Study on the Liberation of Organic Macerals in Coal by Liquid Nitrogen Quenching Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
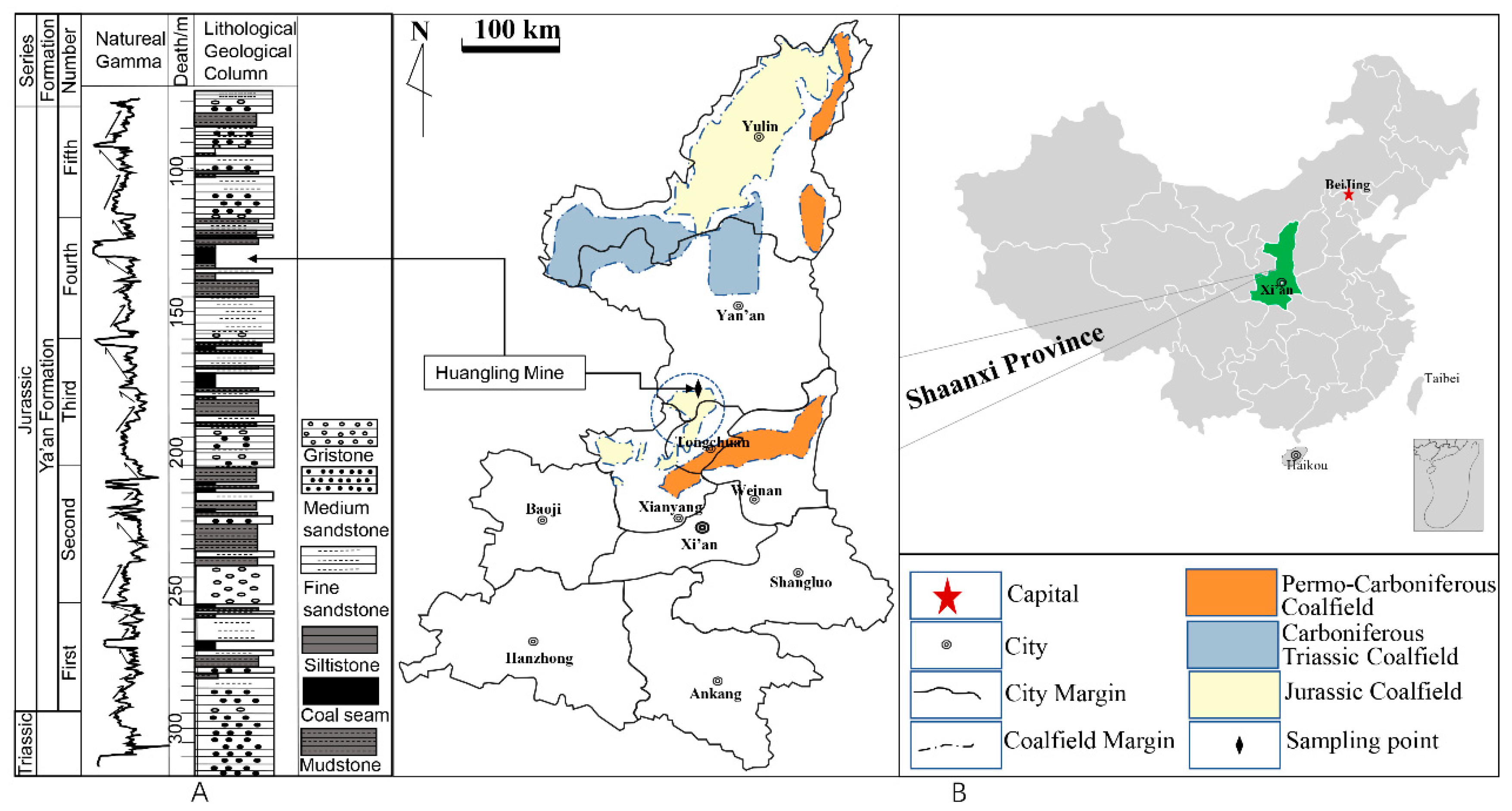
2.1. Materials
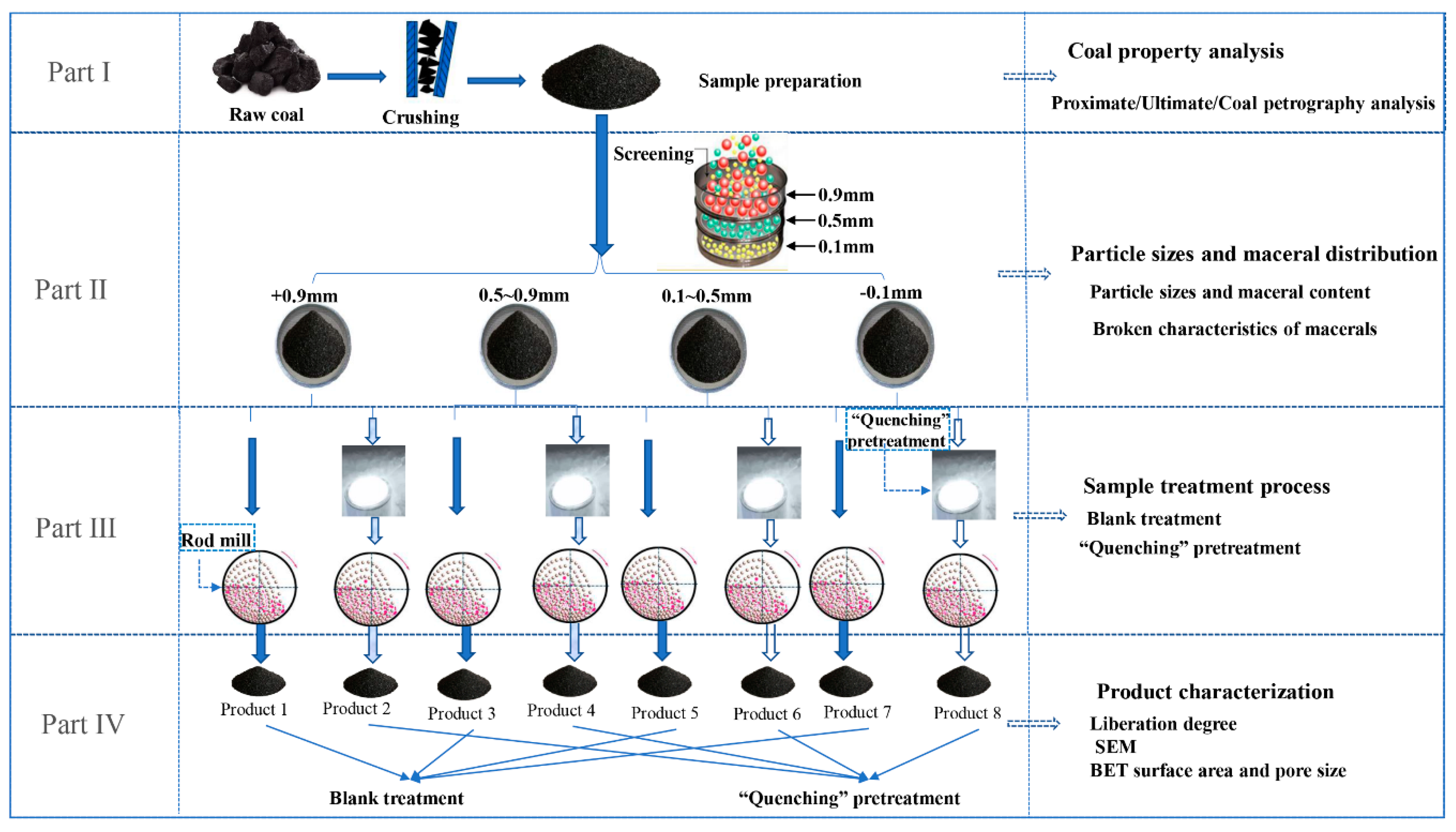
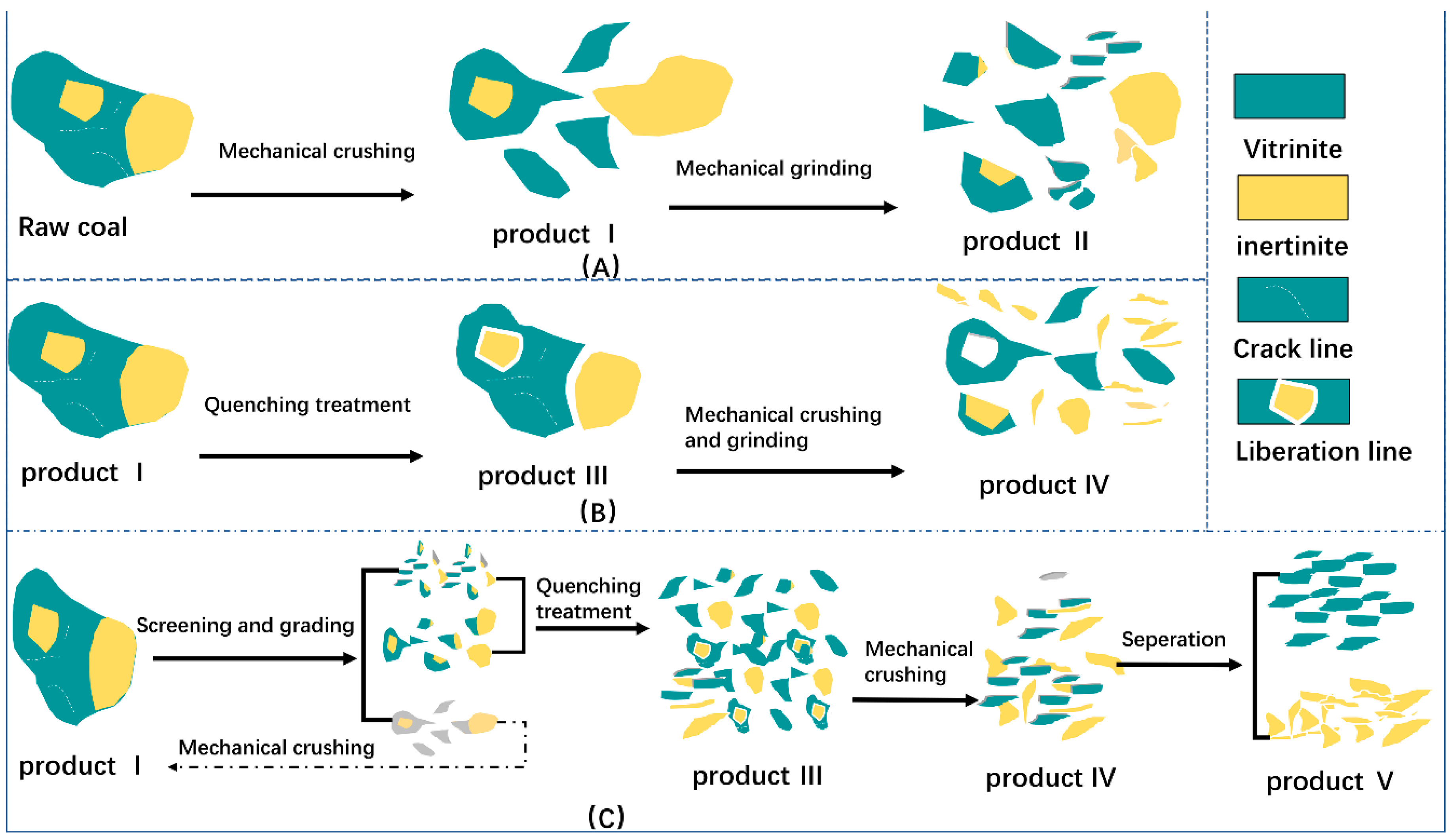
2.2. Experimental Process
- Fa: Monomer liberation degree of macerals in coal;
- fa: Number of liberated monomer particles;
- fb: Number of unliberated monomer particles.
3. Results and Discussion
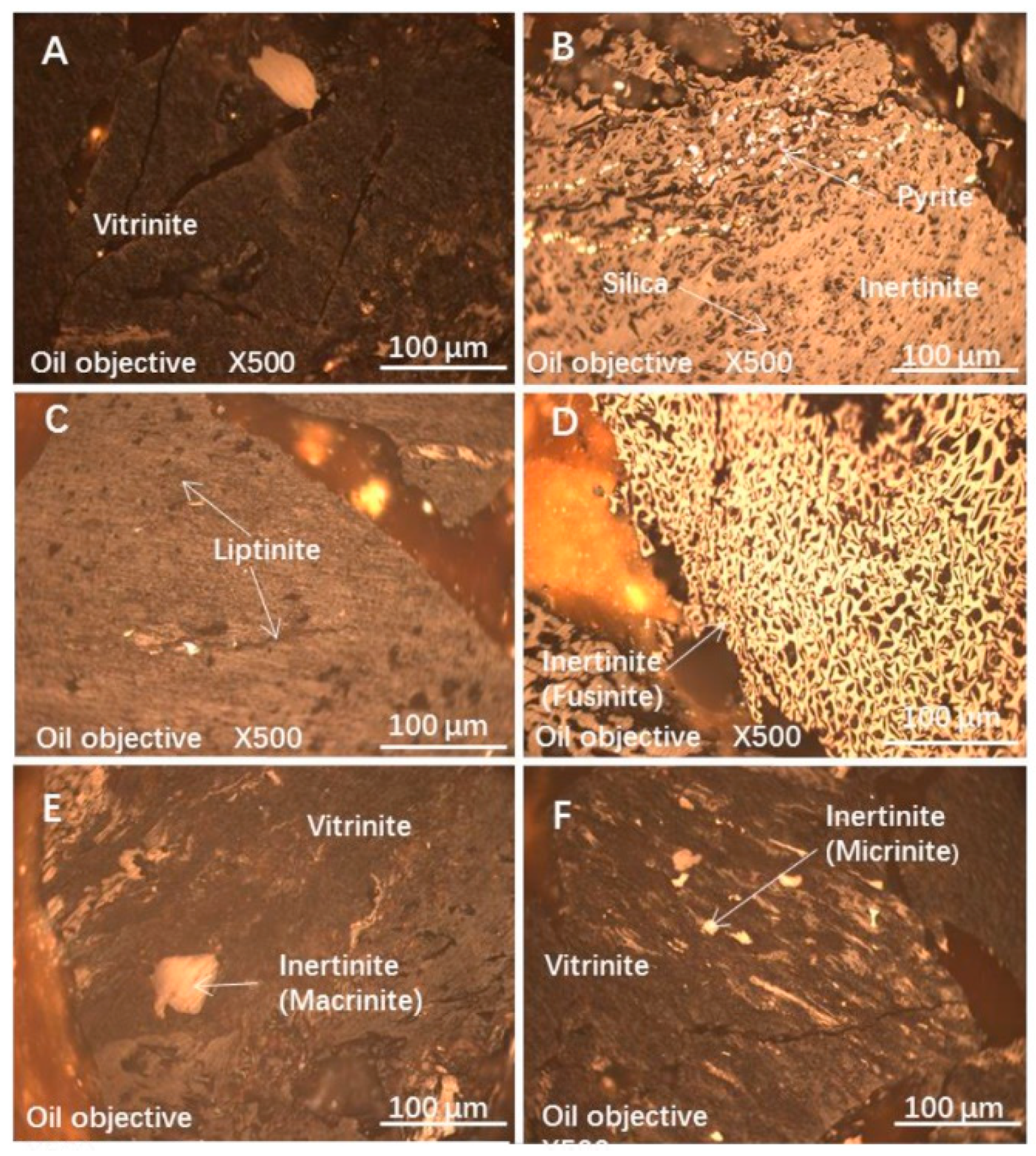
3.1. Coal Property Analysis
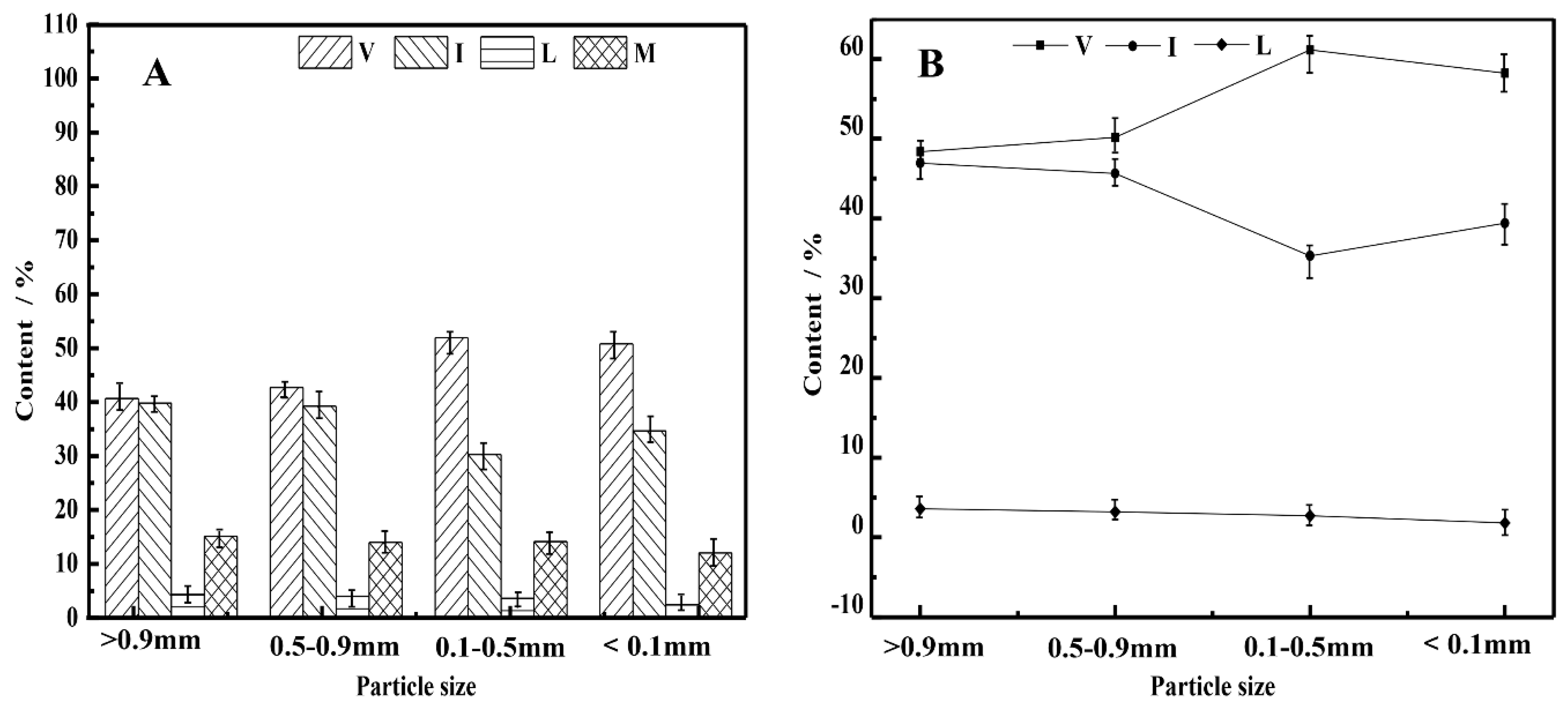
3.2. Particle Sizes and Maceral Distribution
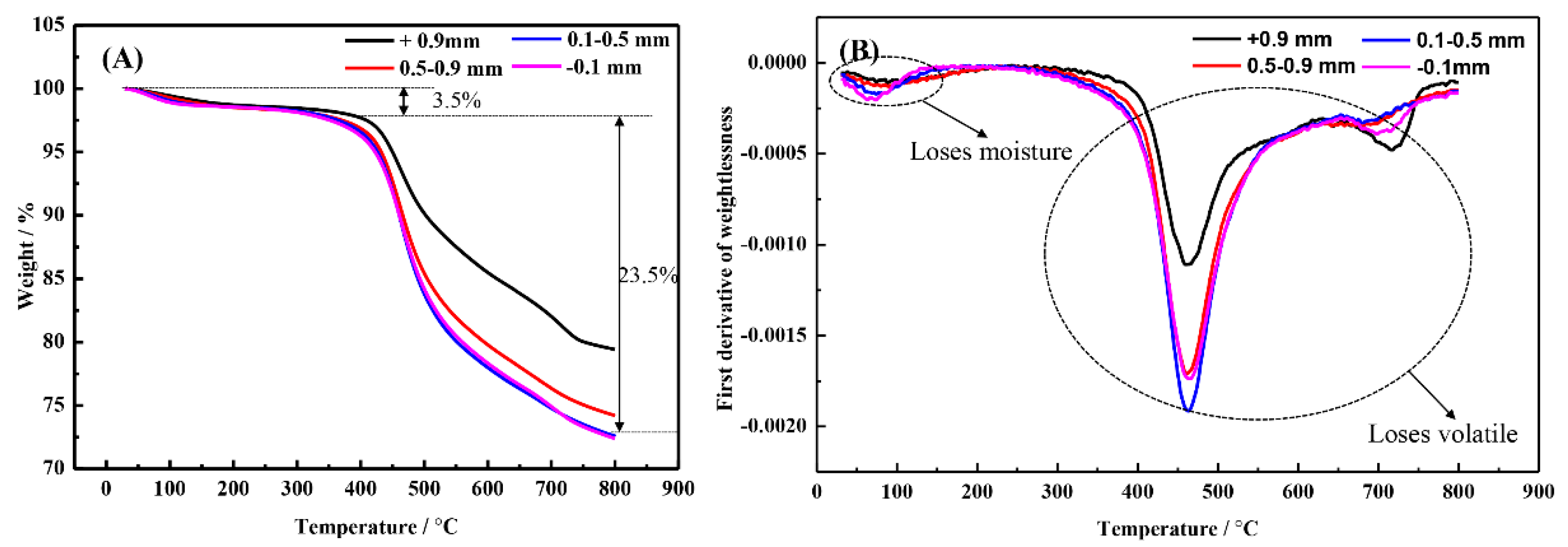
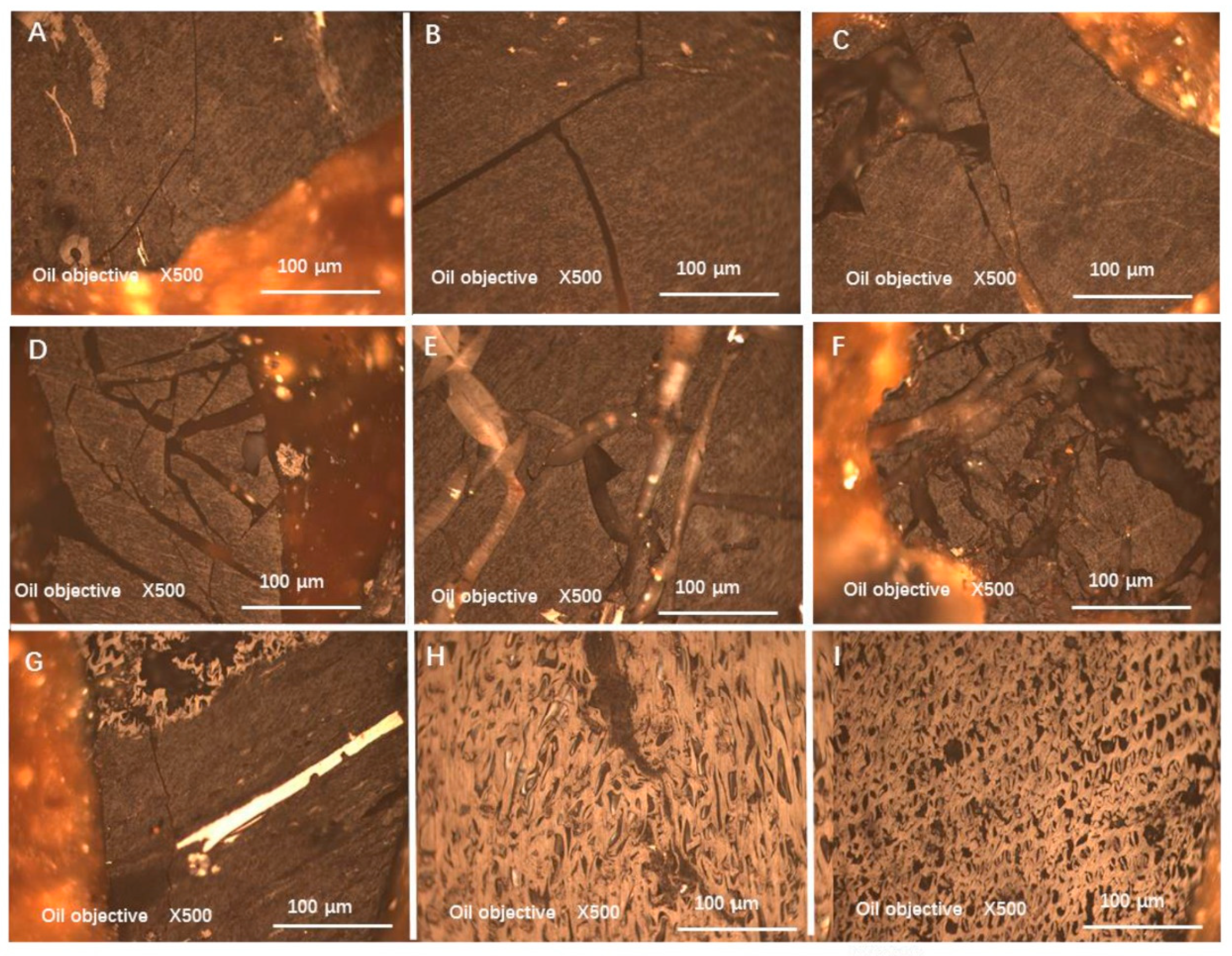
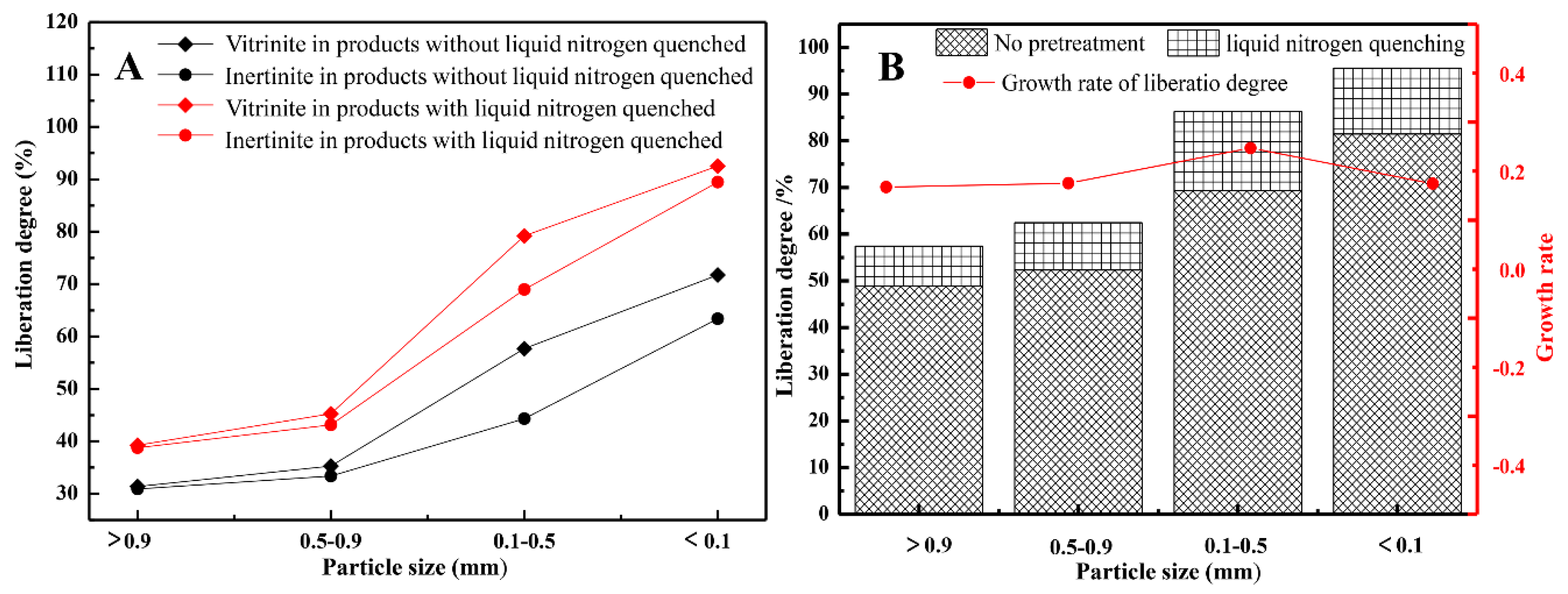
3.3. Pretreatment with “Quenching”
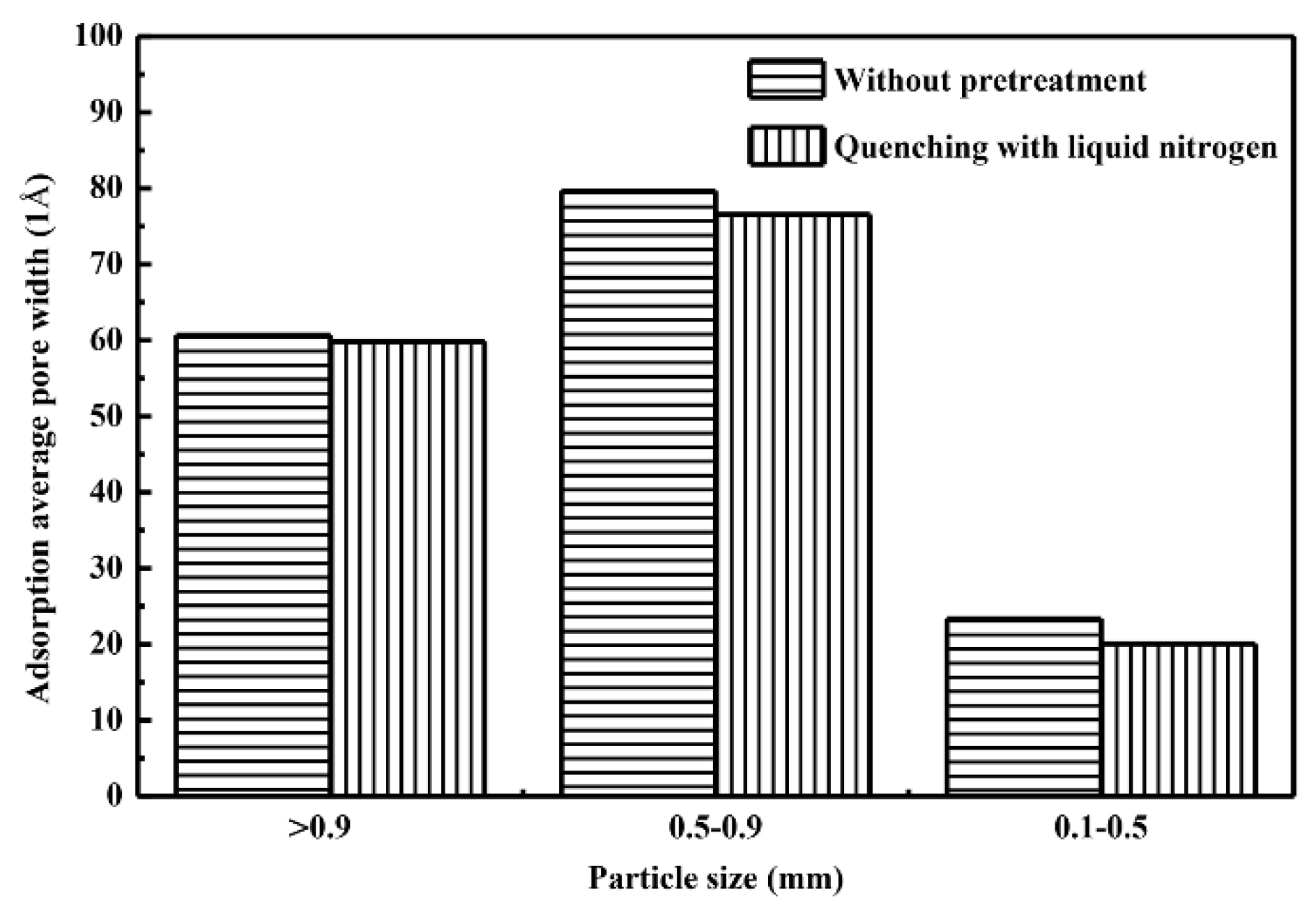
3.4. BET Surface Area and Pore Size
3.5. Model of Maceral Liberation and Optimization
4. Conclusions
- (1)
- Huangling coal is a low-rank bituminous coal with low sulfur, low ash and medium calorific value, and it is rich in inertinite, which may be the reason for its relatively higher calorific value;
- (2)
- Screening can preconcentrate macerals because the distribution of macerals is related to the particle size, such as inertinite with a high content in coarse particles and vitrinite with a high content in fine particles;
- (3)
- Liquid nitrogen quenching pretreatment can promote the liberation of different macerals before crushing and grinding. Compared with mechanical crushing alone, the liquid nitrogen quenching pretreatment yields different macerals that exhibit different microscopic characteristics and this affects the liberation or pre-liberation of different macerals from coal. Moreover, the liberation pattern is mainly arc-shaped, which is different from line-shaped liberation induced by mechanical fracturing methods alone;
- (4)
- Along with the above results, we believed that liquid nitrogen quenching pretreatment has the best liberation effect for samples with a particle size of 0.1–0.5 mm and that this presents an optimized model for the liberation of macerals based on the combination of screening, liquid nitrogen quenching pretreatment and re-crushing.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chai, J.; Du, M.; Liang, T.; Sun, X.C.; Yu, J.; Zhang, Z.G. Coal consumption in China: How to bend down the curve? Energy Econ. 2019, 80, 38–47. [Google Scholar] [CrossRef]
- Stach, E.; Mackowsky, M.T.; Teichmuller, M.; Taylor, G.H.; Teichmüller, R. Stach’s Textbook of Coal Petrology, 3rd ed.; Gebruder Borntraeger: Berlin, Germany, 1982; pp. 87–100. [Google Scholar]
- Guo, Y.; Bustin, R.M. Micro-FTIR spectroscopy of liptinite macerals in coal. Int. J. Coal Geol. 1998, 36, 259–275. [Google Scholar] [CrossRef]
- George, A.M.; Mackay, G.H. The Science of Victorian Brown Coal, 1st ed.; Butterworth-Heinemann: Oxford, UK, 1991; pp. 45–102. [Google Scholar]
- Hower, J.C.; O’Keefe, J.M.K.; Eble, C.F.; Raymond, A.; Valentim, B.; Volk, T.J.; Richardson, A.R.; Satterwhite, A.B.; Hatch, R.; Stucker, J.D.; et al. Notes on the origin of inertinite macerals in coal: Evidence for fungal and arthropod transformations of degraded macerals. Int. J. Coal Geol. 2011, 86, 231–240. [Google Scholar] [CrossRef]
- Gupta, R. Advanced coal characterization: A review. Energy Fuels 2007, 21, 451–460. [Google Scholar] [CrossRef]
- Roberts, M.J.; Everson, R.C.; Neomagus, H.W.J.P.; Niekerk, D.V.; Mathews, J.P.; Branken, D.J. Influence of maceral composition on the structure, properties and behaviour of chars derived from South African coals. Fuel 2015, 142, 9–20. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yamashita, H.; Tomita, A. Gasification reactivities of coal macerals. Fuel Process. Technol. 1991, 29, 75–84. [Google Scholar] [CrossRef]
- Keogh, R.A.; Taulbee, D.N.; Hower, J.C.; Chawla, B.; Davis, B.H. Liquefaction characteristics of the three major maceral groups separated from a single coal. Energy Fuels 1992, 6, 614–618. [Google Scholar] [CrossRef]
- Machnikowska, H.; Krztoń, A.; Machnikowski, J. The characterization of coal macerals by diffuse reflectance infrared spectroscopy. Fuel 2002, 81, 245–252. [Google Scholar] [CrossRef]
- Dyrkacz, G.R.; Bloomquist, C.A.A.; Ruscic, L. Chemical variations in coal macerals separated by density gradient centrifugation. Fuel 1984, 63, 1166–1173. [Google Scholar] [CrossRef]
- Marotovaler, M.M.; Taulbee, D.N.; Andrésen, J.M.; Hower, J.C.; Snape, C.E. Quantitative 13C NMR study of structural variations within the vitrinite and inertinite maceral groups for a semifusinite-rich bituminous coal. Fuel 1998, 77, 805–813. [Google Scholar] [CrossRef]
- Das, T.K. Thermogravimetric characterisation of maceral concentrates of Russian coking coals. Fuel 2001, 80, 97–106. [Google Scholar] [CrossRef]
- Roy, M.M. Studies on coal macerals, [Part] 4, Chlorination of exinite and fusinite. Econ. Geol. 1965, 60, 1404–1410. [Google Scholar] [CrossRef]
- Taulbee, D.; Poe, S.H.; Robl, T.; Keogh, B. Density gradient centrifugation separation and characterization of maceral groups from a mixed maceral bituminous coal. Energy Fuels 1989, 3, 662–670. [Google Scholar] [CrossRef]
- Ding, L.P. Investigation of bituminous coal hydrophobicity and its influence on flotation. Energy Fuels 2009, 23, 5536–5543. [Google Scholar] [CrossRef]
- Louw, E.B.; Mitchell, G.D.; Wang, J.; Winans, R.E.; Mathews, J.P. Constitution of drop-tube-generated coal chars from vitrinite-and inertinite-rich South African coals. Energy Fuels 2015, 30, 112–120. [Google Scholar] [CrossRef]
- Guo, H.; Lj, S.; Gx, X.B.H. Effects of coal macerals on the pore structure and electrochemical performances of activated carbon. J. China Coal Soc. 2014, 39, 2328–2334. [Google Scholar]
- Duxbury, J. Prediction of coal pyrolysis yields by maceral separation. J. Anal. Appl. Pyrolysis 1997, 40, 233–242. [Google Scholar] [CrossRef]
- Dyrkacz, G.R.; Horwitz, E.P. Separation of coal macerals. Fuel 1982, 61, 3–12. [Google Scholar] [CrossRef]
- Stankiewicz, B.A.; Kruge, M.A.; Crelling, J.C.; Salmon, G.L. Density gradient centrifugation: Application to the separation of macerals of type I, II, and III sedimentary organic matter. Energy Fuels 1994, 8, 1513–1521. [Google Scholar] [CrossRef]
- Pugmire, R.J.; Zilm, K.W.; Woolfenden, W.R.; Grant, D.M.; Dyrkacz, G.R.; Bloomquist, C.A.A.; Horwitz, E.P. Carbon-13 NMR spectra of macerals separated from individual coals. Org. Geochem. 1982, 4, 79–84. [Google Scholar] [CrossRef]
- Dyrkacz, G.R.; Bloomquist, C.A.A.; Ruscic, L.; Crelling, J.C. An investigation of the vitrinite maceral group in microlithotypes using density gradient separation methods. Energy Fuels 1991, 5, 155–163. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Johns, R.B.; Dyrkacz, G.R.; Buchanan, A.S. Separation and preliminary characterization of high-purity maceral group fractions from an Australian bituminous coal. Energy Fuels 1988, 2, 657–662. [Google Scholar] [CrossRef]
- Yan, Y.; Qi, Y.; Marshall, M.; Jackson, W.R.; Chaffee, A.L. Separation and analysis of maceral concentrates from Victorian brown coal. Fuel 2019, 242, 232–242. [Google Scholar] [CrossRef]
- Dyrkacz, G.R.; Bloomquist, C.A.A.; Ruscic, L. Investigations into the process of maceral separation by centrifugal techniques. 3. Continuous flow and sink/float maceral separation of-100-mesh coal. Energy Fuels 1993, 7, 655–660. [Google Scholar] [CrossRef]
- Dyrkacz, G.R.; Ruscic, L.; Fredericks, J. An investigation into the process of centrifugal sink/float separations of micronized coals. 1. Some inferences for coal maceral separations. Energy Fuels 1992, 6, 720–742. [Google Scholar] [CrossRef]
- Jorjani, E.; Esmaeili, S.; Khorami, M.T. The effect of particle size on coal maceral group’s separation using flotation. Fuel 2013, 114, 10–15. [Google Scholar] [CrossRef]
- Honaker, R.Q.; Mohanty, M.K.; Crelling, J.C. Coal maceral separation using column flotation. Miner. Eng. 1996, 9, 449–464. [Google Scholar] [CrossRef]
- Kopparthi, P.; Singh, R.; Nag, D.; Mukherjee, A.K. Vitrinite maceral separation using column flotation. Int. J. Coal Prep. Util. 2018, 38, 13–29. [Google Scholar] [CrossRef]
- Cronauer, D.C.; Joseph, J.T.; Davis, A.; Quick, J.C.; Luckie, P.T. The beneficiation of Martin Lake Texas lignite. Fuel 1992, 71, 65–73. [Google Scholar] [CrossRef]
- Cho, H.; Luckie, P.T. Evaluation of liberation characteristics of coal organic components. Min. Metall. Explor. 1998, 15, 29–35. [Google Scholar] [CrossRef]
- Gómez Rojas, O.P.; Carmona Lopez, I.; Bustamante Rúa, M.O. Analysis of liberation of the groups of Macerales of the coal: Colombian coals. Boletín Cienc. Tierra 2007, 21, 14. [Google Scholar]
- Zhang, L.; Hower, J.C.; Honaker, R.Q.; Liu, W.; Men, D. Flotation rates and zeta potentials of maceral concentrates of bituminous coals. Min. Metall. Explor. 2015, 32, 129–137. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Zhou, A.; Zhu, C.; Yang, C.; Shen, N.; Yang, C. Effect of multi-intensification on the liberation of maceral components in coal. Fuel 2019, 237, 1003–1012. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Z.; Zhou, A.; Xiong, S.; Yang, C. Evaluation of coal component liberation upon impact breakage by MLA. Fuel 2019, 258, 116136. [Google Scholar] [CrossRef]
- Evans, C.L.; Wightman, E.M.; Yuan, X. Quantifying mineral grain size distributions for process modelling using x-ray micro-tomography. Miner. Eng. 2015, 82, 78–83. [Google Scholar] [CrossRef]
- Ueda, T.; Oki, T.; Koyanaka, S. Statistical effect of sampling particle number on mineral liberation assessment. Miner. Eng. 2016, 98, 204–212. [Google Scholar] [CrossRef]
- Guntoro, P.I.; Tiu, G.; Ghorbani, Y.; Lund, C.; Rosenkranz, J. Application of machine learning techniques in mineral phase segmentation for x-ray microcomputed tomography (µct) data. Miner. Eng. 2019, 142, 105882. [Google Scholar] [CrossRef]
- Fan, J.; Du, M.; Liu, L. Study on the correlation between trace elements in coal and coal-forming plants: A case study. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Pierre, M.G. The sampling of particulate materials—A general theory. Int. J. Miner. Process. 1976, 3, 289–312. [Google Scholar]
- China National Coal Association. Method for Manual Sampling of Commercial Coal, 1st ed.; China National Standard Press: Beijing, China, 2008; pp. 1–30. [Google Scholar]
- China National Coal Association. Method for Preparation of Coal Sample, 1st ed.; China National Standard Press: Beijing, China, 2008; pp. 1–29. [Google Scholar]
- China National Coal Association. Proximate Analysis of Coal, 1st ed.; China National Standard Press: Beijing, China, 2008; pp. 1–14. [Google Scholar]
- China National Coal Association. Ultimate Analysis of Coal, 1st ed.; China National Standard Press: Beijing, China, 2015; pp. 1–12. [Google Scholar]
- China National Coal Association. Determination of Calorific Value of Coal, 1st ed.; China National Standard Press: Beijing, China, 2009; pp. 1–28. [Google Scholar]
- China National Coal Association. Determination of Maceral Group Composition and Minerals in Coal, 1st ed.; China National Standard Press: Beijing, China, 2014; pp. 1–12. [Google Scholar]
- Zhou, L. Process Mineralogy, 3rd ed.; Metallurgical Industry Press: Beijing, China, 2002; pp. 243–263. [Google Scholar]
- China National Coal Association. Classification for Quality of Coal—Part 1: Ash, 1st ed.; China National Standard Press: Beijing, China, 2018; pp. 1–8. [Google Scholar]
- China National Coal Association. Classification of Quality of Coal—Part 2: Sulfur Content, 1st ed.; China National Standard Press: Beijing, China, 2018; pp. 1–8. [Google Scholar]
- China National Coal Association. Classification of Quality of Coal—Part 3: Calorific Value, 1st ed.; China National Standard Press: Beijing, China, 2010; pp. 1–8. [Google Scholar]
- Fu, Y.; Guo, Y.H.; Zhang, K.X. Effect of three different catalysts (Kcl, Cao, and Fe2O3) on the reactivity and mechanism of low-rank coal pyrolysis. Energy Fuels 2016, 30, 2428–2433. [Google Scholar] [CrossRef]
- Solomon, P.R.; Fletcher, T.H.; Pugmire, R.J. Progress in coal pyrolysis. Fuel 1993, 72, 587–597. [Google Scholar] [CrossRef]
- Sun, Q.; Li, W.; Chen, H.; Li, B. The variation of structural characteristics of macerals during pyrolysis☆. Fuel 2013, 82, 669–676. [Google Scholar] [CrossRef]
- Oki, T.; Tanaka, J.; Harada, T. Cause of Coal Grindability. Correlation of coal rank, maceral composition, hardness, and brittleness. Min. Mater. Process. Inst. Jpn. 1996, 112, 37–42. [Google Scholar]
- Falcon, L.M.; Falcon, R.M.S. The petrographic composition of Southern African coals in relation to friability, hardness, and abrasive indices. J. South. Afr. Inst. Min. Metall. 1987, 87, 323–336. [Google Scholar]
- Cardott, B.J.; Curtis, M.E. Identification and nanoporosity of macerals in coal by scanning electron microscopy. Int. J. Coal Geol. 2018, 190, 205–217. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Tomas, J.; Schreier, M.; Gröger, T.; Ehlera, S. Impact crushing of concrete for liberation and recycling. Powder Technol. 1999, 105, 39–51. [Google Scholar] [CrossRef]
- Badzioch, S.; Gregory, D.; Field, M. Investigation of the temperature variation of the thermal conductivity and thermal diffusivity of coal. Fuel 1964, 43, 267. [Google Scholar]
- Chu, Z.; Zhou, G.; Bi, S. Meso-characterization of the effective thermal conductivity of selected typical geomaterials in an underground coal mine. Energy Explor. Exploit. 2018, 36, 488–508. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Li, Q.; Zhang, J. Relationship between Microstructure and Thermal Conductivity in Coal Slags with Variable Silica and Alumina. Energy Fuels 2019, 33, 6226–6233. [Google Scholar] [CrossRef]
- Rezaei, H.R.; Gupta, R.P.; Bryant, G.W.; Hart, J.T.; Liu, G.; Bailey, C.W.; Wall, T.F.; Miyamae, S.; Makino, K.; Endo, Y. Thermal conductivity of coal ash and slags and models used. Fuel 2000, 79, 1697–1710. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, P.; Dong, S.; Huang, Y.; Zhang, M. Brittleness index analysis of coal samples. Acta Geophysica. 2019, 67, 789–797. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Hu, N.; Yang, H.; Lu, J. Calculation model of coal comminution energy consumption. Miner. Eng. 2016, 92, 21–27. [Google Scholar] [CrossRef]



| Proximate Analysis (%) | Ultimate Analysis (%) | Calorific Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | FCad | Cdaf | Hdaf | Ndaf | Odaf | St,d | Qgr.d/mJ·kg−1 |
| 3.61 | 14.60 | 27.97 | 59.90 | 58.80 | 3.71 | 0.73 | 36.04 | 0.72 | 23.89 |
| Vitrinite (%) | Inertinite (%) | Liptinite (%) | Sulfide Minerals (%) | Quartz Minerals (%) | Clay Minerals (%) | Carbonate Minerals (%) | Vitrinite Reflectances (%) |
|---|---|---|---|---|---|---|---|
| 44.05 | 38.60 | 2.35 | 7.28 | 3.62 | 2.16 | 1.94 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Du, M.; Liu, L. Study on the Liberation of Organic Macerals in Coal by Liquid Nitrogen Quenching Pretreatment. Minerals 2020, 10, 911. https://doi.org/10.3390/min10100911
Fan J, Du M, Liu L. Study on the Liberation of Organic Macerals in Coal by Liquid Nitrogen Quenching Pretreatment. Minerals. 2020; 10(10):911. https://doi.org/10.3390/min10100911
Chicago/Turabian StyleFan, Jinwen, Meili Du, and Lei Liu. 2020. "Study on the Liberation of Organic Macerals in Coal by Liquid Nitrogen Quenching Pretreatment" Minerals 10, no. 10: 911. https://doi.org/10.3390/min10100911
APA StyleFan, J., Du, M., & Liu, L. (2020). Study on the Liberation of Organic Macerals in Coal by Liquid Nitrogen Quenching Pretreatment. Minerals, 10(10), 911. https://doi.org/10.3390/min10100911




