Abstract
Bifunctional chiral 2-aminobenzimidazole derivatives 1 and 2 catalyze the enantioselective stereodivergent α-chlorination of β-ketoesters and 1,3-diketone derivatives with up to 50% ee using N-chlorosuccinimide (NCS) or 2,3,4,4,5,6-hexachloro-2,5-cyclohexadien-1-one as electrophilic chlorine sources.
1. Introduction
Currently, about 85 percent of all pharmaceuticals (prescription and over-the-counter) contain or are manufactured using chlorine. Some of the top-selling pharmaceutically active compounds contain chlorine, since chlorinated analogues of drugs have been often shown to provide metabolic stability as compared with their parent compounds without loss in substrate binding affinity [1]. Chlorinated drugs are used to treat many illnesses and diseases, including hypertension, allergy (Chlorphenamine), anxiety (Alprazolam), Alzheimer’s disease, cancer, and bacterial infections (Cefaclor, Loracarbef). From a synthetic point of view, optically active bi-functionalized compounds such as α-chlorinated carbonyls are especially attractive due to their high value as linchpin derivatives [2,3,4]. Due to the potential of these compounds, their catalytic asymmetric synthesis has been intensively investigated in the past few years [5,6,7]. Despite the breakthrough asymmetric organocatalysis has experienced during the last fifteen years, not only from a synthetic point of view [8], but also as a strategy for the preparation of natural-occurring and pharmaceutically active compounds [9,10,11], only a few privileged catalysts have been shown to be highly selective in the electrophilic α-chlorination of carbonyl compounds. In 2004, MacMillan [12], Lectka [13], and Jørgensen [14] reported the first catalytic highly enantioselective α-chlorination of aldehydes, acid chlorides, and ketones, respectively, using perchloroquinones and N-chlorosuccinimide (NCS) as chlorination reagents. After these preliminary works, the enantioselective α-chlorination of ketones [15] and aldehydes [16,17,18,19,20] has remained under study, other derivatives such as 1,3-dicarbonyl compounds [21,22,23,24,25,26,27], oxindoles [28,29], and silyl ketene acetals [30], being recently added to the nucleophile pool of this transformation. Regarding 1,3-dicarbonyl compounds, the best enantioselectivities have been obtained for cyclic β-ketoesters, which have been chlorinated in the α-position employing chiral alkaloid derivatives [21,25,27], chiral N,N′-dioxides [22], chiral amino diol derivatives [23], and chiral phase transfer catalysts [24,26].
As part of our program aimed at developing broadly useful organic catalysts for enantioselective transformations, we have recently shown that chiral 2-aminobenzimidazole derivatives 1 and 2 are very active and selective organocatalysts for the asymmetric functionalization of 1,3-dicarbonyl compounds (Figure 1). Through a bifunctional Brønsted base/hydrogen bonding activation, these catalysts have afforded excellent enantioselectivities in the conjugate addition of malonates, 1,3-diketones, and β-ketoesters to nitroolefins [31] and maleimides [32,33]. Also, catalyst 1 has shown enantioselectivities of up to 92% in the α-amination of cyclic β-ketoesters with azodicarboxylates [34]. Based on the good selectivities obtained so far with our 2-aminobenzimidazole-derived catalysts, in this work we describe an enantioselective procedure for the α-chlorination of cyclic 1,3-dicarbonyl compounds (Figure 1).
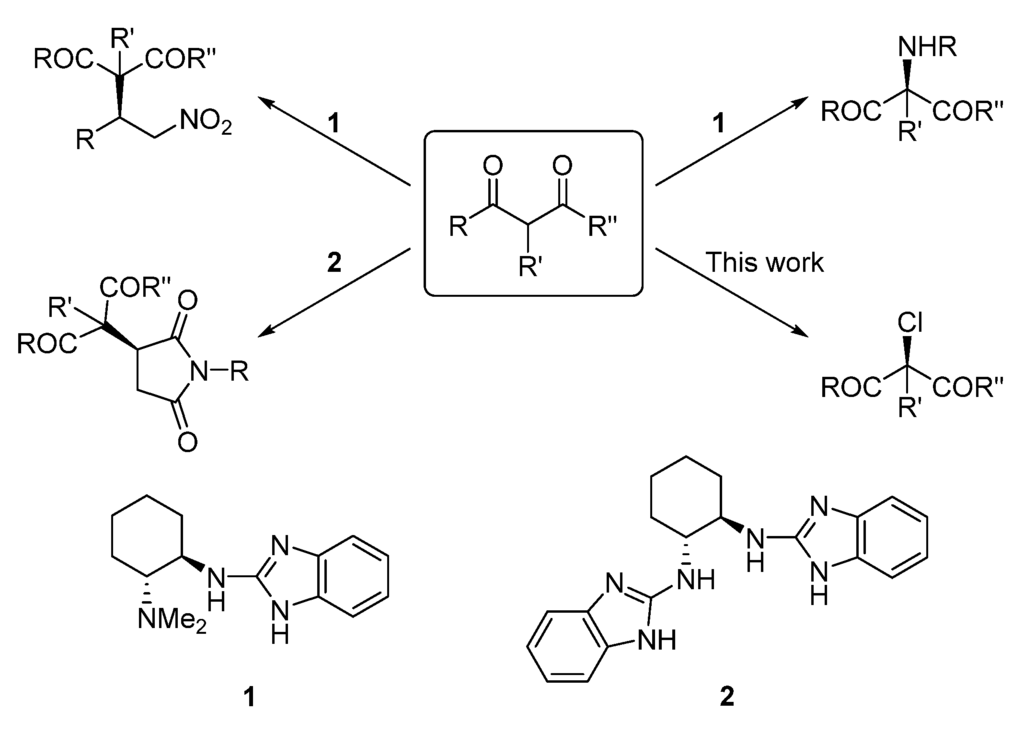
Figure 1.
Chiral 2-aminobenzimidazole-catalyzed reactions.
2. Results and Discussion
To identify the best organocatalyst (0.01 mmol, 10 mol%) and reaction conditions, the chlorination of ethyl 2-oxocyclopentanecarboxylate (0.125 mmol) in toluene with N-chlorosuccinimide (NCS, 0.1 mmol) was chosen as the model reaction (Table 1). Besides chiral benzimidazoles 1 and 2, we also studied catalyst 3, easily prepared from 2-chlorobenzimidazole and (R)-1-phenylethan-1-amine. Catalyst 1 afforded the desired compound 5a in excellent conversion but nearly no enantioselectivity when working at room temperature (Table 1, entry 1). Examination of the effect of temperature revealed that lowering of the temperature to −50 °C significantly improved the enantioselectivity, being 5a obtained with a promising 95% isolated yield and a 40% ee when C2-symmetric chiral benzimidazole 2 was used as catalyst (entry 3). No further improvement was detected at lower temperatures (−78 °C) (Table 1, entry 4).

Table 1.
Chlorination of ethyl 2-oxocyclopentanecarboxylate with NCS. Reaction conditions study. 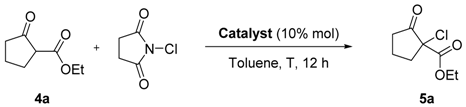

| Entry | Catalyst | T (°C) | Conv. (%) a | ee (%) a |
|---|---|---|---|---|
| 1 | 1 | 20 | 99 | 5 |
| 2 | 1 | −50 | 99 | 9 |
| 3 | 2 | −50 | 99 (95) b | 40 |
| 4 | 2 | −78 | 99 | 40 |
| 5 | 3 | −50 | 99 | 12 |
a Reaction conversion and ee determined by chiral GC (CP-Chirasil-Dex CB); b Isolated yield after flash chromatography. 

We continued our studies of the α-chlorination reaction by screening a number of known chlorinating agents for the functionalization of 4a using catalysts 1 and 2. Representative results are summarized in Table 2. With the aim of studying a plausible favorable catalyst/electrophile interaction through π-π stacking, 4a was subjected to chlorination with N-chlorophthalimide. However, this electrophile gave very similar results to those obtained with NCS, affording, as the best result, 5a in a 99% conversion and a 40% ee when using catalyst 2 (Table 2, entry 2). Polychloroquinone-derivatives are some of the most frequently used chlorinating reagents [9,13,21]. As depicted in entry 3, when using 2,3,4,5,6,6-hexachlorocyclohexa-2,4-dien-1-one as electrophile, chiral benzimidazole 1 afforded compound 5a with a 30% ee. This result seems to corroborate the expected lower enantioselectivity of this chlorinated agent with the more reactive carbanions derived from 1,3-dicarbonyl compounds [35]. Although full conversion was also obtained using catalyst 2 (Table 2, entry 4), the selectivity of the process was much lower (10% ee). The rest of the tested chlorinating reagents 1,3-dichloro-5,5-dimethylimidazolidine-2,4-dione, 1,3,5-trichloro-1,3,5-triazinane-2,4,6-trione, and chloramine-T (Table 2, entries 5–10), afforded ethyl 1-chloro-2-oxocyclopentanecarboxylate (5a) with very low enantioselectivities regardless of the chiral catalyst employed.

Table 2.
Chlorination of ethyl 2-oxocyclopentanecarboxylate with NCS. Reaction conditions study. 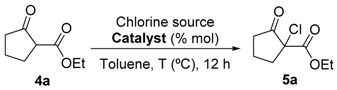

| Entry | Catalyst (mol%) | Cocatalyst (mol%) | Chlorine Source | T (°C) | Conv. (%) a | ee (%) a |
|---|---|---|---|---|---|---|
| 1 | 1 (10) | – | 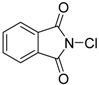 | −50 | 99 | 7 |
| 2 | 2 (10) | – | −50 | 99 | 40 | |
| 3 | 1 (10) | – | 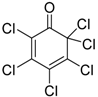 | −50 | 99 | 31 |
| 4 | 2 (10) | – | −50 | 99 | 10 | |
| 5 | 1 (10) | – | 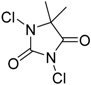 | −50 | 99 | 8 |
| 6 | 2 (10) | – | −50 | 99 | 14 | |
| 7 | 1 (10) | – | 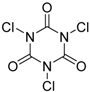 | −50 | 99 | 8 |
| 8 | 2 (10) | – | −50 | 99 | 1 | |
| 9 | 1 (10) | – | 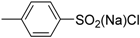 | −50 | <5 | – |
| 10 | 2 (10) | – | −50 | <5 | – | |
| 11 | 1 (10) | – | 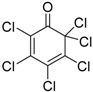 | −78 | 99 | 31 |
| 12 | 1 (5) | – | −50 | 99 | 27 | |
| 13 | 1 (20) | – | −50 | 99 | 31 | |
| 14 | 2 (20) | – | 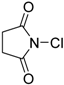 | −50 | 99 | 47 |
| 15 | 2 (20) | TFA (20) | −50 | 99 | 5 | |
| 16 | 2 (20) | NaHCO3 (100) | −50 | 99 | 28 | |
| 17 | 2 (10) | TEA (10) | −50 | 99 | 2 |
a Reaction conversion and ee determined by chiral GC (CP-Chirasil-Dex CB).
The results obtained so far demonstrate that the studied catalytic chlorination reaction is quite sensitive to the catalyst/electrophile combination, obtaining the best results when combining catalyst 1 with 2,3,4,5,6,6-hexachlorocyclohexa-2,4-dien-1-one and catalyst 2 with NCS or N-chlorophthalimide. With these two catalytic systems in our hands, we continued the optimization of the reaction conditions which led to the best enantioselectivity (47% ee) using a 20 mol% of catalyst 2 at −50 °C and using NCS as chlorinating reagent (entry 14). Unfortunately, this result could not be improved any further by using other different solvents, such as methylene chloride, hexane, diethyl ether, and methanol, acid additives such as TFA (Table 2, entry 15), or other basic additives such as, NaHCO3 and TEA which have been previously demonstrated to accelerate the turnover of the catalyst [21].
After the exhaustive search for the optimal reaction conditions, and in view of the results, two procedures for the asymmetric chlorination of 1,3-dicarbonyl compounds were established, namely methods A and B [36]. The first one (Method A) implies the use of 10 mol% of catalyst 1 and 2,3,4,5,6,6-hexachlorocyclohexa-2,4-dienone as an electrophilic chlorine source, while Method B involves a 10 mol% of catalyst 2 in combination with NCS as a chlorinating agent [37]. In both methods the reaction was performed in toluene at −50 °C. Next, with these optimal conditions in place, the scope of the reaction was evaluated (Table 3).
As previously described, ketoester 4a was effectively chlorinated under both methods although with moderate enantioselectivities (Table 3, entries 1 and 2). The influence of a bulkier substituent in the ester moiety was next evaluated. Thus, the tert-butyl derivative 4b was submitted to the optimized reaction conditions achieving high yields in both cases although with an opposite behavior in the enantioselectivity. Thus, whereas the use of this substrate produced a drop in the ee compared to the less sterically crowded analogue 4a when Method A conditions were applied, a small enhancement of the optical purity was observed for the case of Method B (Table 3, entries 3 and 4). By the contrary, six-membered ketoester 4c only rendered the corresponding chlorinated product 5c with some enantioselectivity (39% ee) when Method A conditions were employed (Table 3, entries 5 and 6). Benzocondensed β-ketoesters were next taken into account. When compound 4d was submitted to chlorination, high yields and poor to moderate enantioselectivities were obtained in both cases, being slightly better the results obtained under Method A conditions (Table 3, entries 7 and 8). Next, methyl ester 4e was tested obtaining high yield and moderate enantioselectivity (40% ee) using Method A (Table 3, entry 9). A parallel behavior to that observed with the non-benzocondensed analogue was obtained with Method B, namely excellent yield and poor enantioselectivity (Table 3, entry 10). The replacement of methyl by an ethyl group in the ester moiety produced an enhancement in the enantioselectivity in both methods (Table 3, entries 11 and 12), reaching up to 50% ee when Method A was used. Next, the more reactive 1,3-diketones were essayed. As somewhat expected due to the high reactivity of this type of nucleophiles, almost no enantioselection was observed when compounds 4g and 4h were tested regardless of the method employed (Table 3, entries 13–16). Obtaining the best enantioselectivities when ketoester 4f was employed as a nucleophile led us to test 2-acetyl-1-tetralone 4i as a substrate. Unfortunately, only poor enantioselectivities at best were also obtained (Table 3, entries 17 and 18).

Table 3.
Chlorination of 1,3-dicarbonyl compounds. Substrate scope. a 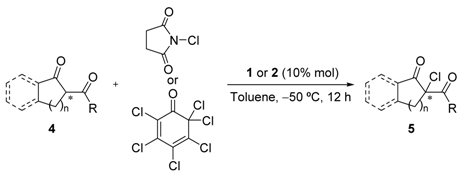

| Entry | Nucleophile | No. | Method | Product | |||
|---|---|---|---|---|---|---|---|
| Structure | No. | Yield (%) b | ee (%) c | ||||
| 1 | 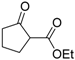 | 4a | A | 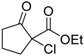 | 5a | 99 | 31 |
| 2 | 4a | B | 5a | 99 | 40 | ||
| 3 | 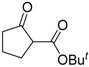 | 4b | A | 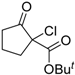 | 5b | 97 | 21 |
| 4 | 4b | B | 5b | 95 | 47 | ||
| 5 | 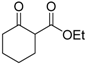 | 4c | A | 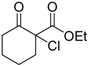 | 5c | 93 | 39 |
| 6 | 4c | B | 5c | 92 | <5 | ||
| 7 | 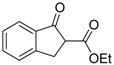 | 4d | A | 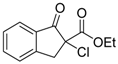 | 5d | 98 | 39 |
| 8 | 4d | B | 5d | 96 | 21 | ||
| 9 | 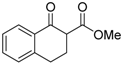 | 4e | A | 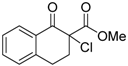 | 5e | 98 | 40 |
| 10 | 4e | B | 5e | 98 | 15 | ||
| 11 | 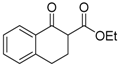 | 4f | A | 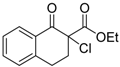 | 5f | 97 | 50 |
| 12 | 4f | B | 5f | 99 | 34 | ||
| 13 | 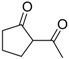 | 4g | A | 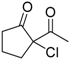 | 5g | 99 | <5 |
| 14 | 4g | B | 5g | 99 | 5 | ||
| 15 |  | 4h | A | 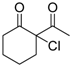 | 5h | 98 | 10 |
| 16 | 4h | B | 5h | 98 | 5 | ||
| 17 | 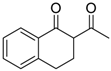 | 4i | A |  | 5i | 96 | 14 |
| 18 | 4i | B | 5i | 94 | 8 | ||
a Unless otherwise stated, reactions conditions were: 4 (0.18 mmol, 1.25 equiv.), NCS (Method A) or 2,3,4,5,6,6-hexachlorocyclohexa-2,4-dienone (Method B) (0.15 mmol), 10 mol% catalyst 1 (Method A) or 2 (Method B) in PhMe (1.5 mL) at −50 °C; b Isolated yields after flash chromatography; c Determined by GC or HPLC analysis on chiral column (see experimental section for details).
Remarkably, from experimental evidence it was observed that the opposite configuration in the chlorinated product was obtained when using method A and B, despite both catalysts 1 and 2 being derived from the same (1R,2R)-cyclohexane-1,2-diamine. Thus, Method A rendered the (S)-configured product, whereas Method B gave rise to the chlorinated (R)-product. However, this switch in the final configuration does not seem to be a consequence of the catalysts configuration but of the nature of the chlorinating agent which would produce a different arrangement of the rather participating species in the transition state. This assumption was based on the fact that the reaction using catalyst 1 with NCS and 2,3,4,5,6,6-hexachlorocyclohexa-2,4-dienone as chlorine source rendered the corresponding product 5a with opposite configurations. The same stereodivergent [38,39,40,41,42] behavior was observed with catalyst 2.
Finally, and based on previous studies from our group on which chiral 2-aminobenzimidazole-derived organocatalysts were employed in different reactions, tentative mechanisms can be proposed for methods A and B (Figure 2 and Figure 3). In both cases the organocatalysts have a bifunctional role. Firstly, the benzimidazole derivatives would act as a Brønsted base deprotonating the 1,3-dicarbonyl compound generating the corresponding enolate which could be coordinated through a dual hydrogen bond as shown in intermediates I and III. Next, the chlorinating agent would be activated by hydrogen bond either with the ammonium moiety (II, Figure 2) or with the second benzimidazol moiety (IV, Figure 3) favoring a tight transition state which would render the final product, regenerating the catalyst. On the other hand, an alternative and previously suggested [35,43,44] initial N-chlorination of the catalysts and subsequent chlorine inner transfer to the coordinated dicarbonyl compound seems to be discarded, since no chlorine incorporation to the organocatalysts was detected by ESI-MS after mixing them with the chlorinating reagents.
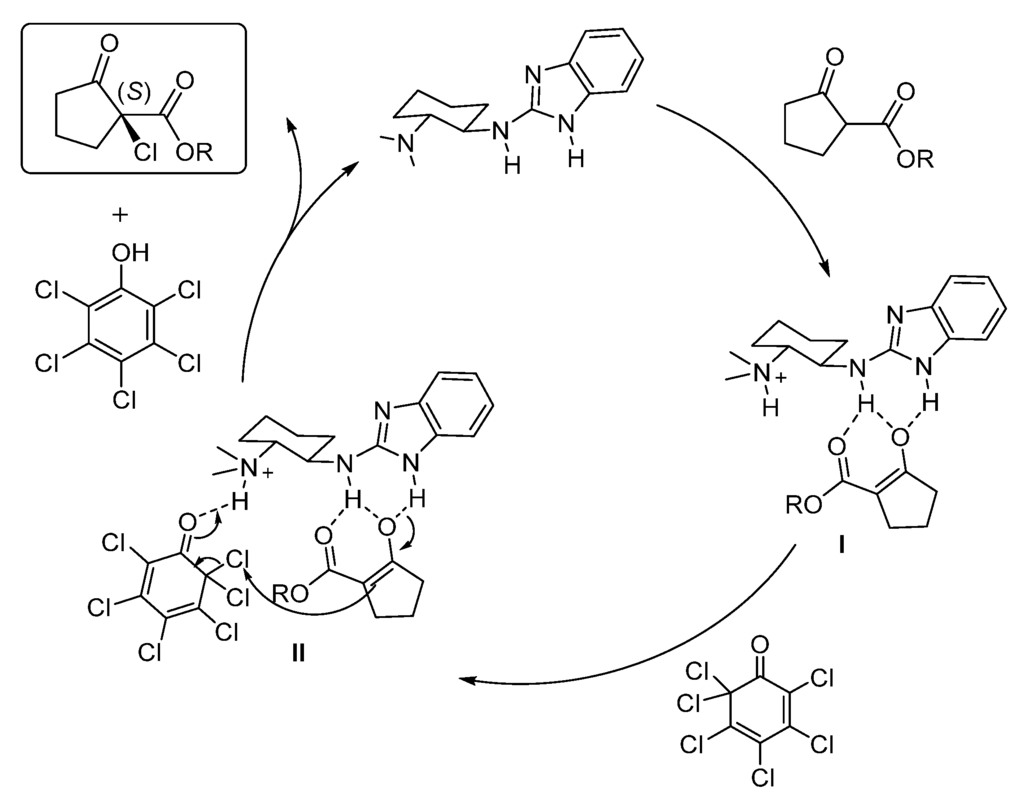
Figure 2.
Plausible mechanism for the asymmetric chlorination using Method A.
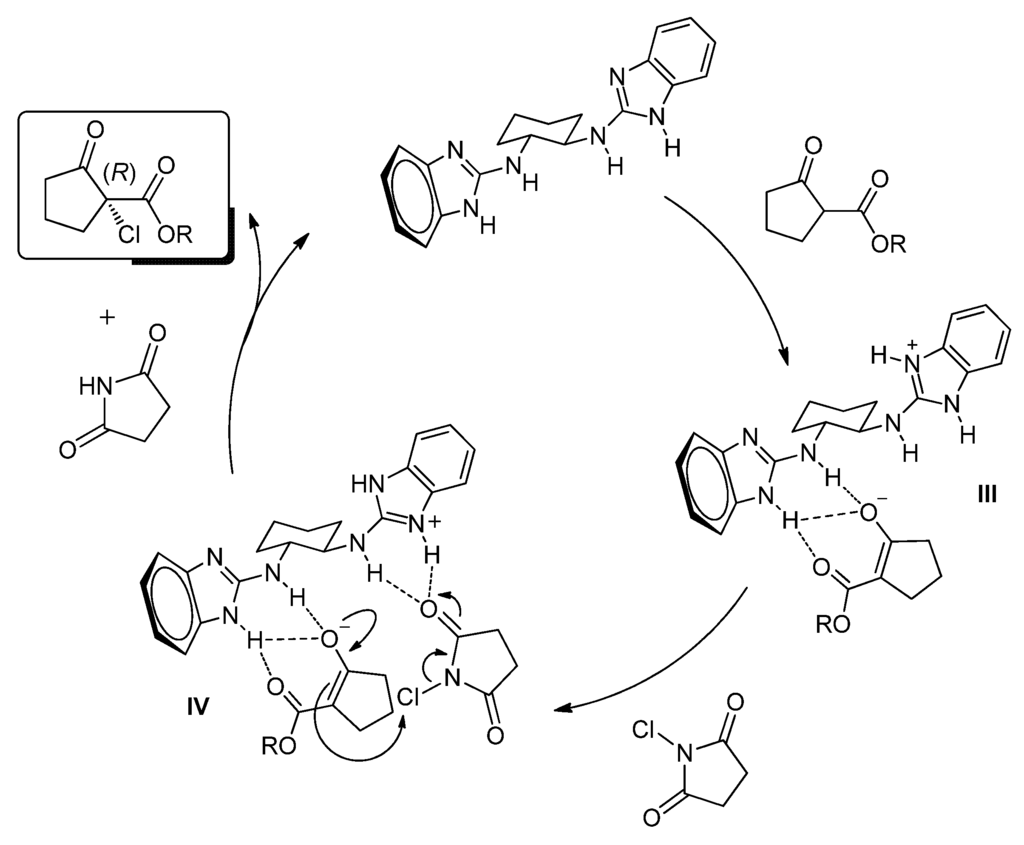
Figure 3.
Plausible mechanism for the asymmetric chlorination using Method B.
3. Experimental Section
3.1. General Remarks
All reagents were purchased from commercial sources and used without further purification. Substrates which were not commercially available were synthesized according to known procedures from the literature. Catalysts 1 and 2 were synthesized as described in the literature and the spectroscopical data fully agreed with the reported values [31,32,33,34]. Conversions were measured by GC chromatography employing a HP-6890 equipped with a WCOT HP-5 silica column (30 m × 0.25 mm × 0.25 µm) with 5% PHME siloxane as stationary phase. IR spectra were recorded on a Jasco FT-IR 4100 LE (Pike Miracle ATR) (Jasco Analitica Spain S.L., Madrid, Spain) and only the structurally most relevant peaks are listed. NMR spectra were performed on a Bruker AC-300 or Bruker Avance-400 (Bruker Corporation, Billerica, MA, USA) using CDCl3 as solvent and TMS as internal standard unless otherwise stated. Low-resolution electron impact (EI) mass spectra were obtained at 70 eV on Agilent GC/MS-5973N apparatus equipped with a HP-5MS column (Agilent technologies, 30 m × 0.25 mm). Optical rotations were measured on a Jasco P-1030 Polarimeter (Jasco Analitica Spain S.L., Madrid, Spain) with a 5 cm cell (c given in g/100 mL). Enantioselectivities were determined by HPLC analysis (Agilent 1100 Series HPLC) (Agilent Technologies, Santa Clara, CA, USA) equipped with a G1315B diode array detector and a Quat Pump G1311A equipped with the corresponding Daicel chiral column or by Chiral GC employing an Agilent GC Series 7820A chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with Chirasil-Dex CB (25 m × 0.25 mm × 0.25 µm) or Cyclosil-B (30 m × 0.25 mm × 0.25 µm) columns. The retention time of the major enantiomer is highlighted in bold. Analytical TLC was performed on Merck silica gel plates (Merck Millipore, Darmstadt, Germany) and the spots visualized with UV light at 254 nm. Flash chromatography was performed using Merck silica gel 60 (0.040–0.063 mm) using hexanes and ethyl acetate as eluents.
3.2. General Procedure for the Asymmetric Chlorination of 1,3-Dicarbonyl Compounds
Catalysts 1 or 2 and 1.5 mL of toluene were added to an open air round bottom tube (15 µmol, 10 mol%). The solution was stirred for 5 min in a thermostatized bath at −50 °C and then the corresponding 1,3-dicarbonyl compound (0.18 mmol, 1.25 equiv.) was added. After stirring for 5 additional minutes, the chlorinating agent was added in a single portion (0.15 mmol). The mixture was allowed to react for 12 h. After this time water (5 mL) and ethyl acetate (5 mL) were added and the organic phase was separated. The aqueous layer was re-extracted twice with ethyl acetate (2 × 5 mL). The organic phases were dried (MgSO4), filtered and evaporated under vacuum. The crude compound was then purified by flash chromatography using hexanes and ethyl acetate mixtures.
In the case of using co-catalysts, those were added together with the catalysts.
The analytical data shown below corresponds to those enantioenriched products as representative compounds. All the compounds are described in the literature. Therefore, only 1H NMR, MS (EI) and enantiomeric excess determination conditions are listed.
(R)-Ethyl 1-chloro-2-oxociclopentanecarboxilate (4a) [21]
Light yellow oil; = +12.1 (c = 0.5, CHCl3, 40% ee); 1H-NMR (300 MHz, CDCl3) δH = 1.32 (t, 3H, J = 7.1 Hz), 2.05–2.25 (m, 2H), 2.35–2.48 (m, 2H), 2.50–2.65 (m, 1H), 2.7–2.8 (m, 1H), 4.3 (q, 2H, J = 7.1 Hz); MS (EI) m/z 162 (M+, 61%), 145 (12), 134 (25), 127 (32), 117 (44), 109 (46), 107 (100), 99 (46), 89 (35); Chiral CG, CP-Chirasil-DEX, isotherm 90 °C, tR: (S) = 41.5 min, (R) = 46 min.
(S)-tert-Butyl 1-chloro-2-oxociclopentanecarboxilate (4b) [45]
Pale orange oil; = +4.7 (c = 0.3, CHCl3, 21% ee); 1H-NMR (300 MHz, CDCl3) δH = 1.49 (s, 9H), 2.20–2.08 (m, 2H), 2.43–2.29 (m, 2H), 2.60–2.47 (m, 1H), 2.76–2.67 (m, 1H). MS (EI) m/z 218 (M+, 5%), 203 (9), 162 (53), 145 (38), 134 (59), 89 (27), 57 (100); Chiral CG, Cyclosil-B, 50–70 °C (1 °C/min)/70 °C (15 min)/70–80 °C (0.5 °C/min)/80 (65 min), tR: (S) = 121 min, (R) = 126 min.
(S)-Ethyl 1-chloro-2-oxociclohexanecarboxilate (4c) [21]
Pale yellow oil; = −14.4 (c = 0.5, CHCl3, 39% ee); 1H-NMR (300 MHz, CDCl3) δH = 1.33–1.28 (t, 3H, J = 7.14 Hz), 1.60–1.78 (m, 1H), 1.81–2.00 (m, 3H), 2.09–2.21 (m, 1H), 2.40–2.50 (m, 1H), 2.75–2.90 (m, 2H), 4.25–4.32 (q, 2H, J = 7.2 Hz). MS (EI) m/z 204 (M+, 11%), 176 (7), 169 (13), 141 (100), 131 (16), 122 (33), 113 (58), 107 (27), 95 (33); Chiral CG, Cyclosil-B, isotherm 90 °C, tR: (S) = 154 min, (R) = 159 min.
(R)-Ethyl 2-chloro-1-oxo-2,3-dihydro-1H-indene-2-carboxylate (4d) [22]
Orange oil; = −8.6 (c = 1, CHCl3, 20% ee); 1H-NMR (300 MHz, CDCl3) δH = 1.26 (t, 3H, J = 7.1 Hz), 3.52 (d, 1H, J = 18.0 Hz), 4.1 (d, 1H, J = 18.0 Hz), 4.25 (c, 2H, J = 7.1 Hz), 7.30–7.80 (m, 4H); MS (EI) m/z 203 (M+, 70%), 175 (17), 165 (19), 157 (100), 147 (20), 131 (41), 130 (24), 102 (25), 77 (9); Chiral HPLC, Chiralpak OJ (Chiral, Henrietta, New York, NY, USA), f = 1 mL/min, 96:4 (hexane/2-propanol), tR: (S) = 19.5 min, (R) = 27.6 min.
(S)-Methyl 2-chloro-1-oxo-1,2,3,4-tetrahydronaphthalene-2-carboxylate (4e) [22]
Yellow sticky oil; = +7.2 (c = 1.3, CHCl3, 40% ee); 1H-NMR (300 MHz, CDCl3) δH = 2.52–2.57 (m, 1H), 2.99–3.05 (m, 2H), 3.24–3.31 (m, 1H), 3.86 (s, 3H), 7.29 (m, 1H), 7.36 (t, 1H, J = 7.6 Hz), 7.55 (td, 1H, J = 7.5, 1.5 Hz), 8.09 (dd, 1H, J = 7.9, 1.2 Hz); MS (EI) m/z 203 (M+,40%), 171 (36), 144 (16), 118 (100), 115 (53), 90 (75). Chiral HPLC, Chiralpak OD-H, f = 1 mL/min, 95:5 (hexane/2-propanol), tR: (R) = 8.9 min, (S) = 11.3 min.
(S)-Ethyl 2-chloro-1-oxo-1,2,3,4-tetrahydronaphthalene-2-carboxylate (4f) [22]
Orange sticky oil; = +14.2 (c = 0.8, CHCl3, 50% ee); 1H-NMR (300 MHz, CDCl3) δH = 1.27–1.31 (t, 3H, J = 7.1 Hz), 2.50–2.55 (m, 1H), 2.98–3.05 (m, 2H), 3.25–3.30 (m, 1H), 4.30–4.32 (q, 2H, J = 7.1 Hz), 7.40–7.55 (m, 3H), 8.08–8.10 (d, 1H, J = 7.9 Hz); MS (EI) m/z 252 (M+, 5%), 218 (23), 218 (36), 171 (41), 144 (28), 118 (100), 115 (53), 90 (42); Chiral HPLC, Chiralpak OD-H, f = 0.8 mL/min, 95:5 (hexane/2-propanol), tR: (R) = 9.7 min, (S) = 11.8 min.
4. Conclusions
In conclusion, in this work the asymmetric chlorination of cyclic 1,3-dicarbonyl compounds has been disclosed using benzimidazole-derived organocatalysts. In this sense, catalysts 1 and 2 have been proven to be very efficient for such a purpose, rendering the corresponding chlorinated compounds in high yields and producing enantioselectivites ranging from poor to moderate. The organocatalysts seems to play a bifunctional role in activating both the dicarbonyl compound and the chlorinating agent. Interestingly, this process turned out to be stereodiveregent, since the opposite configuration can be obtained in the final product using the same catalyst and simply varying the electrophilic chlorine source.
Acknowledgments
Financial support from the University of Alicante (VIGROB-173, VIGROB-285, GRE12-03, UAUSTI13-01, UAUSTI13-02), and Spanish Ministerio de Economía y Competitividad (CTQ2011-24151) is acknowledged.
Author Contributions
Daniel Serrano Sánchez performed the synthetic works. Alejandro Baeza and Diego A. Alonso designed the experiments of the project and supervised the whole studies reported in the manuscript.; Daniel Serrano Sánchez, Alejandro Baeza and Diego A. Alonso wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Thomas, G. Medicinal Chemistry: An Introduction; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- De Kimpe, N.; Verhé, R. The Chemistry of α-Haloketones, α-Haloaldehydes, and α-Haloimines; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Cornil, J.; Guérinot, A.; Cossy, J. Linchpin dienes: Key building-blocks in the synthesis of polyenic frameworks. Org. Biomol. Chem. 2015, 13, 4129–4142. [Google Scholar] [CrossRef] [PubMed]
- St. Denis, J.D.; He, Z.; Yudin, A.K. Amphoteric α-boryl aldehyde linchpins in the synthesis of heterocycles. ACS Catal. 2015, 5, 5373–5379. [Google Scholar] [CrossRef]
- Marigo, M.; Jørgensen, K.A. Organocatalytic direct asymmetric α-heteroatom functionalization of aldehydes and ketones. Chem. Commun. 2006, 2015, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Kano, T.; Maruoka, K. Organocatalyzed direct asymmetric α-halogenation of carbonyl compounds. Org. Biomol. Chem. 2009, 7, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Shibatomi, K.; Narayama, A. Catalytic enantioselective α-chlorination of carbonyl compounds. Asian J. Org. Chem. 2013, 2, 812–823. [Google Scholar] [CrossRef]
- Dalko, P.I. Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- De Figueiredo, R.M.; Christmann, M. Organocatalytic Synthesis of Drugs and Bioactive Natural Products. Eur. J. Org. Chem. 2007, 2007, 2575–2600. [Google Scholar] [CrossRef]
- Marqués-López, E.; Herrera, R.P.; Christmann, M. Asymmetric organocatalysis in total synthesis—A trial by fire. Nat. Prod. Rep. 2010, 27, 1138–1167. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.; Cabrera, S. Applications of asymmetric organocatalysis in medicinal chemistry. Chem. Soc. Rev. 2013, 42, 774–793. [Google Scholar] [CrossRef] [PubMed]
- Brochu, M.P.; Brown, S.P.; MacMillan, D.W.C. Direct and enantioselective organocatalytic α-chlorination of aldehydes. J. Am. Chem. Soc. 2004, 126, 4108–4109. [Google Scholar] [CrossRef] [PubMed]
- France, S.; Wack, H.; Taggi, A.E.; Hafez, A.M.; Wagerle, T.R.; Shah, M.H.; Dusich, C.L.; Lectka, T. Catalytic, Asymmetric α-chlorination of acid halides. J. Am. Chem. Soc. 2004, 126, 4245–4255. [Google Scholar] [CrossRef] [PubMed]
- Marigo, M.; Bachmann, S.; Halland, N.; Braunton, A.; Jørgensen, K.A. Highly enantioselective direct organocatalytic α-chlorination of ketones. Angew. Chem. Int. Ed. 2004, 43, 5507–5510. [Google Scholar] [CrossRef] [PubMed]
- Bergeron-Briek, M.; Teoh, T.; Britton, R. A tandem organocatalytic α-chlorination-aldol reaction thet proceeds with dynamic kinetic resolution: A powerful tool for carbohydrate synthesis. Org. Lett. 2013, 15, 3554–3557. [Google Scholar] [CrossRef] [PubMed]
- Halland, N.; Braunton, A.; Bachmann, S.; Marigo, M.; Jørgensen, K.A. Direct Organocatalytic Asymmetric α-Chlorination of Aldehydes. J. Am. Chem. Soc. 2004, 126, 4790–4791. [Google Scholar] [CrossRef] [PubMed]
- Fadeyi, O.O.; Schulte, M.L.; Lindsley, C.W. General Access to Chiral N-Alkyl Terminal Aziridines via Organocatalysis. Org. Lett. 2010, 12, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, C.; Curran, D.P.; Zhang, W. Enantioselective α-Chlorination of Aldehydes with Recyclable Fluorous (S)-Pyrrolidine-Thiourea Bifunctional Organocatalyst. Synlett 2010, 3, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.; Swatschek, J.; Willout, M.; Radtke, L.; Olbrisch, T.; Schäfer, A.; Christmann, M. Transforming Terpene-derived Aldehydes into 1,2-Epoxides via Asymmetric α-chlorination: Subsequent epoxide opening with carbon nucleophiles. Chem. Commun. 2011, 47, 12200–12202. [Google Scholar] [CrossRef] [PubMed]
- Johannes, M.; Brimble, M.A. Synthesis of an Azido Precursor to (2S,5R)-5-Hydroxylysine Using an Asymmetric Organocatalytic Chlorination/Reduction Sequence. J. Org. Chem. 2013, 78, 12809–12813. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, G.; Bosco, M.; Carlone, A.; Locatelli, M.; Melchiorre, P.; Sambri, L. Organocatalytic Asymmetric α-Halogenation of 1,3-Dicarbonyl Compounds. Angew. Chem. Int. Ed. 2005, 44, 6219–6222. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, W.; Shen, K.; Wang, J.; Hu, X.; Lin, L.; Liu, X.; Feng, X. Highly α-Chlorination of Cyclic β-Ketoesters Catalyzed by N,N′-Dioxide Using NCS as the Chlorice Source. Chem. Commun. 2010, 46, 1250–1252. [Google Scholar] [CrossRef] [PubMed]
- Etayo, P.; Badorrey, R.; Díaz-de-Villegas, M.D.; Gálvez, J.A. Chral Amino Diol Derivatives as New Modular Organocatalysts for the Enantioselective α-Chlorination of Cyclic β-Keto Esters. Adv. Synth. Catal. 2010, 352, 3329–3338. [Google Scholar] [CrossRef]
- Shirakawa, S.; Tokuda, T.; Kasai, A.; Maruoka, K. Design of Chiral Bifunctional Quaternary Phosphonium Bromide Catalysts Possessing an Amide Moiety. Org. Lett. 2013, 15, 3350–3353. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, F.; Meng, Q.; Gao, Z. Enantioselective α-Chlorination of β-Oxo Esters Catalyzed by Chiral Diterpenoid Alkaloid Derivatives. Tetrahedron Asymmetry 2014, 25, 1215–1220. [Google Scholar] [CrossRef]
- Novacek, J.; Monkowius, U.; Himmelsbach, M.; Waser, M. Asymmetric α-Chlorination of β-Ketoesters Using Bifunctional Ammonium Salt Catalysis. Monatsh. Chem. 2015. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, S.-G.; Liang, X.-W.; Gao, D.W.; Zheng, J. You, S.-L. Organocatalytic Asymmetric Chlorinative Dearomatization of Naphthols. Chem. Sci. 2015, 6, 4179–4183. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Zhang, Z.-W.; Chen, M.-X.; Tang, W.-H.; Shi, M. Cinchona Alkaloid Catalyzed Enantioselective Chlorination of 3-Aryloxindoles. Eur. J. Org. Chem. 2011, 2011, 3001–3008. [Google Scholar] [CrossRef]
- Gao, X.; Han, J.; Wang, L. Design of Highly Stable Iminophosphoranes as Recyclable Organocatalysts: Application to Asymmetric Chlorinations of Oxindoles. Org. Lett. 2015, 17, 4596–4599. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Y.; Wasa, M.; Jacobsen, E.N. Enantioselective Synthesis of α-Chloro Esters by non-Covalent Catalysis. Tetrahedron Lett. 2015, 56, 3428–3430. [Google Scholar] [CrossRef] [PubMed]
- Almaşi, D.; Alonso, D.A.; Gómez-Bengoa, E.; Nájera, C. Chiral 2-Aminobenzimidazoles as Recoverable Organocatalysts for the Addition of 1,3-Dicarbonyl Compounds to Nitroalkenes. J. Org. Chem. 2009, 74, 6163–6168. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torres, E.; Alonso, D.A.; Gómez-Bengoa, E.; Nájera, C. Conjugate Addition of 1,3-Dicarbonyl Compounds to Maleimides Using a Chiral C2-Symmetric Bis(2-aminobenzimidazole) as Recyclable Organocatalyst. Org. Lett. 2011, 13, 6106–6109. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torres, E.; Alonso, D.A.; Gómez-Bengoa, E.; Nájera, C. Enantioselective Synthesis of Succinimides by Michael Addition of 1,3-Dicarbonyl Compounds to Maleimides Catalyzed by a Chiral Bis(2-aminobenzimidazole) Organocatalyst. Eur. J. Org. Chem. 2013, 2013, 1434–1440. [Google Scholar] [CrossRef]
- Trillo, P.; Gómez-Martínez, M.; Alonso, D.A.; Baeza, A. 2-Aminobenzimidazole Organocatalyzed Asymmetric Amination of Cyclic 1,3-Dicarbonyl Compounds. Synlett 2015, 26, 95–100. [Google Scholar]
- Duan, X.-H.; Mayr, H. Electrophilicities of α-Chlorinating Agents Used in Organocatalysis. Org. Lett. 2010, 12, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- We have also corroborated an important contribution of the non-chiral background reaction for methods A (85% conversion by GC after 12 h) and B (94% conversion by GC after 12 h).
- Since only a 7% improvement of ee was obtained using 20 mol% of catalyst loading, we decided to carry out the substrate scope with a 10 mol% of the corresponding chiral catalyst.
- Northrup, A.B.; MacMillan, D.W.C. Two-Step Synthesis of Carbohydrates by Selective Aldol Reactions. Science 2004, 305, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ferrándiz, J.; Fiser, B.; Gómez-Bengoa, E.; Chinchilla, R. Solvent-Induced Reversal of Enantioselectivity in the Synthesis of Succinimides by the Addition of Aldehydes to Maleimides Catalysed by Carbamate-Monoprotected 1,2-Diamines. Eur. J. Org. Chem. 2015, 2015, 1218–1225. [Google Scholar] [CrossRef]
- Moteki, S.A.; Han, J.; Arimitsu, S.; Akakura, M.; Nakayama, K.; Maruoka, K. An Achiral-Acid-Induced Switch in the Enantioselectivity of a Chiral cis-Diamine-Based Organocatalyst for Asymmetric Aldol and Mannich Reactions. Angew. Chem. Int. Ed. 2012, 51, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.T.; Luparia, M.; Audisio, D.; Maulide, N. Dual Catalysis Becomes Diastereodivergent. Angew. Chem. Int. Ed. 2013, 52, 13149–13152. [Google Scholar] [CrossRef] [PubMed]
- Krautwald, S.; Sarlah, D.; Schafroth, M.A.; Carreira, E.M. Enantio- and Diastereodivergent Dual Catalysis: α-Allylation of Branched Aldehydes. Science 2013, 340, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Halland, N.; Lie, M.A.; Kjærsgaard, A.; Marigo, M.; Schiøtt, B.; Jørgensen, K.A. Mechanistic Investigation of the 2,5-Diphenylpyrrolidine-Catalyzed Enantioselective α-Chlorination of Aldehydes. Chem. Eur. J. 2005, 11, 7083–7090. [Google Scholar] [CrossRef] [PubMed]
- Marquez, C.A.; Fabbretti, F.; Metzger, J.O. Electrospray Ionization Mass Spectrometric Study on the Direct Organocatalytic α-Halogenation of Aldehydes. Angew. Chem. Int. Ed. 2007, 46, 6915–6917. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, L.; Togni, A. Catalytic Enantioselective Chlorination and Bromination of β-Keto Esters. Helv. Chim. Acta 2000, 83, 2425–2435. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).