
- Collinet, M.; Grove, T. Incremental melting in the ureilite parent body: Initial composition, melting temperatures, and compositions. Meteorit. Planet. Sci. 2020, 4, 832–856. [Google Scholar] [CrossRef]
- Mittlefehldt, D.W.; McCoy, T.J.; Goodrich, C.A.; Kracher, A. Non-chondritic meteorites from asteroidal bodies. Planet. Mater. 1998, 36, 1–195. [Google Scholar]
- Stöffler, D.; Hamann, C.; Metzlet, K. Shock metamorphism of planetary silicate rocks and sediments: Proposal for an updated classification system. Meteorit. Planet. Sci. 2018, 53, 5–49. [Google Scholar] [CrossRef]
- Gillet, P.; El Goresy, A. Shock Events in the Solar System: The message from minerals in Terrestrial Planets and Asteroids. Annu. Rev. Earth Planet. Sci. 2013, 41, 247–285. [Google Scholar] [CrossRef]
- Flynn, G.J.; Moore, L.B.; Klöck, W. Density and Porosity of Stone Meteorites: Implication for the Density, Porosity, Cratering, and Collisional Disruption of Asteroids. Icarus 1999, 142, 97–105. [Google Scholar] [CrossRef]
- Corrigan, C.M.; Zolensky, M.E.; Long, M.; Weir, J. Comparison of Porosity and Permeability in Chondritic Materials. Meteorit. Planet. Sci. 1996, 31, A32. [Google Scholar]
- Zolensky, M.E.; Herrin, J.S.; Mikouchi, T.; Ohsumi, K.; Friedrich, J.; Steele, A.; Rumble, D.; Fries, M.; Sandford, S.; Milam, S.; et al. Mineralogy and petrography of the Almahata Sitta ureilite. Meteorit. Planet. Sci. 2010, 45, 1618–1637. [Google Scholar] [CrossRef]
- Efremov, V.P.; Zakatilova, E.I.; Maklashova, I.V.; Shevchenko, N.V. Thermal stability of detonation-produced micro and nanodiamonds. J. Phys. Conf. Ser. 2018, 946, 012107. [Google Scholar] [CrossRef]
- Efremov, V.P.; Zakatilova, E.I. Spectroscopic methods of investigation and the thermal stability of detonation nanodiamonds. J. Phys. Conf. Ser. 2019, 1238, 012013. [Google Scholar] [CrossRef]
- Popov, V.A.; Egorov, A.V.; Savilov, S.V.; Lunin, V.V.; Kirichenko, A.N.; Denisov, V.N.; Blank, V.D.; Vyaselev, O.M.; Sagalova, T.B. Features of the transformation of detonation nanodiamonds into onion-like carbon nanoparticles. J. Surf. Invest. X-ray Synchrotron Neutron Tech. 2013, 7, 1034–1043. [Google Scholar] [CrossRef]


| D-Band | G-Band | I(D)/I(G) | La(nm) |
|---|---|---|---|
| AhS 209 b1 | |||
| 127,900 | 399,714 | 0.32 | 138 |
| 220,935 | 479,647 | 0.46 | 96 |
| 318,675 | 710,620 | 0.45 | 98 |
| 201,393 | 397,684 | 0.51 | 87 |
| 102,706 | 351,800 | 0.29 | 151 |
| AhS 209b3 | |||
| 338,898 | 653,892 | 0.52 | 84 |
| 360,516 | 578,409 | 0.62 | 70 |
| 206,073 | 433,157 | 0.48 | 92 |
| 321,808 | 605,128 | 0.53 | 83 |
| 280,470 | 519,306 | 0.54 | 81 |
| AhS 209b4 | |||
| 668,370 | 1,164,605 | 0.57 | 77 |
| 203,554 | 355,261 | 0.57 | 77 |
| 282,967 | 417,484 | 0.68 | 65 |
| 211,874 | 463,592 | 0.46 | 96 |
| 287,755 | 442,705 | 0.65 | 68 |
| AhS 72 | |||
| 321,884 | 550,667 | 0.58 | 75 |
| 285,483 | 476,515 | 0.60 | 73 |
| 89,546 | 127,387 | 0.70 | 63 |
| 384,023 | 491,833 | 0.78 | 56 |
| 317,754 | 479,550 | 0.66 | 66 |
| AhS A135A | |||
| 20,946 | 37,139 | 0.56 | 78 |
| 23,887 | 35,184 | 0.68 | 65 |
| 23,911 | 44,283 | 0.54 | 81 |
| 8174 | 18,598 | 0.44 | 100 |
| 9394 | 21,367 | 0.46 | 95 |
| G-Band Center | G-Band ΓG | G-Band ΓG Corrected | D-Band Center | D-Band ΓG | D′-Band Center | D′-Band ΓG | Tmax (°C) |
|---|---|---|---|---|---|---|---|
| AhS 209B | |||||||
| b1 | |||||||
| 1582 | 22 | 11 | 1356 | 41 | 1615 | 26 | 1360 |
| 1582 | 27 | 13 | 1354 | 49 | 1618 | 25 | 1310 |
| 1582 | 35 | 18 | 1352 | 47 | 1619 | 37 | 1212 |
| 1582 | 27 | 13 | 1355 | 46 | 1618 | 40 | 1309 |
| 1582 | 29 | 15 | 1355 | 47 | 1618 | 29 | 1285 |
| b3 | |||||||
| 1585 | 45 | 23 | 1354 | 55 | 1618 | 31 | 1103 |
| 1583 | 33 | 17 | 1354 | 50 | 1620 | 28 | 1237 |
| 1581 | 28 | 14 | 1355 | 50 | 1620 | 28 | 1300 |
| 1583 | 29 | 15 | 1355 | 48 | 1620 | 27 | 1284 |
| 1583 | 32 | 16 | 1355 | 57 | 1621 | 28 | 1254 |
| b4 | |||||||
| 1581 | 43 | 22 | 1355 | 60 | 1613 | 44 | 1122 |
| 1582 | 26 | 13 | 1355 | 49 | 1619 | 31 | 1313 |
| 1582 | 25 | 12 | 1354 | 47 | 1618 | 30 | 1332 |
| 1580 | 35 | 17 | 1353 | 51 | 1619 | 24 | 1219 |
| 1580 | 22 | 11 | 1353 | 52 | 1611 | 58 | 1357 |
| AhS 72 | |||||||
| 1577 | 33 | 13 | 1352 | 53 | 1616 | 37 | 1245 |
| 1579 | 40 | 16 | 1351 | 54 | 1616 | 31 | 1166 |
| 1581 | 29 | 20 | 1353 | 51 | 1606 | 68 | 1283 |
| 1584 | 32 | 15 | 1352 | 50 | 1619 | 33 | 1246 |
| 1583 | 30 | 14 | 1353 | 50 | 1620 | 29 | 1274 |
| AhS A135A | |||||||
| 1582 | 25 | 13 | 1355 | 48 | 1619 | 20 | 1320 |
| 1583 | 28 | 14 | 1356 | 51 | 1618 | 26 | 1288 |
| 1582 | 22 | 10 | 1357 | 41 | 1620 | 25 | 1350 |
| 1580 | 21 | 12 | 1353 | 57 | 1614 | 30 | 1361 |
| 1582 | 23 | 11 | 1357 | 38 | 1620 | 23 | 1340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbaro, A.; Domeneghetti, M.C.; Goodrich, C.A.; Meneghetti, M.; Litti, L.; Fioretti, A.M.; Jenniskens, P.; Shaddad, M.H.; Nestola, F. Graphite-Based Geothermometry on Almahata Sitta Ureilitic Meteorites. Minerals 2020, 10, 1005. https://doi.org/10.3390/min10111005
Barbaro A, Domeneghetti MC, Goodrich CA, Meneghetti M, Litti L, Fioretti AM, Jenniskens P, Shaddad MH, Nestola F. Graphite-Based Geothermometry on Almahata Sitta Ureilitic Meteorites. Minerals. 2020; 10(11):1005. https://doi.org/10.3390/min10111005
Chicago/Turabian StyleBarbaro, Anna, M. Chiara Domeneghetti, Cyrena A. Goodrich, Moreno Meneghetti, Lucio Litti, Anna Maria Fioretti, Peter Jenniskens, Muawia H. Shaddad, and Fabrizio Nestola. 2020. "Graphite-Based Geothermometry on Almahata Sitta Ureilitic Meteorites" Minerals 10, no. 11: 1005. https://doi.org/10.3390/min10111005
APA StyleBarbaro, A., Domeneghetti, M. C., Goodrich, C. A., Meneghetti, M., Litti, L., Fioretti, A. M., Jenniskens, P., Shaddad, M. H., & Nestola, F. (2020). Graphite-Based Geothermometry on Almahata Sitta Ureilitic Meteorites. Minerals, 10(11), 1005. https://doi.org/10.3390/min10111005






