Interaction Between FeOOH and NaCl at Extreme Conditions: Synthesis of Novel Na2FeCl4OHx Compound
Abstract
1. Introduction
2. Materials and Methods
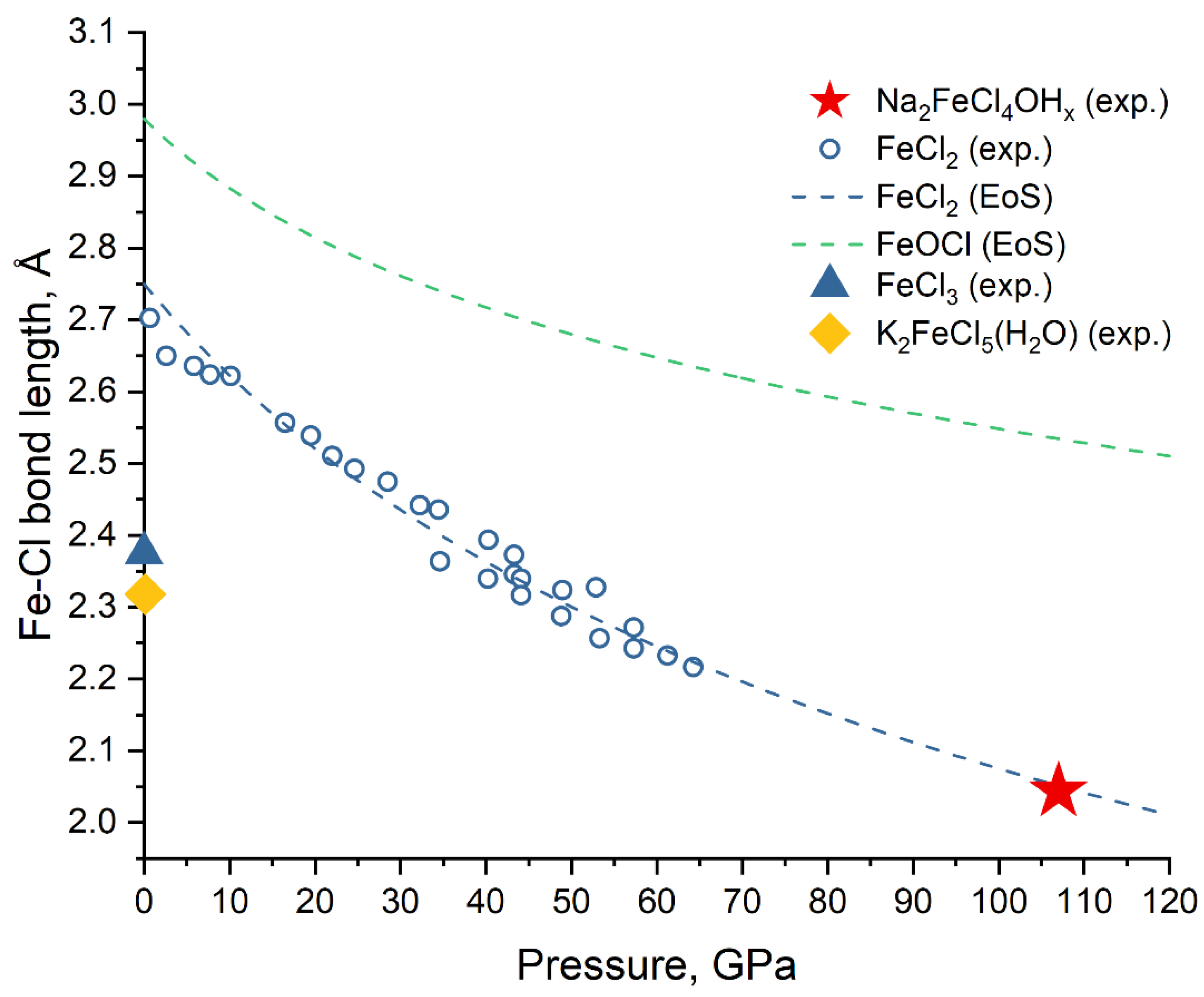
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peslier, A.H.; Schönbächler, M.; Busemann, H.; Karato, S.I. Water in the Earth’s interior: Distribution and origin. Space Sci. Rev. 2017, 212, 1–68. [Google Scholar]
- Hirschmann, M.M. Water, melting, and the deep Earth H2O cycle. Annu. Rev. Earth Planet. Sci. 2006, 34, 629–653. [Google Scholar] [CrossRef]
- Hu, Q.; Kim, D.Y.; Yang, W.; Yang, L.; Meng, Y.; Zhang, L.; Mao, H.K. FeO2 and FeOOH under deep lower-mantle conditions and Earth’s oxygen-hydrogen cycles. Nature 2016, 534, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Kim, D.Y.; Liu, J.; Meng, Y.; Yang, L.; Zhang, D.; Mao, W.L.; Mao, H. Dehydrogenation of goethite in Earth’s deep lower mantle. Proc. Natl. Acad. Sci. USA 2017, 114, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.K.; Hu, Q.; Yang, L.; Liu, J.; Kim, D.Y.; Meng, Y.; Zhang, L.; Prakapenka, V.B.; Yang, W.; Mao, W.L. When water meets iron at Earth’s core-mantle boundary. Natl. Sci. Rev. 2017, 4, 870–878. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Bi, W.; Yang, L.; Xiao, Y.; Chow, P.; Meng, Y.; Prakapenka, V.B.; Mao, H.K.; Mao, W.L. Altered chemistry of oxygen and iron under deep Earth conditions. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Kuwayama, Y.; Tsuchiya, J.; Tsuchiya, T. The pyrite-Type high-pressure form of FeOOH. Nature 2017, 547, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Streltsov, S.S.; Shorikov, A.O.; Skornyakov, S.L.; Poteryaev, A.I.; Khomskii, D.I. Unexpected 3+ valence of iron in FeO2, a geologically important material lying “in between” oxides and peroxides. Sci. Rep. 2017, 7, 13005. [Google Scholar] [CrossRef] [PubMed]
- Boulard, E.; Harmand, M.; Guyot, F.; Lelong, G.; Morard, G.; Cabaret, D.; Boccato, S.; Rosa, A.D.; Briggs, R.; Pascarelli, S.; et al. Ferrous iron under oxygen-rich conditions in the deep mantle. Geophys. Res. Lett. 2019, 46, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Kantor, I.; Prakapenka, V.; Kantor, A.; Dera, P.; Kurnosov, A.; Sinogeikin, S.; Dubrovinskaia, N.; Dubrovinsky, L. BX90: A new diamond anvil cell design for X-ray diffraction and optical measurements. Rev. Sci. Instrum. 2012, 83, 125102. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Ricolleau, A.; Frank, M.; Mibe, K.; Shen, G.; Prakapenka, V. High-pressure geoscience special feature: Toward an internally consistent pressure scale. Proc. Natl. Acad. Sci. USA 2007, 104, 9182–9186. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, Y.; Hirao, N.; Sata, N.; Hirose, K.; Takata, M. Highly intense monochromatic X-ray diffraction facility for high-pressure research at SPring-8. High Press. Res. 2008, 28, 163–173. [Google Scholar] [CrossRef]
- Liermann, H.P.; Konôpková, Z.; Morgenroth, W.; Glazyrin, K.; Bednarčik, J.; McBride, E.E.; Petitgirard, S.; Delitz, J.T.; Wendt, M.; Bican, Y.; et al. The extreme conditions beamline P02.2 and the extreme conditions science infrastructure at PETRA III. J. Synchrotron Radiat. 2015, 22, 908–924. [Google Scholar] [CrossRef]
- Bykova, E. Single-crystal X-ray Diffraction at Extreme Conditions in Mineral Physics and Material Sciences. Ph.D. Thesis, Universität Bayreuth, Bayreuth, Germany, 2015. [Google Scholar]
- CrysalisPRO (Rigaku Products Website). Available online: https://www.rigaku.com/products/smc/crysalis (accessed on 3 January 2020).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Hölsä, J.; Säilynoja, E.; Koski, K.; Rahiala, H.; Valkonen, J. X-ray powder diffraction study of the stability of solid solutions in (La1−xGdx)OCl. Powder Diffr. 1996, 11, 129–133. [Google Scholar] [CrossRef]
- Rozenberg, G.K.; Pasternak, M.P.; Gorodetsky, P.; Xu, W.M.; Dubrovinsky, L.S.; Le Bihan, T.; Taylor, R.D. Pressure-induced structural, electronic, and magnetic phase transitions in FeCl2 studied by X-ray diffraction and resistivity measurements. Phys. Rev. B 2009, 79, 1–7. [Google Scholar] [CrossRef]
- Bykov, M. Structural Aspects of Pressure- and Temperature-Induced Phase Transitions in Low-Dimensional Systems. Ph.D. Thesis, Bayreuth University, Bayreuth, Germany, June 2015. [Google Scholar]
- Troyanov, S.I. Crystalline structure of FeCl3 polytype modifications. Zhurnal Neorganicheskoi Khimii 1993, 38, 1946–1949. [Google Scholar]
- Schultz, A.J.; Carlin, R.L. Single-crystal pulsed neutron diffraction structure of the antiferromagnet K2[FeCl5(H2O)] with and without applied pressure. Acta Crystallogr. Sect. B 1995, 51, 43–47. [Google Scholar] [CrossRef]

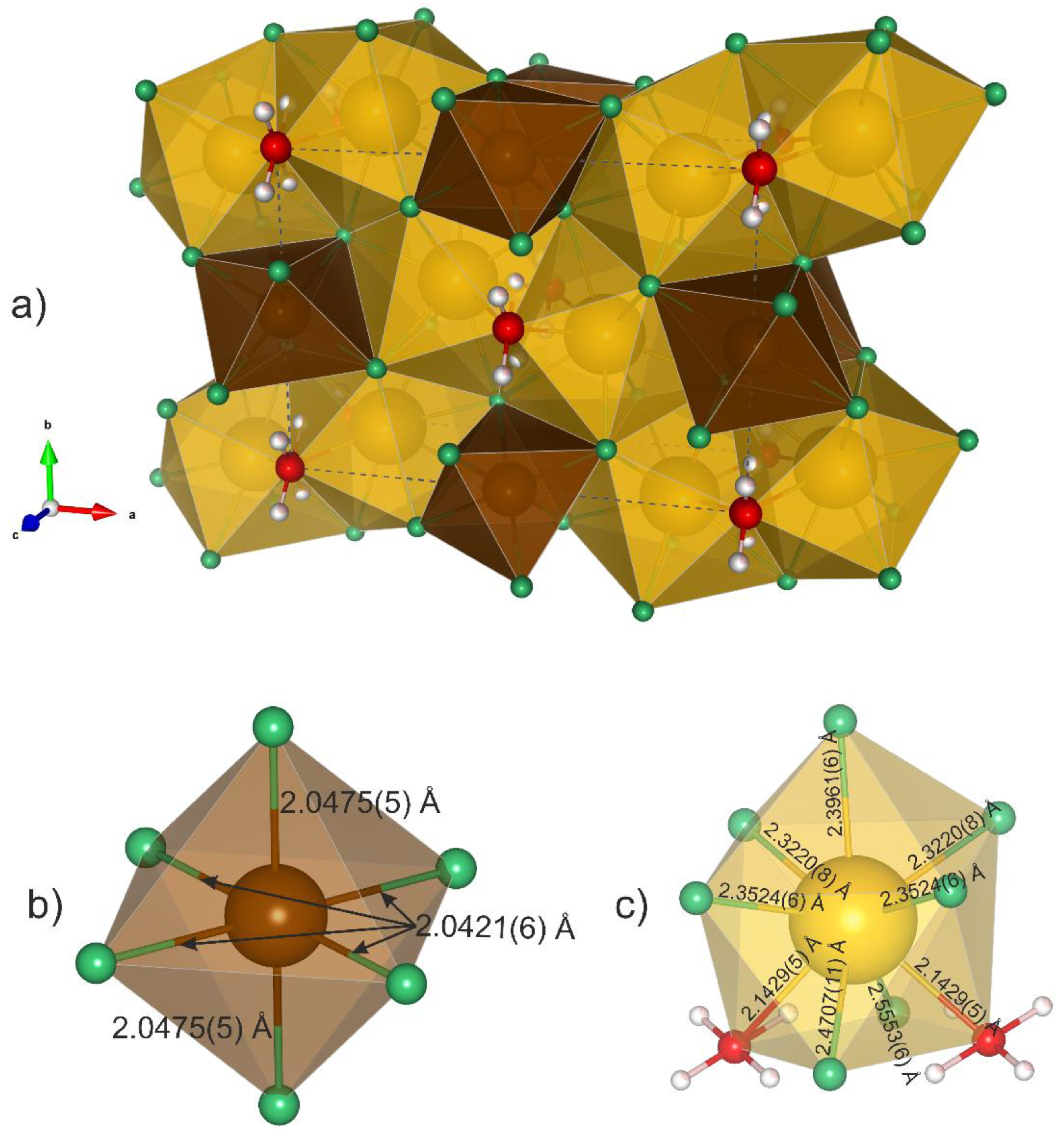
| Crystal Data | |
|---|---|
| Chemical formula | Na2FeCl4OHx |
| Mr (g/mol) | 260.6 |
| Crystal system, space group | Orthorhombic, Pbam (#55) |
| Temperature (K), Pressure (GPa) | 293, 107(2) |
| a,b,c (Å) | 8.725 (2), 6.180 (3), 3.0679 (12) |
| V (Å3) | 165.41 (11) |
| Z | 2 |
| Radiation type | Synchrotron, λ = 0.2892 Å |
| μ (mm−1) | 0.61 |
| Crystal shape | Cube |
| Crystal size (mm) | 0.01 × 0.01 × 0.01 |
| Data collection | |
| Diffractometer | P02.2, DESY |
| Absorption correction | Multi-scan (ABSPACK, [15]) |
| Tmin, Tmax | 0.538, 1 |
| No. of measured, independent and observed [I > 3σ(I)] reflections | 894, 432, 315 |
| Rint | 0.038 |
| θ values (°) | θ = 3.2–18.4 |
| (sin θ/λ)max (Å-1) | 1.089 |
| Range of h, k, l | h = −16→13, k = −8→10, l = −5→4 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.070, 0.071, 2.93 |
| No. of reflections | 315 |
| No. of parameters | 27 |
| Δρmax, Δρmin (eÅ−3) | 2.07, –1.63 |
| Atom Number | Atom Name | Atomic Coordinates (x, y, z) | Occupancy | Isotropic Atomic Displacement Parameter (U, Å2) | Wyckoff |
|---|---|---|---|---|---|
| 1 | Fe1 | 0.5, 0.0, 0.0 | 1 | 0.01 | 2 c |
| 2 | Cl1 | 0.2076, 0.3455, 0.0 | 1 | 0.012 | 4 g |
| 3 | Cl2 | 0.4251, 0.1907, 0.5 | 1 | 0.011 | 4 h |
| 4 | Na1 | 0.1645, 0.0701, 0.5 | 1 | 0.012 | 4 h |
| 5 | O1 | 0.0, 0.0, 0.0 | 1 | 0.002 | 2 a |
| 6 | H1 | 0.00380, 0.11683, 0.17495 | 0.25 | 0.0 | 8 i |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koemets, E.; Yuan, L.; Bykova, E.; Glazyrin, K.; Ohtani, E.; Dubrovinsky, L. Interaction Between FeOOH and NaCl at Extreme Conditions: Synthesis of Novel Na2FeCl4OHx Compound. Minerals 2020, 10, 51. https://doi.org/10.3390/min10010051
Koemets E, Yuan L, Bykova E, Glazyrin K, Ohtani E, Dubrovinsky L. Interaction Between FeOOH and NaCl at Extreme Conditions: Synthesis of Novel Na2FeCl4OHx Compound. Minerals. 2020; 10(1):51. https://doi.org/10.3390/min10010051
Chicago/Turabian StyleKoemets, Egor, Liang Yuan, Elena Bykova, Konstantin Glazyrin, Eiji Ohtani, and Leonid Dubrovinsky. 2020. "Interaction Between FeOOH and NaCl at Extreme Conditions: Synthesis of Novel Na2FeCl4OHx Compound" Minerals 10, no. 1: 51. https://doi.org/10.3390/min10010051
APA StyleKoemets, E., Yuan, L., Bykova, E., Glazyrin, K., Ohtani, E., & Dubrovinsky, L. (2020). Interaction Between FeOOH and NaCl at Extreme Conditions: Synthesis of Novel Na2FeCl4OHx Compound. Minerals, 10(1), 51. https://doi.org/10.3390/min10010051






