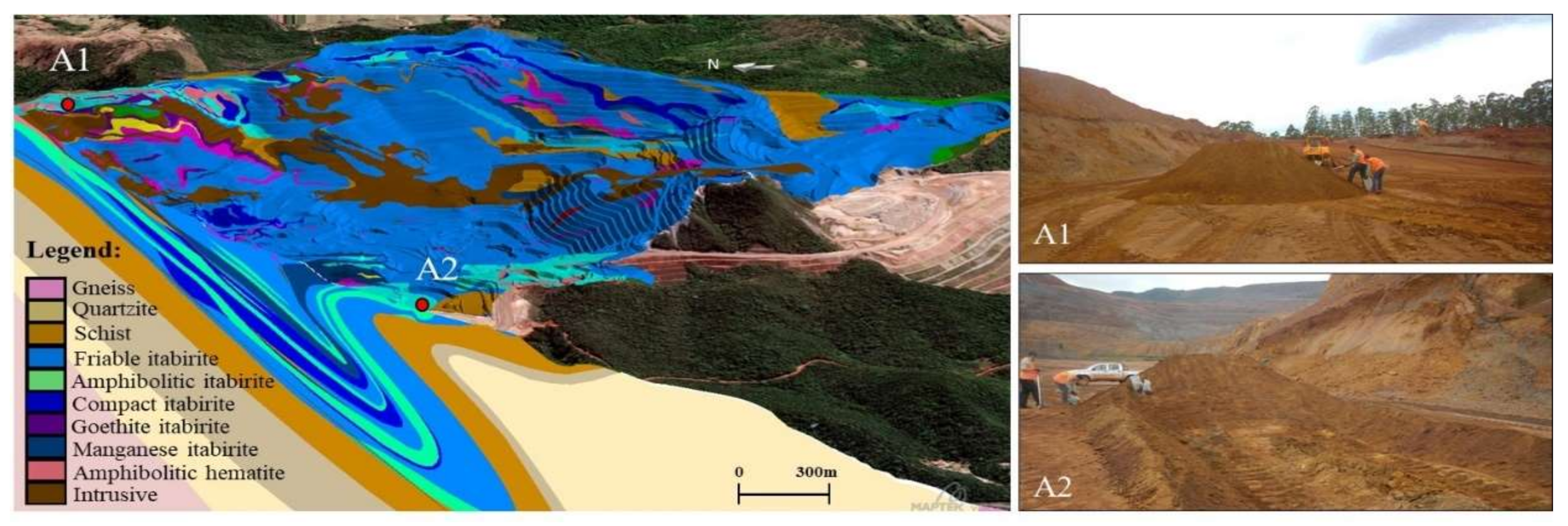
Proposal for an Environmentally Sustainable Beneficiation Route for the Amphibolitic Itabirite from the Quadrilátero Ferrífero-Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Characterization
2.2. Chemical Characterization and Loss on Ignition (LOI)
2.3. Mineralogical Characterization
2.4. Magnetic Concentration and Flotation Tests
3. Results and Discussion
3.1. Physical Characterization
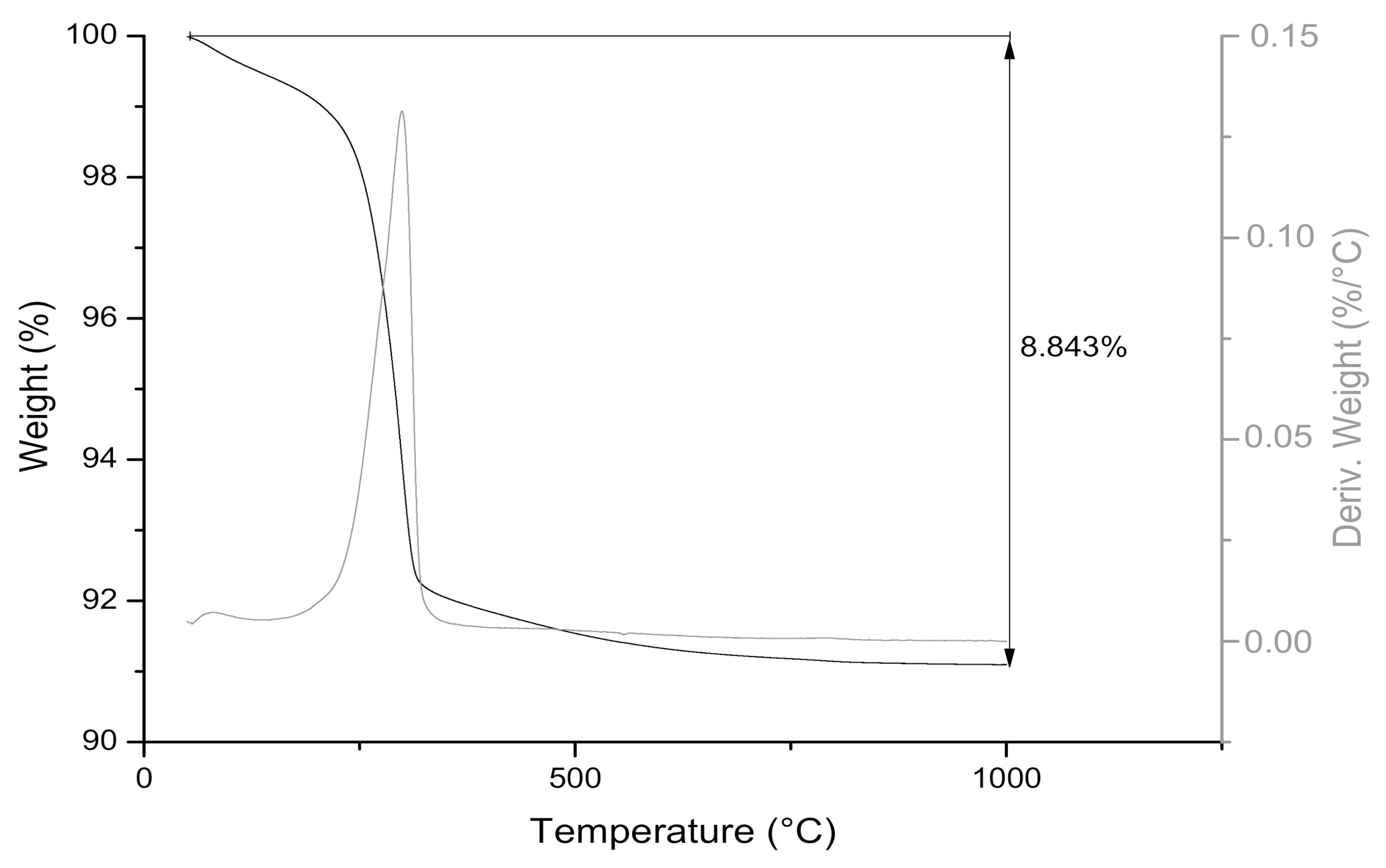
3.2. Chemical Characterization
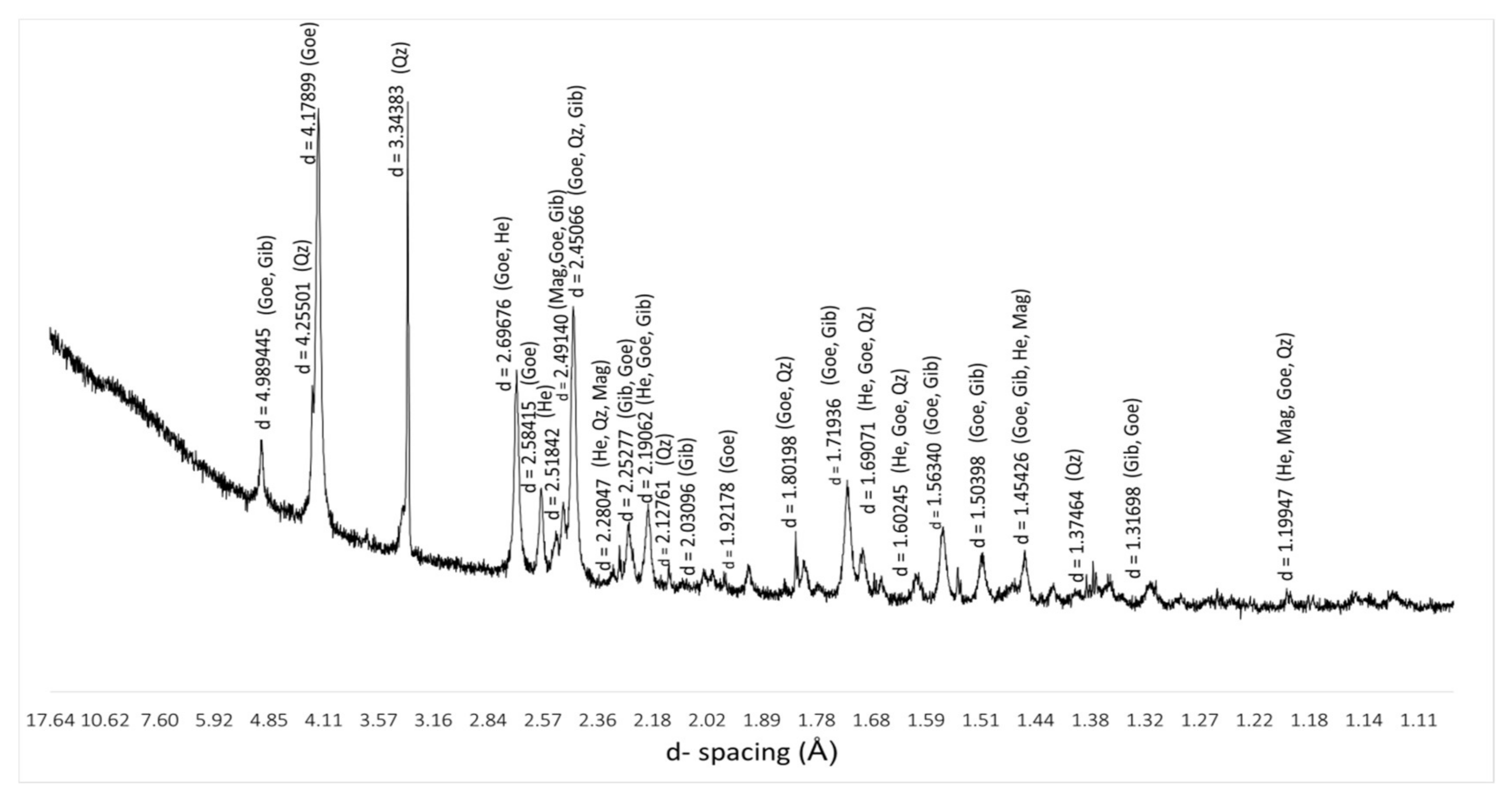
3.3. Mineralogical Characterization
3.4. Proposal of the Beneficiation Route
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohiuddin, K.; Strezovand, V.; Nelson, P.F. Industrial and environmental sustainability of ironmaking technologies. In Advances in Environmental Research; Daniels, J.A., Ed.; Nova Publishers: New York, NY, USA, 2011; Volume 12, pp. 131–152. [Google Scholar]
- Serviço de Apoio às micro e Pequenas Empresas do Rio Grande do Norte. Boletim de Comércio Exterior Período 2012 a 2016. Natal. Available online: www.rn.sebrae.com.br/ (accessed on 20 July 2018).
- Silva, M.S.S.; Lima, M.M.F.; Graça, L.M.; Lima, R.M.F. Bench-scale calcination and sintering of a goethite iron ore sample. Int. J. Miner. Process. 2016, 150, 54–64. [Google Scholar] [CrossRef]
- Veloso, C.H.; Filippov, L.O.; Filippova, I.V.; Araujo, A.C. Investigation of the interaction mechanism of depressants in the reverse cationic flotation of complex iron ores. Miner. Eng. 2018, 125, 133–139. [Google Scholar] [CrossRef]
- USGS. Iron ore statistics and information. In US Geological Survey Minerals Information; US Department of Interior: Washington, DC, USA, 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-iron-ore.pdf (accessed on 4 April 2020).
- Dauce, P.D.; Castro, G.B.; Lima, M.M.F.; Lima, R.M.F. Characterisation and magnetic concentration of an iron ore tailings. J. Mater. Res. Technol.-JMR&T 2019, 8, 1052–1059. [Google Scholar]
- Lima, R.M.F.; Abreu, F.P.V.F. Characterization and concentration by selective flocculation/magnetic separation of iron ore slimes from a dam of Quadrilátero Ferrífero/Brazil. J. Mater. Res. Technol.-JMR&T 2020, 9, 2021–2027. [Google Scholar]
- Silva, M.B.; Luz, J.A.M. Magnetic scavenging of ultrafine hematite from itabirites. Revista Escola de Minas-REM 2013, 66, 499–505. [Google Scholar] [CrossRef]
- Sales, C.G. Rotas de Beneficiamento para Recuperação de Minerais Portadores de Ferro do Underflow do Espessador de Lamas da Usina de Brucutu. Master’s Thesis, Universidade Federal de Minas Gerais—UFMG, Belo Horizonte, Brazil, 2012. [Google Scholar]
- Alkmim, F.F.; Amorim, L.Q. New ore types from the Cauê banded iron-formation. Quadrilátero Ferrífero. Minas Gerais. Brazil—Responses to the growing demand. In Proceedings of the IRON ORE CONFERENCE, Perth, Australia, 11–13 July 2011; p. 13. [Google Scholar]
- Rocha, J.M.P. Definição da Tipologia e Caracterização Mineralógica e Microestrutural dos Itabiritos Anfibolíticos das Minas de Alegria da Samarco Mineração S.A.—Minas Gerais. Ph.D. Thesis, Universidade Federal de Minas Gerais, UFMG, Belo Horizonte, Brazil, 2008. [Google Scholar]
- Roberto, J.B. Influência dos Diversos Tipos Litológicos nas Operações de Concentração da Instalação de Beneficiamento de Brucutu. Master’s Thesis, Universidade Federal de Minas Gerais, UFMG, Belo Horizonte, Brazil, 2010. [Google Scholar]
- Gonçalves, G.M.C. Caracterização Tecnológica do Itabirito Anfibolítico da Mina de Brucutu-MG. Master’s Thesis, Universidade Federal de Ouro Preto, UFOP, Ouro Preto, Brazil, 2020. [Google Scholar]
- Lima, R.M.F.; Lopes, G.M.; Gontijo, C.F. Aspectos mineralógicos. físicos e químicos na flotação catiônica de minérios de ferro de baixos teores do Quadrilátero Ferrífero-MG. Tecnol. em Metal. Mater. e Mineração 2011, 8, 126–131. [Google Scholar] [CrossRef]
- Breuil, C.J.; Perroton, A.; Blancher, B.S.; Rodriguez, C.; Meschi-Daniel, A.F.; Wallmach, T. Iron oxides evolution along the lateritic profile of mabounié carbonatite (Gabon): A key point to understand magnetic separation processes. Miner. Resour. Sustain. World 2015, 4, 1369–1372. [Google Scholar]
- Stradling, A.W. Development of a mathematical model of a crossbelt magnetic separator. Miner. Eng. 1991, 4, 733–745. [Google Scholar] [CrossRef]
- Santos, P.A. Estudo de Densidade de Rochas e Comparação de Técnicas de Medição na Região do Quadrilátero Ferrífero. Minas Gerais. Brasil. Bachelor’s Thesis, Instituto Superior de Educação de Itabira—ISEI, Itabira, Brazil, 2006; p. 70. [Google Scholar]
- Caldeira, E.; Angeli, G.; Cunha, E.; Roldão, D. Revisão de recursos da mina de Brucutu. Relat. Interno Vale 2011, 1, 43–45. [Google Scholar]
- Freitas, L.S. Avaliação dos Minérios Itabiritos Compactos e Semi-Compactos em um Circuito de Britagem da Samarco Mineração S/A. Master’s Thesis, Universidade Federal de Minas Gerais—UFMG, Belo Horizonte, Brazil, 2014. [Google Scholar]
- Queiroz, L.A.; Brandão, P.R.G. Aspectos mineralógicos relacionados à concentração magnética de minério de ferro itabirítico. Metal. Mater. 2009, 65, 148–154. [Google Scholar]
- Neumann, R.; Avelar, A.N.; da Costa, G.M. Refinement of the isomorphic substitutions in goethite and hematite by the Rietveld method, and relevance to bauxite characterisation and processing. Miner. Eng. 2014, 55, 80–86. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Xie, Q.; Zou, X.; Qing, C.; Frost, R. Kinetic study of goethite dehydration and the effect of aluminium substitution on the dehydrate. Thermochim. Acta 2012, 545, 20–25. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Chang, J.; Zou, X.; Frost, R. The effect of hydroxyl groups and surface area of hematite derived from annealing goethite for phosphate removal. J. Colloid Interface Sci. 2013, 398, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R. Thermal treatment of natural goethite: Thermal transformation and physical properties. Thermochim. Acta 2013, 568, 115–121. [Google Scholar] [CrossRef]
- Costa, G.M.; Novack, K.M.; Elias, M.M.; Cunha, C.R.F. Quantification of moisture contents in iron and manganese ores. ISIJ Int. 2013, 53, 1732–1738. [Google Scholar] [CrossRef]
- Weissenborn, P.K.; Dunn, J.G.; Warren, L.J. Quantitative thermogravimetric analysis of haematite. goethite and kaolinite in western Australian iron ores. Thermochim. Acta 1994, 239, 147–156. [Google Scholar] [CrossRef]
- Schwertmann, U. The double dehydroxylation peak of goethite. Thermochim. Acta 1984, 78, 39–46. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Temuujin, J.; Okada, K. Thermal decomposition of mechanically activated gibbsite. Thermochim. Acta 1999, 327, 103–108. [Google Scholar] [CrossRef]
- Magalhães, M.S.; Brandão, P.R.; Tavares, R.P. Types of goethite from Quadrilátero Ferrifero’s iron ores and their implications in the sintering process. Trans. Inst. Min. Metall. 2007, 116, 54–64. [Google Scholar] [CrossRef]
- Song, S.; Lu, S.; Lopez-Valdivieso, A. Magnetic separation of hematite and limonite fines as hydrophobic flocs from iron ores. Miner. Eng. 2002, 15, 415–422. [Google Scholar] [CrossRef]
- Araujo, A.C.; Amarante, S.C.; Souza, C.C.; Silva, R.R.R. Ore mineralogy and its relevance for selection of concentration methods in processing of Brazilian iron ores. Miner Process. Extr. Metall. 2003, 112, C54–C64. [Google Scholar] [CrossRef]
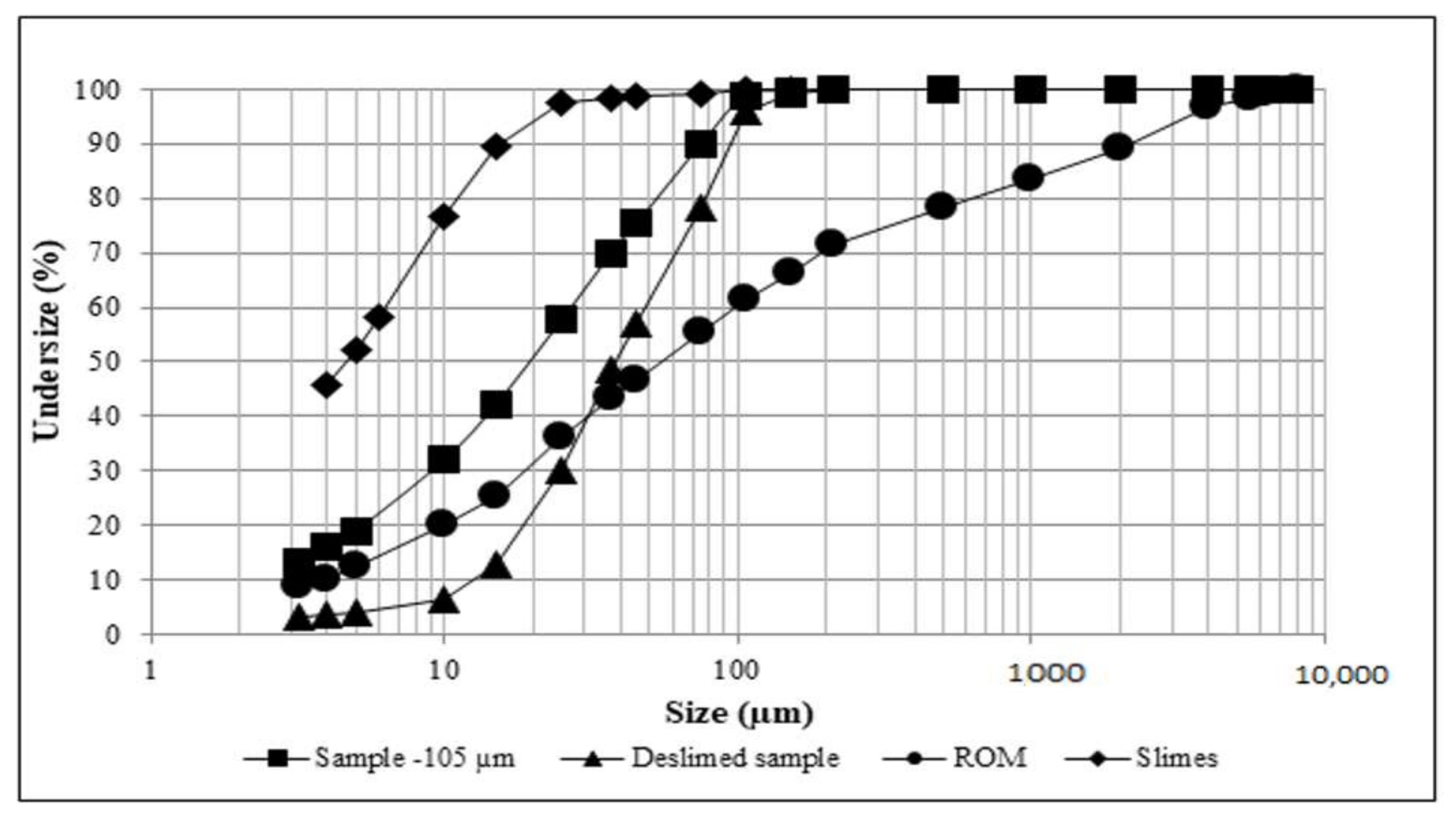

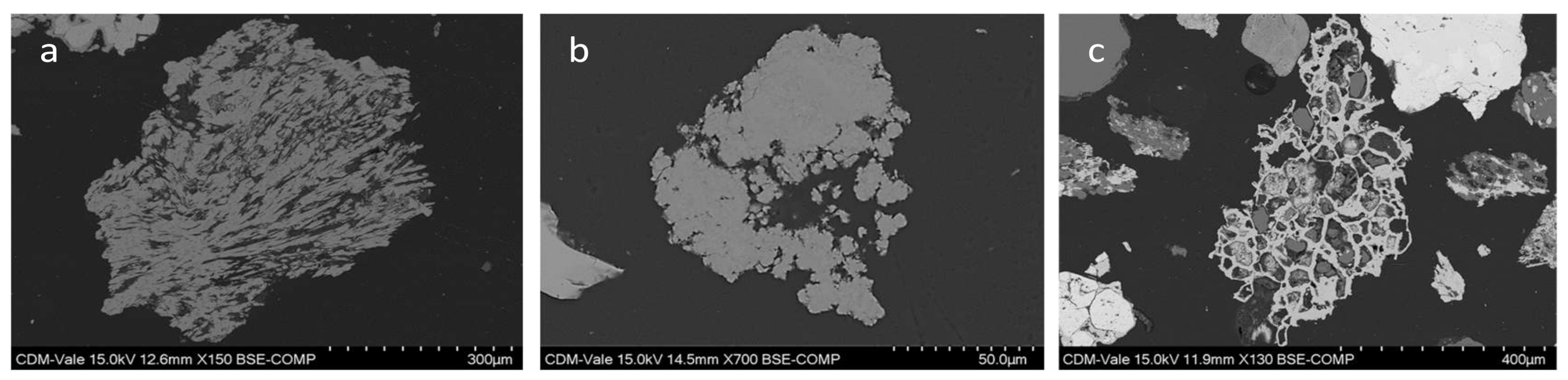
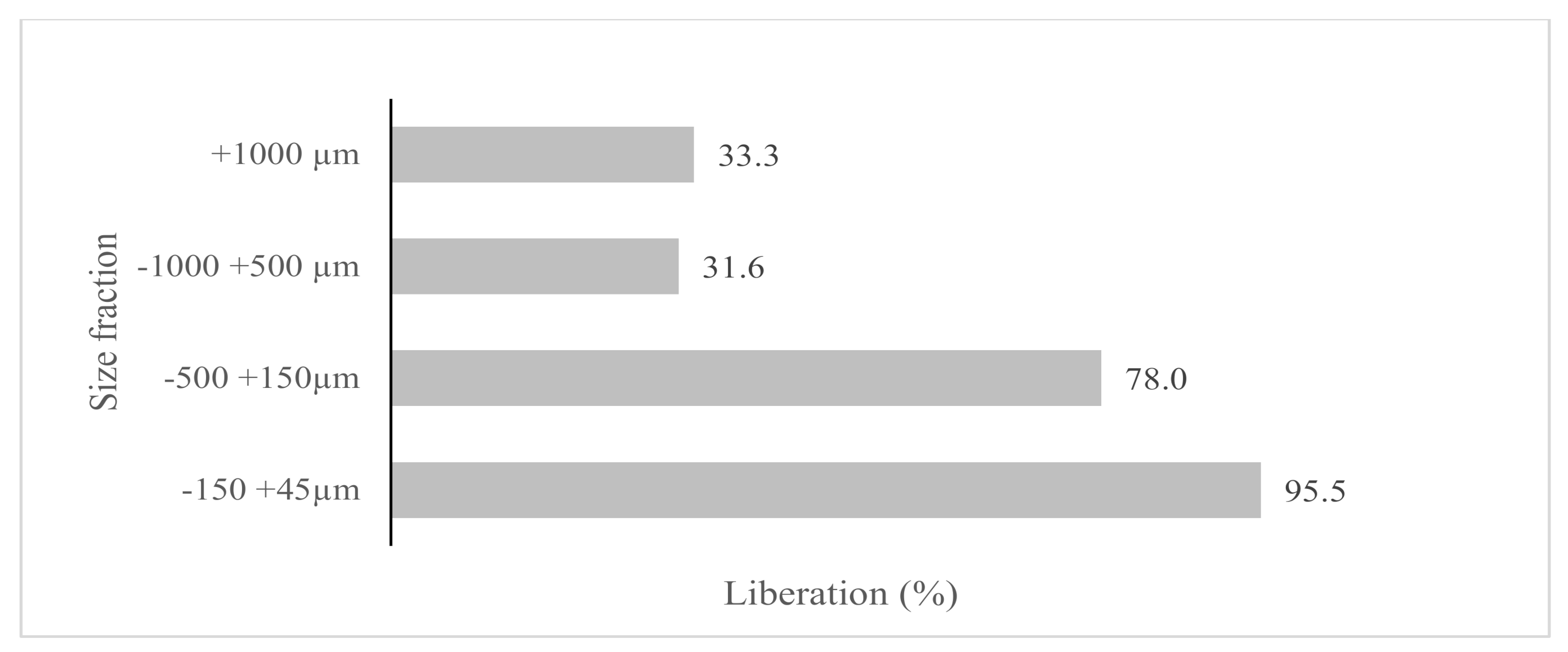
| Size Fraction (µm) | Weight (%) | Grade (%) | Distribution (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FeTotal | Fe3O4 | SiO2 | P | Al2O3 | Mn | CaO | MgO | TiO2 | LOI | Fe | SiO2 | P | LOI | ||
| +1000 | 17.80 | 41.50 | 0.26 | 31.55 | 0.14 | 0.82 | 0.20 | 0.02 | 0.19 | 0.02 | 5.62 | 16.0 | 22.0 | 17.9 | 14.9 |
| −1000 +500 | 4.90 | 45.10 | 0.78 | 27.09 | 0.15 | 0.88 | 0.41 | 0.02 | 0.16 | 0.04 | 6.66 | 4.8 | 5.2 | 5.3 | 4.9 |
| −500 +150 | 13.50 | 47.90 | 1.20 | 26.90 | 0.10 | 0.67 | 0.20 | 0.01 | 0.09 | 0.06 | 4.32 | 14.0 | 14.2 | 9.7 | 8.8 |
| −150 +45 | 21.70 | 37.80 | 1.10 | 40.93 | 0.08 | 0.49 | 0.08 | 0.02 | 0.08 | 0.04 | 3.98 | 17.8 | 34.8 | 12.5 | 12.9 |
| −45 | 42.20 | 51.70 | 0.99 | 14.32 | 0.18 | 1.34 | 0.13 | 0.02 | 0.17 | 0.07 | 9.28 | 47.3 | 23.7 | 54.6 | 58.5 |
| Total | 100.0 | 46.04 | 0.90 | 25.47 | 0.14 | 0.95 | 0.15 | 0.02 | 0.14 | 0.05 | 6.69 | 100.0 | 100.0 | 100.0 | 100.0 |
| PDF-2 Ref. Code | Mineral | d-spacing (Å) |
|---|---|---|
| 96-901-6407 | Goethite (Goe) | 4.98000; 4.17986; 2.69307; 2.58415; 2.49000; 2.44951; 2.24334; 2.19031; 1.92196; 1.80149; 1.77368; 1.71910; 1.69052; 1.66000; 1.60380; 1.56442; 1.51150; 1.45506; 1.42142; 1.37989; 1.36866; 1.35592; 1.31809; 1.19941; 1.15119; 1.12679 |
| 96-591-0083 | Hematite (He) | 2.68150; 2.50400; 2.27450; 2.19363; 1.83013; 1.68362; 1.59390; 1.44569; 1.19824 |
| 96-900-2327 | Magnetite (Mag) | 2.47661; 1.45204; 1.19824; 1.15019; 2.12739; 2.02866; 1.77653; 1.71492; 1.68179; 1.65838; 1.60134; 1.56167; |
| 96-901-3322 | Quartz (Qz) | 4.25478; 3.34321; 2.45650; 2.28123; 1.81777; 1.80167; 1.65906; 1.45281; 1.38198; 1.37487; 1.19771 |
| 96-101-1082 | Gibbsite (Gb) | 4,84454; 4,30676; 2.69498; 2.49837; 2.46084; 2.18702; 1.68179; 1.65838; 1.60134; 1.53953; 1.50229; 1.45643; 1.38128; 1.31527 |
| Mineral | Chemical Formula | Weight (%) |
|---|---|---|
| Goethite | FeO·OH | 64.5 |
| Quartz | SiO2 | 25.5 |
| Hematite | Fe2O3 | 6.8 |
| Gibbsite | Al(OH)3 | 1.1 |
| Magnetite | Fe3O4 | 0.9 |
| Others | - | 1.2 |
| Sample | Weight (%) | Grade (wt%) | Distribution (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | SiO2 | P | Al2O3 | Mn | LOI | Fe | SiO2 | P | Al2O3 | LOI | ||
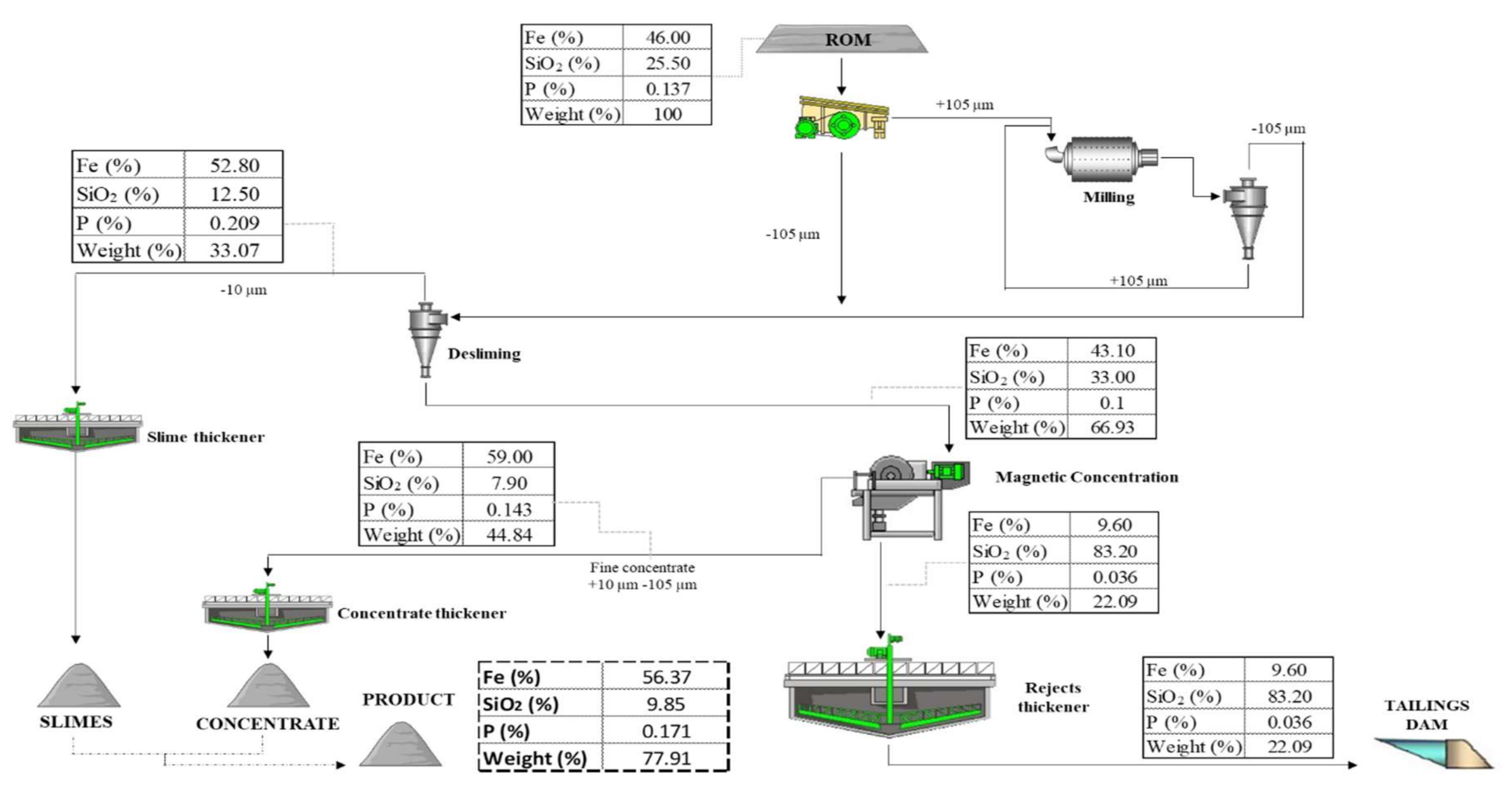
| Deslimed (−105 µm) | 66.9 | 43.1 | 33.0 | 0.100 | 0.56 | 0.128 | 5.03 | 62.3 | 84.2 | 49.2 | 41.1 | 51.1 |
| Slimes | 33.1 | 52.8 | 12.5 | 0.209 | 1.62 | 0.084 | 9.73 | 37.7 | 15.8 | 50.8 | 58.9 | 48.9 |
| Non-deslimed (−105 µm) | 100.0 | 46.0 | 25.5 | 0.137 | 0.95 | 0.156 | 6,69 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Magnetic Concentration | ||||||
|---|---|---|---|---|---|---|
| Sample | Magnetic Field (T) | Pulp (wt%) | Mass Recovery (%) | Fe Recovery (%) | Concentrate Grade (%) | |
| Fe | SiO2 | |||||
| Non-deslimed (−105 µm) | 0.9 | 40 | 26.6 | 27.5 | 61.2 | 6.8 |
| Non-deslimed (−105 µm) | 1.1 | 30 | 20.0 | 26.7 | 60.7 | 6.9 |
| Deslimed | 0.9 | - | 67.0 | 92.61 | 59.0 | 7.9 |
| Flotation (Deslimed Sample) | ||||||
| Amine (g/ton) | Mass Recovery (%) | Fe Recovery (%) | Concentrate Grade (%) | |||
| Fe | SiO2 | |||||
| 170 | 36.7 | 53.9 | 61.7 | 2.2 | ||
| 200 | 34.8 | 50.0 | 62.7 | 1.8 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, G.M.C.; Lima, R.M.F. Proposal for an Environmentally Sustainable Beneficiation Route for the Amphibolitic Itabirite from the Quadrilátero Ferrífero-Brazil. Minerals 2020, 10, 897. https://doi.org/10.3390/min10100897
Gonçalves GMC, Lima RMF. Proposal for an Environmentally Sustainable Beneficiation Route for the Amphibolitic Itabirite from the Quadrilátero Ferrífero-Brazil. Minerals. 2020; 10(10):897. https://doi.org/10.3390/min10100897
Chicago/Turabian StyleGonçalves, Gizele Maria Campos, and Rosa Malena Fernandes Lima. 2020. "Proposal for an Environmentally Sustainable Beneficiation Route for the Amphibolitic Itabirite from the Quadrilátero Ferrífero-Brazil" Minerals 10, no. 10: 897. https://doi.org/10.3390/min10100897
APA StyleGonçalves, G. M. C., & Lima, R. M. F. (2020). Proposal for an Environmentally Sustainable Beneficiation Route for the Amphibolitic Itabirite from the Quadrilátero Ferrífero-Brazil. Minerals, 10(10), 897. https://doi.org/10.3390/min10100897




