Critical Elements in Supergene Phosphates: The Example of the Weathering Profile at the Gavà Neolithic Mines, Catalonia, Spain
Abstract
1. Introduction
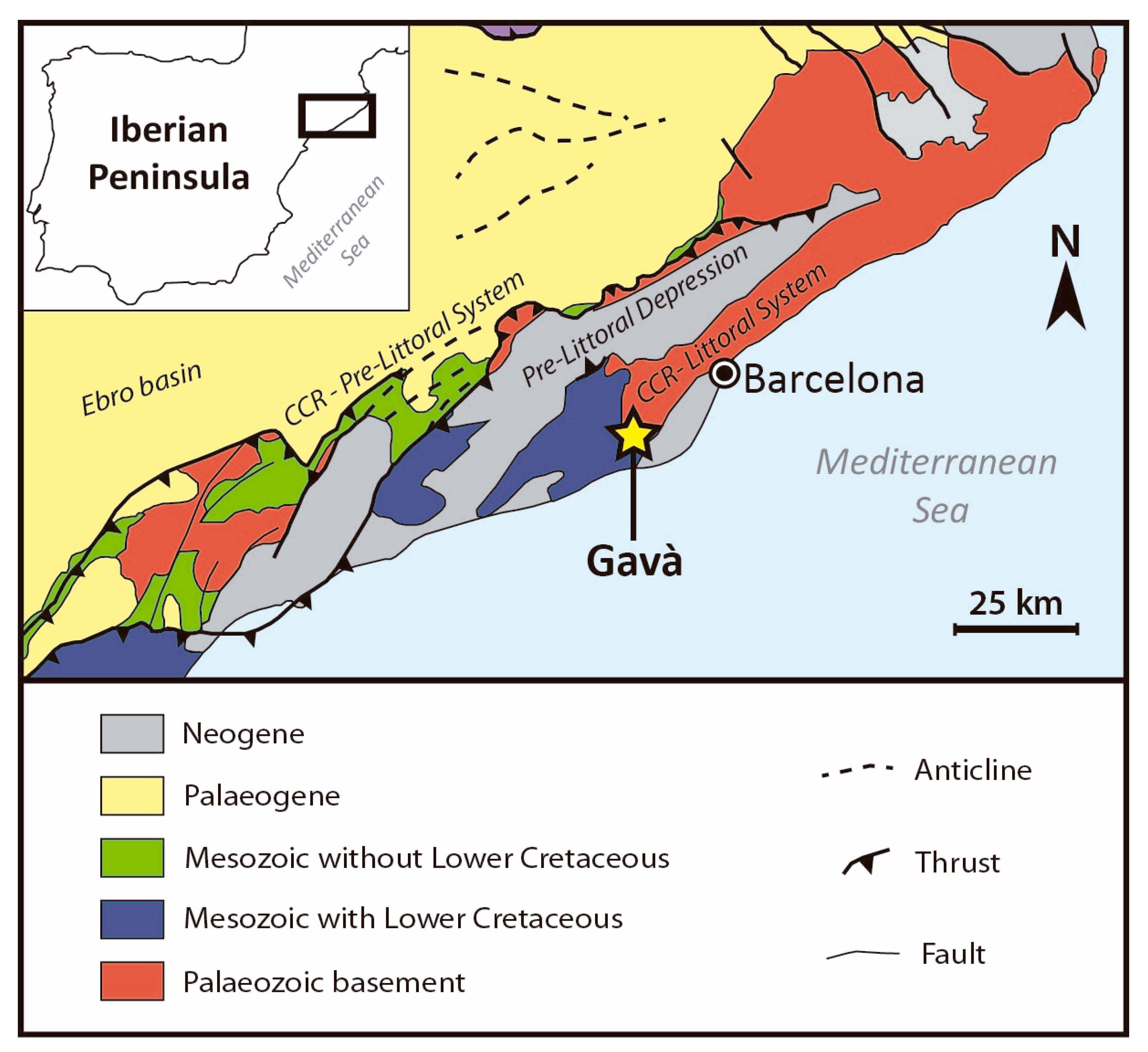
2. Geological Setting
3. Materials and Methods
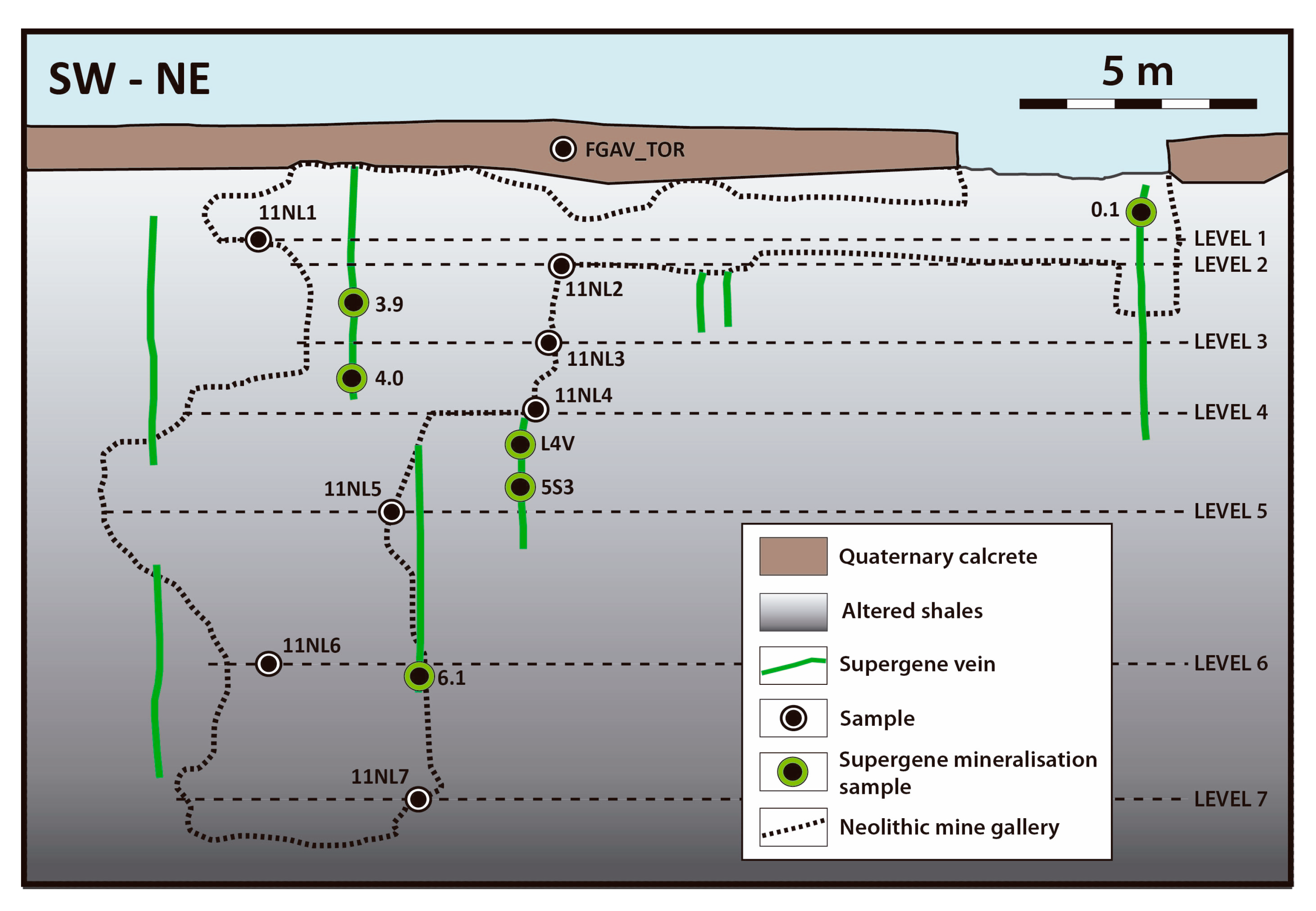
3.1. Sampling
3.2. X-ray Diffraction (XRD)
3.3. Scanning Electron Microscopy (SEM) and Field Emission Scanning Electron Microscopy (FE-SEM)
3.4. Electron Probe Microanalysis (EPMA)
3.5. Bulk Rock Geochemistry
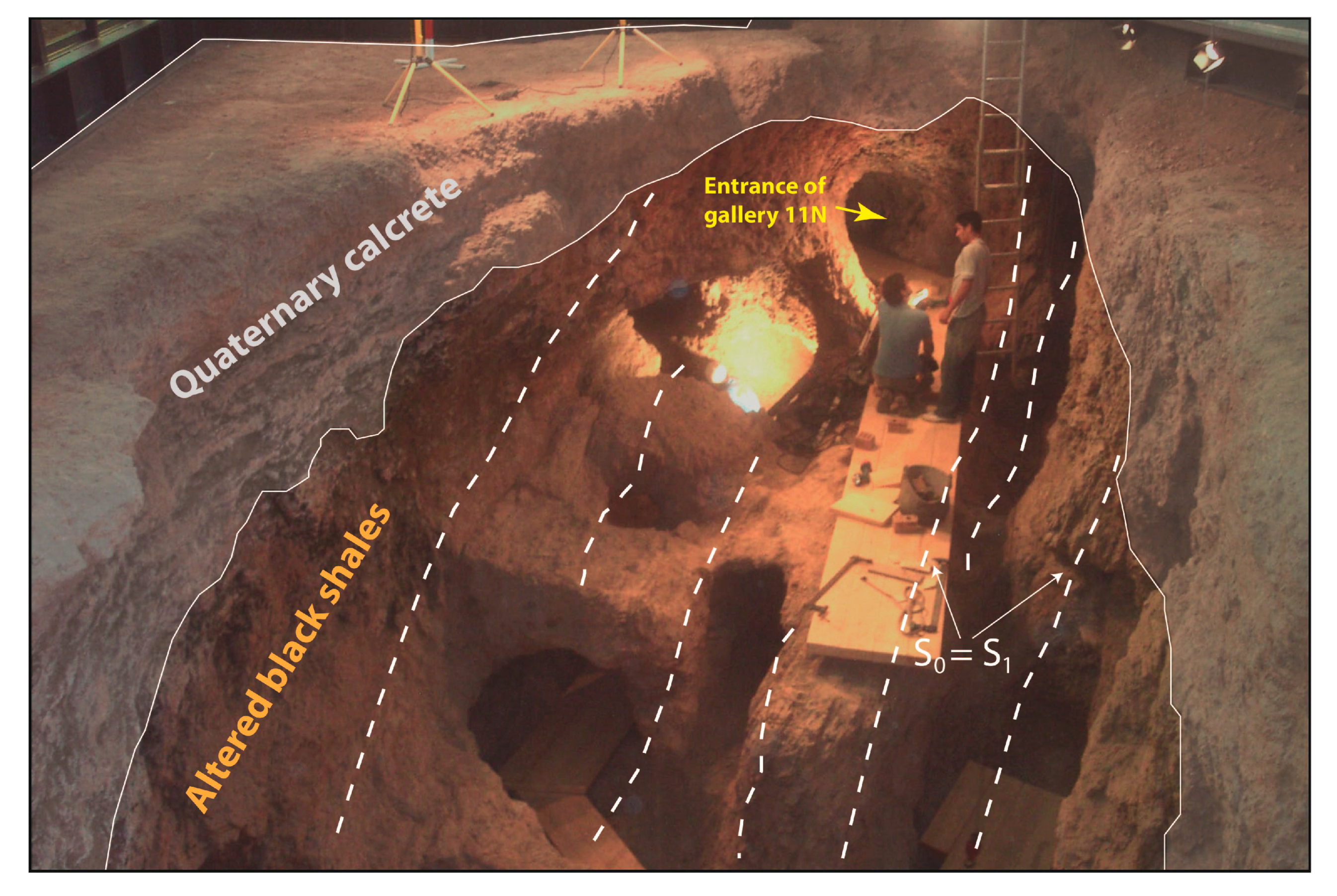
4. Structure of the Deposit
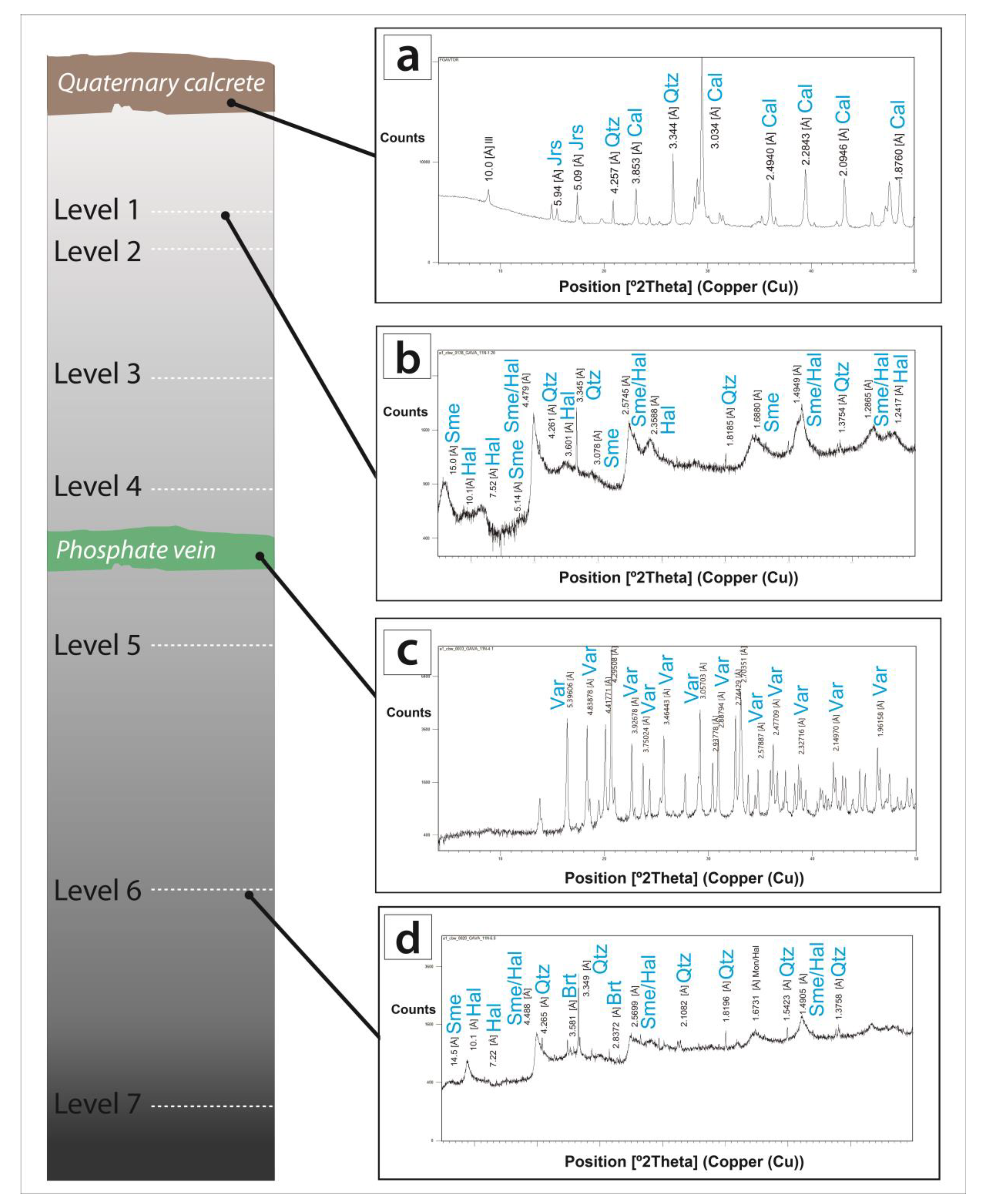
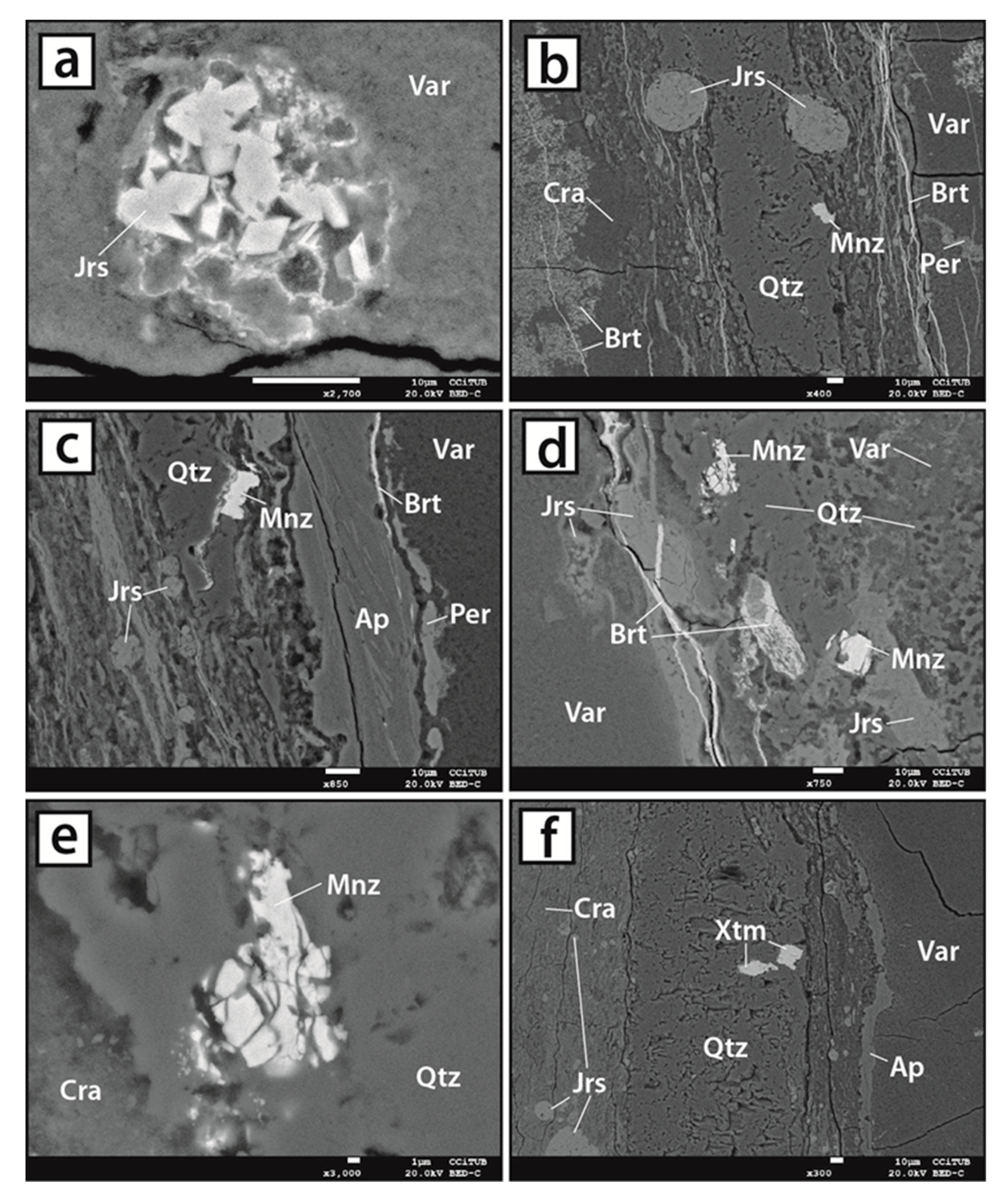
5. Mineral Characterization
5.1. Host Rocks
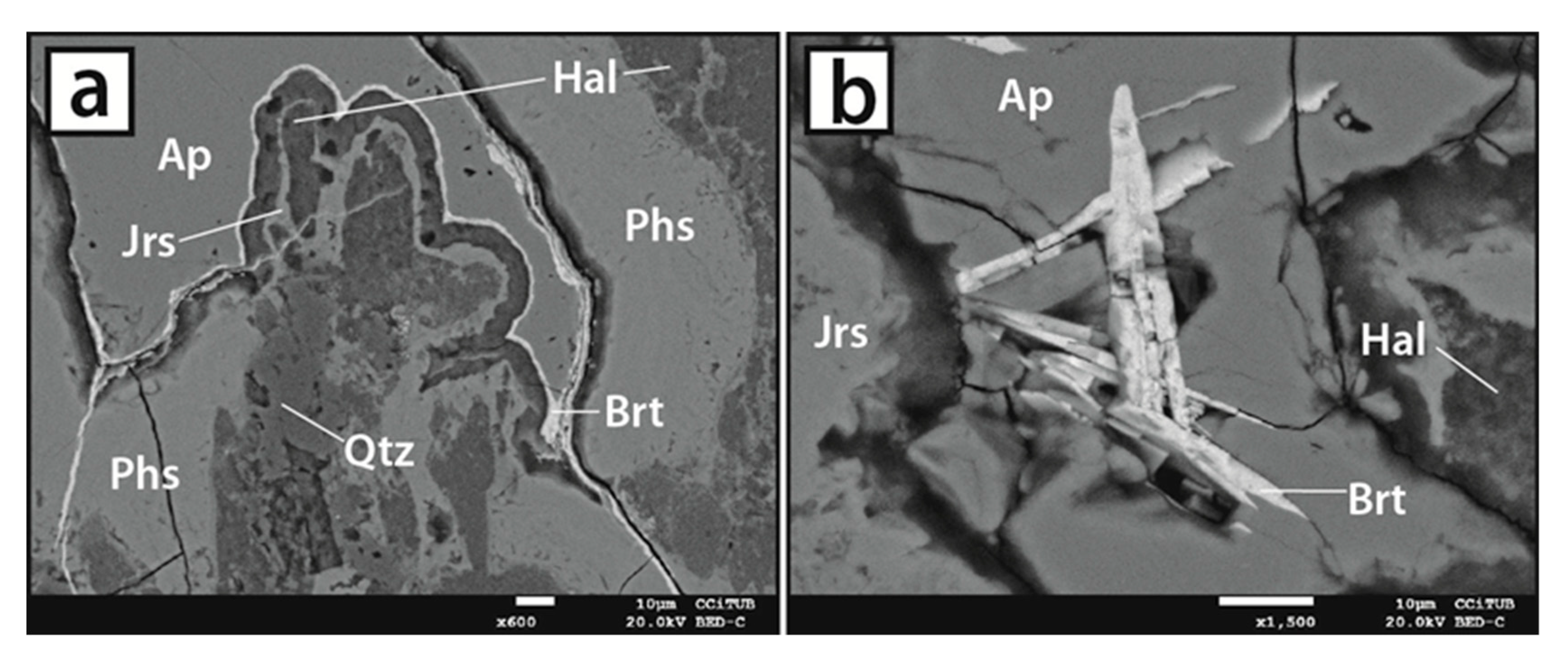
5.2. Vein-like Supergene Mineralisation
5.3. Mineral Chemistry
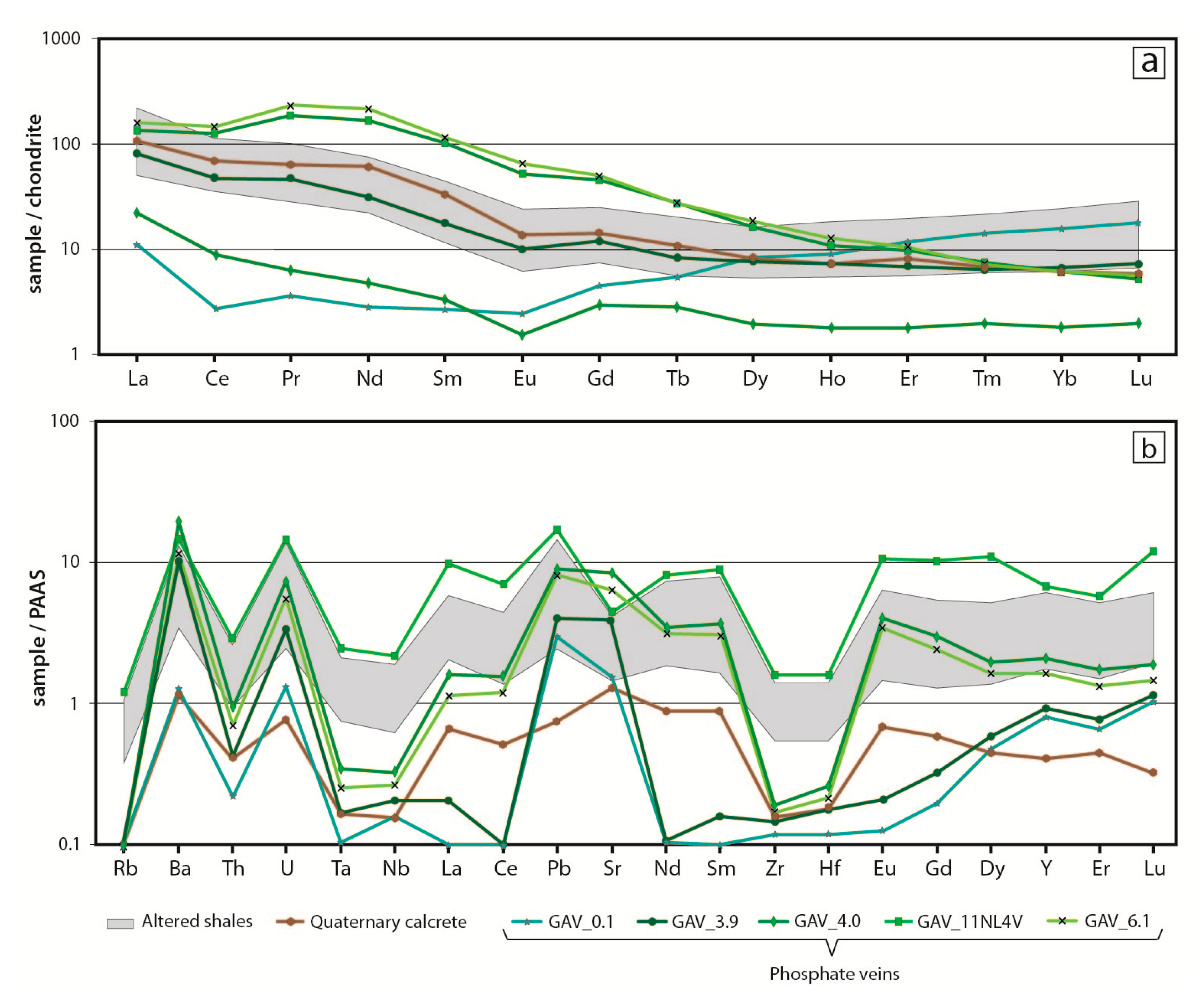
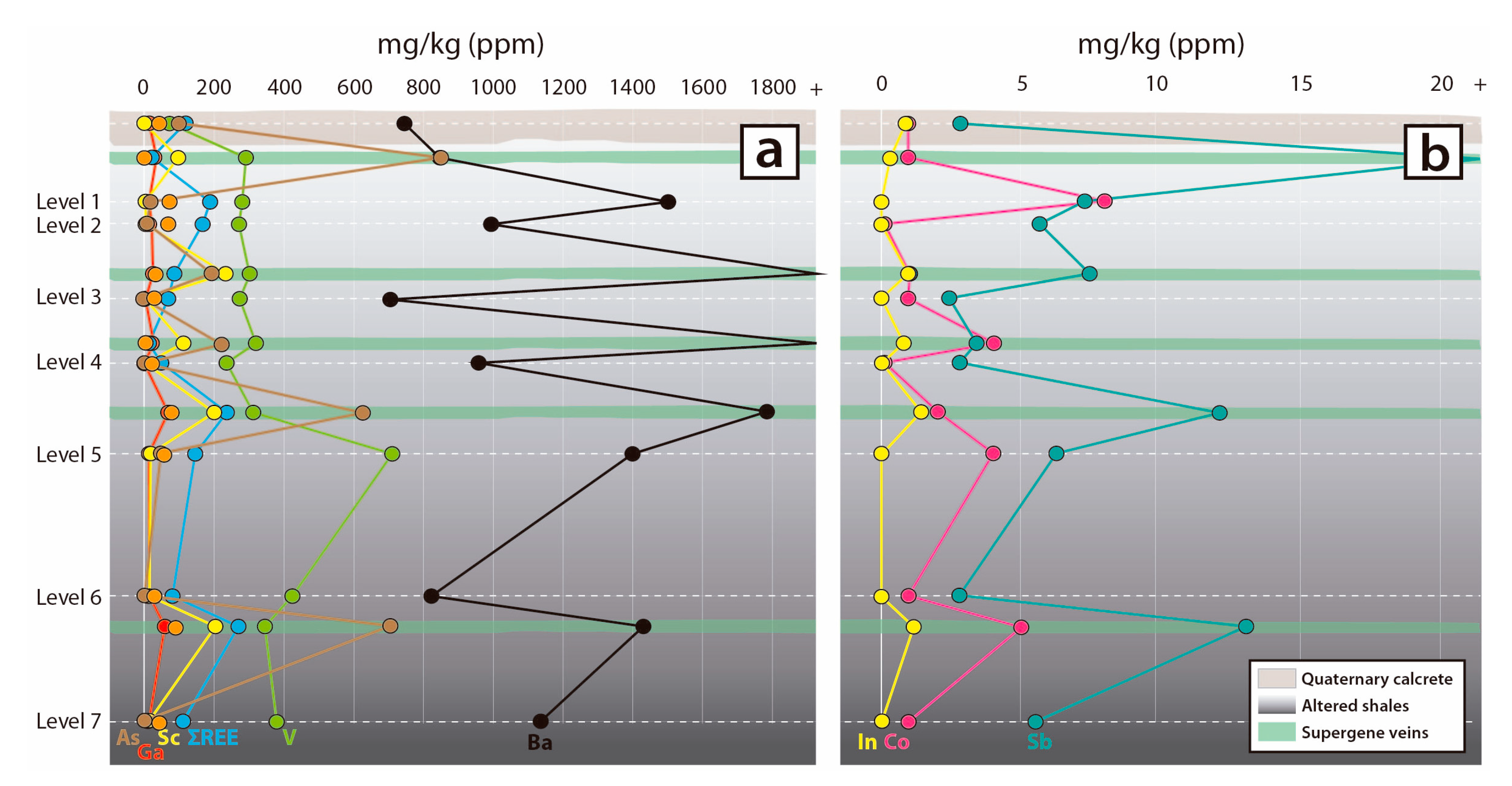
6. Bulk Rock Geochemistry
7. Discussion
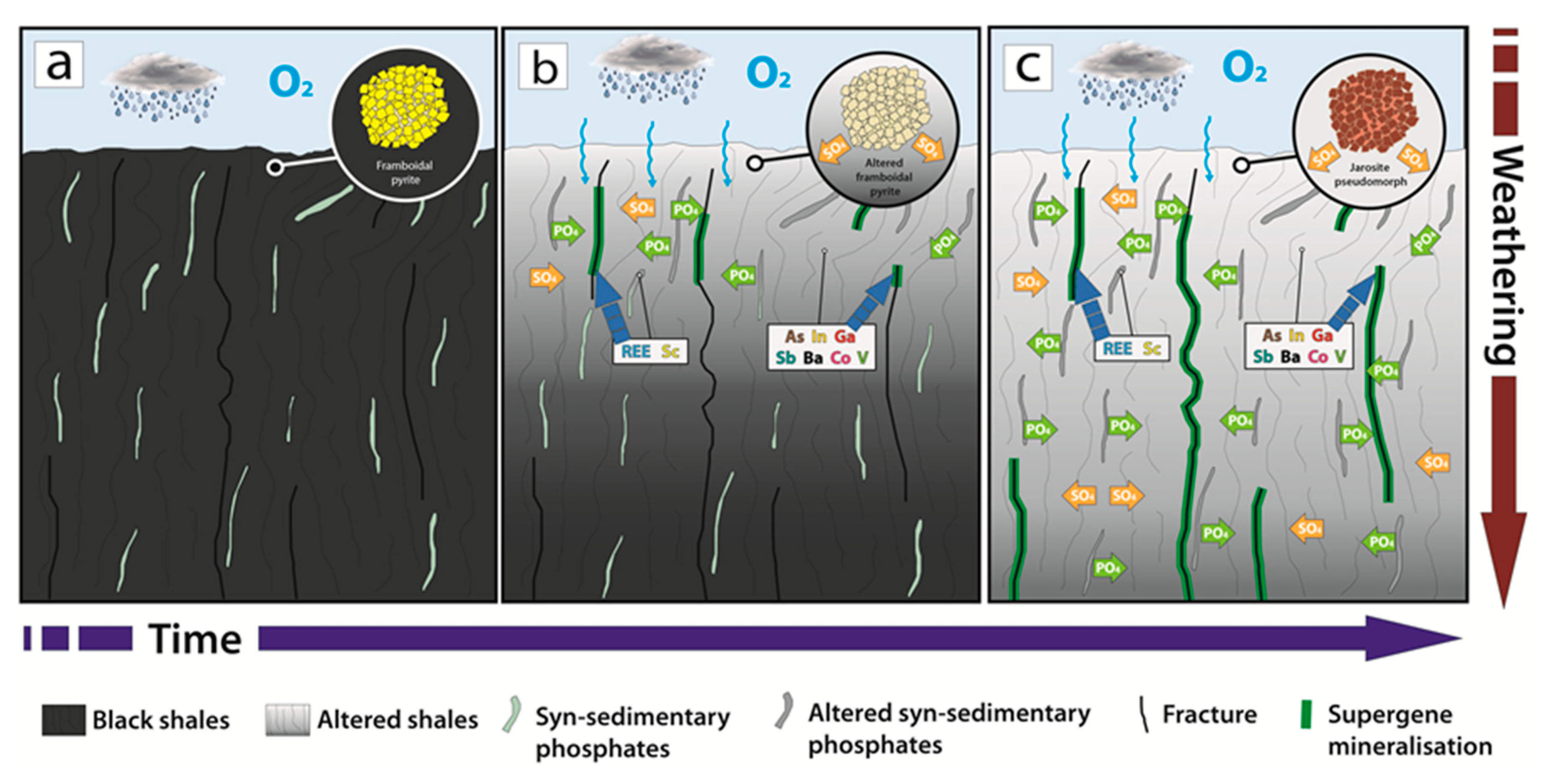
7.1. Formation of the Gavà Supergene Mineralisations
7.2. Distribution of Critical Elements in Supergene Mineralisation
7.3. Weathered Phosphates as Non-Conventional Source of Critical Elements
8. Conclusions
- In the Gavà area, significant weathering processes involving acid and oxidising meteoric fluids occurred during the early Pleistocene affecting a series of Llandoverian black shales and associated syn-sedimentary phosphates.
- Acid fluids would promote the alteration of phyllosilicates and disseminated framboidal pyrite in Llandoverian shales as well as associated beds of syn-sedimentary phosphates, producing a general enrichment of fluids in sulphate, Fe, Al and phosphate, respectively.
- The deposits of the Gavà area include supergene mineralisation distributed among veins that are hosted in weathered and bleached Llandoverian shales. They are mainly comprised of supergene phosphates (e.g., variscite, perhamite, crandallite, phosphosiderite), sulphates (e.g., jarosite, alunite) and associated clay minerals (e.g., halloysite and smectite).
- The supergene mineralisation is significantly enriched in certain CE. Such anomalous contents are related to the action of these acid and oxidised fluids associated with weathering.
- Precipitation of supergene vein-like mineralisation is related to an increase of pH conditions in deeper parts of the profile. CE are hosted in the crystal lattice of supergene minerals.
- Weathering processes and corresponding enrichment of CE in supergene mineralisation at the Gavà deposit can be used as an example to explore CE in primary phosphates affected by weathering worldwide.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Report on critical raw materials or the EU. In Report of the Ad hoc Working Group on Defining Critical Raw Materials; European Commission: Brussels, Belgium, 2017; Available online: http://ec.europa.eu/DocsRoom/documents/10010/attachments/1/translations/en/renditions/native (accessed on 20 November 2019).
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Rare earth elements: Minerals, mines, magnets (and more). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A detailed assessment of global rare earth element resources: Opportunities and challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare earths and the balance problem: How to deal with changing markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic settin. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Aiglsperger, T.; Proenza, J.A.; Zaccarini, F.; Lewis, J.F.; Garuti, G.; Labrador, M.; Longo, F. Platinum group minerals (PGM) in the Falcondo Ni laterite deposit, Loma Caribe peridotite (Dominican Republic). Mineral. Dep. 2015, 50, 105–123. [Google Scholar] [CrossRef]
- Aiglsperger, T.; Proenza, J.A.; Lewis, J.F.; Labrador, M.; Svojtka, M.; Rojas-Purón, A.; Longo, F.; Durisová, J. Critical metals (REE, Sc, PGE) in Ni laterites from Cuba and the Dominican Republic. Ore Geol. Rev. 2016, 73, 127–147. [Google Scholar] [CrossRef]
- Berger, A.; Janots, E.; Gnos, E.; Frei, R.; Bernier, F. Rare earth element mineralogy and geochemistry in a laterite profile from Madagascar. Appl. Geochem. 2014, 41, 218–228. [Google Scholar] [CrossRef]
- Eliopoulos, D.; Economou, G.; Tzifas, I.; Papatrechas, C. The potential of rare earth elements in Greece. In Proceedings of the 1st European Rare Earth Resources Conference ERES, Milos, Greece, 4–7 September 2014; pp. 308–316. [Google Scholar]
- Gamaletsos, P.N.; Godelitsas, A.; Filippidis, A.; Pontikes, Y. The Rare Earth Elements Potential of Greek Bauxite Active Mines in the Light of a Sustainable REE Demand. J. Sustain. Metall. 2018, 5, 1–28. [Google Scholar] [CrossRef]
- Hoshino, M.; Sanematsu, K.; Watanabe, Y. REE mineralogy and resources. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 129–291. [Google Scholar]
- Mihajlovic, J.; Rinklebe, J. Rare earth elements in German soils—A review. Chemosphere 2018, 205, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, N.; Proenza, J.A.; Villanova-de-Benavent, C.; Aiglsperger, T.; Bover-Arnal, T.; Torró, L.; Salas, R.; Dziggel, A. Geochemistry and Mineralogy of Rare Earth Elements (REE) in Bauxitic Ores of the Catalan Coastal Range, NE Spain. Minerals 2018, 8, 562. [Google Scholar] [CrossRef]
- Tzifas, I.T.; Godelitsas, A.; Magganas, A.; Androulakaki, E.; Eleftheriou, G.; Mertzimekis, T.J.; Perraki, M. Uranium-bearing phosphatized limestones of NW Greece. J. Geo. Exp. 2004, 143, 62–73. [Google Scholar] [CrossRef]
- Walter, A.V.; Nahon, D.; Flicoteaux, R.; Girard, J.P.; Melfi, A. Behaviour of major and trace elements and fractionation of REE under tropical weathering of a typical apatite-rich carbonatite from Brazil. Earth Planet. Sci. Lett. 1995, 136, 591–596. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A review on the potentiality of Rare Earth Elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Sanematsu, K.; Moriyama, T.; Sotouky, L.; Watanabe, Y. Mobility of rare earth elements in basalt-derived laterite at the Bolaven Plateau, southern Laos. Resour. Geol. 2011, 61, 140–158. [Google Scholar] [CrossRef]
- Camprubí, A.; Costa, F.; Melgarejo, J.C. Mineralizaciones de fosfatos férrico-alumínicos de Gavà (Catalunya): Tipología. Bol. Geol. Min. 1994, 105, 444–453. [Google Scholar]
- Camprubí, A.; Melgarejo, J.C.; Proenza, J.A.; Costa, F.; Bosch, J.; Estrada, A.; Borrell, F.; Yushkin, N.P.; Andreichev, V.L. Mining and geological knowledge during the Neolithic: A geological study on the variscite mines at Gavà, Catalonia. Episodes 2003, 26, 295–301. [Google Scholar] [CrossRef][Green Version]
- Costa, F.; Camprubí, A.; Melgarejo, J.C. Aproximación geológica a las minas neolíticas de fosfatos férrico-alumínicos de Gavà (Cataluña). Bol. Geol. Min. 1994, 105, 436–443. [Google Scholar]
- Gimeno, D.; Fernández-Turiel, J.L.; Villalba, M.J.; Edo, M.; Blasco, A. Complejo minero de Can Tintorer, Gavà: Geología y técnicas de explotación en el IV milenio. Rubricatum 1995, 1, 259–263. [Google Scholar]
- Melgarejo, J.C.; Cirera, M.C.; Proenza, J. Estudi geològic i mineralògic de mines de l’època neolítica a la Serra de Les Ferreres (Mines de Gavà). Rubricatum 2009, 4, 47–61. [Google Scholar]
- Díaz-Acha, Y.; Melgarejo, J.C.; Bosch, J.; Andreazini, A.; Pastor, M.; Pujol-Solà, N.; Campeny, M.; Torró, L.; Villanova-de-Benavent, C.; Castillo-Oliver, M.; et al. The Neolithic variscite mines of Gavà, Catalonia: Criteria for mineral exploration and exploitation in the Prehistory. Bol. Soc. Geol. Mex. 2019, 71, 83–319. [Google Scholar] [CrossRef]
- Alonso, M.; Edo, M.; Gordo, L.; Millán, M.; Villalba, M.J. Explotación minera neolítica en Can Tintoré (Gavà, Barcelona). Pyrenae 1978, 13–14, 7–14. [Google Scholar]
- Villalba, M.J.; Bañolas, L.; Arenas, J.; Alonso, M. Les mines neolítiques de can Tintorer. Gavà. Excavacions 1978-1980: Barcelona, Catalonia, Departament de Cultura de la Generalitat de Catalunya. Excavacions Arqueològiques a Catalunya 1986, 6, 203. [Google Scholar]
- Villalba, M.J.; Blasco, A.; Edo, M.; Bañolas, L.; Arenas, J. Minería neolítica: Can Tintorer, una aportación fundamental. Revista de Arqueología 1989, 96, 13–24. [Google Scholar]
- Villalba, M.J.; Bañolas, L.; Arenas, J. Can Tintorer (Gavà, Catalunya): Une exploitation néolithique de phosphates et silicates. Cahiers du Quaternaire 1990, 17, 275–285. [Google Scholar]
- Blasco, A.; Edo, M.; Villalba, M.J. Les perles en Callaïs du Sud de la France proviennent-elles des Mines de Can Tintorer? Le Chalcolithique en Languedoc, ses relations extra-regionales (Saint Mathieu de Tréviers, 1990). In Archéologie en Languedoc; Fédération Archéologique de l’Hérault: Hérault, France, 1991; pp. 279–289. [Google Scholar]
- Blasco, A.; Villalba, M.J.; Edo, M. Cronologia del complex miner de Can Tintorer. Aportacions a la periodització del Neolític mitjà català. In 9è Col·loqui Internacional d’Arqueologia de Puigcerdà; Institut d’Estudis Ceretans: Puigcerdà, Spain, 1992; pp. 215–219. [Google Scholar]
- Bosch, J.; Estrada, A.; Noain, M.J. Minería neolítica en Gavá (Baix Llobregat, Barcelona). Trabajos de Prehistoria 1996, 53, 59–71. [Google Scholar] [CrossRef][Green Version]
- Villalba, M.J. Le gîte de variscite de Can Tintorer: Production, transformation, et circulation du minéral vert. In Matériaux, Productions, Circulations du Néolithique à L’âge du Bronze; Guilaine, J., Ed.; Éditions Errance: Paris, France, 2002; pp. 115–129. [Google Scholar]
- Borrell, F.; Estrada, A. Elements ornamentals neolítics de variscita trobats a les mines 83 i 85 de Gavà, in. In Intervencions arqueològiques a les Mines de Gavà (sector serra de les Ferreres). Anys 1998–2009. De la variscita al ferro: Neolític i antiguitat; Bosch, J., Borrell, F., Eds.; Rubricatum: Barcelona, Spain, 2009; Volume 4, pp. 171–181. [Google Scholar]
- Borrell, F.; Orri, E. Excavacions arqueològiques a la serra de les Ferreres, Mines Prehistòriques de Gavà, in L’Arqueologia a Gavà. Homenatge a Alícia Estrada: Gavà, Catalonia, Ajuntament de Gavà/Amics del Museu de Gavà. Collecció La Nostra Gent 2009, 5, 67–86. [Google Scholar]
- Borrell, F.; Bosch, J. Las minas de variscita de Gavà (Barcelona) y las redes de circulación en el Neolítico. Rubricatum 2012, 5, 315–322. [Google Scholar]
- Blasco, M.; Borrell, M.; Bosch, J. Las minas prehistóricas de Gavà (Barcelona): Un ejemplo de estudio, conservación y presentación pública de un yacimiento arqueológico. Trabajos de Prehistoria 2000, 57, 77–87. [Google Scholar] [CrossRef]
- Díaz-Acha, Y.; Campeny, M.; Melgarejo, J.C.; Bosch, J.; Lehbib, S.; Torró, L.; Proenza, J.A.; Castillo-Oliver, M.; Camprubí, A.; Villanova-de-Benavent, C.; et al. Geological context of the historic and prehistoric iron mines in the Gavà area, Catalonia, NE Iberian peninsula. Bol. Soc. Geol. Mex. 2019, 71, 321–342. [Google Scholar]
- Anadón, P.; Colombo, F.; Esteban, M.; Marzo, M.; Robles, S.; Santanach, P.; Solé Sugrañés, L. Evolución tectonoestratigráfica de los Catalánides. Acta Geol. Hisp. 1979, 14, 242–270. [Google Scholar]
- Guimerà, J. Palaeogene evolution of deformation in the north-eastern Iberian peninsula. Geol. Mag. 1984, 121, 413–420. [Google Scholar] [CrossRef]
- Santanach, P.; Casas, J.M.; Gratacós, O.; Liesa, M.; Muñoz, J.A.; Sàbat, F. Variscan and Alpine structure of the hills of Barcelona: Geology in an urban area. J. Iber. Geol. 2011, 37, 121–136. [Google Scholar]
- Julivert, M.; Duran, H. Paleozoic stratigraphy of the Central and Northern part of the Catalonian Coastal Ranges (NE Spain). Acta Geol. Hisp. 1990, 25, 3–12. [Google Scholar]
- Julivert, M.; Duran, H.; Rickards, R.B.; Chapman, A.J. Siluro–Devonian graptolite stratigraphy of the Catalonian Coastal Ranges. Acta Geol. Hisp. 1985, 20, 199–207. [Google Scholar]
- Salvador, P.; Fayos, J. Some aspects of the structural relationship between “Messbach-type” and “Lucin-type” variscites. Am. Miner. 1972, 57, 36–44. [Google Scholar]
- Taylor, S.R. McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985. [Google Scholar]
- Leythaeuser, D. Effects of weathering on organic matter in shales. Geochim. Cosmochim. Acta 1973, 37, 113–120. [Google Scholar] [CrossRef]
- Clayton, J.L.; Swetland, P.J. Subaerial weathering of sedimentary organic matter. Geochim. Cosmochim. Acta 1978, 42, 305–312. [Google Scholar] [CrossRef]
- Littke, R.; Klussmann, U.; Krooss, B.; Leythaeuser, D. Quantification of loss of calcite, pyrite and organic matter due to weathering of Toarcian black shales and effects on kerogen and bitumen characteristics. Geochim. Cosmochim. Acta 1991, 55, 3369–3378. [Google Scholar] [CrossRef]
- Dill, H.G.; Busch, K.; Blum, N. Chemistry and origin o veinlike phosphate mineralization, Nuba Mts. (Sudan). Ore. Geol. Rev. 1991, 6, 9–24. [Google Scholar] [CrossRef]
- Dill, H.G. The geology of aluminium phosphates and sulphates of the alunite group minerals: A review. Earth Sci. Rev. 2001, 53, 25–93. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L. Formation processes of framboidal pyrite. Geoch. Cosm. Ac. 1996, 61, 323–339. [Google Scholar] [CrossRef]
- Soliman, M.F.; El Goresy, A. Framboidal and idiomorphic pyrite in the upper Maastrichtian sedimentary rocks at Gabal Oweina, Nile Valley, Egypt: Formation processes, oxidation products and genetic implications to the origin of framboidal pyrite. Geoch. Cosm. Ac. 2012, 90, 195–220. [Google Scholar] [CrossRef]
- Stroffregen, R.E.; Alpers, C.N. Woodhouseite and svanbergite in hydrothermal ore deposits: Products of apatite destruction during advanced alteration. Can. Min. 1987, 25, 201–211. [Google Scholar]
- Petsch, S.T.; Berner, R.A.; Eglinton, T.I. A field study of the chemical weathering of ancient sedimentary organic matter. Org. Geochem. 2000, 31, 475–487. [Google Scholar] [CrossRef]
- Petsch, S.T.; Edwards, K.J.; Eglinton, T.I. Microbial transformation of organic matter in black shale and implications for global biogeochemical cycles. Palaeogeog. Palaeoclimatol. Palaeoecol. 2005, 219, 157–170. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Tang, X.; Yang, C.; Tang, S. Weathering characteristics of the Lower Paleozoic black shale in northwestern Guizhou Province, south China. J. Earth Syst. Sci. 2016, 125, 1061–1078. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. In Sulfate Minerals—Crystallography, Geochemistry and Environmental Significance; Reviews in Mineralogy & Geochemistry; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Mineralogical Society of America: Washington, DC, USA, 2000; Volume 40, pp. 305–393. [Google Scholar]
- Liu, F.; Liu, Z.; Li, Y.; Wilson, B.P.; Lundström, M. Behavior of gallium and germanium associated with zinc sulfide concentrate in oxygen pressure leaching. Phys. Prob. Min. Process. 2017, 53, 1047–1060. [Google Scholar]
- Tuttle, M.L.W.; Breig, G.N.; Goldhaber, M.B. Weathering of the New Albany Shale, Kentucky: II. Redistribution of minor and trace elements. App. Geochem. 2009, 24, 1565–1578. [Google Scholar] [CrossRef]
- Seward, T.M.; Williams-Jones, A.; Migdisov, A. The Chemistry of Metal Transport and Deposition by Ore-Forming Hydrothermal Fluids. Treatise Geochem. 2014, 13, 29–57. [Google Scholar]
- Wood, S.A. The aqueous geochemistry of the rare-earth elements and yttrium. 1. Review of available low-temperature data for inorganic complexes and the inorganic REE speciation of natural waters. Chem. Geol. 1990, 82, 159–186. [Google Scholar] [CrossRef]
- Migdisov, A.; Williams-Jones, A.; Brugger, J.; Caporuscio, F. Hydrothermal transport, deposition, and fractionation of the REE: Experimental data and thermodynamic calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef]
- Migdisov, A.; Guo, X.; Nisbet, H.; Xu, H.; Williams-Jones, A. Fractionation of REE, U, and Th in natural ore-forming hydrothermal systems: Thermodynamic modeling. J. Chem. Thermodyn. 2019, 128, 305–319. [Google Scholar] [CrossRef]
- Andersson, S. Formation of Hydrothermal REE-Phosphate Deposits; Helsingin Yliopistö: Helsinki, Finland, 2019; p. 53. [Google Scholar]
- Christmann, P. A forward look into rare earth supply and demand: A role for sedimentary phosphate deposits? Procedia Eng. 2014, 83, 19–26. [Google Scholar] [CrossRef]
- Sherman, D.M.; Ragnarsdottir, K.V.; Oelkers, E.H. Antimony transport in hydrothermal solutions: An EXAFS study of antimony (V) complexation in alkaline sulfide and sulfide-chloride brines at temperatures from 25 °C to 300 °C at Psat. Chem. Geol. 2000, 167, 161–167. [Google Scholar] [CrossRef]
- Krupka, K.M.; Serne, R.J. Geochemical Factors Affecting the Behavior of Antimony, Cobalt, Europium, Technetium, and Uranium in Vadose Sediments; Pacific Northwest National Laboratory: Washington, DC, USA, 2002; Volume PNNL-14126, p. 95. [Google Scholar]
- Kim, Y.; Wolf, A.S.; Becker, U. Thermodynamic mixing properties of alunite supergroup minerals: Quantum-mechanical modeling and thermodynamic analysis of sulfate, chromate, selenate, phosphate, and arsenate solid solutions, as well as uranyl incorporation. Geochim. Cosmochim. Acta 2019, 248, 138–160. [Google Scholar] [CrossRef]
- Orris, G.J.; Grauch, R.I. Rare Earth Element Mines, Deposits, and Occurrences; Open-File Report 02-189; US Geological Survey: Reston, VA, USA, 2002; p. 167.
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Lixin, J.; Ma, L.; Dere, A.; White, T.; Mathur, R.; Brantley, S. REE mobility and fractionation during shale weathering along climate gradient. Chem. Geol. 2017, 466, 352–379. [Google Scholar]
- Mariano, A.N.; Mariano, A.N., Jr. Rare earth mining and exploration in North America. Elements 2012, 8, 369–376. [Google Scholar] [CrossRef]
- Emsbo, P.; McLaughin, P.I.; Breit, G.N.; du Bray, E.A.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Rare-earth element mineralization within the Mt. Weld carbonatite laterite, Western Australia. Lithos 1990, 24, 151–167. [Google Scholar] [CrossRef]
- Bao, Z.; Zhao, Z. Geochemistry of mineralization with exchangeable REY in the weathering crusts of granitic rocks in South China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar] [CrossRef]
- Torró, L.; Proenza, J.A.; Aiglsperger, T.; Bover-Arnal, T.; Villanova-de-Benavent, C.; Rodríguez-García, D.; Ramírez, A.; Rodríguez, J.; Mosquea, L.A.; Salas, R. Geological, geochemical and mineralogical characteristics of REE-bearing Las Mercedes bauxite deposit, Dominican Republic. Ore Geol. Rev. 2017, 89, 114–131. [Google Scholar] [CrossRef]
- Nechaev, V.P.; Chekryzhov, I.Y.; Vysotskiy, S.V.; Ignatiev, A.V.; Velivetskaya, T.A.; Tarasenko, I.A.; Agoshkov, A.I. Isotopic signatures of REY mineralization associated with lignite basins in South Primorye, Russian Far East. in: Special Issue on the Metallogeny of the Russian Far East. Ore Geol. Rev. 2018, 103, 68–77. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhang, Q.; Zhang, Y.; Li, Y. Genesis of REE minerals in the karstic bauxite in western Guangxi, China, and its constraints on the deposit formation conditions. Ore Geol. Rev. 2016, 75, 100–115. [Google Scholar] [CrossRef]
- Proenza, J.A.; Aiglsperger, T.; Villanova-de-Benavent, C.; Torró, L.; Rodríguez, D.; Ramírez, A.; Rodríguez, J. Discovery of REE minerals hosted in karst bauxite ores from the Sierra de Bahoruco, Pedernales, Dominican Republic. In Proceedings of the 14th SGA Biennial Meeting, Québec City, QC, Canada, 20–23 August 2017; Volume 4, p. 5. [Google Scholar]
- Giovannini, A.L.; Bastos, A.C.; Porto, C.G.; Pereira, V.P.; Takehara, L.; Barbanson, L.; Bastos, P.H.S. Mineralogy and geochemistry laterites from the Morro dos Seis Lagos Nb (Ti,REE) deposit (Amazonas, Brazil). Ore Geol. Rev. 2017, 88, 461–480. [Google Scholar] [CrossRef]
- Makeev, B.A.; Makeev, A.B. Rare Earth and Strontium Aluminophosphates from the Vol–Vym Ridge of the Middle Timan. Geol. Ore Depos. 2011, 53, 657–662. [Google Scholar] [CrossRef]
- Schlüter, J.; Malcherek, T.; Mihailova, B. Galloplumbogummite from Tsumeb, Namibia, a new member of the alunite group with tetravalent charge balance. N. Jahrb. Mineral. Abh. 2014, 191, 301–309. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Jenkins, R.A.; Downs, R.T.; Costin, G. Kolbeckite, ScPO4·2H2O, isomorphous with metavariscite. Acta Cryst. 2007, 63, 91–92. [Google Scholar] [CrossRef]
- Bayliss, P.; Kolitsch, U.; Nickel, E.H.; Pring, A. Alunite supergroup: Recommended nomenclature. Min. Mag. 2010, 74, 919–992. [Google Scholar] [CrossRef]
- Shields, G.A.; Stille, P. Diagenetic constraints on the use of cerium anomalies as palaeoseawater redox proxies: An isotopic and REE study of Cambrian phosphorites. Chem. Geol. 2001, 175, 29–48. [Google Scholar] [CrossRef]
- Shields, G.A.; Webb, G.E. Has the REE composition of seawater changed over geological time? Chem. Geol. 2004, 204, 103–107. [Google Scholar] [CrossRef]
- Slansky, M.; Lallemand, A.; Millot, G. La sédimentation et l’altération latéritique des formations phosphatées du gisement de Taïba (République du Sénégal). Bull. Serv. Géol. Alsace Lorraine 1965, 17, 311–324. [Google Scholar] [CrossRef]
- Zanin, Y.N. Zones of lateritic weathering of secondary phosphorites of Altay-Sayan region. Int. Geol. Rev. 1968, 10, 1119–1127. [Google Scholar] [CrossRef]
- Flicoteaux, R.; Nahon, D.; Paquet, H. Génèse des phosphates alumineux à partir des sédiments argilo-phosphatés du Tertiaire de Lam-Lam (Sénégal): Suite minéralogique. Permanences et changements de structures. Sci. Géol. Bull. 1977, 30, 153–174. [Google Scholar] [CrossRef]
- Flicoteaux, R. Genese des phosphates alumineux du Sengal occidental, etapes et guides de l’alteration. Sci. Geol. Mem. 1982, 67, 292–317. [Google Scholar]
- Nriagu, J.O.; Moore, P.B. Phosphate Minerals; Springer: Berlin, Germany, 1984; 442p. [Google Scholar]
- Zohar, E.; Moshkovitz, S. A Campanian-Maastrichtian unconformity in the Arad Basin, NE Negev, Israel. Geol. Sur. Israel Cur. Res. 1983, 1984, 56–59. [Google Scholar]
- Van Kauwenbergh, S.J.; Cathcart, J.B.; McClelian, G.H. Mineralogy and alteration of the phosphate deposits of Florida. US Geol. Surv. Bull. 1990, 1914, 45. [Google Scholar]
- Abed, A.M.; Kraishan, G.M. Evidence for shallow marine origin of a Monterey-Formation-Type chert phosphorite dolomite sequence: Amman Formation (Late Cretaceous) central Jordan. Facies 1991, 24, 25–38. [Google Scholar] [CrossRef]
- Trappe, J. Stratigraphy, facies distinction and paleogeography of the marine Paleogene from the western High Atlas, Morocco. Ne. Jah. Geol. Pal. Abh. 1991, 180, 279–321. [Google Scholar]
- Prian, J.P. Phosphate deposits of the Senegal-Mauritania-Guinea Basin (West Africa): A review. Procedia Eng. 2014, 83, 27–36. [Google Scholar] [CrossRef]

| Level | Variscite | Phosphosiderite | Crandallite | Jarosite | Baryte | Halloysite 10Å | Halloysite 7Å | Alunite | Smectite | Quartz | Pyrite | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altered shales | 1 | xxx | xx | m | m | |||||||
| 2 | x | xx | x | m | ||||||||
| 3 | xx | x | x | m | ||||||||
| 4 | x | xx | xx | x | m | m | ||||||
| 5 | x | xx | x | m | ||||||||
| 6 | m | x | x | x | m | m | ||||||
| 7 | x | x | m | |||||||||
| Supergene veins | 1 | x | x | x | m | xx | x | xxx | x | m | ||
| 2 | x | x | xx | m | x | xxx | x | m | ||||
| 4 | xxx | x | x | x | x | |||||||
| 6 | xx | x | m | x | xx | x | x | m | ||||
| 7 | xxx | xx | x | m |
| Analysis Number | Variscite | Phosphosiderite | Halloysite | Perhamite | Apatite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11S34.5.5 | 11S5.1 | 11S34.1 | 11S34.5.3 | 11S5.1 | 11S34.5.4 | 71.2 | 71.2 | 11S2.5L3 | 11N1.20 | 6.7 | 11S34.5.5 | 11S34.5.5 | 573.1 | |
| wt. % | ||||||||||||||
| SiO2 | 7.33 | 0.59 | 9.52 | 2.29 | 2.45 | 0.71 | 2.64 | 0.91 | 43.72 | 44.50 | 40.91 | 20.47 | 26.23 | 0.02 |
| Al2O3 | 30.24 | 30.90 | 25.83 | 28.52 | 29.88 | 2.32 | 2.82 | 2.48 | 32.59 | 32.21 | 28.32 | 21.64 | 22.69 | 0.05 |
| FeO | 0.59 | 1.51 | 1.90 | 1.82 | 1.47 | 32.22 | 32.77 | 33.72 | 2.18 | 1.32 | 2.27 | 0.77 | 0.77 | 0 |
| Fe2O3 calculated | 0.66 | 1.68 | 2.11 | 2.02 | 1.63 | 35.81 | 36.42 | 37.47 | 2.42 | 1.47 | 2.52 | 0.86 | 0.85 | 0 |
| MgO | 0 | 0 | 0.09 | 0.11 | 0.12 | 0.17 | 0.09 | 0 | 0.13 | 0.22 | 0.14 | 0.32 | 0.27 | 0.24 |
| CaO | 0.79 | 0.28 | 0.98 | 0.39 | 2.31 | 4.21 | 1.89 | 0.41 | 0.65 | 0.52 | 0.42 | 11.06 | 10.99 | 49.59 |
| K2O | 0.42 | 0.03 | 0.73 | 0.27 | 0.07 | 0.04 | 0.01 | 0 | 0.09 | 0.14 | 0.17 | 0.28 | 0.23 | 0.01 |
| P2O5 | 39.60 | 45.26 | 39.08 | 44.23 | 41.01 | 33.58 | 33.96 | 36.23 | 0.18 | 0.08 | 0.12 | 21.81 | 16.20 | 35.58 |
| SO3 | 0.03 | 0.06 | 0.05 | 0.14 | 0.16 | 0.05 | 0.07 | 0.04 | 0.01 | 0.03 | 0.03 | 0.06 | 0.07 | 0.19 |
| Sum | 79.22 | 78.85 | 78.57 | 78.00 | 77.78 | 73.47 | 74.36 | 73.86 | 79.91 | 79.68 | 72.67 | 76.65 | 77.58 | 86.21 |
| Si | 0.191 | 0.015 | 0.251 | 0.060 | 0.066 | 0.023 | 0.084 | 0.029 | 2.052 | 2.094 | 2.108 | 8.691 | 11.030 | 0.002 |
| Al | 0.926 | 0.946 | 0.801 | 0.885 | 0.943 | 0.090 | 0.106 | 0.093 | 1.803 | 1.786 | 1.720 | 10.829 | 11.245 | 0.005 |
| Fe3+ | 0.013 | 0.033 | 0.042 | 0.040 | 0.033 | 0.884 | 0.873 | 0.897 | 0.086 | 0.052 | 0.098 | 0.275 | 0.270 | 0 |
| Mg | 0 | 0 | 0.003 | 0.004 | 0.005 | 0.008 | 0.004 | 0 | 0.010 | 0.015 | 0.010 | 0.203 | 0.171 | 0.033 |
| Ca | 0.022 | 0.008 | 0.028 | 0.011 | 0.066 | 0.148 | 0.065 | 0.014 | 0.033 | 0.026 | 0.023 | 5.031 | 4.952 | 4.929 |
| K | 0.014 | 0.001 | 0.025 | 0.009 | 0.003 | 0.002 | 0 | 0 | 0.006 | 0.008 | 0.011 | 0.153 | 0.122 | 0.002 |
| P | 0.872 | 0.996 | 0.873 | 0.986 | 0.929 | 0.933 | 0.916 | 0.976 | 0.007 | 0.003 | 0.005 | 7.839 | 5.767 | 2.794 |
| S | 0.001 | 0.001 | 0.001 | 0.003 | 0.003 | 0.001 | 0.002 | 0.001 | 0 | 0.001 | 0.001 | 0.018 | 0.022 | 0.013 |
| Sum | 2.038 | 2.000 | 2.026 | 1.998 | 2.047 | 2.089 | 2.050 | 2.010 | 3.996 | 3.985 | 3.978 | 33.039 | 33.579 | 7.778 |
| Oxygens | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 7 | 7 | 7 | 59 | 59 | 12 |
| Sample | Quaternary Calcrete | Altered Shales | Supergene Phosphates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FGAV_TOR | 11NL1 | 11NL2 | 11NL3 | 11NL4 | 11NL5 | 11NL6 | 11NL7 | 0.1 | 3.9 | 4.0 | L4V | 6.1 | |
| wt. % | |||||||||||||
| SiO2 | 12.24 | 81.31 | 89.63 | 92.83 | 91.69 | 82.49 | 92.26 | 88.05 | 5.49 | 14.04 | 15.03 | 9.40 | 7.08 |
| TiO2 | 0.20 | 0.47 | 0.30 | 0.17 | 0.20 | 0.28 | 0.20 | 0.22 | 0.31 | 0.25 | 0.22 | 0.14 | 0.14 |
| Al2O3 | 2.98 | 8.61 | 4.76 | 3.27 | 4.72 | 7.23 | 4.07 | 5.27 | 3.26 | 20.28 | 22.37 | 24.61 | 23.74 |
| Fe2O3 | 5.71 | 1.74 | 0.72 | 0.72 | 0.37 | 4.41 | 1.01 | 0.91 | 32.85 | 8.70 | 5.22 | 6.91 | 8.61 |
| MnO | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| MgO | 0.59 | 0.92 | 0.46 | 0.26 | 0.44 | 0.59 | 0.32 | 0.45 | 0.21 | 0.18 | 0.16 | 0.10 | 0.09 |
| CaO | 38.7 | 0.43 | 0.12 | 0.08 | 0.09 | 0.13 | 0.05 | 0.40 | 3.54 | 1.77 | 2.11 | 0.85 | 0.57 |
| Na2O | 0.05 | 0.08 | 0.02 | 0.05 | 0.02 | 0.03 | 0.03 | 0.05 | 0.15 | 0.30 | 0.15 | 0.09 | 0.10 |
| K2O | 1.78 | 2.35 | 1.24 | 0.8 | 1.17 | 1.80 | 0.98 | 1.25 | 0.03 | 0.20 | 0.30 | 0.49 | 0.70 |
| P2O5 | 0.40 | 0.07 | 0.07 | 0.04 | 0.03 | 0.17 | 0.06 | 0.18 | 30.63 | 32.24 | 29.14 | 33.16 | 34.19 |
| LOI | 33.77 | 4.52 | 1.76 | 1.18 | 1.43 | 2.68 | 1.40 | 2.20 | 22.58 | 21.40 | 23.75 | 22.40 | 23.08 |
| Sum | 96.43 | 100.52 | 99.08 | 99.41 | 100.17 | 99.82 | 100.38 | 98.99 | 99.06 | 99.39 | 98.46 | 98.15 | 98.33 |
| ppm | |||||||||||||
| Sc | 9 | 11 | 6 | 3 | 6 | 8 | 5 | 6 | 49 | 219 | 113 | 208 | 203 |
| V | 76 | 286 | 269 | 275 | 236 | 718 | 423 | 384 | 288 | 300 | 336 | 312 | 363 |
| Cr | 120 | 60 | 50 | 30 | 30 | 70 | 40 | 50 | 790 | 340 | 280 | 770 | 720 |
| Co | 1 | 8 | <1 | 1 | <1 | 4 | 1 | 1 | <1 | 1 | 4 | 2 | 5 |
| Ni | <20 | 30 | <20 | <20 | <20 | 70 | <20 | <20 | < 20 | <20 | 80 | 50 | 40 |
| Cu | 40 | 50 | 10 | 20 | 10 | 150 | 30 | 40 | 230 | 40 | 340 | 90 | 100 |
| Zn | <30 | <30 | <30 | <30 | <30 | < 30 | <30 | 80 | <30 | <30 | 70 | <30 | <30 |
| Ga | 14 | 17 | 9 | 7 | 8 | 13 | 9 | 10 | 29 | 20 | 15 | 72 | 69 |
| Ge | <1 | 5 | 3 | 1 | 2 | 2 | 2 | 2 | <1 | <1 | 1 | 1 | <1 |
| As | 98 | 16 | 10 | <5 | <5 | 51 | <5 | 6 | 852 | 192 | 219 | 631 | 714 |
| Rb | 26 | 74 | 37 | 22 | 34 | 51 | 28 | 37 | <2 | 7 | 4 | 5 | 5 |
| Sr | 256 | 29 | 32 | 17 | 13 | 37 | 19 | 47 | 309 | 442 | 452 | 430 | 491 |
| Y | 11 | 37 | 28 | 11 | 10 | 33 | 16 | 19 | 22 | 13 | 3 | 19 | 20 |
| Zr | 33 | 85 | 49 | 27 | 27 | 46 | 30 | 37 | 25 | 4 | 5 | 5 | 5 |
| Nb | 3 | 9 | 6 | 4 | 4 | 5 | 4 | 5 | 3 | 1 | <1 | <1 | <1 |
| Mo | 10 | 6 | 8 | 4 | 2 | 10 | 4 | 7 | 16 | 19 | 18 | 15 | 17 |
| In | 0.8 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | 0.3 | 0.8 | 0.7 | 1.2 | 1.0 |
| Sb | 2.8 | 7.2 | 5.7 | 2.4 | 2.8 | 6.3 | 2.8 | 5.4 | 51.2 | 7.5 | 3.5 | 12.1 | 13.1 |
| Cs | 1.2 | 2.3 | 1.1 | 0.5 | 1.3 | 1.3 | 0.8 | 1.0 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Ba | 747 | 1494 | 990 | 711 | 961 | 1398 | 822 | 1136 | 842 | 5150 | 5877 | 1785 | 1434 |
| La | 25.5 | 52.1 | 43.6 | 15.0 | 11.9 | 31.2 | 17.3 | 22.2 | 2.6 | 18.7 | 5.2 | 32.0 | 36.9 |
| Ce | 41.7 | 69.3 | 65.6 | 27.1 | 21.7 | 55.6 | 28.6 | 41.8 | 1.7 | 28.9 | 5.5 | 76.0 | 87.7 |
| Pr | 5.9 | 8.7 | 9.3 | 3.4 | 2.6 | 7.1 | 3.8 | 5.4 | 0.4 | 4.3 | 0.6 | 17.2 | 21.4 |
| Nd | 29 | 31 | 34 | 14 | 10 | 27 | 15 | 23 | 1 | 14 | 2 | 77 | 96 |
| Sm | 5.0 | 4.4 | 5.8 | 2.5 | 1.7 | 6.6 | 3.4 | 5.7 | 0.4 | 2.6 | 0.5 | 15.0 | 16.9 |
| Eu | 0.8 | 0.9 | 1.0 | 0.4 | 0.4 | 1.3 | 0.8 | 1.3 | 0.1 | 0.6 | 0.1 | 2.9 | 3.6 |
| Gd | 2.8 | 3.2 | 4.0 | 1.8 | 1.5 | 4.6 | 2.9 | 5.0 | 0.9 | 2.4 | 0.6 | 8.9 | 10.0 |
| Tb | 0.4 | 0.6 | 0.6 | 0.3 | 0.2 | 0.7 | 0.4 | 0.6 | 0.2 | 0.3 | <0.1 | 1.0 | 1.0 |
| Dy | 2.0 | 4.0 | 3.9 | 1.7 | 1.3 | 4.5 | 2.6 | 3.3 | 2.1 | 1.9 | 0.5 | 4.0 | 4.5 |
| Ho | 0.4 | 0.9 | 0.8 | 0.3 | 0.3 | 1.0 | 0.5 | 0.6 | 0.5 | 0.4 | 0.1 | 0.6 | 0.7 |
| Er | 1.3 | 3.1 | 2.6 | 1.1 | 0.9 | 2.8 | 1.5 | 1.8 | 1.9 | 1.1 | 0.3 | 1.6 | 1.7 |
| Tm | 0.2 | 0.5 | 0.4 | 0.2 | 0.2 | 0.4 | 0.2 | 0.3 | 0.4 | 0.2 | <0.1 | 0.2 | 0.2 |
| Yb | 1.0 | 3.9 | 2.7 | 1.3 | 1.0 | 3.1 | 1.5 | 2.0 | 2.5 | 1.1 | 0.3 | 1.0 | 1.0 |
| Lu | 0.14 | 0.7 | 0.4 | 0.2 | 0.2 | 0.5 | 0.2 | 0.3 | 0.4 | 0.2 | 0.1 | 0.1 | 0.1 |
| Hf | 0.9 | 1.8 | 1.2 | 0.6 | 0.6 | 1.1 | 0.6 | 0.9 | 0.6 | 0.2 | 0.3 | <0.2 | 0.2 |
| Ta | 0.2 | 0.7 | 0.4 | 0.2 | 0.3 | 0.4 | 0.3 | 0.4 | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Pb | 15 | 34 | 31 | 15 | 27 | 42 | 17 | 58 | 60 | 22 | 20 | 110 | 81 |
| Th | 6 | 7.8 | 5.5 | 3.0 | 3.2 | 5.0 | 3.5 | 4.0 | 3.1 | 2.5 | 3.1 | 3.8 | 4.0 |
| U | 2.4 | 5.2 | 4.9 | 3.7 | 2.5 | 7.0 | 3.8 | 5.5 | 4.2 | 6.0 | 6.4 | 12.8 | 8.3 |
| ΣREE | 201.1 | 183.7 | 174.4 | 68.9 | 53.9 | 146.5 | 78.9 | 113.2 | 15.4 | 76.9 | 16.0 | 237.9 | 281.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Acha, Y.; Campeny, M.; Tauler, E.; Bosch, J.; Melgarejo, J.C.; Camprubí, A.; Villanova-de-Benavent, C.; Jorge-Villar, S.E.; Díaz-Ontiveros, I.; Fernández-Lluch, D.; et al. Critical Elements in Supergene Phosphates: The Example of the Weathering Profile at the Gavà Neolithic Mines, Catalonia, Spain. Minerals 2020, 10, 3. https://doi.org/10.3390/min10010003
Díaz-Acha Y, Campeny M, Tauler E, Bosch J, Melgarejo JC, Camprubí A, Villanova-de-Benavent C, Jorge-Villar SE, Díaz-Ontiveros I, Fernández-Lluch D, et al. Critical Elements in Supergene Phosphates: The Example of the Weathering Profile at the Gavà Neolithic Mines, Catalonia, Spain. Minerals. 2020; 10(1):3. https://doi.org/10.3390/min10010003
Chicago/Turabian StyleDíaz-Acha, Yael, Marc Campeny, Esperança Tauler, Josep Bosch, Joan Carles Melgarejo, Antoni Camprubí, Cristina Villanova-de-Benavent, Susana E. Jorge-Villar, Iria Díaz-Ontiveros, David Fernández-Lluch, and et al. 2020. "Critical Elements in Supergene Phosphates: The Example of the Weathering Profile at the Gavà Neolithic Mines, Catalonia, Spain" Minerals 10, no. 1: 3. https://doi.org/10.3390/min10010003
APA StyleDíaz-Acha, Y., Campeny, M., Tauler, E., Bosch, J., Melgarejo, J. C., Camprubí, A., Villanova-de-Benavent, C., Jorge-Villar, S. E., Díaz-Ontiveros, I., Fernández-Lluch, D., Proenza, J. A., Andreazini, A., & Pastor, M. (2020). Critical Elements in Supergene Phosphates: The Example of the Weathering Profile at the Gavà Neolithic Mines, Catalonia, Spain. Minerals, 10(1), 3. https://doi.org/10.3390/min10010003






