Permeability and Adsorption–Desorption Behavior of Rare Earth in Laboratory Leaching Tests
Abstract
:1. Introduction
2. Methodology
2.1. Sample Preparation
2.1.1. Experimental Materials
2.1.2. Sample Remodeling
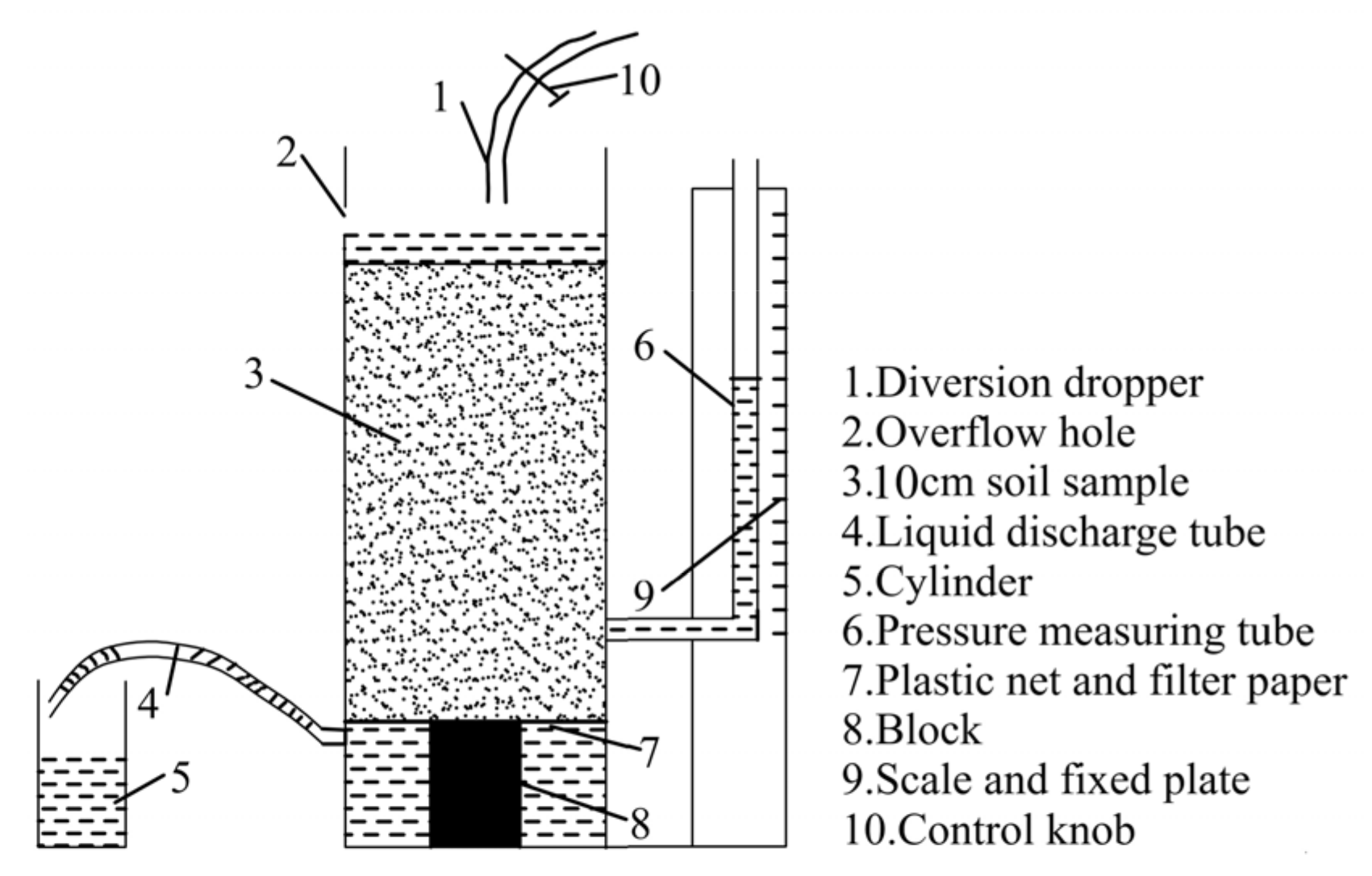
2.2. Experimental Apparatus
2.2.1. Column Leaching Test
2.2.2. NMR Analysis
2.2.3. Inductively Coupled Plasma Mass Spectrometer (ICP-MS) Analysis
2.2.4. SEM and Energy Dispersive X-ray Spectroscopy (EDS) Analysis
3. Results
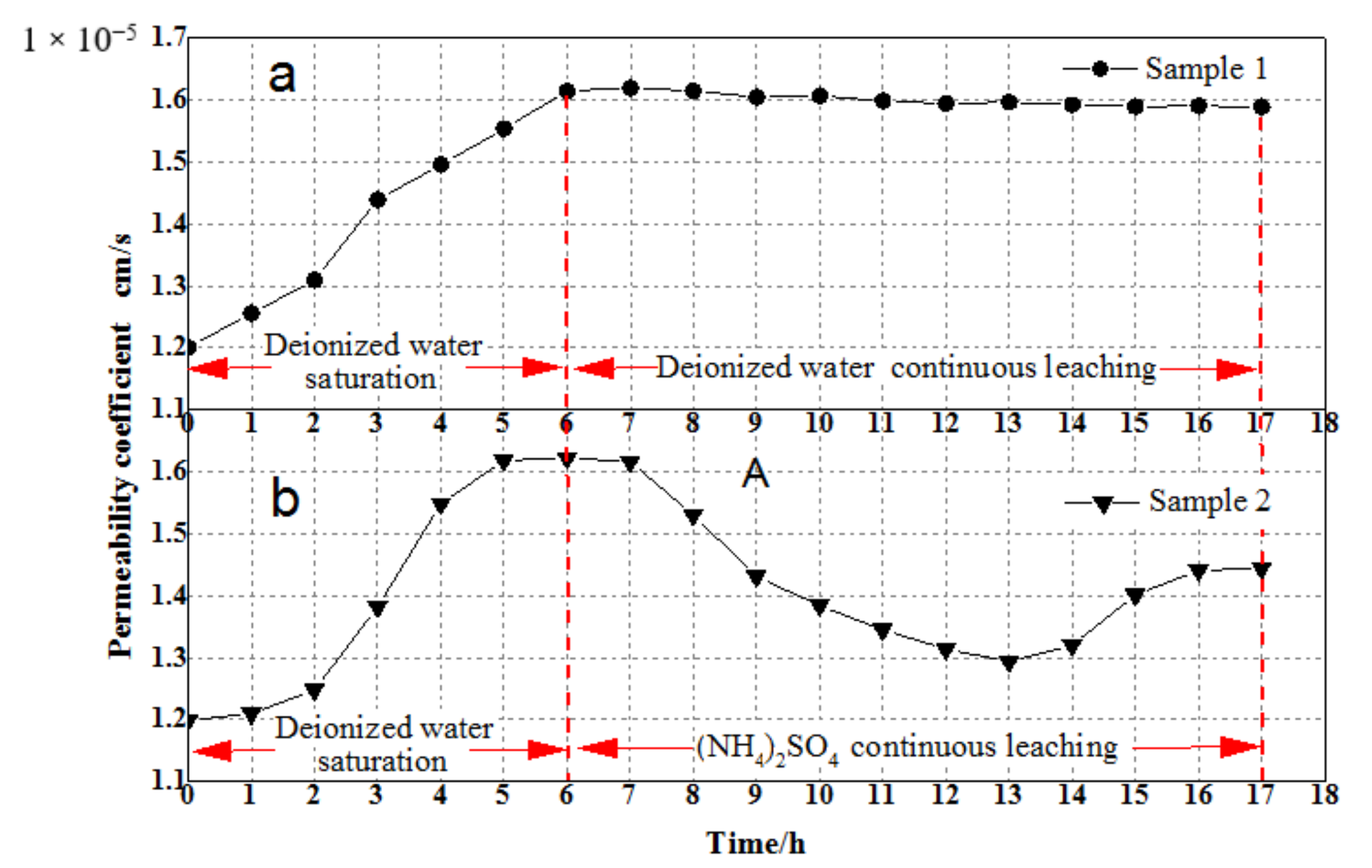
3.1. Permeability Coefficient Analysis
3.2. Pore Structure Characterization
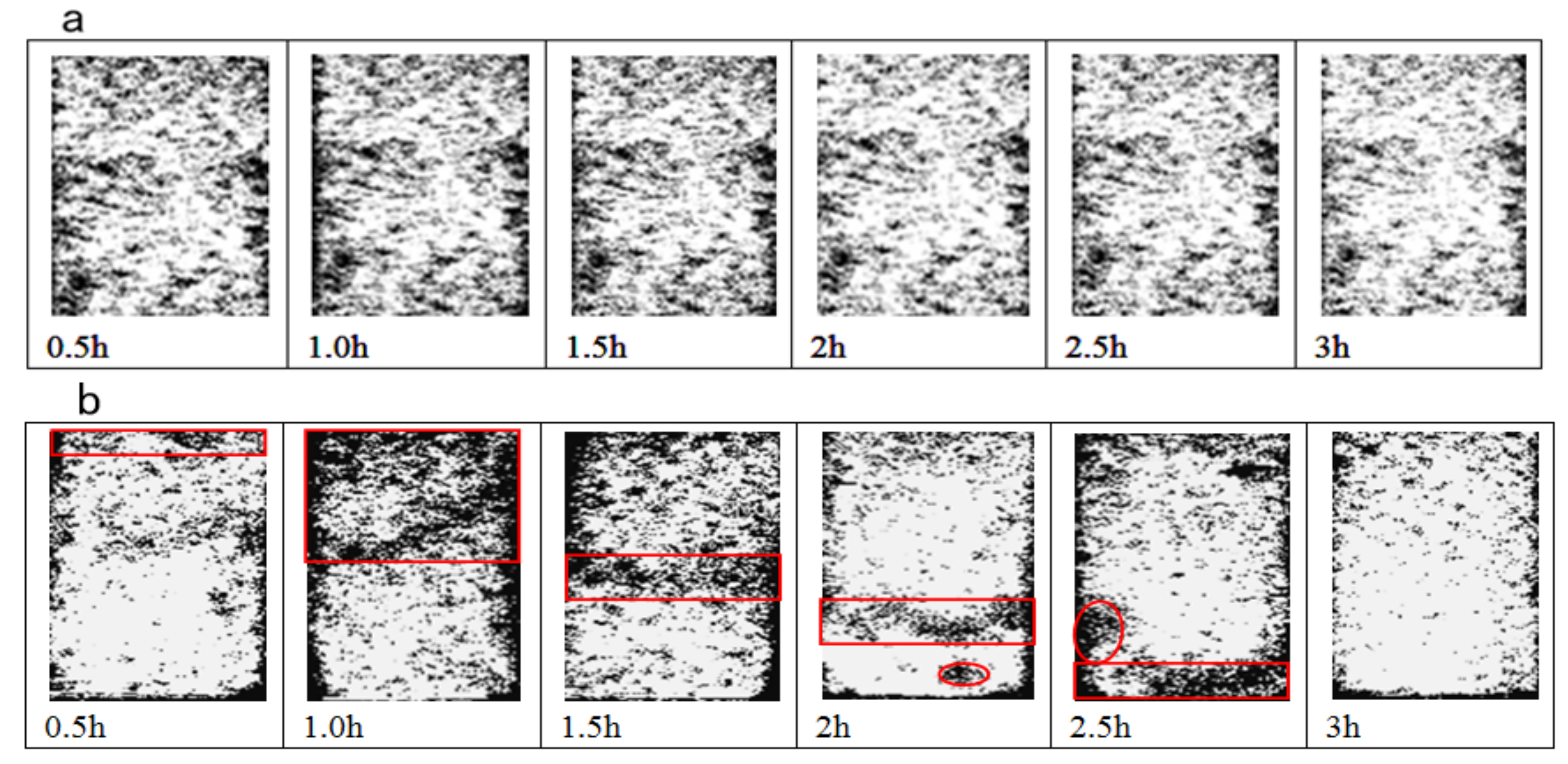
3.2.1. Inversion Image Analysis
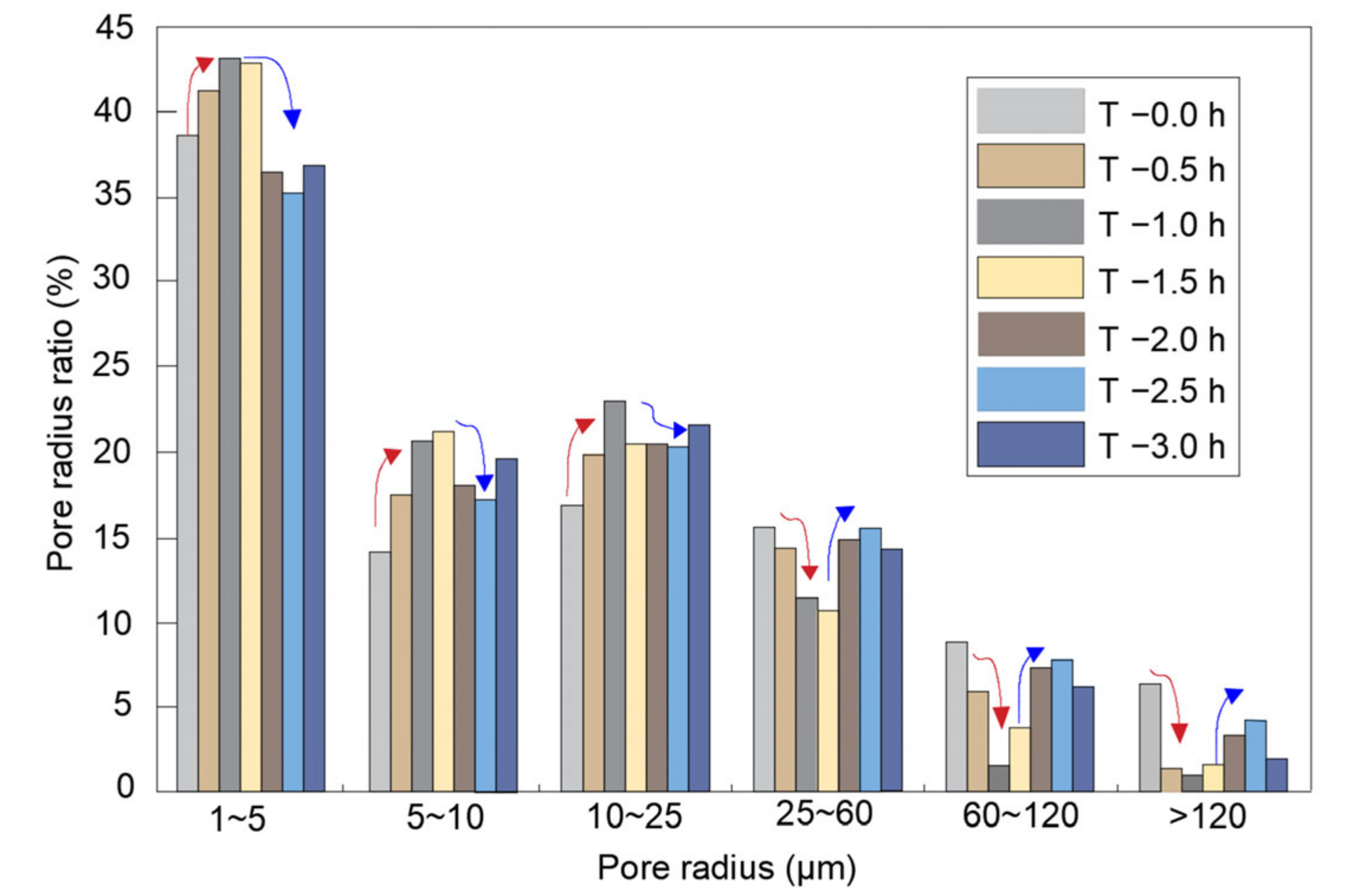
3.2.2. Pore Size Distribution Analysis
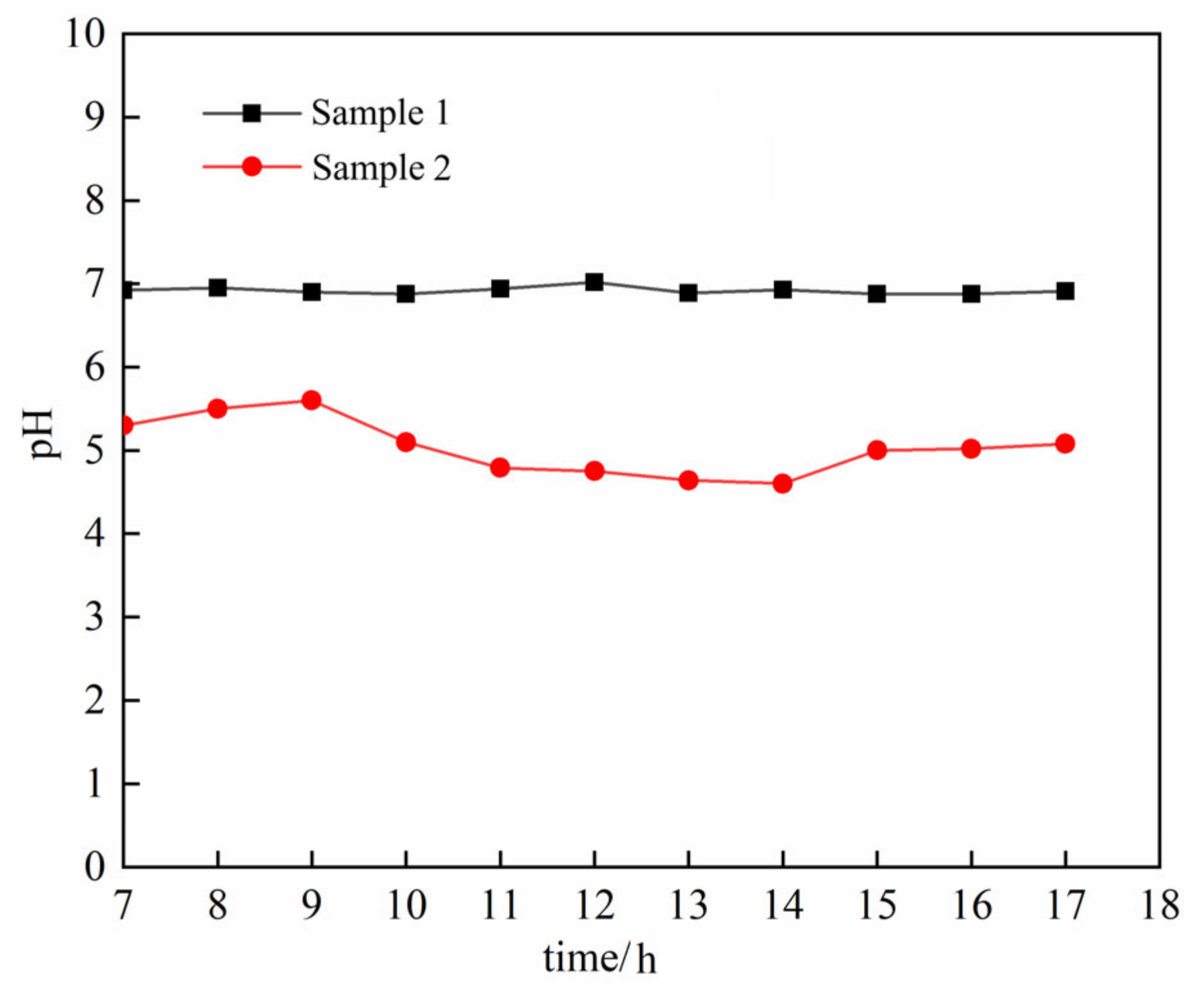
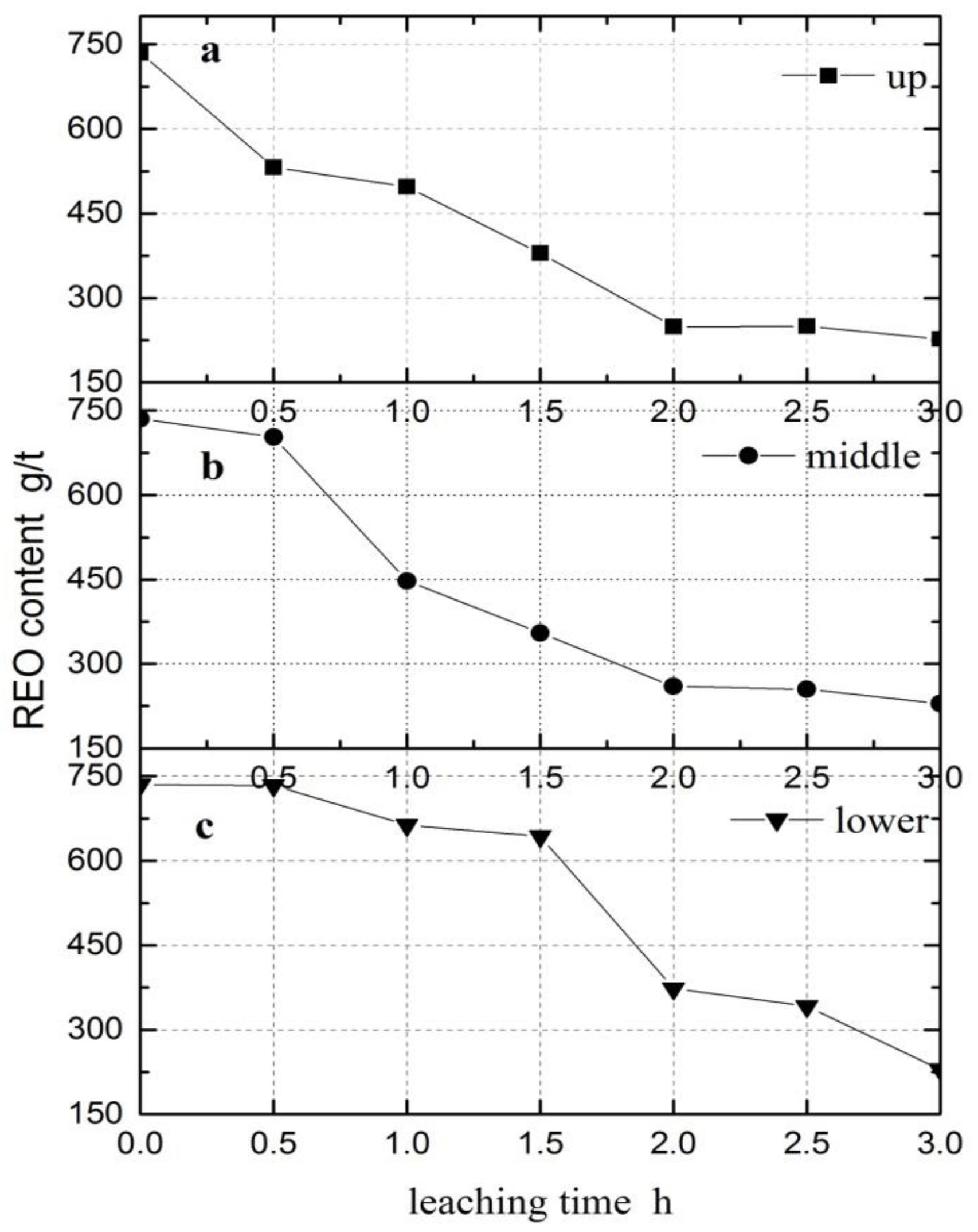
3.3. REO Content Analysis
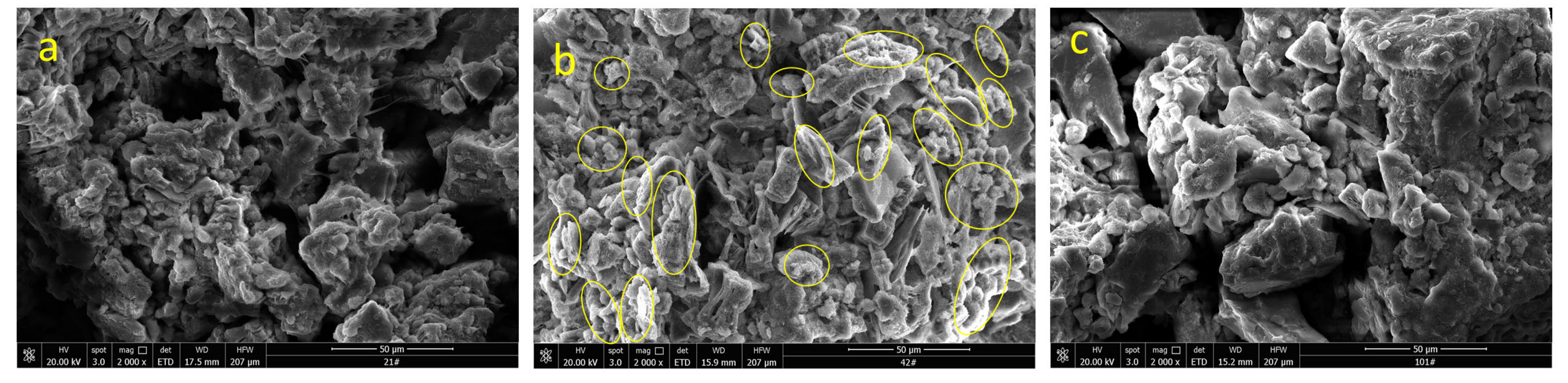
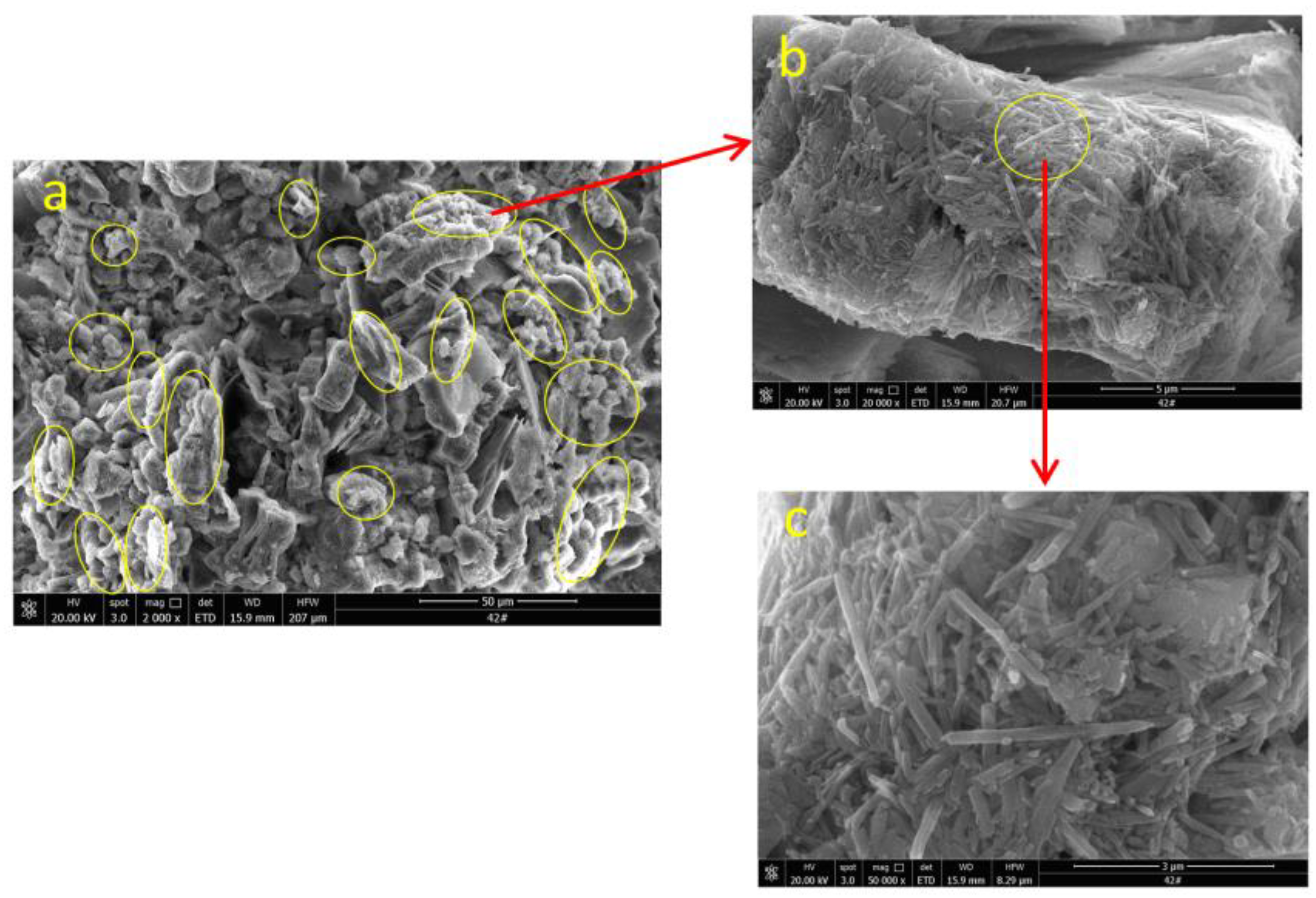
3.4. Surface Morphology Characterization
4. Discussion
5. Conclusions
- By comparing the data obtained from the comparative experiment, it can be concluded that the ion exchange reaction between the leaching solution and the rare earth ore promotes a decrease in the permeability coefficient of the sample. With the completion of the ion exchange reaction, the permeability coefficient begins to recover.
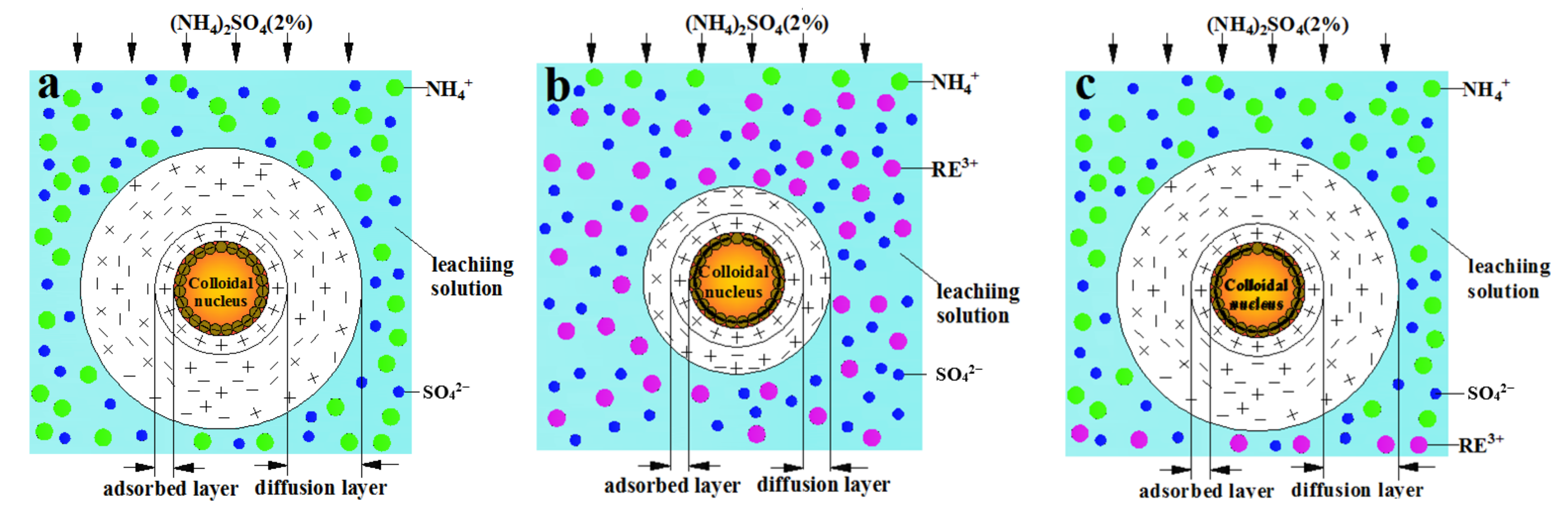
- The ion exchange reaction between NH4+ in the leaching solution and RE3+ adsorbed in the rare earth ore leads to the adsorption of a large number of microparticles inside the sample. After the completion of the ion exchange reaction, the microparticles deposited on the ore body are released under the continuous seepage of the leaching solution. The chemical composition analysis suggests that the microparticles are colloidal clay particles. Their adsorption and desorption phenomenon is closely related to the ion exchange reaction during the leaching process.
- The ion exchange reaction during the leaching process leads to the exchange of NH4+ against RE3+, which causes the rise of the ionic strength of the leaching solution, resulting in a large amount of adsorption of colloidal clay particles. With the completion of the ion exchange reaction, RE3+ in the leaching solution is gradually replaced by NH4+ due to the seepage of the (NH4)2SO4 leaching solution, which induces the reduction of the ionic strength of the leaching solution, leading to the desorption of the clay colloidal particles. This process promotes the dynamic change in the pore structure inside the sample, leading to the fluctuation of the permeability coefficient of the ore sample during the (NH4)2SO4 leaching test.
- The fluctuation of the permeability coefficient is observed on the remodeled ore samples. Through the sample remodeling process, it can be found that the remodeled ore samples have similar physical properties and the same chemical composition as the undisturbed ore sample. Therefore, the observed effect in the laboratory leaching tests has certain applicability to the undisturbed ore sample.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Tao, K.; Yang, Z. Study on material composition and REE-host forms of ion-type RE deposits in South China. J. Rare Earths 1995, 13, 37–41. [Google Scholar]
- Moldoveanu, G.A.; Papangelakis, V.G. An overview of rare-earth recovery by ion-exchange leaching from ion-adsorption clays of various origins. Mineral. Mag. 2016, 80, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Georgiana, A.; Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on clay minerals: I. Desorption mechanism. Hydrometallurgy 2012, 117, 71–78. [Google Scholar] [CrossRef]
- Georgiana, A.; Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on clay minerals: II. Leaching with ammonium sulfate. Hydrometallurgy 2013, 131, 158–166. [Google Scholar] [CrossRef]
- Li, Y. Ion Adsorption Rare Earth Resources and Their Green Extraction; Chemical Industry Press: Beijing, China, 2014; 193p. [Google Scholar]
- Zhou, F.; Liu, Q.; Feng, J.; Su, J.; Liu, X.; Chi, R. Role of initial moisture content on the leaching process of weathered crust elution-deposited rare earth ores. Sep. Purif. Technol. 2019, 217, 24–30. [Google Scholar] [CrossRef]
- Aberg, B. Void ratio of noncohesive soils and similar materials. J. Geotech. Eng. 1992, 118, 1315–1334. [Google Scholar] [CrossRef]
- Wu, A.X.; Yin, S.H.; Li, J.F. Influential factors of permeability rule of leaching solution in ion-absorbed rare earth deposits with in-situ leaching. J. Cent. South. Univ. Sci. Technol. 2005, 36, 506–510. [Google Scholar] [CrossRef]
- Yin, S.H.; Qi, Y.; Xie, F.F.; Chen, X.; Wang, L.M. Permeability characteristic of weathered crust elution-deposited rare earth ores under different pore structures. Chin. J. Nonferrous Met. 2018, 28, 1043–1049. [Google Scholar] [CrossRef]
- Luo, S.H.; Huang, Q.Q.; Wang, G.S.; Shi-Li, H.U.; Hong, B.G. Permeability change rule of ion-adsorption rare-earth in ore leaching process. Nonferrous Met. Sci. Eng. 2014, 597, 95–99. [Google Scholar] [CrossRef]
- Zuo, H.; Wang, Y.; Jiang, H.; Chen, X. Seepage properties of leaching solution in ion-absorbed rare earth deposits under effect of electric field. J. Chin. Soc. Rare Earths 2007, 25, 80–84. [Google Scholar] [CrossRef]
- Xue, Z.; Gan, D.; Zhang, Y.; Liu, Z.; Duan, X.; Huang, M. Liquid spread mechanisms in high-temperature underground stope leaching. Miner. Eng. 2020, 156, 106497. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, J.; Liu, Q. A study on infiltration, transportation and transformation of Pb and Cd in compacted clayliner. Res. Environ. Sci. 1997, 10, 56–60. [Google Scholar] [CrossRef]
- Tang, X.W.; Ying, F.; Kou, N.Y.; Wang, Z.Q. Effects of cation adsorption on properties of silt clay and mixed dredged soil. Rock Soil Mech. 2010, 31, 2519–2524. [Google Scholar] [CrossRef]
- Yong, R.N.; Ouhadi, V.R.; Goodarzi, A.R. Effect of Cu2+ ions and buffering capacity on smectite microstructure and performance. J. Geotech. Geoenvironmental Eng. 2009, 135, 1981–1985. [Google Scholar] [CrossRef]
- Li, Z.; Katsumi, T.; Inui, T. Hydraulic conductivity of kaolin permeated with salt solution. In Advances in Unsaturated Soil, Geo-Hazard, and Geo-Environmental Engineering, Proceedings of the GeoHunan International Conference 2011, Hunan, China, 9–11 June 2011; Bulut, R., Tsukamoto, Y., Deng, A., Katsumi, T., Kokusho, T., Eds.; ASCE: New York, NY, USA, 2011; pp. 34–41. [Google Scholar]
- Zhang, Z.H.; Li, H.Y.; Chen, J.Y.; Lei, Y. Permeability of saturated clay eroded by mixed heavy metal ions. Rock Soil Mech. 2016, 37, 2467–2476. [Google Scholar] [CrossRef]
- He, L.; Feng, T.; Fu, S.; Zhu, D. Study on extraction of rare earth from ion type rare earth ore by ammonium sulfate leaching. Chin. Rare Earths 1983, 3, 1–5. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Z.; Luo, X.; Su, J. Leaching of rare earth elements from contaminated soils using saponin and rhamnolipid bio-surfactant. J. Rare Earths 2017, 35, 911–919. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, X.P.; Qian, Q.H. Fracture characterization and permeability prediction by pore scale variables extracted from X-ray CT images of porous geomaterials. Sci. China Technol. Sci. 2020, 63, 755–767. [Google Scholar] [CrossRef]
- Wang, G.; Qin, Y.; Shen, J.; Chen, S.; Han, B.; Zhou, X. Dynamic-change laws of the porosity and permeability of low- to medium-rank coals under heating and pressurization treatments in the eastern Junggar Basin, China. J. Earth Sci. 2018, 29, 607–615. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, J.; Luo, X.; Zhong, Z. Percolation leaching behavior of ion-adsorption-type rare earth ore. Chin. J. Rare Met. 2018, 35, 531–536. [Google Scholar] [CrossRef]
- Jing, Q.; Chai, L.; Huang, X.; Tang, C.; Guo, H.; Wang, W. Behavior of ammonium adsorption by clay mineral halloysite. Trans. Nonferrous Met. Soc. China 2017, 27, 1627–1635. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, X.; Zhang, B.; Zhao, Y.; Zhai, R.; Liu, J.; Chen, R. Rapid adsorption of Cr (VI) on modified halloysite nanotubes. Desalination 2010, 259, 22–28. [Google Scholar] [CrossRef]
- de Jonge, L.W.; Kjaergaard, C.; Moldrup, P. Colloids and colloid-facilitated transport of contaminants in soils: An introduction. Vadose Zo. J. 2004, 3, 321–325. [Google Scholar] [CrossRef]
- Bekhit, H.M.; Hassan, A.E. Two-dimensional modeling of contaminant transport in porous media in the presence of colloids. Adv. Water Resour. 2005, 28, 1320–1335. [Google Scholar] [CrossRef]
- Stumm, W. Chemical interaction in particle separation. Environ. Sci. Technol. 1977, 11, 1066–1070. [Google Scholar] [CrossRef]
- McCarthy, J.F.; Zachara, J.M. ES&T Features: Subsurface Transport of Contaminants. Environ. Sci. Technol. 1989, 23, 496–502. [Google Scholar] [CrossRef]
- Roy, S.B.; Dzombak, D.A. Colloid release and transport processes in natural and model porous media. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 245–262. [Google Scholar] [CrossRef]
- Sen, T.K.; Khilar, K.C. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv. Colloid Interface Sci. 2006, 119, 71–96. [Google Scholar] [CrossRef]
- McCarthy, J.F.; McKay, L.D. Colloid transport in the subsurface: Past, present, and future challenges. Vadose Zo. J. 2004, 3, 326–337. [Google Scholar] [CrossRef]
- Grolimund, D.; Borkovec, M. Long-term release kinetics of colloidal particles from natural porous media. Environ. Sci. Technol. 1999, 33, 4054–4060. [Google Scholar] [CrossRef]
- Saiers, J.E.; Hornberger, G.M. The influence of ionic strength on the facilitated transport of cesium by kaolinite colloids. Water Resour. Res. 1999, 35, 1713–1727. [Google Scholar] [CrossRef]
- McDowell-Boyer, L.M. Chemical mobilization of micron-sized particles in saturated porous media under steady flow conditions. Environ. Sci. Technol. 1992, 26, 586–593. [Google Scholar] [CrossRef]
- Bergendahl, J.; Grasso, D. Colloid generation during batch leaching tests: Mechanics of disaggregation. Colloids Surf. A Physicochem. Eng. Asp. 1998, 135, 193–205. [Google Scholar] [CrossRef]
- Bergendahl, J.; Grasso, D. Prediction of colloid detachment in a model porous media: Thermodynamics. AIChE J. 1999, 45, 475–484. [Google Scholar] [CrossRef]
- Wikiniyadhanee, R.; Chotpantarat, S.; Ong, S.K. Effects of kaolinite colloids on Cd2+ transport through saturated sand under varying ionic strength conditions: Column experiments and modeling approaches. J. Contam. Hydrol. 2015, 182, 146–156. [Google Scholar] [CrossRef]
- Nocito-Gobel, J.; Tobiason, J.E. Effects of ionic strength on colloid deposition and release. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 223–231. [Google Scholar] [CrossRef]
- Grolimund, D.; Borkovec, M. Colloid-facilitated transport of strongly sorbing contaminants in natural porous media: Mathematical modeling and laboratory column experiments. Environ. Sci. Technol. 2005, 39, 6378–6386. [Google Scholar] [CrossRef]
- García-García, S.; Wold, S.; Jonsson, M. Effects of temperature on the stability of colloidal montmorillonite particles at different pH and ionic strength. Appl. Clay Sci. 2009, 43, 21–26. [Google Scholar] [CrossRef]
- Torkzaban, S.; Bradford, S.A.; Vanderzalm, J.L.; Patterson, B.M.; Harris, B.; Prommer, H. Colloid release and clogging in porous media: Effects of solution ionic strength and flow velocity. J. Contam. Hydrol. 2015, 181, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.Y.; Weisbrod, N. Colloid-facilitated transport of lead in natural discrete fractures. Environ. Pollut. 2009, 157, 2266–2274. [Google Scholar] [CrossRef]
- Hogg, R.; Healy, T.W.; Fuerstenau, D.W. Mutual coagulation of colloidal dispersions. Trans. Faraday Soc. 1966, 62, 1638–1651. [Google Scholar] [CrossRef]
- Machado, S.L.; da Silva-Paes-Cardoso, L.; de Oliveira, I.B.; de Faria-Mariz, D.; Karimpour-Fard, M. Modeling soil permeability when percolated by different soil. Transp. Porous Media 2016, 111, 763–793. [Google Scholar] [CrossRef]
- Ishiguro, M.; Nakaishi, K.; Nakajima, T. Saturated hydraulic conductivity of a volcanic ash soil affected by repulsive potential energy in a multivalent anionic system. Colloids Surf. A Physicochem. Eng. Asp. 2003, 230, 81–88. [Google Scholar] [CrossRef]
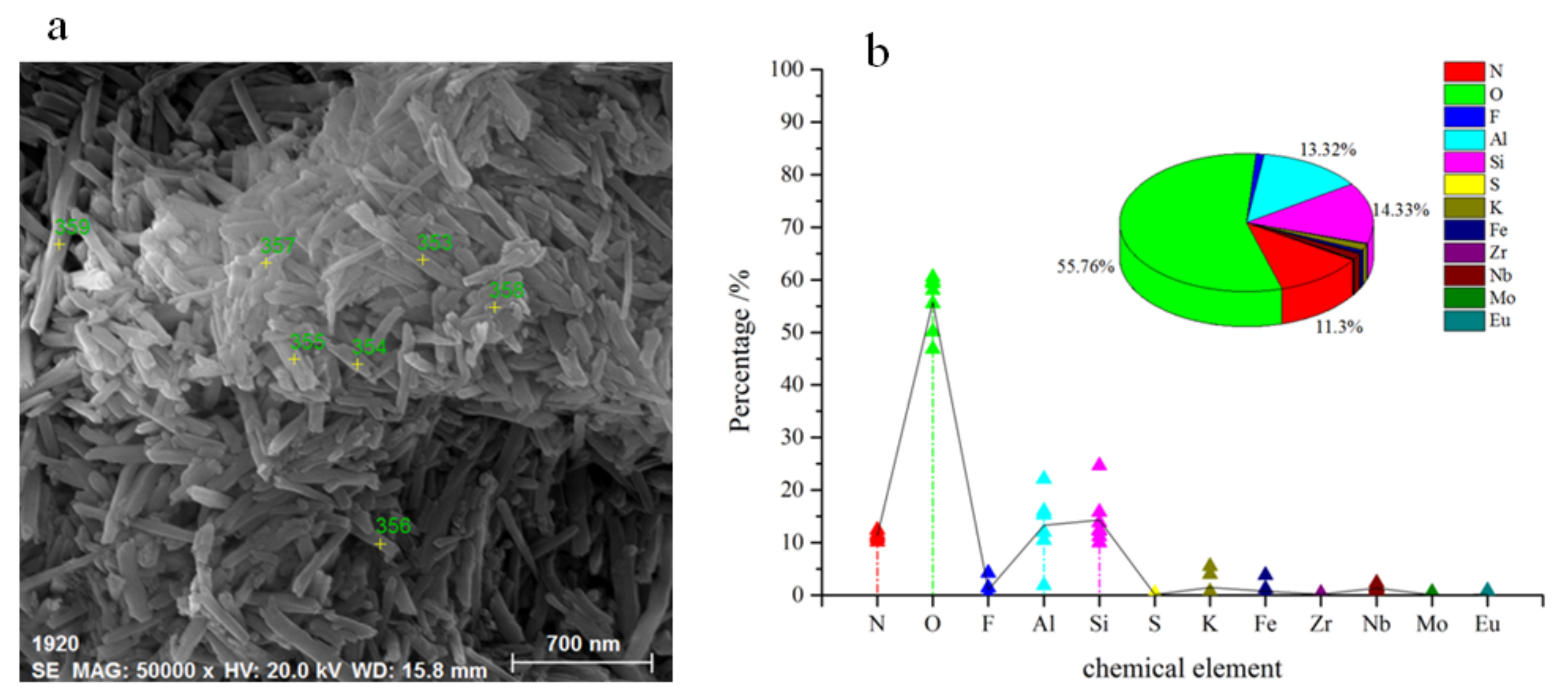
| Size (mm) | >5 | 1–5 | 0.5–1 | 0.1–0.5 | 0.075–0.1 | 0.075–0.005 | <0.005 |
|---|---|---|---|---|---|---|---|
| Percentage (%) | 2.51 | 17.28 | 16.36 | 39.06 | 9.82 | 10.61 | 4.36 |
| Parameter | Density | Moisture Content | Void Ratio | Specific Gravity |
|---|---|---|---|---|
| (g/cm3) | (%) | (%) | ||
| Value | 1.75 | 13 | 51 | 2.675 |
| Parameter | Density | Moisture Content | Void Ratio | Diameter | Height | REO Content |
|---|---|---|---|---|---|---|
| (g/cm3) | (%) | (%) | (mm) | (mm) | (g/t) | |
| Value | 1.75 | 13 | 48 | 50 | 100 | 700±30 |
| Leaching Time (h) | REO Content (Upper) (g/t) | REO Content (Middle) (g/t) | REO Content (Lower) (g/t) |
|---|---|---|---|
| 0 | 727 | 735 | 744 |
| 0.5 | 531.8 | 702.9 | 733.5 |
| 1 | 497.4 | 447.6 | 663.5 |
| 1.5 | 379.4 | 354.9 | 643 |
| 2 | 249.3 | 260 | 373 |
| 2.5 | 249.6 | 255.1 | 341.8 |
| 3 | 227 | 229.2 | 229.4 |
| Test Point Number | N (%) | O (%) | F (%) | Al (%) | Si (%) | S (%) | K (%) | Fe (%) | Zr (%) | Nb (%) | Mo (%) | Eu (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 | 11.46 | 59.36 | 0 | 15.38 | 12.44 | 0.24 | 0.21 | 0.08 | 0 | 0.73 | 0 | 0.1 |
| 354 | 10.85 | 60.53 | 4.26 | 12.04 | 10 | 0.24 | 0.07 | 0.02 | 0.42 | 1.55 | 0 | 0.02 |
| 355 | 10.19 | 46.91 | 1.46 | 22.14 | 15.84 | 0 | 0.03 | 0.03 | 0.43 | 2.37 | 0.6 | 0 |
| 356 | 11.07 | 50.2 | 1.41 | 16.02 | 13.86 | 0 | 4.01 | 1.25 | 0.3 | 1.64 | 0.07 | 0.17 |
| 357 | 12.42 | 59.69 | 0 | 1.89 | 24.65 | 0.05 | 0 | 0.04 | 0 | 1.18 | 0 | 0.08 |
| 358 | 11.47 | 55.54 | 0 | 10.47 | 11.21 | 0.2 | 5.54 | 3.86 | 0 | 0.79 | 0 | 0.92 |
| 359 | 11.61 | 58.06 | 0 | 15.29 | 12.28 | 0.39 | 0.74 | 0.13 | 0.06 | 1.4 | 0 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, H.; Sui, C.; Zhou, L.; Feng, X.; Huang, C.; Zhao, K.; Zhong, W.; Hu, K. Permeability and Adsorption–Desorption Behavior of Rare Earth in Laboratory Leaching Tests. Minerals 2020, 10, 889. https://doi.org/10.3390/min10100889
Wang X, Wang H, Sui C, Zhou L, Feng X, Huang C, Zhao K, Zhong W, Hu K. Permeability and Adsorption–Desorption Behavior of Rare Earth in Laboratory Leaching Tests. Minerals. 2020; 10(10):889. https://doi.org/10.3390/min10100889
Chicago/Turabian StyleWang, Xiaojun, Hao Wang, Can Sui, Lingbo Zhou, Xiao Feng, Chengguang Huang, Kui Zhao, Wen Zhong, and Kaijian Hu. 2020. "Permeability and Adsorption–Desorption Behavior of Rare Earth in Laboratory Leaching Tests" Minerals 10, no. 10: 889. https://doi.org/10.3390/min10100889
APA StyleWang, X., Wang, H., Sui, C., Zhou, L., Feng, X., Huang, C., Zhao, K., Zhong, W., & Hu, K. (2020). Permeability and Adsorption–Desorption Behavior of Rare Earth in Laboratory Leaching Tests. Minerals, 10(10), 889. https://doi.org/10.3390/min10100889




