Gold Partitioning in a Model Multiphase Mineral-Hydrothermal Fluid System: Distribution Coefficients, Speciation and Segregation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Analytical Methods
2.3. Physicochemical Modelling
3. Results
3.1. Experimental Results
3.2. Computational Results
4. Discussion
4.1. Au Interphase Distribution and Evaluation of Gold Solubility in Minerals
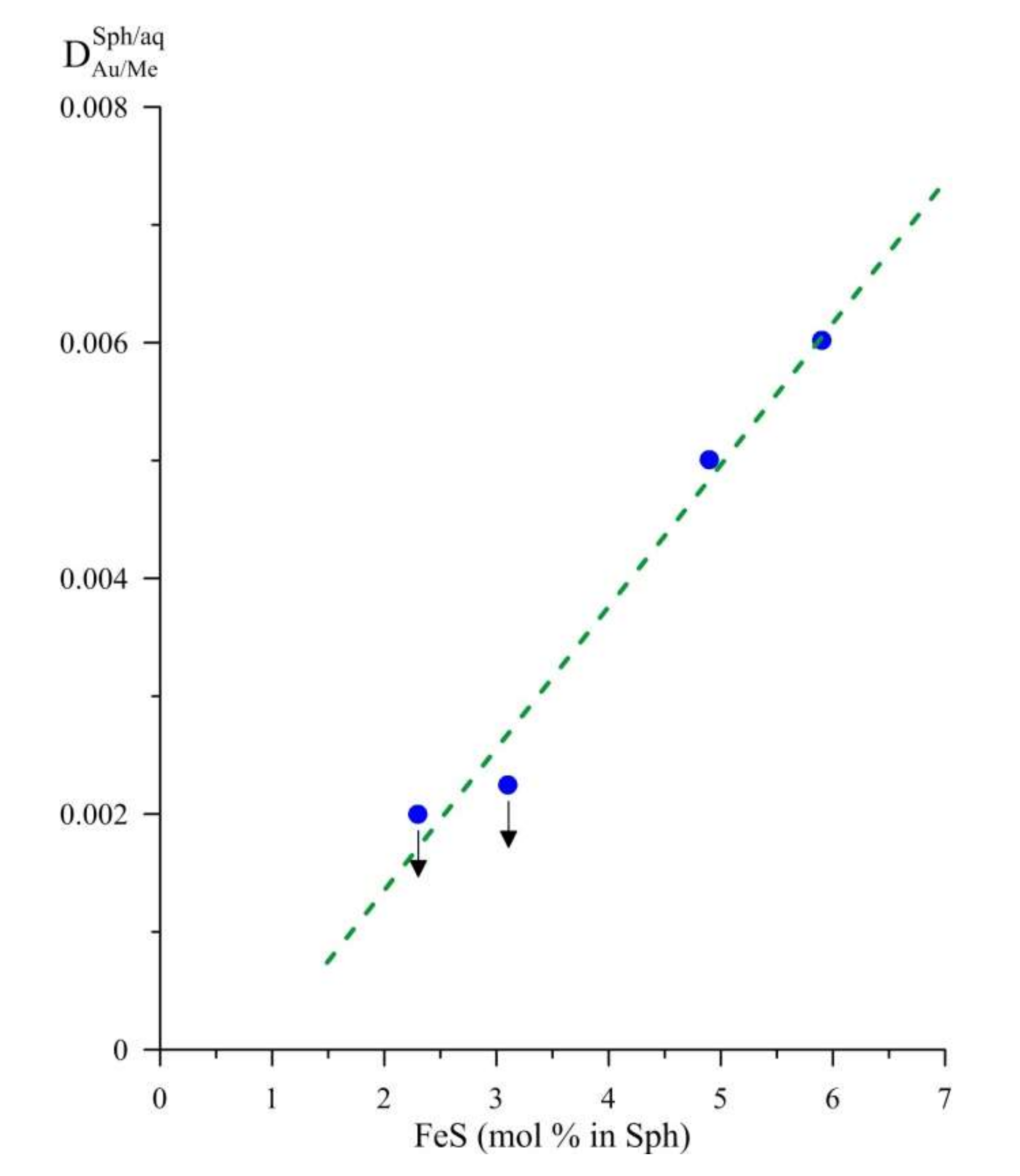
4.2. Au/Me Cocrystallisation Coefficients and Their Variations
4.3. Gold/Metal Ratios in Ore-Forming Fluids
4.4. Dualism of Gold Partitioning
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, X.-M.; Lentz, D.R.; Sylvester, P.J. Gold contents of sulfide minerals in granitoids from southwestern New Brunswick, Canada. Miner. Depos. 2006, 41, 369–386. [Google Scholar] [CrossRef]
- Cook, N.J.; Chryssoulis, S.L. Concentrations of “invisible gold” in the common sulfides. Can. Mineral. 1990, 28, 1–16. [Google Scholar]
- Cabri, L.J. The distribution of trace precious metals in minerals and mineral products. Mineral. Mag. 1992, 56, 289–308. [Google Scholar] [CrossRef]
- Tauson, V.L.; Mironov, A.G.; Smagunov, N.V.; Bugaeva, N.G.; Akimov, V.V. Gold in sulfides: State of the art occurrence and horizons of experimental studies. Russ. Geol. Geophys. 1996, 37, 1–11. [Google Scholar]
- El-Bouseily, A.M.; El-Dahhar, M.A.; Arslan, A.I. Ore-microscopic and geochemical characteristics of gold-bearing sulfide minerals, El Sid Gold Mine, Easern Desert, Egypt. Miner. Depos. 1985, 20, 194–200. [Google Scholar] [CrossRef]
- Wohlgemuth-Ueberwasser, C.C.; Viljoen, F.; Petersen, S.; Vorster, C. Distribution and solubility limits of trace elements in hydrothermal black smoker sulfides: An in-situ LA-ICP-MS study. Geochim. Cosmochim. Acta 2015, 159, 16–41. [Google Scholar] [CrossRef]
- Tauson, V.L.; Lipko, S.V.; Smagunov, N.V.; Kravtsova, R.G. Trace element partitioning dualism under mineral-fluid interaction: Origin and geochemical significance. Minerals 2018, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Tauson, V.L.; Lipko, S.V.; Smagunov, N.V.; Kravtsova, R.G.; Arsent’ev, K.Y. Distribution and segregation of trace elements during the growth of ore mineral crystals in hydrothermal systems: Geochemical and mineralogical implications. Russ. Geol. Geophys. 2018, 59, 1718–1732. [Google Scholar] [CrossRef]
- Tauson, V.L.; Lipko, S.V.; Arsent’ev, K.Y.; Smagunov, N.V. Crystal growth through the medium of nonautonomous phase: Implications for element partitioning in ore systems. Crystallogr. Rep. 2019, 64, 496–507. [Google Scholar] [CrossRef]
- Zajacz, Z.; Hanley, J.J.; Heinrich, C.A.; Halter, W.E.; Guillong, M. Diffusive reequilibration of quartz-hosted silicate melt and fluid inclusions: Are all metal concentrations unmodified? Geochim. Cosmochim. Acta 2009, 73, 3013–3027. [Google Scholar] [CrossRef]
- Karpov, I.K.; Chudnenko, K.V.; Bychinskii, V.A.; Kulik, D.A.; Avchenko, O.V. Minimization of Gibbs free energy in geochemical systems by convex programming. Geochem. Int. 2001, 39, 1108–1119. [Google Scholar]
- Chudnenko, K.V. Thermodynamic Modeling in Geochemistry: Theory, Algorithms, Software, Applications; Geo Publications: Novosibirsk, Russia, 2010. [Google Scholar]
- Tauson, V.L.; Smagunov, N.V.; Akimov, V.V.; Datkov, V.A. Mechanisms and species of gold incorporation into crystals of cadmium, lead, and iron sulfides. Russ. Geol. Geophys. 2008, 49, 594–601. [Google Scholar] [CrossRef]
- Tauson, V.L.; Smagunov, N.V.; Lipko, S.V. Cocrystallization coefficients of Cr, V, and Fe in hydrothermal ore systems (from experimental data). Russ. Geol. Geophys. 2017, 58, 949–955. [Google Scholar] [CrossRef]
- Abramovich, M.G.; Shmakin, B.M.; Tauson, V.L.; Akimov, V.V. Mineral topochemistry: Anomalous trace-element concentrations in solid solutions with defect structures. Int. Geol. Rev. 1990, 32, 608–615. [Google Scholar] [CrossRef]
- Chouinard, A.; Paquette, J.; Williams-Jones, A.E. Crystallographic controls on trace-element incorporation in auriferous pyrite from the Pascua epithermal high-sulfidation deposit, Chile-Argentina. Can. Mineral. 2005, 43, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Tauson, V.L.; Babkin, D.N.; Lustenberg, E.E.; Lipko, S.V.; Parkhomenko, I.Y. Surface typochemistry of hydrothermal pyrite: Electron spectroscopic and scanning probe microscopic data. I. Synthetic pyrite. Geochem. Int. 2008, 46, 565–577. [Google Scholar] [CrossRef]
- Tauson, V.L. Gold solubility in the common gold-bearing minerals: Experimental evaluation and application to pyrite. Eur. J. Mineral. 1999, 11, 937–947. [Google Scholar] [CrossRef]
- Tauson, V.L.; Babkin, D.N.; Akimov, V.V.; Lipko, S.V.; Smagunov, N.V.; Parkhomenko, I.Y. Trace elements as indicators of the physicochemical conditions of mineral formation in hydrothermal sulfide systems. Russ. Geol. Geophys. 2013, 54, 526–543. [Google Scholar] [CrossRef]
- Tauson, V.L.; Smagunov, N.V.; Pastushkova, T.M. Gold incorporation into pyrrhotite and the influence of nonautonomous phases on its distribution. Geochem. Int. 2005, 43, 86–89. [Google Scholar]
- Lepetit, P.; Bente, K.; Doering, T.; Luckhaus, S. Crystal chemistry of Fe-containing sphalerites. Phys. Chem. Miner. 2003, 30, 185–191. [Google Scholar] [CrossRef]
- Tonkacheev, D.E.; Chareev, D.A.; Abramova, V.D.; Kovalchuk, E.V.; Vikentyev, I.V.; Tagirov, B.R. The substitution mechanism of Au in In-, Fe- and In-Fe-bearing synthetic crystals of sphalerite, based on the data from EPMA and LA-ICP-MS study. Litosfera 2019, 19, 148–161. [Google Scholar] [CrossRef]
- Asadi, H.H.; Voncken, J.H.L.; Hale, M. Invisible gold at Zarshuran. Iran. Econ. Geol. 1999, 94, 1367–1374. [Google Scholar] [CrossRef]
- Vikentyev, I.V. Invisible and microscopic gold in pyrite: Methods and new data for massive sulfide ores of the Urals. Geol. Ore Depos. 2015, 57, 237–265. [Google Scholar] [CrossRef]
- Mironov, A.G.; Al’mukhamedov, A.I.; Geletii, V.F.; Glyuk, D.S.; Zhatnuev, N.S.; Zhmodik, S.M.; Konnikov, E.G.; Medvedev, A.Y.; Plyusnin, A.M. Experimental Studies of Gold Geochemistry Using the Method of Radioisotope Indicators; Nauka: Novosibirsk, Russia, 1989. (In Russian) [Google Scholar]
- Mironov, A.G.; Geletii, V.F. Study of gold distribution in synthetic pyrites using Au-195 radioisotope. Dokl. Akad. Nauk. SSSR 1978, 241, 1428–1431. [Google Scholar]
- Bugaeva, N.G. Finely Dispersed (“Invisible”) Gold in Sulfides: An Experimental Study of the Formation Mechanism; LAP Lambert Academic Pub.: Saarbrucken, Germany, 2011. [Google Scholar]
- Wilson, G.C.; Rucklidge, J.C.; Kilius, L.R. Sulfide gold content of skarn mineralization at Rossland, British Columbia. Econ. Geol. 1990, 85, 1252–1259. [Google Scholar] [CrossRef]
- Hinchey, J.G.; Wilton, D.H.C.; Tubrett, M.N. A LAM-ICP-MS study of the distribution of gold in arsenopyrite from the Lodestar prospect, Newfoundland, Canada. Can. Mineral. 2003, 41, 353–364. [Google Scholar] [CrossRef]
- Pope, J.G.; Brown, K.L.; McConchie, D.M. Gold concentrations in springs at Waiotapu, New Zealand: Implications for precious metal deposition in geothermal systems. Econ. Geol. 2005, 100, 677–687. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kokh, M.A.; Proux, O.; Hazemann, J.-L.; Bazarkina, E.F.; Testemale, D.; Escoda, C.; Boiron, M.-C.; Blanchard, M.; Aigouy, T.; et al. The nature and partitioning of invisible gold in the pyrite-fluid system. Ore Geol. Rev. 2019, 109, 545–563. [Google Scholar] [CrossRef]
- Tauson, V.L.; Rychagov, S.N.; Akimov, V.V.; Lipko, S.V.; Smagunov, N.V.; Gerasimov, I.N.; Davletbaev, R.G.; Loginov, B.A. Role of surface phenomena in concentrating incompatible elements: Au in pyrite from hydrothermal clays at thermal fields in Southern Kamchatka. Geochem. Int. 2016, 53, 973–986. [Google Scholar] [CrossRef]
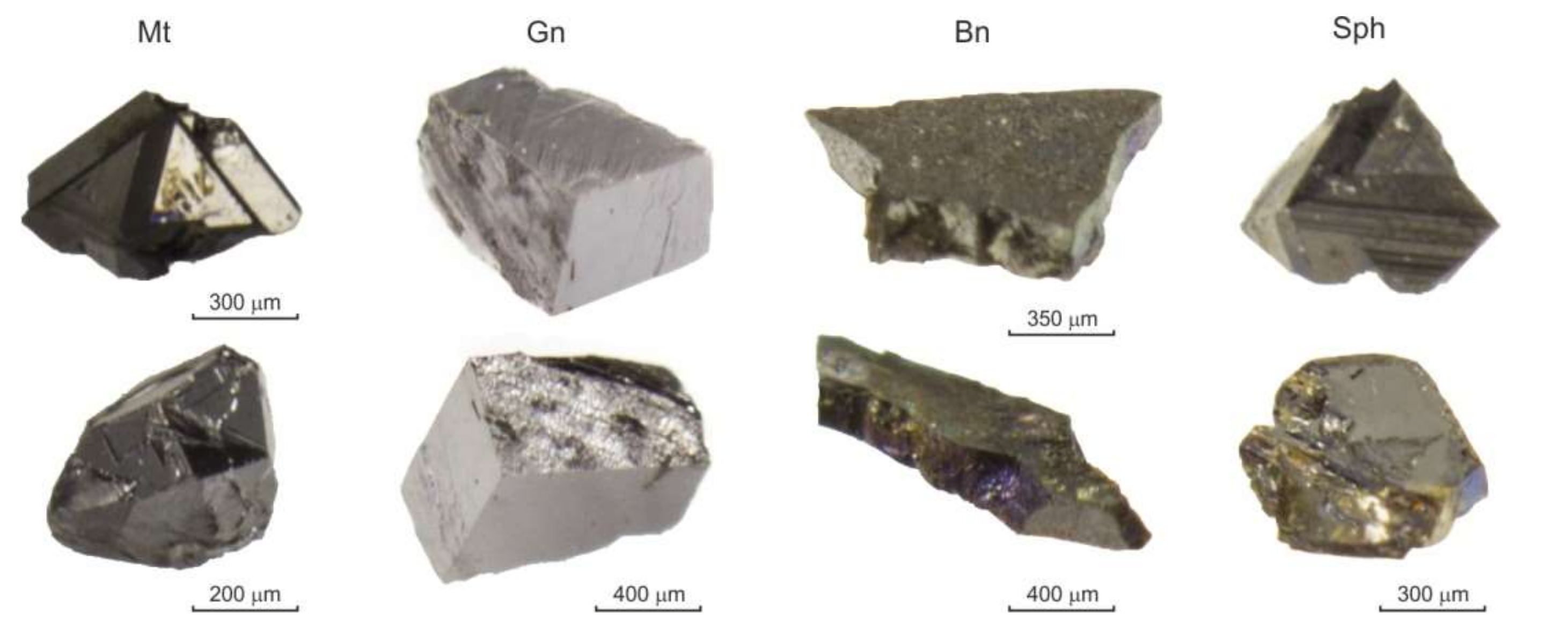
| Experiment No. | Solution | Batch Composition (g) a | Phases Obtained b | Solution in Sampler | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition | Insert Volume (cm3) | Fluid Density (g/cm3) | ZnS | PbS | Cu2S | Fe | S | pH | Au (µg/g) | Zn (wt%) | Fe (wt%) | Cu (wt%) | Pb (wt%) c | ||
| 1 | 5% NH4Cl | 48.8 | 0.65 | 1.8 | 1.8 | 1.2 | 1.2 | - | Sph, Mt, Gn, Bn, Cpy d | 7.9 | 1.0 | 1.48 | 0.62 | 0.033 | >0.19 |
| 2 | 10% NH4Cl | 54.5 | 0.69 | 1.8 | 1.8 | 1.2 | 1.2 | - | Sph, Mt, Bn, Gn, Cpy | 7.5 | 0.61 | 1.24 | 1.0 | 0.027 | >0.06 |
| 3 | 10% NH4Cl + 2% K2Cr2O7 | 55.4 | 0.7 | 1.8 | 1.8 | 1.2 | 1.2 | - | Sph, Mt, Gn | 7.6 | Not determined (not enough fluid trapped) | ||||
| 4 | 5% NH4Cl | 61.9 | 0.65 | 1.8 | 1.8 | 0.6 | 0.9 | 0.9 | Sph, Gn, Py, Cpy | 6.5 | 1.94 | 2.31 | 0.62 | 0.084 | >0.61 |
| 5 | 10% NH4Cl | 58.3 | 0.69 | 1.8 | 1.8 | 0.6 | 0.9 | 0.9 | Sph, Gn, Py, Bn | 6.5 | 2.56 | 3.21 | 0.22 | 0.055 | >0.26 |
| Experiment No. | Fe in Sph (wt% AAS) | Au ± σ, µg/g (LA-ICP-MS) a | |||||
|---|---|---|---|---|---|---|---|
| Sph | Mt | Gn | Cpy | Bn | Py | ||
| 1 | 2.8 | 0.16 ± 0.02 | 0.15 ± 0.02 | 73 ± 5 | n.d. b | 42 ± 10 | - |
| 2 | 3.4 | 0.11 ± 0.02 | ≤0.1 | 28 ± 1 | 13±5 | 17 ± 3 | - |
| 3 | 4.1 | 0.14 ± 0.03 | 0.12 ± 0.02 | 47 ± 2 | - | - | - |
| 4 | 1.8 | ≤0.1 | - | 600 ± 30 c | 15 ± 2 | - | 2 ± 2 |
| 5 | 1.3 | ≤0.1 | - | 9.9 ± 0.3 | - | 10.2 ± 0.2 | 9.7 ± 0.2 d |
| Experiment No. | ||||||
|---|---|---|---|---|---|---|
| Sphalerite | Magnetite | Galena | Chalcopyrite | Bornite | Pyrite | |
| 1 | 0.16 ± 0.04 | 0.15 ± 0.03 | 73 ± 12 | n.d. | 42 ± 14 | - |
| 2 | 0.18 ± 0.05 | ≤0.16 | 45 ± 6 | 21 ± 10 | 27 ± 7 | - |
| 4 | ≤0.05 | - | 310 ± 50 a | 8 ± 2 | - | 1 ± 1 |
| 5 | ≤0.04 | - | 3.9 ± 0.5 | - | 4.0 ± 0.5 | 3.8 ± 0.4 b |
| Experiment No. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphalerite | Magnetite | Chalcopyrite | Bornite | Pyrite | |||||||
| Zn + Fe | Zn | Fe | Fe | Cu + Fe | Cu | Fe | Cu + Fe | Cu | Fe | Fe | |
| 1 | 5.01 × 10−3 | 3.68 × 10−3 | 3.54 × 10−2 | 1.29 × 10−3 | - | - | - | 0.37 | 2.19 × 10−2 | 2.34 | - |
| 2 | 6.02 × 10−3 | 3.51 × 10−3 | 5.30 × 10−2 | ≤2.27 × 10−3 | 0.34 | 1.66 × 10−2 | 0.70 | 0.38 | 1.19 × 10−2 | 2.50 | - |
| 4 | ≤2.25 × 10−3 | ≤1.82 × 10−3 | ≤1.78 × 10−2 | - | 8.37 × 10−2 | 1.88 × 10−2 | 0.16 | - | - | - | 1.37 × 10−2 |
| 5 | ≤2.0 × 10−3 | ≤1.91 × 10−3 | ≤6.61 × 10−3 | - | - | - | - | 1.47 × 10−2 | 3.46 × 10−3 | 7.88 × 10−2 | 1.79 × 10−2 |
| Mineral | ||||
|---|---|---|---|---|
| Sphalerite | 2.5 ± 1.1 | ≤0.1 | 1.0 ± 0.4 | ≤0.04 |
| Pyrite | 11.6 ± 7.3 | 9.7 ± 0.2 | 4.5 ± 2.8 | 3.8 ± 0.4 |
| Galena | 470 ± 30 | 9.9 ± 0.3 | 184 ± 12 | 3.9 ± 0.5 |
| Bornite | 2450 ± 320 | 10.2 ± 0.2 | 957 ± 42 | 4.0 ± 0.5 |
| Experiment No. | pH | Eh (V) | -Log Gas Fugacity (Bar) | Main Species of Metals in Fluid (Molality) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.87 | −0.05 | 3.59 | 20.0 | 0.40 | 1.58 | 1.39 | 4.66 | 1.47 | 3.54 | 1.42 | 3.07 | 1.88 |
| 2 | 3.90 | −0.06 | 3.75 | 20.19 | 0.39 | 2.57 | 1.23 | 8.26 | 2.60 | 3.78 | 2.02 | 4.95 | 4.28 |
| 4 | 3.53 | −0.0005 | 2.42 | 20.09 | −0.23 | 3.09 | 5.36 | 2.05 | 1.50 | 4.01 | 1.44 | 3.10 | 1.90 |
| 5 | 3.40 | 0.01 | 1.92 | 20.26 | −0.56 | 5.91 | 10.2 | 1.54 | 2.27 | 4.06 | 1.81 | 4.12 | 3.17 |
| Mineral | Formula | SAu (µg/g) | Ref. |
|---|---|---|---|
| Low-Fe Sphalerite | (Zn, Fe)S | 0.7 | This work |
| High-Fe Sphalerite | (Zn, Fe)S | 5 | This work and [4] |
| Magnetite | Fe3O4 | 1 | This work |
| Pyrite | FeS2 | 3 | [18] |
| Pyrite-Mn | (Fe, Mn)S2 | 7 | [19] |
| Pyrite-Cu | (Fe, Cu)S2 | 10 | This work |
| Pyrrhotite | Fe1−xS | 21 | [20] |
| Chalcopyrite | CuFeS2 | 110 | This work |
| Bornite | Cu5FeS4 | 140 | This work |
| Galena | PbS | 240 | [13] |
| Deposit | Age, Setting, Type | Mineral | CAu (ppm) | Au/Me in Ore-Forming Fluid a | |
|---|---|---|---|---|---|
| Au/Fe | Au/Cu | ||||
| Estrades, Quebec [2] | Archean, Superior Province, volcanic-sedimentary sequence | Pyrite | 0.67 ± 0.53 | (9 ± 7) × 10−5 | - |
| Chalcopyrite | 0.13 ± 0.09 | (1 ± 0.7) × 10−6 | (2.1 ± 1.4) × 10−5 | ||
| Mobrun, Quebec [2] | -“- | Pyrite | 1.41 ± 0.23 | (1.9 ± 0.3) × 10−4 | - |
| Chalcopyrite | 1.1 ± 0.8 | (8 ± 6) × 10−6 | (1.8 ± 1.3) × 10−4 | ||
| HW, British Columbia [2] | Triassic, Coastal Insular belt volcanic-sedimentary hosted massive sulfides | Pyrite | 0.25 ± 0.09 | (3.4 ± 1.2) × 10−5 | |
| Chalcopyrite (coarse) | 0.19 ± 0.10 | (1.5 ± 0.8) × 10−6 | (3.0 ± 1.6) × 10−5 | ||
| Chalcopyrite (fine) | 3.0 ± 1.3 | (2.3 ± 1) × 10−5 | (4.8 ± 2.1) × 10−4 | ||
| Bornite | 0.67 ± 0.20 | (2.5 ± 0.8) × 10−6 | (6.2 ± 1.9) × 10−5 | ||
| Crown Point, British Columbia [28] | Jurassic, Skarn-hosted mineralization at Rossland | Chalcopyrite b | 0.011 0.027 | 0.8 × 10−7 2.1 × 10−7 | 1.8 × 10−6 4.3 × 10−6 |
| Chalcopyrite c | 0.095 | 7.3 × 10−7 | 1.5 × 10−5 | ||
| Magnetite (crack) d | 0.015 0.17 | 1.6 × 10−5 1.8 × 10−4 | - | ||
| Magnetite (matrix) d | 0.003 0.015 | 3.2 × 10−6 1.6 × 10−5 | - | ||
| Lodestar Prospect, Newfoundland [29] | Neoproterozoic, magmatic-hydrothermal breccia related to a porphyry intrusive system | Pyrite | 0.73 | 9.8 × 10−5 | - |
| Chalcopyrite | 1.48 | 1.1 × 10−5 | 2.4 × 10−4 | ||
| Pyrite | 1.33 | 1.8 × 10−4 | - | ||
| Chalcopyrite | 0.63 | 4.8 × 10−6 | 1.0 × 10−4 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipko, S.; Tauson, V.; Bychinskii, V. Gold Partitioning in a Model Multiphase Mineral-Hydrothermal Fluid System: Distribution Coefficients, Speciation and Segregation. Minerals 2020, 10, 890. https://doi.org/10.3390/min10100890
Lipko S, Tauson V, Bychinskii V. Gold Partitioning in a Model Multiphase Mineral-Hydrothermal Fluid System: Distribution Coefficients, Speciation and Segregation. Minerals. 2020; 10(10):890. https://doi.org/10.3390/min10100890
Chicago/Turabian StyleLipko, Sergey, Vladimir Tauson, and Valeriy Bychinskii. 2020. "Gold Partitioning in a Model Multiphase Mineral-Hydrothermal Fluid System: Distribution Coefficients, Speciation and Segregation" Minerals 10, no. 10: 890. https://doi.org/10.3390/min10100890
APA StyleLipko, S., Tauson, V., & Bychinskii, V. (2020). Gold Partitioning in a Model Multiphase Mineral-Hydrothermal Fluid System: Distribution Coefficients, Speciation and Segregation. Minerals, 10(10), 890. https://doi.org/10.3390/min10100890






