Effect of the Cooling Regime on the Mineralogy and Reactivity of Belite-Sulfoaluminate Clinkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. X-Ray Powder Diffraction
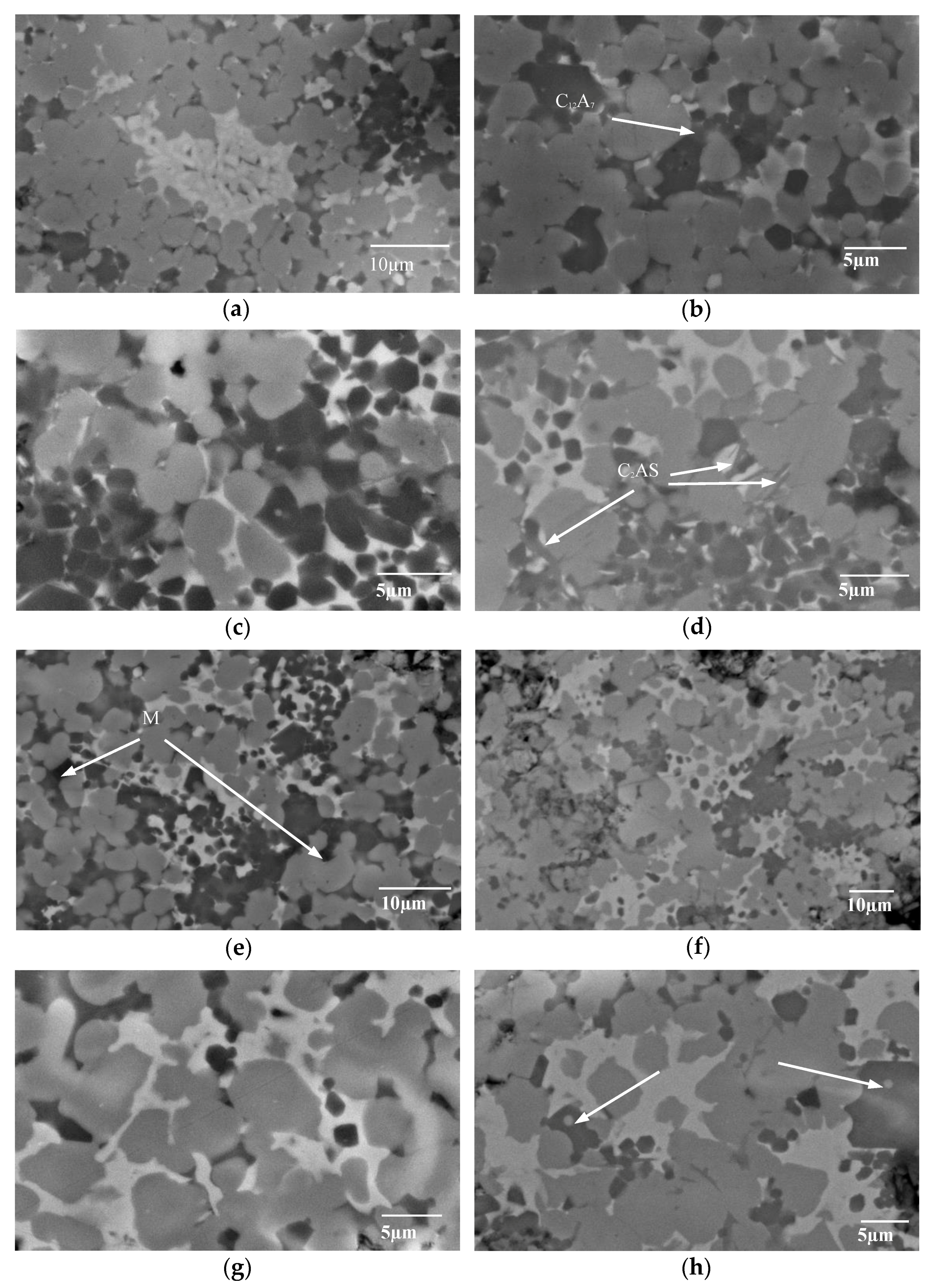
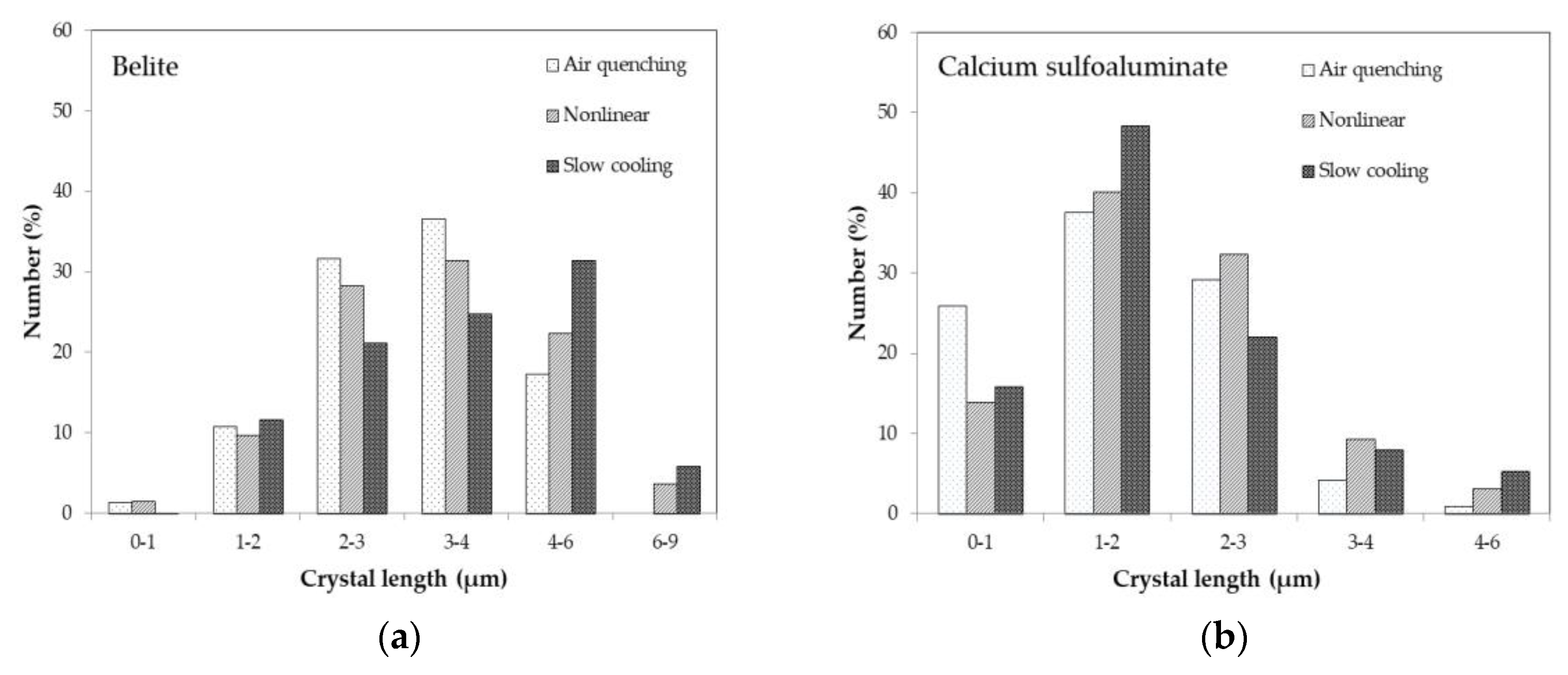
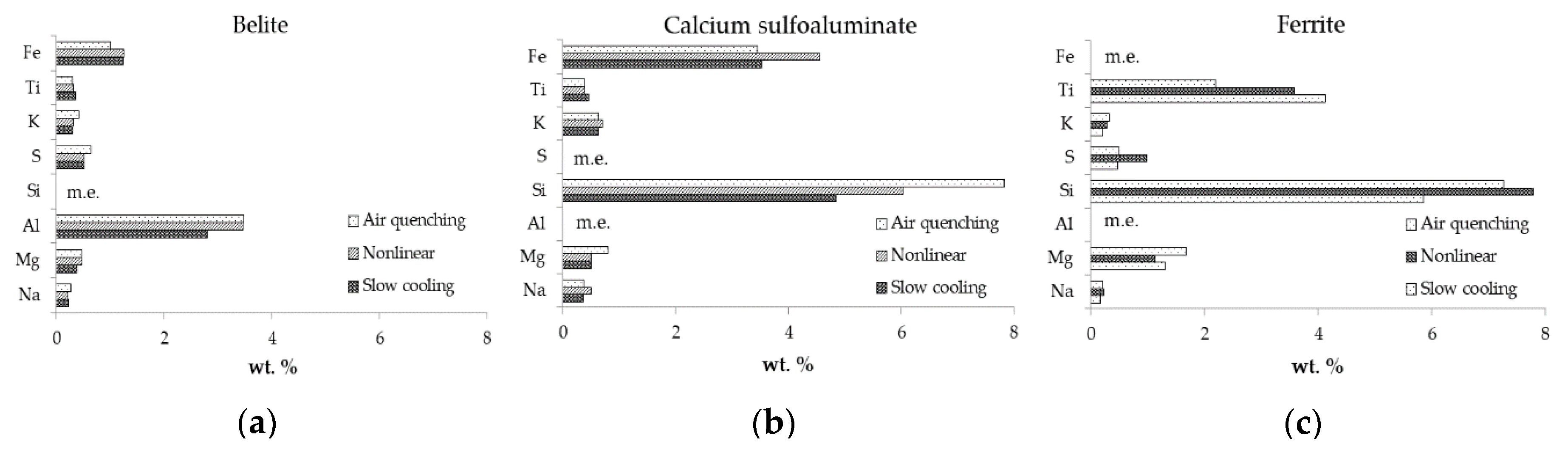
3.2. SEM/EDS
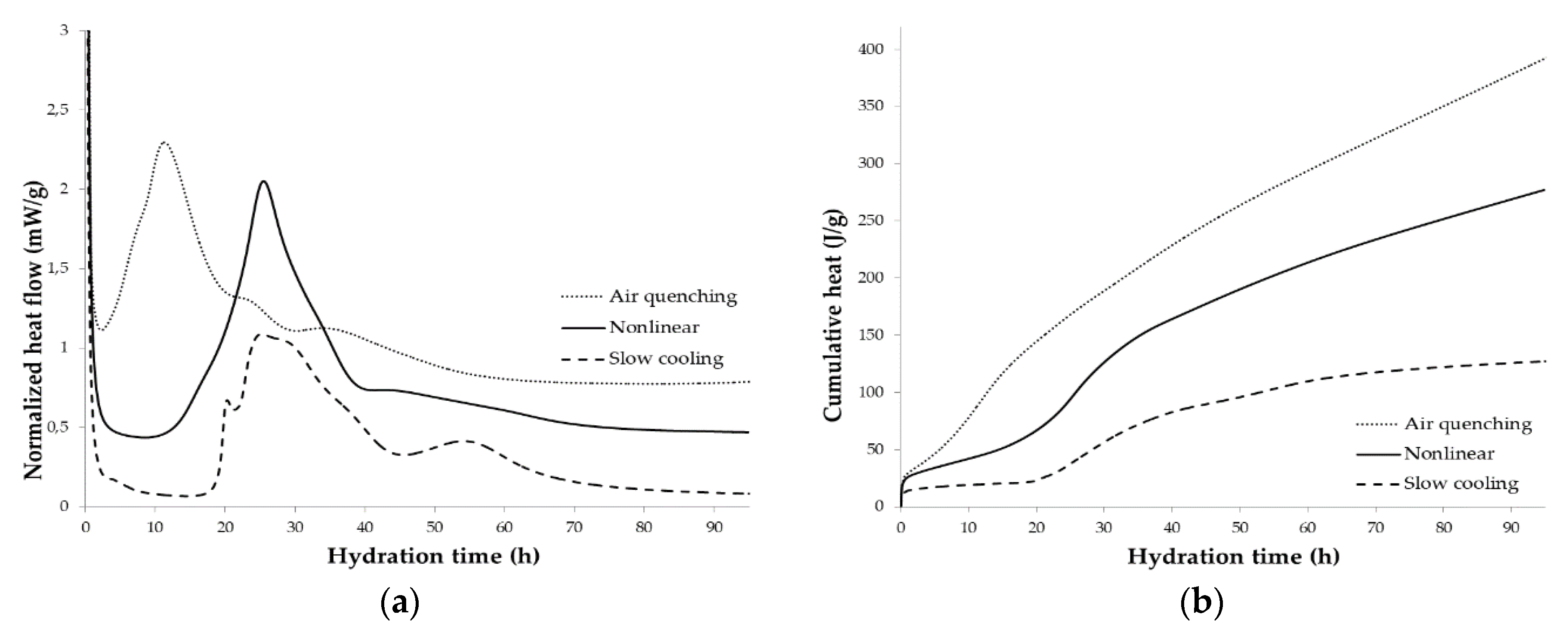
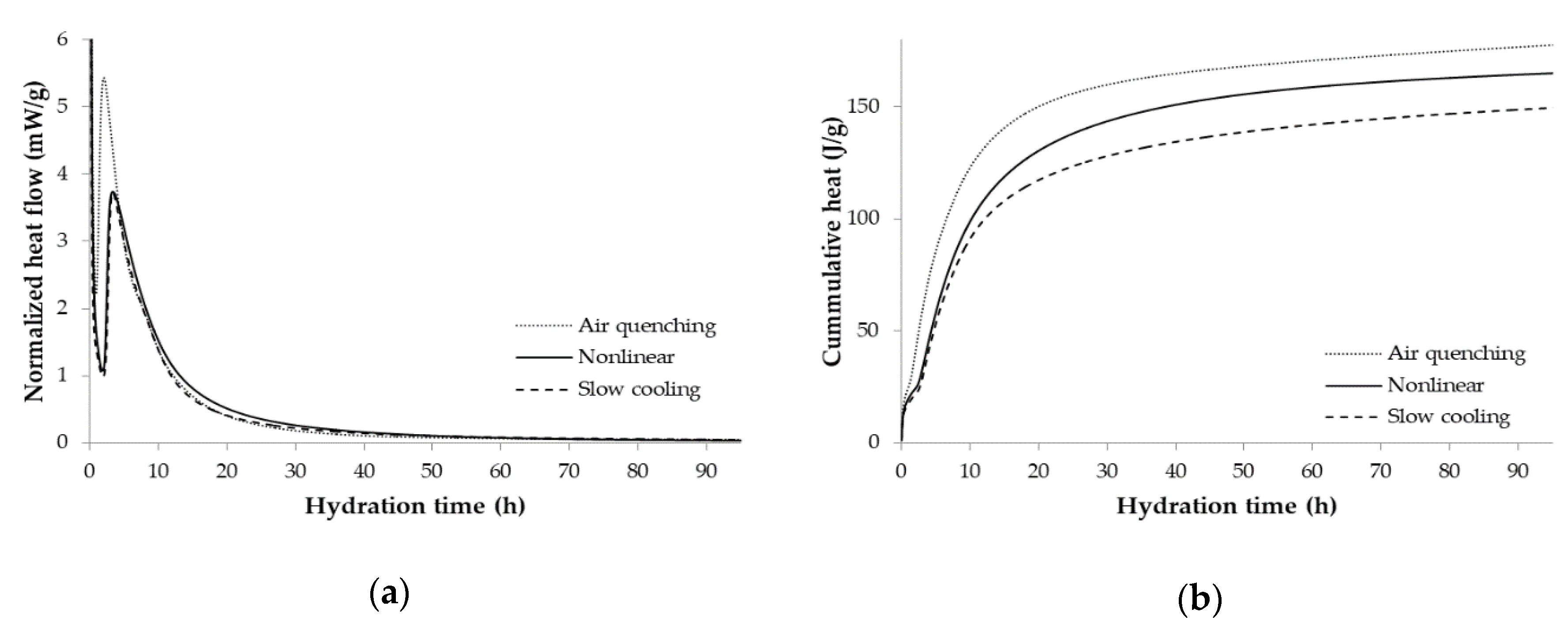
3.3. Isothermal Conduction Calorimetry
3.4. Compressive Strength
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ono, Y. Microscopical Observations of Clinker for the Estimation of Burning Condition. Grindability, and Hydraulic Activity. In Proceedings of the Third International Conference on Cement Microscopy, International Cement Microscopy Association, Houston, TX, USA, 19–21 March 1981; pp. 198–210. [Google Scholar]
- Bullard, R.A. Effect of Cooling Rates on Mineralization in Portland Cement Clinker. Ph.D. Thesis, University of Missouri-Columbia, Columbia, MO, USA, 2015. [Google Scholar]
- Telschow, S.; Dam-Johansen, K.; Jappe Frandsen, F.; Wedel, S.; Theisen, K. Clinker Burning Kinetics and Mechanism. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2012. [Google Scholar]
- Staněk, T.; Sulovský, P. The Impact of Basic Minor Oxides on the Clinker Formation. Mater. Sci. Forum 2017, 908, 3–9. [Google Scholar] [CrossRef]
- Ward, G.W. Effect of heat treatment and cooling rate on the microscopic structure of portland cement clinker. J. Res. Natl. Bur. Stan. 1941, 26, 49. [Google Scholar] [CrossRef]
- Hong, H.; Fu, Z.; Min, X. Effect of cooling performance on the mineralogical character of Portland cement clinker. Cem. Concr. Res. 2001, 31, 287–290. [Google Scholar] [CrossRef]
- Sazonova, N.A.; Skripnikova, N.K. Using the low-temperature plasma in cement production. J. Phys. Conf. Ser. 2015, 652, 012063. [Google Scholar] [CrossRef]
- Maki, I. Processing Conditions of Portland Cement Clinker as Viewed from the Fine Textures of the Constituent Minerals. Ceram. Trans. 1994, 40, 3–17. [Google Scholar]
- Ichikawa, M.; Ikeda, S.; Komukai, Y. Effect of cooling rate and Na20 content on the character of the interstitial materials in portland cement clinker. Cem. Concr. Res. 1994, 24, 5. [Google Scholar]
- Fukuda, K.; Maki, I.; Ikeda, S.; Ito, S. Microtextures Formed by the Remelting Reaction in Belite Crystals. J. Am. Ceram. Soc. 1993, 76, 2942–2944. [Google Scholar] [CrossRef]
- Martín-Sedeño, M.C.; Cuberos, A.J.M.; De la Torre, Á.G.; Álvarez-Pinazo, G.; Ordónez, L.M.; Gateshki, M.; Aranda, M.A.G. Aluminum-rich belite sulfoaluminate cements: Clinkering and early age hydration. Cem. Concr. Res. 2010, 40, 359–369. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Shen, X.; Mao, Y.; Huang, H. Enhancing the addition of fly ash from thermal power plants in activated high belite sulfoaluminate cement. Constr. Build. Mater. 2014, 52, 261–266. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- De la Torre, Á.G.; Cuberos, A.J.M.; Álvarez-Pinazo, G.; Cuesta, A.; Aranda, M.A.G. In situ powder diffraction study of belite sulfoaluminate clinkering. J. Synchrotron Rad. 2011, 18, 506–514. [Google Scholar] [CrossRef]
- Ma, S.; Snellings, R.; Li, X.; Shen, X.; Scrivener, K.L. Alite-ye’elimite cement: Synthesis and mineralogical analysis. Cem. Concr. Res. 2013, 45, 15–20. [Google Scholar] [CrossRef]
- Quillin, K. Performance of belite–sulfoaluminate cements. Cem. Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Liu, G.Q.; Yang, Q.X.; Jiang, L.; Xue, P.; Zhang, X.L.; Han, F.L. Sintering characteristics of BCSAF cement clinker with added wastes from production of manganese and magnesium metals. Adv. Cem. Res. 2017, 1–9. [Google Scholar] [CrossRef]
- Cuberos, A.J.M.; De la Torre, Á.G.; Álvarez-Pinazo, G.; Martín-Sedeño, M.C.; Schollbach, K.; Pöllmann, H.; Aranda, M.A.G. Active Iron-Rich Belite Sulfoaluminate Cements: Clinkering and Hydration. Environ. Sci. Technol. 2010, 44, 6855–6862. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.A.G.; Cuberos, A.J.M.; Cuesta, A.; Alvarez-Pinazo, G.; De la Torre, A.G.; Schollbach, K.; Pollmann, H. Hydrating behaviour of activated belite sulfoaluminate cements. In Proceedings of the 13th International Congress on the Chemistry of Cement, Madrid, Spain, 3–8 July 2011. [Google Scholar]
- Senff, L.; Castela, A.; Hajjaji, W.; Hotza, D.; Labrincha, J.A. Formulations of sulfobelite cement through design of experiments. Constr. Build. Mater. 2011, 25, 3410–3416. [Google Scholar] [CrossRef]
- Da Costa, E.B.; Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Gobbo, L.A.; Kirchheim, A.P. Production and hydration of calcium sulfoaluminate-belite cements derived from aluminium anodising sludge. Constr. Build. Mater. 2016, 122, 373–383. [Google Scholar] [CrossRef]
- Dieneman, W.; Schmitt, D.; Bullerjahn, F.; Ben Haha, M. Belite-Calciumsulfoaluminate-Ternesite (BCT)-A new low-carbon clinker Technology. Cem. Int. 2013, 11, 100–109. [Google Scholar]
- Arjunan, P.; Silsbee, M.R.; Della, M. Roy Sulfoaluminate-belite cement from low-calcium fly ash and sulfur-rich and other industrial by-products. Cem. Concr. Res. 1999, 29, 1305–1311. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Synthesis and hydration of calcium sulfoaluminate-belite cements with varied phase compositions. J. Mater. Sci. 2011, 46, 2568–2577. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; la Torre, A.G.D.; León-Reina, L.; Aranda, M.A.G. Rietveld quantitative phase analysis of Yeelimite-containing cements. Cem. Concr. Res. 2012, 42, 960–971. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhu, J.; Wang, P.; Ma, C. Experimental Investigation and Modeling of Sulfoaluminate Cement Preparation Using Desulfurization Gypsum and Red Mud. Ind. Eng. Chem. Res. 2013, 52, 1261–1266. [Google Scholar] [CrossRef]
- Borštnar, M.; Daneu, N.; Dolenec, S. Phase development and hydration kinetics of belite-calcium sulfoaluminate cements at different curing temperatures. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; T. Telford: London, UK, 1997; ISBN 978-0-7277-2592-9. [Google Scholar]
- Cuesta, A.; De la Torre, A.G.; Losilla, E.R.; Peterson, V.K.; Rejmak, P.; Ayuela, A.; Frontera, C.; Aranda, M.A.G. Structure, Atomistic Simulations, and Phase Transition of Stoichiometric Yeelimite. Chem. Mater. 2013, 25, 1680–1687. [Google Scholar] [CrossRef]
- Hargis, C.W.; Moon, J.; Lothenbach, B.; Winnefeld, F.; Wenk, H.-R.; Monteiro, P.J.M. Calcium Sulfoaluminate Sodalite (Ca4Al6O12SO4) Crystal Structure Evaluation and Bulk Modulus Determination. J. Am. Ceram. Soc. 2014, 97, 892–898. [Google Scholar] [CrossRef]
- Pedersen, M.T.; Jensen, F.; Skibsted, J. Structural Investigation of Ye’elimite, Ca4Al6O12SO4, by 27Al MAS and MQMAS NMR at Different Magnetic Fields. J. Phys. Chem. C 2018, 122, 12077–12089. [Google Scholar] [CrossRef]
- Muzhen, S.; Junan d Zongdao, W.; Xiaoxin, L. Research on the chemical composition and microstructures of sulho-aluminate cement clinker.
- Kohl, R.F. Effects of Cooling Rate; Portland Cement Association: Skokie, IL, USA, 1979; Kiln Paper No. 24; pp. 1–23. [Google Scholar]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: Cham, The Netherlands, 2014; ISBN 978-94-007-7944-0. [Google Scholar]
- Cuesta, A.; Aranda, M.A.G.; Sanz, J.; de la Torre, Á.G.; Losilla, E.R. Mechanism of stabilization of dicalcium silicate solid solution with aluminium. Dalton Trans. 2014, 43, 2176–2182. [Google Scholar] [CrossRef]
- Moteki, T.; Chaikittisilp, W.; Shimojima, A.; Okubo, T. Silica Sodalite without Occluded Organic Matters by Topotactic Conversion of Lamellar Precursor. J. Am. Chem. Soc. 2008, 130, 15780. [Google Scholar] [CrossRef]
- Fortes, G.M.; Lourenço, R.R.; Montini, M.; Gallo, J.B.; Rodrigues, J.D.A. Synthesis and Mechanical Characterization of Iron Oxide Rich Sulfobelite Cements Prepared Using Bauxite Residue. Mat. Res. 2016, 19, 276–284. [Google Scholar] [CrossRef]
- Idrissi, M.; Diouri, A.; Damidot, D.; Greneche, J.M.; Talbi, M.A.; Taibi, M. Characterisation of iron inclusion during the formation of calcium sulfoaluminate phase. Cem. Concr. Res. 2010, 40, 1314–1319. [Google Scholar] [CrossRef]
- Gartner, E.M.; Young, J.F.; Damidot, D.A.; Jawed, I. Hydration of Portland cement. In Structure and Performance of Cements, 2nd ed.; Bensted, J., Barnes, P., Eds.; Spon Press: London, UK, 2002; pp. 83–84. [Google Scholar]
- Odigure, J.O. Kinetic modelling of cement raw mix containing iron particles and clinker microstructure. Cem. Concr. Res. 1996, 26, 1435–1442. [Google Scholar] [CrossRef]
- Moir, G.K.; Glasser, F.P. Mineralisers, modifiers and activators in the clinkering process, Mineralisers, modifiers and activators in the clinkering process. In Proceedings of the 9th International Congress on the Chemistry of Cement, Delhi, India, 23–28 November 1992; Volume 1, pp. 125–152. [Google Scholar]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, X.; Zhang, W.; Li, X. Effect of ternesite on the hydration and properties of calcium sulfoaluminate cement. J. Therm. Anal. Calorim. 2019, 136, 687–695. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Müller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- West, A.R. Solid State Chemistry and Its Applications; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2014; p. 584. [Google Scholar]
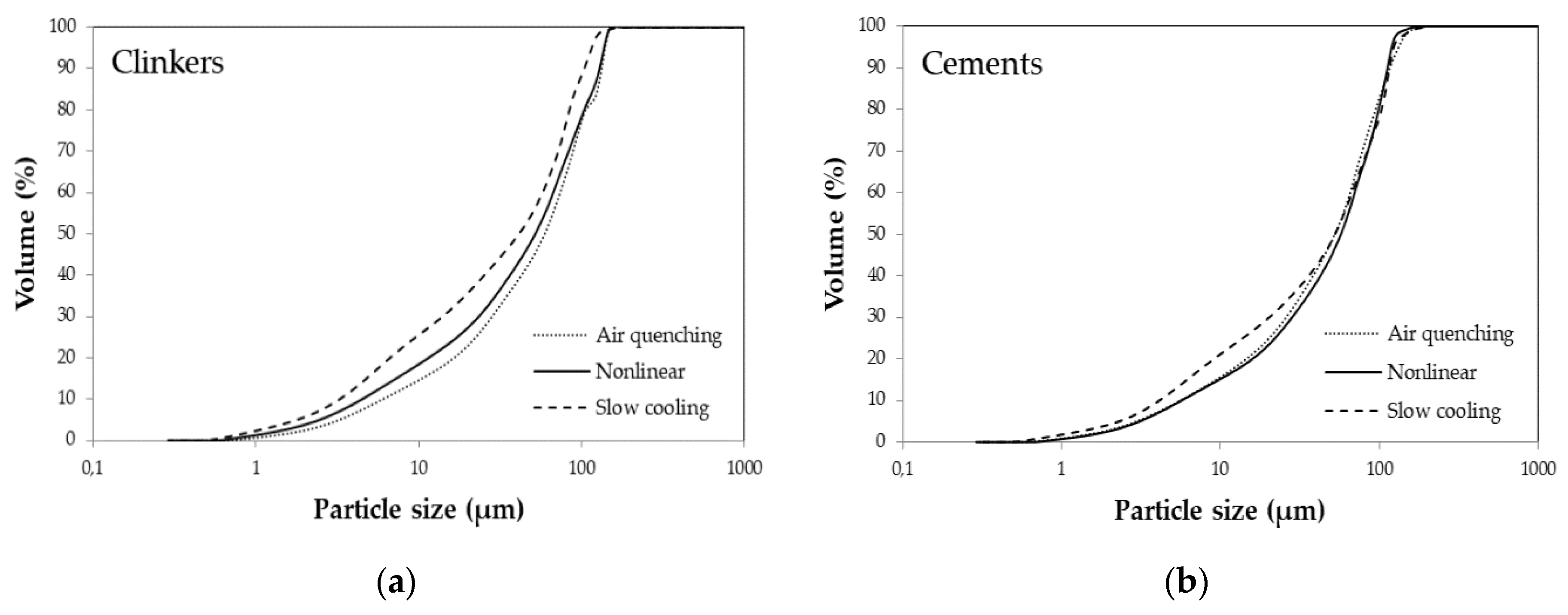
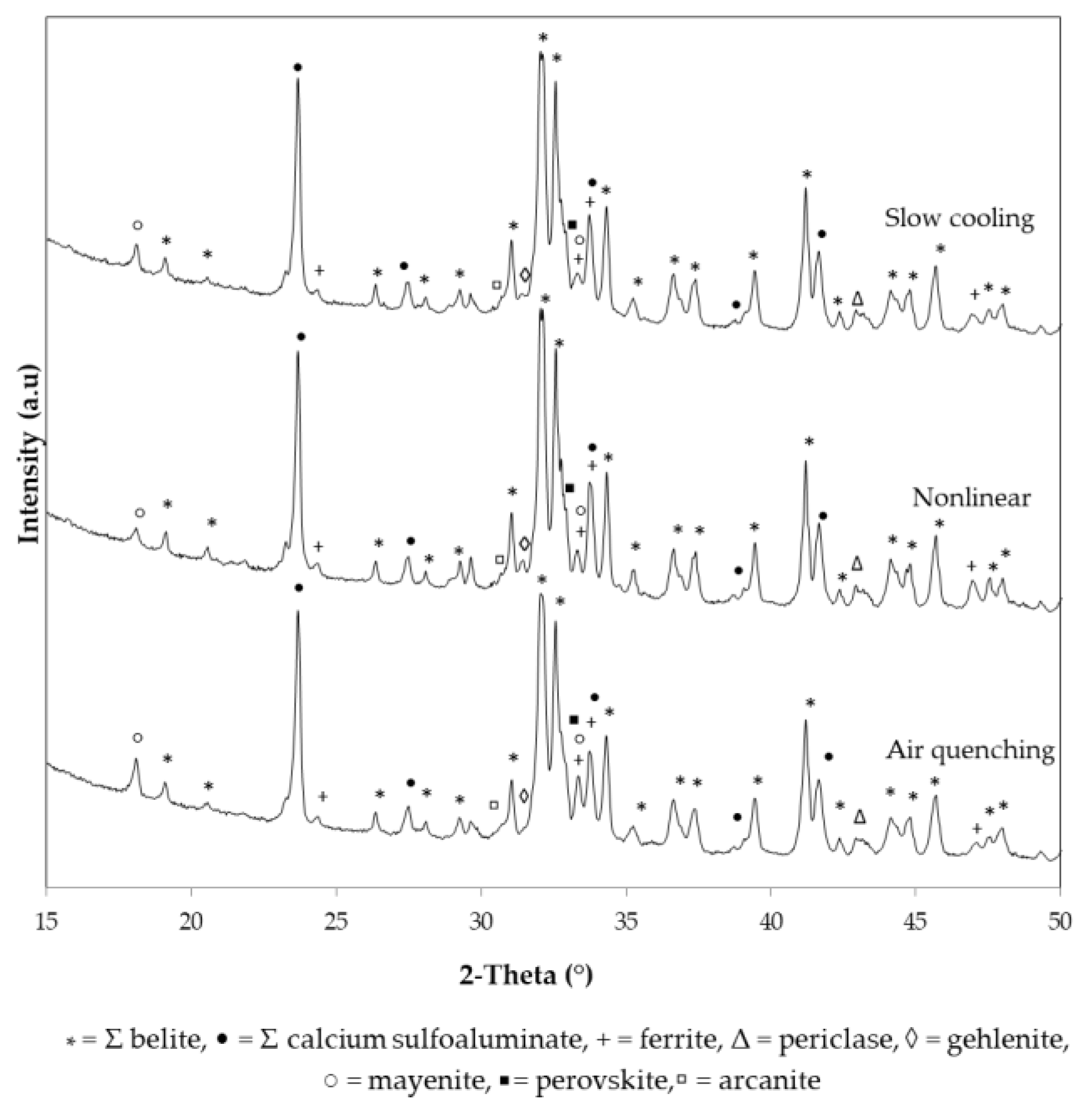
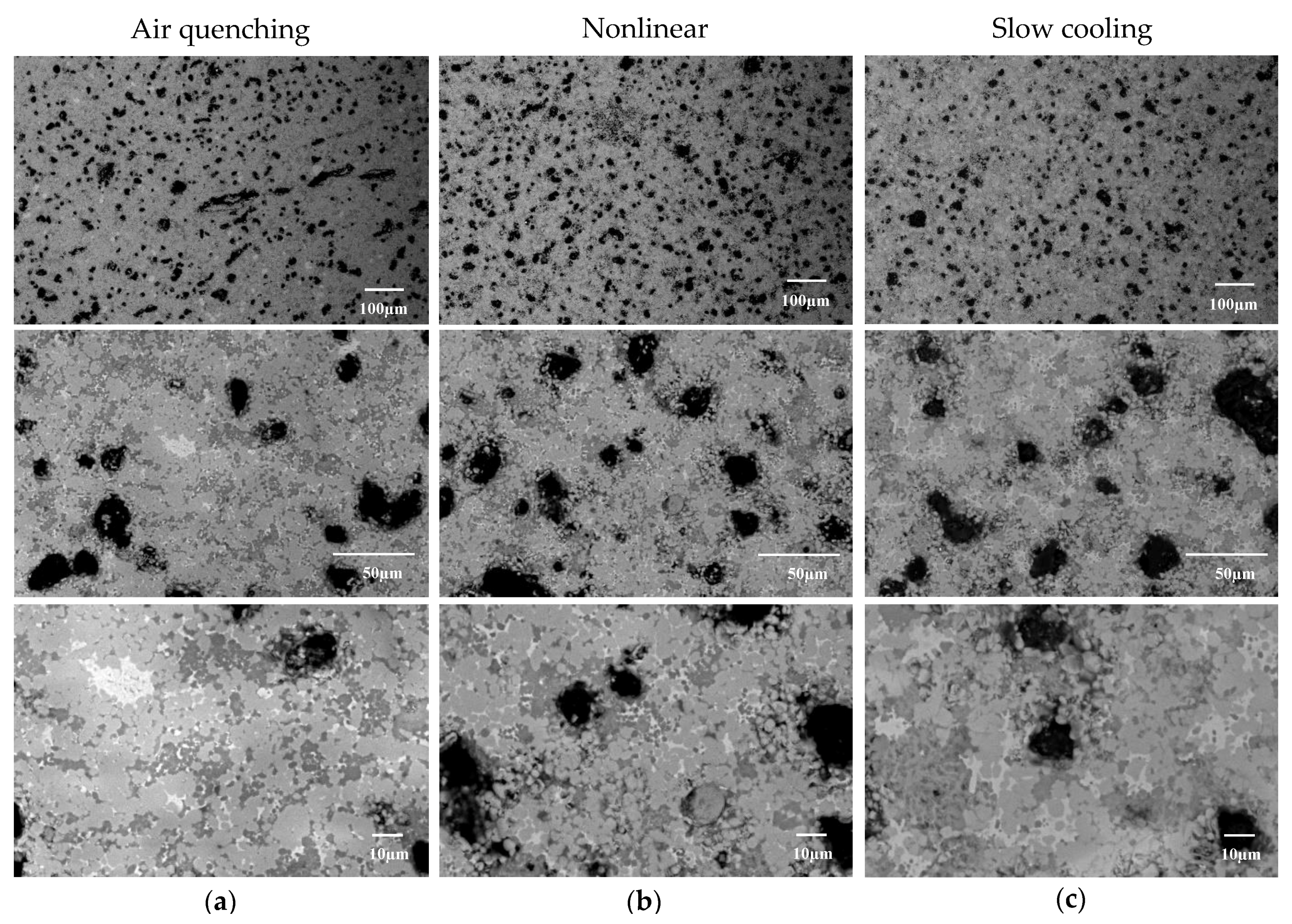
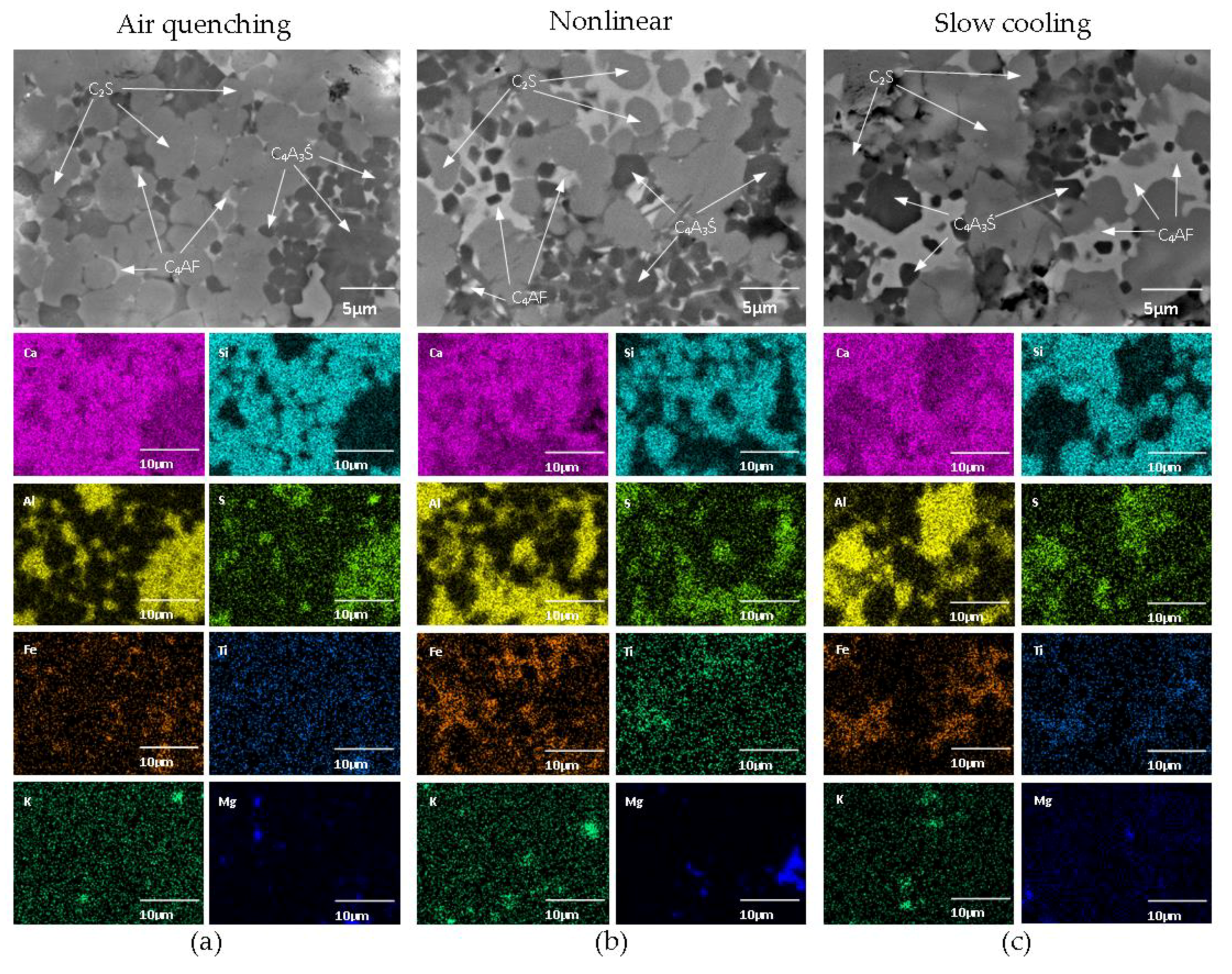
| Chemical Composition | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | TiO2 | Na2O | LOI | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 35.45 | 16.28 | 9.51 | 2.44 | 1.25 | 1.09 | 0.58 | 0.43 | 0.30 | 30.62 | 97.95 |
| Blaine SSA | Clinkers | Cements | ||||
|---|---|---|---|---|---|---|
| Air Quenching | Nonlinear | Slow Cooling | Air Quenching | Nonlinear | Slow Cooling | |
| SSA cm2/g | 2740 | 2760 | 3270 | 2420 | 2580 | 2960 |
| Phases | Air Quenching | Nonlinear | Slow Cooling |
|---|---|---|---|
| β-C2S | 67.3 | 65.6 | 65.7 |
| γ-C2S | 1.4 | 3.0 | 5.0 |
| ∑ C2S | 68.7 | 68.6 | 70.7 |
| o-C4A3Ś | 11.0 | 9.9 | 11.5 |
| c-C4A3Ś | 7.6 | 7.1 | 5.5 |
| ∑ C4A3Ś | 18.6 | 17.0 | 17.0 |
| C4AF | 5.3 | 8.1 | 6.8 |
| CT | 1.9 | 1.0 | 0.8 |
| C12A7 | 2.9 | 1.4 | 0.2 |
| C2AS | 0.2 | 1.4 | 2.1 |
| KŚ | 1.6 | 1.5 | 1.5 |
| M | 0.8 | 0.9 | 0.9 |
| Phase | Cooling | Na | Mg | Al | Si | S | K | Ca | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Belite | Air quenching | 0.27 | 0.46 | 3.47 | 25.88 | 0.65 | 0.41 | 68.25 | 0.30 | 1.00 |
| Nonlinear | 0.22 | 0.46 | 3.46 | 23.97 | 0.51 | 0.31 | 69.53 | 0.31 | 1.24 | |
| Slow cooling | 0.22 | 0.39 | 2.80 | 24.75 | 0.51 | 0.29 | 69.44 | 0.36 | 1.24 | |
| Calcium sulfoaluminate | Air quenching | 0.36 | 0.79 | 32.94 | 7.82 | 5.39 | 0.63 | 48.22 | 0.38 | 3.45 |
| Nonlinear | 0.50 | 0.50 | 32.78 | 6.03 | 5.50 | 0.70 | 49.05 | 0.37 | 4.56 | |
| Slow cooling | 0.35 | 0.50 | 35.97 | 4.85 | 6.57 | 0.62 | 47.07 | 0.45 | 3.52 | |
| Ferrite | Air quenching | 0.21 | 1.68 | 15.73 | 7.26 | 0.49 | 0.32 | 53.12 | 2.20 | 19.57 |
| Nonlinear | 0.22 | 1.13 | 14.17 | 7.78 | 0.99 | 0.28 | 52.00 | 3.58 | 20.20 | |
| Slow cooling | 0.17 | 1.30 | 12.26 | 5.85 | 0.47 | 0.20 | 51.12 | 4.13 | 24.50 |
| Compressive Strength | Air Quenching | Nonlinear | Slow Cooling |
|---|---|---|---|
| N/mm2 | 18.8 ± 0.7 | 17.9 ± 1.2 | 11.5 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolenec, S.; Šter, K.; Borštnar, M.; Nagode, K.; Ipavec, A.; Žibret, L. Effect of the Cooling Regime on the Mineralogy and Reactivity of Belite-Sulfoaluminate Clinkers. Minerals 2020, 10, 910. https://doi.org/10.3390/min10100910
Dolenec S, Šter K, Borštnar M, Nagode K, Ipavec A, Žibret L. Effect of the Cooling Regime on the Mineralogy and Reactivity of Belite-Sulfoaluminate Clinkers. Minerals. 2020; 10(10):910. https://doi.org/10.3390/min10100910
Chicago/Turabian StyleDolenec, Sabina, Katarina Šter, Maruša Borštnar, Klara Nagode, Andrej Ipavec, and Lea Žibret. 2020. "Effect of the Cooling Regime on the Mineralogy and Reactivity of Belite-Sulfoaluminate Clinkers" Minerals 10, no. 10: 910. https://doi.org/10.3390/min10100910
APA StyleDolenec, S., Šter, K., Borštnar, M., Nagode, K., Ipavec, A., & Žibret, L. (2020). Effect of the Cooling Regime on the Mineralogy and Reactivity of Belite-Sulfoaluminate Clinkers. Minerals, 10(10), 910. https://doi.org/10.3390/min10100910






