Abstract
Bioactive molecules are playing essential role in the field of drug discovery and various pharmaceutical applications. Vibrational spectral investigations of the anti-Candida agent ({[(1E)-3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)(4-methylphenyl)methanone ((1E)-IPMM) have been recorded and analyzed to understand its structural geometry, inter- and intra-molecular interactions. The equilibrium geometry, harmonic vibrational wavenumber, natural bond orbital (NBO) and Frontier orbital energy analyses have been carried out with the help of density functional theory with B3LYP/6-311++G(d,p) level of theory. The detailed vibrational assignments for the title molecule were performed on the basis of potential energy distribution analysis in order to unambiguously predict its modes. The calculated wavenumbers had good agreement with the experimental values. NBO analysis has confirmed the intramolecular charge transfer interactions. The predicted docking binding energy gave insight into the possible biological activity of the title molecule.
1. Introduction
Fungi are eukaryotic organisms and some of them are important human pathogens causing diseases such as aspergillosis, candidiasis and cryptococcosis. Actually, the incidence of severe fungal infections has increased in an alarming way over the past few decades. An antifungal drug is an agent that selectively attacks fungal pathogens with minimal toxicity to the host. Azoles are nitrogen-containing five-member heterocyclic ring system. They constitute the largest family of antifungal drugs and have been and are still widely used to treat superficial mucosal as well as deep and disseminated fungal infections. However, their extensive use gives rise to the development of resistance and resulted in therapeutic failure [1]. Azole antifungal agents inhibit biosynthesis of cell membrane sterols [2,3]. Single crystal X-ray structure and anti-Candida activity (minimum inhibitory concentration value = 0.3752 μmol/mL toward Candida albicans) of the titled azole-containing molecule, namely ({[(1E)-3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)(4-methylphenyl)methanone ((1E)-IPMM) were previously reported [4,5].
The present work deals with the FT-IR and FT-Raman spectral investigations of (1E)-IPMM to understand its structural geometry, inter- and intra-molecular interactions, hydrogen bonding, highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) energy and the natural bond orbital (NBO) analysis with the aid by density functional theory (DFT) calculations. The work also explores the biological activity of the title molecule by molecular docking approach.
2. Experimental
2.1. General
Melting point was performed using Gallenkamp melting point device, and it is uncorrected. Crystallographic data have been deposited with the Cambridge Crystallographic Data Center (supplementary publication number CCDC-1006859).
2.2. Synthesis
A solution containing N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI·HCl, 7.3 mmol), 4-dimethylaminopyridine (DMAP, 400 mg), and 4-methylbenzoic acid (7 mmol) in dichloromethane (75 mL) was stirred at room temperature. (1E)-N-Hydroxy-3-(1H-imidazol-1-yl)-1-phenylpropan-1-imine (6.9 mmol) [5] was added to the stirred reaction mixture and stirring was continued for further 18 h at ambient temperature. The reaction blend was washed successively with water (2 × 20 mL), 10% NaHCO3 solution (2 × 15 mL) and water (2 × 15 mL). The organic layer was separated, dried (Na2SO4) and evaporated under vacuum. The residue was crystallized from isopropanol to give pale yellow crystals of the title compound 3 (m.p. 398–400 K) which were suitable for X-ray analysis [4]. 1H and 13C NMR as well as the mass spectral data of the title compound 3 are consistent with the previously reported ones [5].
2.3. Spectroscopic Measurements
The Fourier transform infrared spectrum of (1E)-IPMM was recorded using a Perkin Elmer RXL Spectrometer (Waltham, MA, USA) in the region 4000–400 cm−1, with samples in the KBr using pellet press method. The resolution of the spectrum is 2 cm−1. The FT-Raman spectrum of sample in the solid phase was recorded in the range 3500–50 cm−1 using a Bruker RFS 100/S FT-Raman spectrophotometer (Ettlingen, Germany) with a 1064 nm Nd:YAG laser source of 100 mW power (Göettingen, Germany).
2.4. Quantum Chemical Calculations
The quantum chemical computations of (1E)-IPMM have been performed using Gaussian 09 Program Package [6] at the Becke3-Lee-Yang-Parr (B3LYP) level with standard 6-311++G(d,p) basis set [6]. An empirical uniform scaling factor of 0.9673 was used to offset the systematic errors caused by basis set incompleteness, neglect of electron correlation and vibrational anharmonicity [7,8]. The calculated Raman activities (Si) have been converted to relative Raman intensities (Ii) using the following relationship derived from the intensity theory of Raman scattering [9,10].
where ν0 is the exciting wavenumber, νi is the vibrational wavenumber of the ith normal mode, h, c and k are fundamental constants, and f is a suitably chosen common normalization factor for all peak intensities. The simulated IR and Raman spectra have been plotted using pure Lorentzian band shape with a bandwidth of full width half maximum (FWHM) of 10 cm−1.
3. Results and Discussion
3.1. Synthesis
The title molecule 3 was prepared using the commercially available acetophenone as illustrated in Scheme 1.
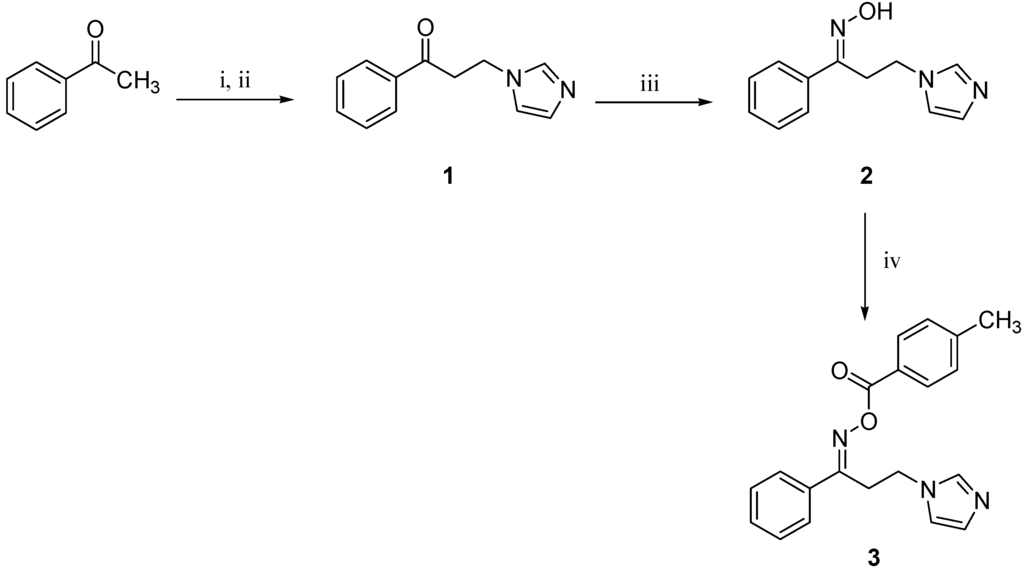
Scheme 1.
Synthetic strategy to prepare the target molecule 3. Reagents and conditions: (i) HN(CH3)2·HCl, (CH2O)n, conc. HCl, ethanol, reflux, 2 h; (ii) imidazole, water, reflux, 5 h; (iii) H2NOH·HCl, KOH, ethanol, reflux, 18 h; (iv) 4-methylbenzoic acid, EDCI·HCl, DMAP, DCM, rt, 18 h.
3.2. Structural Geometry Analysis
The optimized geometry of the molecule is determined by minimizing its energy with respect to all geometrical parameters without imposing molecular symmetry constraints. The optimized molecular structure of the isolated molecule is shown in Figure 1. The optimized bond lengths, bond and dihedral angles are presented in Table 1 in comparison with the experimental values. The calculated bond lengths of C16=O1, and C16–O2 are found to be 1.1993 Å and 1.3828 Å, respectively. The shortening of C16=O1 is due to the presence of double bond character. The calculated bond lengths of C–N bonds are C3–N21 (1.2859 Å), C36–N4 (1.4568 Å), C4–N39 (1.3817 Å), C4–N43 (1.3692 Å), C5–N41 (1.3757 Å), and C5–N43 (1.3131 Å) and the lengthening of the bond C36–N4 is due the conjugation effect of the imidazole moiety. The bond length of C39–H40 (1.0774 Å) is found to be shorter than the other C–H bonds, which is due to the presence of intra-molecular C–H···π bond interaction. In addition, the bond length of C33–C36 (1.5455 Å) appears to be longer than the other C–C bonds and it may be due to the charge transfer effect between the donor to acceptor group. The experimental and calculated value of bond length shows a good agreement in the correlation analysis (R2 = 0.9945). The slight deviations in the bond angle and dihedral angles from the experimental XRD data are probably due to the intermolecular interactions in the crystalline state.

Table 1.
The structural geometry parameters of (1E)-IPMM.
| Bond Length (Å) | Bond Angle (°) | Dihedral Angle (°) | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Calculated | Experimental | Parameters | Calculated | Experimental | Parameters | Calculated | Experimental |
| O1–C16 | 1.1993 | 1.1960 | N3–O2–C16 | 113.03 | 112.71 | C16–O2–N3–C21 | −169.72 | −174.61 |
| O2–C16 | 1.3828 | 1.360 | O2–N3–C21 | 111.26 | 108.99 | N3–O2–C16–O1 | 9.74 | 4.71 |
| O2–N3 | 1.4179 | 1.438 | C36–N4–C39 | 127.25 | 127.11 | N3–O2–C16–C15 | −171.56 | −175.29 |
| N3–C21 | 1.2859 | 1.283 | C36–N4–C43 | 126.44 | 126.39 | O2–N3–C21–C22 | −179.53 | 178.96 |
| N4–C36 | 1.4568 | 1.456 | C39–N4–C43 | 106.29 | 106.49 | O2–N3–C21–C33 | 0.84 | −0.30 |
| N4–C39 | 1.3817 | 1.360 | C41–N5–C43 | 105.23 | 104.26 | C39–N4–C36–C33 | −87.08 | −87.21 |
| N4–C43 | 1.3692 | 1.345 | H7–C6–C8 | 120.88 | 119.87 | C39–N4–C36–H37 | 152.42 | 151.25 |
| N5–C41 | 1.3757 | 1.365 | H7–C6–C15 | 118.76 | 119.79 | C39–N4–C36–H38 | 36.49 | 34.35 |
| N5–C43 | 1.3131 | 1.310 | C8–C6–C15 | 120.36 | 120.35 | C43–N4–C36–C33 | 90.66 | 94.49 |
| C6–H7 | 1.0831 | 0.930 | C6–C8–H9 | 119.42 | 119.36 | C43–N4–C36–H37 | −29.84 | −27.05 |
| C8–H9 | 1.0852 | 0.930 | C6–C8–C10 | 121.1 | 121.28 | C43–N4–C36–H38 | −145.77 | −143.95 |
| C11–H12 | 1.0851 | 0.930 | H9–C8–C10 | 119.48 | 119.36 | C36–N4–C39–H40 | −1.95 | 1.61 |
| C13–H14 | 1.0818 | 0.929 | C8–C10–C11 | 118.14 | 117.89 | C36–N4–C39–C41 | 178.18 | −178.44 |
| C17–H18 | 1.0926 | 0.960 | C8–C10–C17 | 120.78 | 121.19 | C43–N4–C39–H40 | 179.94 | −179.82 |
| C17–H19 | 1.0918 | 0.959 | C11–C10–C17 | 121.08 | 120.92 | C43–N4–C39–C41 | 0.07 | 0.13 |
| C17–H20 | 1.0955 | 0.960 | C10–C11–H12 | 119.44 | 119.22 | C36–N4–C43–N5 | −178.27 | 179.09 |
| C23–H24 | 1.0825 | 0.930 | C10–C11–C13 | 121.19 | 121.53 | C36–N4–C43–H44 | 2.52 | −0.95 |
| C25–H26 | 1.0839 | 0.930 | H12–C11–C13 | 119.37 | 119.26 | C39–N4–C43–N5 | −0.14 | 0.50 |
| C27–H28 | 1.0841 | 0.930 | C11–C13–H14 | 119.9 | 120.03 | C39–N4–C43–H44 | −179.35 | −179.53 |
| C29–H30 | 1.0841 | 0.930 | C11–C13–C15 | 120.2 | 119.88 | C43–N5–C41–C39 | −0.1 | 0.97 |
| C31–H32 | 1.0823 | 0.930 | H14–C13–C15 | 119.89 | 120.10 | C43–N5–C41–H42 | −179.75 | −179.11 |
| C33–H34 | 1.0926 | 0.970 | C6–C15–C13 | 119.02 | 119.02 | C41–N5–C43–N4 | 0.15 | −0.89 |
| C33–H35 | 1.0887 | 0.970 | C6–C15–C16 | 117.54 | 118.37 | C41–N5–C43–H44 | 179.32 | 179.14 |
| C36–H37 | 1.0924 | 0.970 | C13–C15–C16 | 123.43 | 122.51 | H7–C6–C8–H9 | 0.17 | 1.56 |
| C36–H38 | 1.0894 | 0.970 | O1–C16–O2 | 123.93 | 123.87 | H7–C6–C8–C10 | −179.63 | −178.47 |
| C39–H40 | 1.0774 | 0.930 | O1–C16–C15 | 125.36 | 125.55 | C15–C6–C8–H9 | 179.86 | −178.43 |
| C41–H42 | 1.0788 | 0.931 | O2–C16–C15 | 110.69 | 110.58 | C15–C6–C8–C10 | 0.05 | 1.55 |
| C43–H44 | 1.0806 | 0.930 | C10–C17–H18 | 111.36 | 109.45 | H7–C6–C15–C13 | 179.4 | −179.42 |
| C6–C8 | 1.3879 | 1.377 | C10–C17–H19 | 111.49 | 109.47 | H7–C6–C15–C16 | 0.28 | 3.90 |
| C6–C15 | 1.4004 | 1.388 | C10–C17–H20 | 110.72 | 109.44 | C8–C6–C15–C13 | −0.29 | 0.56 |
| C8–C10 | 1.4013 | 1.388 | H18–C17–H19 | 108.26 | 109.44 | C8–C6–C15–C16 | −179.41 | −176.12 |
| C10–C11 | 1.3993 | 1.388 | H18–C17–H20 | 107.27 | 109.53 | C6–C8–C10–C11 | 0.3 | −1.94 |
| C10–C17 | 1.5079 | 1.508 | H19–C17–H20 | 107.55 | 109.50 | C6–C8–C10–C17 | −178.79 | 178.36 |
| C11–C13 | 1.3911 | 1.380 | N3–C21–C22 | 114.73 | 114.19 | H9–C8–C10–C11 | −179.51 | 178.03 |
| C13–C15 | 1.3993 | 1.391 | N3–C21–C33 | 123.56 | 124.71 | H9–C8–C10–C17 | 1.41 | −1.67 |
| C15–C16 | 1.4884 | 1.481 | C22–C21–C33 | 121.72 | 121.09 | C8–C10–C11–H12 | 179.25 | −179.71 |
| C21–C22 | 1.4883 | 1.487 | C21–C22–C23 | 121.7 | 121.76 | C8–C10–C11–C13 | −0.42 | 0.26 |
| C21–C33 | 1.5146 | 1.503 | C21–C22–C31 | 119.81 | 119.94 | C17–C10–C11–H12 | −1.66 | −0.01 |
| C22–C23 | 1.4012 | 1.390 | C23–C22–C31 | 118.48 | 118.29 | C17–C10–C11–C13 | 178.67 | 179.96 |
| C22–C31 | 1.406 | 1.392 | C22–C23–H24 | 120.8 | 119.69 | C8–C10–C17–H18 | −38.01 | −39.32 |
| C23–C25 | 1.3936 | 1.383 | C22–C23–C25 | 120.73 | 120.50 | C8–C10–C17–H19 | −159.05 | −159.26 |
| C25–C27 | 1.3913 | 1.367 | H24–C23–C25 | 118.46 | 119.82 | C8–C10–C17–H20 | 81.25 | 80.72 |
| C27–C29 | 1.396 | 1.377 | C23–C25–H26 | 119.55 | 119.69 | C11–C10–C17–H18 | 142.93 | 140.99 |
| C29–C31 | 1.3883 | 1.378 | C23–C25–C27 | 120.24 | 120.60 | C11–C10–C17–H19 | 21.89 | 21.05 |
| C33–C36 | 1.5455 | 1.520 | H26–C25–C27 | 120.21 | 119.72 | C11–C10–C17–H20 | −97.81 | −98.97 |
| C39–C41 | 1.3711 | 1.348 | C25–C27–H28 | 120.19 | 120.26 | C10–C11–C13–H14 | 179.38 | −178.19 |
| C25–C27–C29 | 119.56 | 119.52 | C10–C11–C13–C15 | 0.18 | 1.81 | |||
| H28–C27–C29 | 120.25 | 120.22 | H12–C11–C13–H14 | −0.29 | 1.78 | |||
| C27–C29–H30 | 120.07 | 119.71 | H12–C11–C13–C15 | −179.49 | −178.22 | |||
| C27–C29–C31 | 120.36 | 120.61 | C11–C13–C15–C6 | 0.17 | −2.21 | |||
| H30–C29–C31 | 119.57 | 119.61 | C11–C13–C15–C16 | 179.24 | 174.32 | |||
| C22–C31–C29 | 120.63 | 120.48 | H14–C13–C15–C6 | −179.02 | 177.8 | |||
| C22–C31–H32 | 118.98 | 119.79 | H14–C13–C15–C16 | 0.05 | −5.67 | |||
| C29–C31–H32 | 120.38 | 119.74 | C6–C15–C16–O1 | 6.16 | 12.52 | |||
| C21–C33–H34 | 108.61 | 108.90 | C6–C15–C16–O2 | −172.53 | −167.47 | |||
| C21–C33–H35 | 110.58 | 108.89 | C13–C15–C16–O1 | −172.92 | −164.03 | |||
| C21–C33–C36 | 113.79 | 113.42 | C13–C15–C16–O2 | 8.39 | 15.97 | |||
| H34–C33–H35 | 107.62 | 107.70 | N3–C21–C22–C23 | 156.34 | 150.54 | |||
| H34–C33–C36 | 107.5 | 108.89 | N3–C21–C22–C31 | −22.63 | −28.46 | |||
| H35–C33–C36 | 108.53 | 108.89 | C33–C21–C22–C23 | −24.03 | −30.17 | |||
| N4–C36–C33 | 114.24 | 114.07 | C33–C21–C22–C31 | 157 | 150.83 | |||
| N4–C36–H37 | 107.49 | 108.76 | N3–C21–C33–H34 | −45.52 | −45.77 | |||
| N4–C36–H38 | 108.62 | 108.77 | N3–C21–C33–H35 | −163.4 | −162.95 | |||
| C33–C36–H37 | 108.55 | 108.73 | N3–C21–C33–C36 | 74.16 | 75.65 | |||
| C33–C36–H38 | 110.28 | 108.73 | C22–C21–C33–H34 | 134.88 | 135.02 | |||
| H37–C36–H38 | 107.42 | 107.59 | C22–C21–C33–H35 | 17 | 17.84 | |||
| N4–C39–H40 | 121.91 | 127.03 | C22–C21–C33–C36 | −105.44 | −103.57 | |||
| N4–C39–C41 | 105.71 | 105.95 | C21–C22–C23–H24 | 0.24 | 1.07 | |||
| H40–C39–C41 | 132.38 | 127.02 | C21–C22–C23–C25 | −178.79 | −178.91 | |||
| N5–C41–C39 | 110.5 | 110.84 | C31–C22–C23–H24 | 179.23 | −179.92 | |||
| N5–C41–H42 | 121.55 | 124.57 | C31–C22–C23–C25 | 0.19 | 0.10 | |||
| C39–C41–H42 | 127.94 | 124.59 | C21–C22–C31–C29 | 179.08 | 178.81 | |||
| N4–C43–N5 | 112.27 | 112.45 | C21–C22–C31–H32 | −1.07 | −1.18 | |||
| N4–C43–H44 | 121.89 | 123.80 | C23–C22–C31–C29 | 0.07 | −0.22 | |||
| N5–C43–H44 | 125.84 | 123.75 | C23–C22–C31–H32 | 179.92 | 179.79 | |||
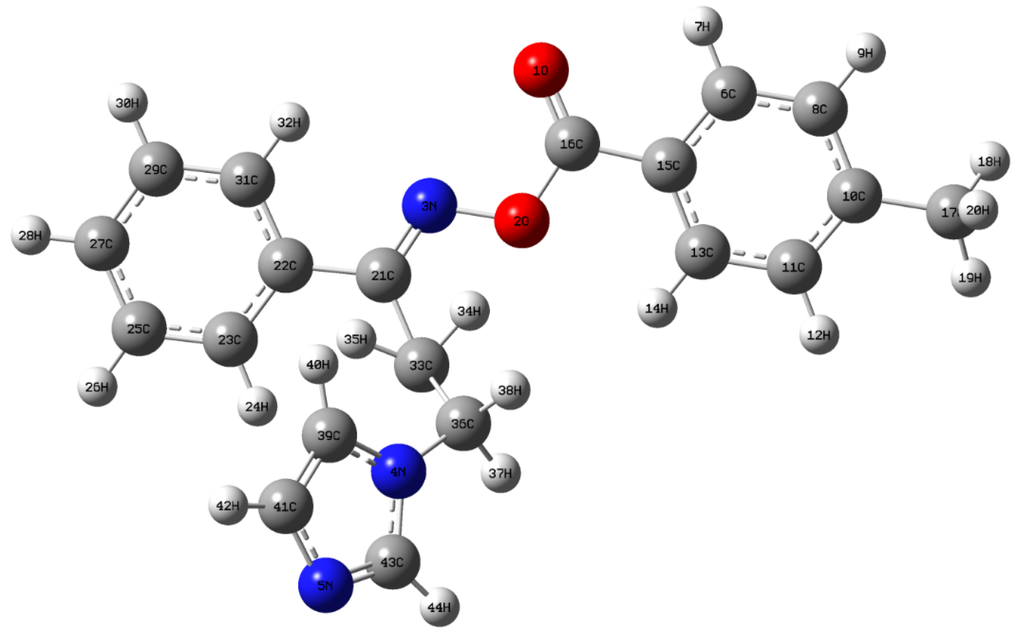
Figure 1.
Optimized structure of (1E)-IPMM.
3.3. Natural Bond Orbital (NBO) Analysis
The natural bond orbital (NBO) calculations were performed using NBO 3.1 program as implemented in the Gaussian 09 package [6] at the DFT level [11]. The corresponding results have been presented in Table 2. A useful aspect of the NBO method is that it gives information about interactions in both filled and virtual orbital spaces, which could enhance the analysis of intra-and intermolecular interactions that will give a measure of the intermolecular delocalization or hyper conjugation. Delocalization of electron density between occupied Lewis-type (bond or lone pair) NBO orbital and formally unoccupied (anti-bond or Rydberg) non-Lewis NBO orbital corresponds to a stabilizing donor-acceptor interaction. The delocalization effect can be described as a charge transfer from the highest occupied bonding orbital into unoccupied anti bonding orbital and their importance can be more quantitatively characterized through a second order perturbative treatment that gives the energy lowering associated with such interaction. The magnitude of these delocalization effects can be determined from an analysis of the off diagonal elements in the Fock matrix of the NBO basis by taking into account all possible donor-acceptor interactions. The hyper conjugative interaction energy was deduced from the second-order perturbation approach as
where <σǀFǀσ>2 or Fij2 is the Fock matrix element between i and j NBO orbital, εσ and εσ* are the energies of σ and σ* NBO’s, and nσ is the population of the donor σ orbital [12]. The larger value of hyperconjugative interaction energy (E(2)) implies that the interaction between electron donors and electron acceptors is more intensive and thus the greater the extent of the conjugation of the whole system. Here, the intra-molecular hyperconjugative interactions are formed by the orbital overlap between π(C–C) and π*(C–C) bond orbital which results in intramolecular charge transfer (ICT), causing stabilization of the system. These interactions are observed as an increase in electron density (ED) in C–C anti bonding orbital that weakens the respective bonds. The ED value of the phenyl rings (∼1.6e) shows strong charge delocalization. However, the two conjugated π bonds (∼1.8e) and π* bonds (∼0.3e) of the imidazole ring clearly demonstrate a lesser degree of conjugation leading to dearomatization. The important interactions between filled (donors) Lewis type NBO and empty (acceptors) non Lewis NBOs are reported. The most important interaction (n-π*) and (n-σ*) energies of LP1N4 π*(N5–C43) and LP2O1 σ*(O2–C16) are 45.86 and 37.90 kcal/mol, respectively. This larger E(2) value reveals the strong ICT interactions of this molecule.

Table 2.
Second order perturbation theory analysis of Fock matrix in natural bond orbital (NBO) basis for (1E)-IPMM.
| Donor (i) | ED (e) | Acceptor (j) | ED (e) | E(2) a (kcal/mol) | E(i)–E(j) b (kcal/mol) | F(i,j) c (kcal/mol) |
|---|---|---|---|---|---|---|
| π(N5–C43) | 1.8696 | π*(C39–C41) | 0.2991 | 20.96 | 0.33 | 0.077 |
| π(C6–C8) | 1.6612 | π*(C10–C11) | 0.3353 | 22.79 | 0.29 | 0.072 |
| π(C6–C8) | 1.6612 | π*(C13–C15) | 0.3800 | 18.34 | 0.28 | 0.065 |
| π(C10–C11) | 1.6285 | π*(C6–C8) | 0.2882 | 17.02 | 0.28 | 0.063 |
| π(C10–C11) | 1.6285 | π*(C13–C15) | 0.3800 | 24.38 | 0.28 | 0.074 |
| π(C13–C15) | 1.6473 | π*(O1–C16) | 0.2324 | 20.53 | 0.27 | 0.069 |
| π(C13–C15) | 1.6473 | π*(C6–C8) | 0.2882 | 20.63 | 0.29 | 0.070 |
| π(C13–C15) | 1.6473 | π*(C10–C11) | 0.3353 | 17.40 | 0.29 | 0.064 |
| π(C22–C23) | 1.6487 | π*(N3–C21) | 0.1570 | 15.73 | 0.27 | 0.061 |
| π(C22–C23) | 1.6487 | π*(C25–C27) | 0.3192 | 19.10 | 0.29 | 0.066 |
| π(C22–C23) | 1.6487 | π*(C29–C31) | 0.2875 | 18.74 | 0.29 | 0.067 |
| π(C25–C27) | 1.6565 | π*(C22–C23) | 0.3739 | 21.13 | 0.28 | 0.069 |
| π(C25–C27) | 1.6565 | π*(C29–C31) | 0.2875 | 18.43 | 0.29 | 0.066 |
| π(C29–C31) | 1.6602 | π*(C22–C23) | 0.3739 | 19.73 | 0.28 | 0.067 |
| π(C29–C31) | 1.6602 | π*(C25–C27) | 0.3192 | 20.94 | 0.28 | 0.069 |
| π(C39–C41) | 1.8516 | π*(N5–C43) | 0.3813 | 15.41 | 0.27 | 0.062 |
| LP(2) O1 | 1.8315 | σ*(O2–C16) | 0.1184 | 37.90 | 0.57 | 0.133 |
| LP(2) O1 | 1.8315 | σ*(C15–C16) | 0.0639 | 17.29 | 0.69 | 0.100 |
| LP(2) O2 | 1.7979 | π*(O1–C16) | 0.2324 | 35.59 | 0.36 | 0.102 |
| LP(2) O2 | 1.7979 | π*(N3–C21) | 0.1570 | 12.38 | 0.36 | 0.060 |
| LP(1) N4 | 1.5610 | π*(N5–C43) | 0.3813 | 45.86 | 0.28 | 0.102 |
| LP(1) N4 | 1.5610 | π*(C39–C41) | 0.2991 | 30.46 | 0.29 | 0.088 |
| LP(1) O2 | 1.9728 | π*(C33–H35) | 0.0128 | 0.72 | 1.04 | 0.025 |
a E(2) means energy of hyperconjugative interactions; Equation (2); b Energy difference between donor and acceptor i and j NBO orbitals; c F(i,j) is the Fock matrix element between i and j NBO orbitals.
3.4. Vibrational Spectral Analysis
The FT-IR and FT-Raman spectra of (1E)-IPMM have been analyzed on the basis of density functional theory calculations. The vibrational modes were assigned on the basis of potential energy distribution (PED) analysis using the VEDA 4 program [13]. The combined experimental and simulated Infrared and Raman spectra are shown in Figure 2 and Figure 3, respectively. The computed wavenumbers are compared with the experimental FT-IR and FT-Raman wavenumbers and their assignments are presented in Table 3. Vibrational analysis is based on the vibrational modes of the groups phenyl ring, methylene, methyl, imidazole ring and skeletal mode.
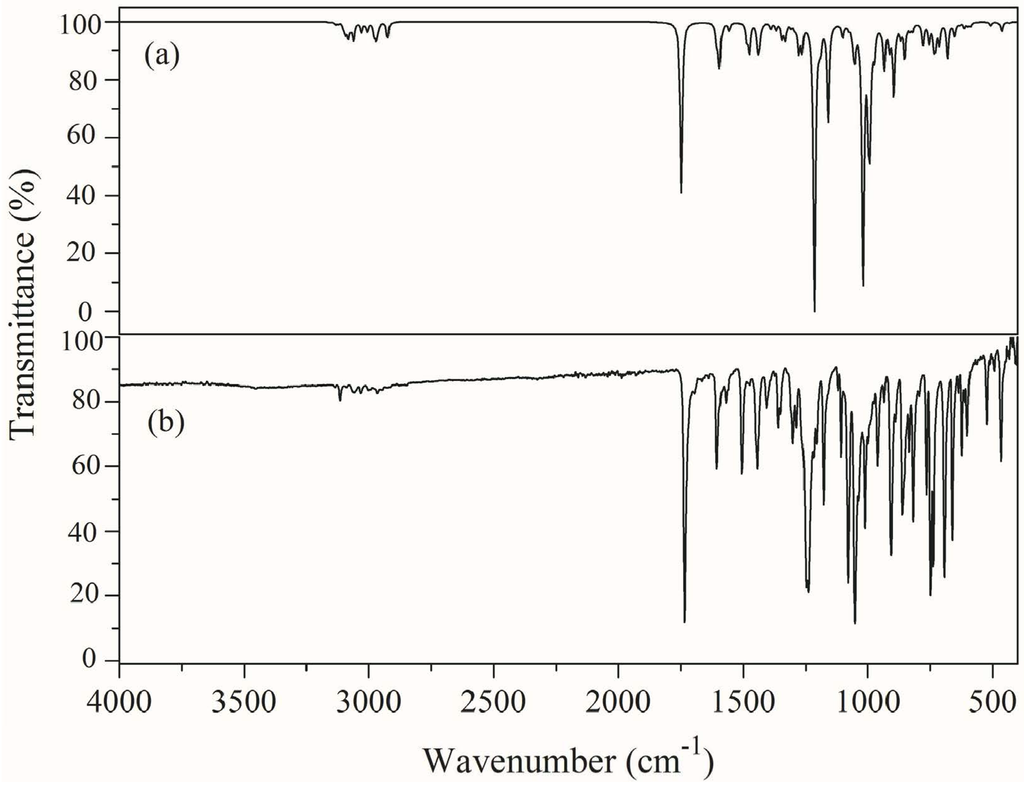
Figure 2.
(a) Simulated (b) Experimental FT-IR spectrum of (1E)-IPMM.
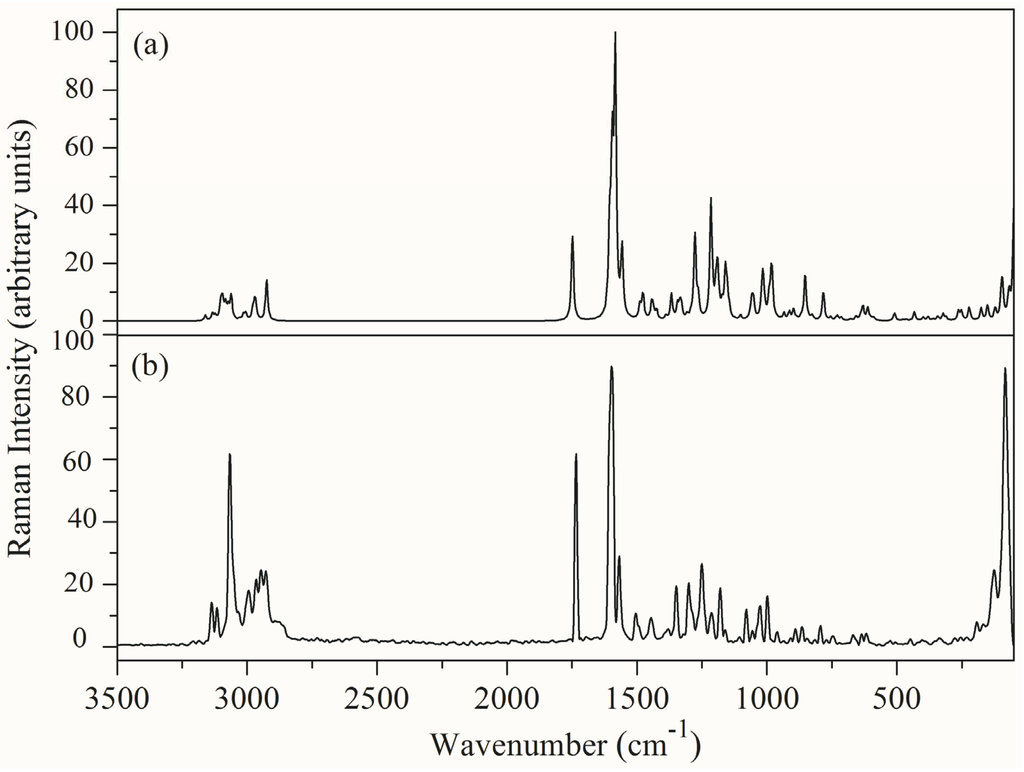
Figure 3.
(a) Simulated (b) Experimental FT-Raman spectrum of (1E)-IPMM.

Table 3.
Vibrational assignment of (1E)-IPMM.
| Calculated Wavenumber (cm−1) | Experimental Wavenumber (cm−1) | IR Intensity | Raman Intensity | Force Constant Mdyne/Å | Assignments with PED% | ||
|---|---|---|---|---|---|---|---|
| Unscaled | Scaled | IR | Raman | ||||
| 3268 | 3161 | – | – | 0.593 | 0.39 | 6.9489 | CH ss (85), CH ss (13) |
| 3239 | 3134 | – | 3136 M | 3.6251 | 0.51 | 6.7536 | CH as (85), CH as (14) |
| 3228 | 3123 | – | – | 2.2934 | 0.35 | 6.7270 | CH v (98) |
| 3208 | 3103 | 3116 VW | 3116 M | 4.7262 | 0.67 | 6.6208 | CH ss (96) |
| 3205 | 3100 | – | – | 2.7163 | 0.64 | 6.6210 | CH ss (88) |
| 3200 | 3095 | – | – | 3.2974 | 0.55 | 6.5918 | CH ss (95) |
| 3198 | 3094 | – | – | 11.246 | 0.8 | 6.5894 | CH ss (69), CH ss (21) |
| 3187 | 3083 | – | – | 21.7901 | 1.12 | 6.5450 | CH as (50), CH as (18) |
| 3177 | 3073 | – | – | 7.1817 | 0.83 | 6.4757 | CH as (45), CH as (44) |
| 3166 | 3063 | – | 3066 S | 0.2433 | 0.31 | 6.4132 | CH as (44), CH as (28), CH as (25) |
| 3165 | 3061 | – | – | 13.691 | 0.67 | 6.4307 | CH ss (77), CH ss (18) |
| 3164 | 3060 | 3058 VW | – | 12.943 | 0.83 | 6.4245 | CH as (77), CH as (19) |
| 3132 | 3029 | 3032 VW | – | 14.3723 | 0.1 | 6.3489 | CH ss (52), CH ss (33) |
| 3116 | 3014 | – | – | 2.0283 | 0.42 | 6.3011 | CH ss (47), CH ss (31), CH ss (13) |
| 3107 | 3005 | 3001 VW | 2994 M | 13.465 | 0.53 | 6.2686 | CH as (62), CH as (36) |
| 3079 | 2978 | – | – | 16.7718 | 0.78 | 6.1126 | CH as (43), CH as (37), CH as (20) |
| 3070 | 2969 | 2965 VW | 2965 M | 23.7511 | 1.58 | 5.9039 | CH ss (49), CH ss (30), CH ss (11), CH ss (10) |
| 3061 | 2961 | 2945 VW | – | 7.8155 | 0.15 | 5.8801 | CH as (51), CH ss (35) |
| 3024 | 2925 | – | 2928 M | 24.0513 | 3.15 | 5.6052 | CH ss (62), CH ss (20), CH ss (17) |
| 2893 W | Overtones and Combination bands | ||||||
| 2878 W | |||||||
| 2858 W | |||||||
| 1807 | 1748 | 1735 VS | 1735 S | 269.1274 | 6.69 | 25.0634 | O=C ss (88) |
| 1660 | 1605 | 1604 M | – | 26.7859 | 6.42 | 14.4429 | vN=C (60) |
| 1649 | 1595 | – | 1597 VS | 64.4175 | 11.64 | 9.3552 | vCC (22), vCC (17) |
| 1637 | 1584 | – | – | 6.3303 | 20.09 | 8.9281 | vCC (28), vCC (16) |
| 1610 | 1558 | 1569 VW | 1568 M | 8.6839 | 4.96 | 8.1884 | vNC (13), vCC (20), vCC (15), vCC (11) |
| 1608 | 1555 | – | – | 3.9604 | 0.32 | 9.0692 | vCC (32) |
| 1539 | 1489 | 1505 M | 1505 W | 1.8657 | 1.04 | 3.4994 | δCCH (18), δCCH (17), δCCH (16) |
| 1536 | 1486 | – | – | 22.6513 | 0.13 | 5.3525 | vN=C (33), vC=C (33), δCCH (13) |
| 1527 | 1477 | – | – | 2.8843 | 1.4 | 2.9785 | δCCH (14), δCCH (13) |
| 1526 | 1476 | – | – | 42.0124 | 0.54 | 3.0799 | vC=C (13), CH2sci (19) |
| 1493 | 1444 | 1443 M | 1446 W | 13.357 | 0.9 | 1.4623 | CH3sci (41), CH3sci (26) |
| 1489 | 1441 | – | – | 18.9506 | 0.28 | 1.6996 | CH2sci (51), δCH2 (47) |
| 1488 | 1440 | – | – | 10.9672 | 0.46 | 1.3911 | CH3sci(38) |
| 1483 | 1435 | – | – | 23.2028 | 0.29 | 1.5608 | CH2sci (61) |
| 1472 | 1424 | 1407 W | – | 2.639 | 0.7 | 2.5501 | δsCCH (24), vCC (13) |
| 1436 | 1389 | – | 1380 W | 8.6605 | 0.28 | 3.0566 | vCC (20), δCCH (12) |
| 1414 | 1367 | – | – | 7.3558 | 0.25 | 2.0790 | NCHtwi (32) |
| 1414 | 1367 | 1360 W | – | 2.2648 | 1.73 | 1.6400 | δsCH3 (22),δCH2 (13) |
| 1390 | 1344 | – | 1349 M | 21.4422 | 1.09 | 2.4537 | vNC (15), CH2wag (15) |
| 1381 | 1336 | – | – | 3.75 | 0.78 | 2.5704 | vNC (27), CH2wag (19) |
| 1375 | 1330 | – | – | 23.9291 | 1.13 | 1.8331 | δCCH (10) |
| 1351 | 1307 | 1302 W | 1301 M | 5.575 | 0.35 | 1.9302 | vCC (12), δsCCH (20) |
| 1337 | 1293 | – | – | 5.0344 | 0.05 | 6.1367 | vCC (31), vCC (27) |
| 1334 | 1291 | – | – | 2.0028 | 0.24 | 1.4769 | δsCCH (22) |
| 1326 | 1283 | 1286 W | – | 0.9504 | 0.14 | 3.7665 | vCC (24) |
| 1320 | 1277 | – | – | 41.0481 | 6.55 | 2.2416 | vCC (26), δCCH (17) |
| 1307 | 1265 | – | 1250 M | 37.7926 | 1.46 | 1.9430 | δsCCH (35), vNC (12) |
| 1259 | 1218 | 1238 S | – | 65.5138 | 0.34 | 1.5270 | δNCH (55), vNC (10) |
| 1256 | 1215 | 1206 W | – | 394.8687 | 8.95 | 3.0905 | vCC (33), δCCH (12) |
| 1237 | 1197 | – | – | 16.8257 | 1.9 | 1.4384 | δCCH (33) |
| 1230 | 1189 | – | – | 27.0607 | 3.94 | 3.2248 | vCC (39), δCCC (12) |
| 1212 | 1173 | 1177 M | 1180 M | 2.3703 | 0.97 | 0.9970 | vCC (11), δsCCH (20) |
| 1199 | 1160 | – | 1161 W | 148.5022 | 3.52 | 1.0149 | δsCCH (20) |
| 1193 | 1154 | – | – | l | 1.51 | 1.1323 | vNC (11), δNCH (14) |
| 1185 | 1146 | – | – | 0.1824 | 0.74 | 0.9300 | δsCCH (33) |
| 1140 | 1103 | 1107 W | 1106 VW | 1.9151 | 0.07 | 1.0086 | vCC (19), δsCCH (20) |
| 1138 | 1101 | – | – | 17.4222 | 0.25 | 1.5829 | vNC (62), δsCCH (20) |
| 1113 | 1077 | 1078 S | – | 5.3066 | 0.03 | 1.1960 | vCC (20), δsCCH (15) |
| 1095 | 1059 | – | – | 27.4394 | 1.54 | 1.0008 | vCC (19), δsCCH (46) |
| 1087 | 1052 | 1051 VS | 1056 M | 42.9985 | 1.43 | 1.8490 | – |
| 1060 | 1025 | – | 1026 M | 10.928 | 0.07 | 1.0692 | δasCCH (62) |
| 1054 | 1020 | – | – | 382.4008 | 1.22 | 1.9040 | vOC (24) |
| 1050 | 1015 | – | – | 16.3115 | 2.41 | 1.7360 | vCC (27) |
| 1046 | 1012 | 1010 M | – | 14.8755 | 1 | 2.0414 | vNC (20), δsCCN (31) |
| 1032 | 998 | – | 998 M | 119.1442 | 0.1 | 2.0580 | δsCCC (24) |
| 1025 | 992 | – | – | 150.1159 | 1.56 | 1.7637 | vCC (15) |
| 1015 | 982 | – | – | 10.5957 | 4.12 | 3.5570 | vCC (14), δsCCC (27) |
| 1010 | 977 | – | – | 0.2509 | 0.09 | 0.7885 | HCCHtwi (47) |
| 1006 | 973 | – | – | 39.6089 | 0.16 | 0.8858 | δsCH3 (55) |
| 1001 | 968 | – | – | 0.7333 | 0.01 | 0.8021 | HCCHtwi (80) |
| 991 | 959 | 960 W | 961 W | 0.145 | 0.08 | 0.7930 | HCCHtwi (40) |
| 980 | 948 | – | – | 3.9185 | 0.04 | 0.7761 | δasCCCH (54) |
| 966 | 934 | 935W | – | 66.3139 | 0.53 | 2.0584 | vCC (36) |
| 945 | 914 | – | – | 33.2026 | 0.63 | 0.9804 | HCCHtwi (24) |
| 928 | 897 | 906 M | – | 111.0668 | 0.83 | 1.0686 | vON (17), HCCHtwi (15) |
| 917 | 887 | – | 890 W | 5.8457 | 0.1 | 3.1525 | δsCNC (70) |
| 899 | 870 | 863 M | 865 W | 18.2761 | 0.18 | 1.8980 | vCC (12), vON (13) |
| 882 | 853 | – | – | 53.555 | 3.69 | 2.6957 | vON (22) |
| 869 | 841 | – | – | 1.649 | 0.15 | 0.6090 | HCCHtwi (80) |
| 861 | 832 | 835 W | – | 8.1045 | 0.01 | 0.6028 | CCCHopb (79) |
| 854 | 826 | – | – | 1.9587 | 0.31 | 0.5388 | HCCHopb (50) |
| 848 | 821 | 819 M | – | 9.9934 | 0.04 | 0.6698 | CCCHopb (50) |
| 810 | 783 | – | 794 W | 3.9159 | 2.27 | 1.8007 | vCC (18), δCCC (30) |
| 806 | 780 | 764 M | – | 31.983 | 0.03 | 0.5309 | NCCNopb (87) |
| 781 | 755 | 749 S | – | 28.9486 | 0.23 | 0.7004 | CCCHopb (53) |
| 761 | 736 | 737 S | – | 33.4661 | 0.13 | 1.0165 | CCCCopb (13), τOCCO (38) |
| 753 | 729 | – | – | 30.0271 | 0.34 | 1.2388 | vCC (15) |
| 738 | 714 | – | – | 30.7013 | 0.22 | 0.3981 | HCNCopb (76) |
| 703 | 680 | 694 S | – | 54.5898 | 0.01 | 0.5922 | CCCCopb (39) |
| 701 | 678 | – | – | 1.2881 | 0.09 | 0.8929 | CCCCopb (19), τOCCO (16) |
| 679 | 657 | – | – | 3.2453 | 0.31 | 1.2364 | δCCC (14) |
| 673 | 651 | – | – | 18.7772 | 0.01 | 0.9418 | NCCNopb (89) |
| 661 | 639 | 636 VW | 636 VW | 0.5431 | 0.36 | 1.0390 | vNC (29), δCCN (13) |
| 652 | 631 | 623 W | 618 VW | 2.8157 | 1.06 | 1.6943 | δCCC (43) |
| 635 | 614 | – | – | 5.4204 | 0.34 | 0.9863 | CCNCopb (44), δCCC (15) |
| 632 | 611 | 612 W | – | 2.7071 | 0.68 | 1.1482 | δCCC (34), τCCNC (25) |
| 620 | 599 | 602 W | – | 5.011 | 0.16 | 1.0360 | δCCC (25), δCC=O (12) |
| 609 | 589 | – | – | 5.5383 | 0.21 | 0.8308 | NCCCopb (13) |
| 526 | 509 | 525 W | – | 5.7184 | 0.59 | 0.8315 | CCNopb (12) |
| 493 | 477 | 493 VW | – | 1.2364 | 0.06 | 0.4729 | CCCCopb (28) |
| 479 | 464 | 466 W | – | 13.7513 | 0.1 | 0.4369 | CCCCopb (36) |
| 448 VW | |||||||
| 447 | 433 | 434 VW | – | 1.5492 | 0.7 | 0.5503 | δCCC (20), δCCN (17) |
| 416 | 403 | 404 VW | 403 VW | 0.0615 | 0 | 0.2964 | CCCCopb (47) |
| 412 | 398 | – | – | 0.1288 | 0.26 | 0.2902 | CCCCopb (48) |
| 393 | 380 | – | – | 2.3925 | 0.3 | 0.3513 | δCCN (31) |
| 372 | 360 | – | – | 1.3636 | 0.11 | 0.5099 | vCC (14) |
| 355 | 344 | – | – | 2.0459 | 0.21 | 0.1924 | CH2opb (14), δCNC (29) |
| 354 | 342 | – | 335 VW | 2.8112 | 0.11 | 0.1949 | CH2opb (37), δCNC (16) |
| 333 | 322 | – | – | 1.1973 | 0.51 | 0.4237 | CCCCopb (11), τCCNO (35) |
| 322 | 311 | – | – | 2.7272 | 0.25 | 0.2073 | CH2opb (28), δCNC (20) |
| 272 | 263 | – | – | 3.6921 | 0.75 | 0.2031 | CCCCopb (46) |
| 260 | 252 | – | – | 2.0039 | 0.71 | 0.2151 | CCCopb (31), δCON (22) |
| 229 | 222 | – | – | 0.4991 | 1.01 | 0.0980 | vOC (10), δCCC (10) |
| 182 | 176 | – | 192 W | 1.7133 | 0.95 | 0.0980 | CNC=Nopb (19), δCCC (30) |
| 157 | 152 | – | – | 0.9648 | 1.13 | 0.0804 | CCCCopb (34) |
| 126 | 122 | – | 125 M | 1.9862 | 0.87 | 0.0521 | δCCC (22), δCCO (17) |
| 103 | 99 | – | – | 1.3736 | 0.72 | 0.0298 | CCCCopb (26), δCNC=N (27) |
| 98 | 95 | – | 83 VS | 0.972 | 2.92 | 0.0174 | δCNC=N (25), τCCCC (23) |
| 71 | 69 | – | – | 1.0649 | 1.91 | 0.0174 | CCNCopb (14), τCCCO (29) |
| 52 | 50 | – | – | 1.6541 | 6.9 | 0.0070 | CCNCopb (40), τCCCO (25) |
| 46 | 45 | – | – | 0.1833 | 1.85 | 0.0064 | δCON (25), δCCO (16) |
| 38 | 37 | – | – | 0.9354 | 6.12 | 0.0034 | τCCCO (28), τCCCN (15) |
| 33 | 32 | – | – | 0.2588 | 2.81 | 0.0007 | C-CH3opb (89) |
| 27 | 26 | – | – | 1.8136 | 9.93 | 0.0023 | CCCNopb (21) |
| 26 | 25 | – | – | 0.0469 | 2.53 | 0.0017 | (29), τCONC (29) |
| 17 | 17 | – | – | 0.7348 | 35 | 0.0009 | τCCCN (25),τCCON (18) |
| 12 | 12 | – | – | 0.9577 | 41.04 | 0.0005 | τCCON (44) |
3.4.1. Phenyl Ring Vibrations
In general, aromatic C–H stretching vibrations absorbs in the region 3080–3010 cm−1 [14]. The intense Raman band is observed 3066 cm−1, which corresponds to C–H stretching vibration. In addition, aromatic C=C stretching vibrations occurs in the region 1625–1430 cm−1 [15]. The intense band is observed at 1597 cm−1 in the Raman spectrum. The in-plane C–H deformation vibration generally appears in the region 1290–1000 cm−1. The C–H out-of-plane deformation expected around 860–800 cm−1 [14]. A medium intense IR band is observed at 819 cm−1 in IR spectrum, which is calculated to be at 821 cm−1 with PED 50% can be attributed to C–H deformation mode.
3.4.2. Methylene Vibrations
The methylene symmetric vibrations expected around in the region 2865–2845 cm−1 [15]. The weak Raman band is observed at 2858 cm−1 can be attributed to CH2 symmetric stretching mode. The –CH2 in-plane vibration expected around in the region 1480–1440 cm−1 [14]. The methylene scissoring mode is observed at 1443 cm−1 (IR) and 1446 cm−1 (Raman). The –CH2 wagging appears around 1411–1174 cm−1 [16]. The Raman band appearing at 1349 cm−1 is assigned to be methylene wagging vibration mode.
3.4.3. Methyl Vibrations
The methyl group asymmetric vibrations generally appear in the regions 2972–2952 cm−1. The methyl asymmetric stretching band is observed at 2965 cm−1, in both IR and Raman spectra. The methyl group symmetric bending modes expected to occur in the region 1365–1385 cm−1 [15]. The observed band at 1360 cm−1 in IR spectrum is calculated to be at 1367 cm−1 with PED 38% can be attributed to methyl bending modes.
3.4.4. Imidazole Ring Vibrations
The imidazole ring CH vibrations expected to occur at 3145–3115 cm−1 [14]. The medium Raman band is observed at 3136 cm−1 assigned to imidazole C–H stretching mode.
3.4.5. Skeletal Mode Vibrations
The C–N and C–C stretching vibrations generally arises in the region 1150–850 cm−1 [17,18,19]. The C–C and N–C stretching modes are observed at 1078 and 1010 cm−1, respectively in the IR spectrum.
3.5. HOMO–LUMO Energy Analysis
The highest occupied molecular orbital (HOMO) energy characterizes the ability of electron giving, whereas the lowest unoccupied molecular orbital (LUMO) energy characterizes the ability of electron accepting. The energy difference between HOMO and LUMO orbitals is called as energy gap, which is an important stability factor for structures. They indicate the electron transport in molecular systems. It is worth noting that HOMOs have an overall π bonding character along with a considerable non-bonding character and LUMOs have an anti-bonding π* character. The strong charge transfer interaction through π conjugated bridge results in substantial ground state donor-acceptor mixing and the appearance of a charge transfer band in the electronic absorption spectrum. Therefore, an electron density (ED) transfer occurs from the more aromatic part of the π conjugated system in the electron-donor side to its electron-withdrawing part. The HOMO-LUMO energy gap for (1E)-IPMM was computed at the B3LYP/6-311++G(d, p) level basis set. The HOMO of the title molecule is located on the imidazole ring and LUMO on phenyl rings. This indicates that the charge transfer between the imidazole ring to phenyl ring system is through the C=N bond. The Eigen values of LUMO (−2.02 eV) and HOMO (−6.36 eV) and their energy gap (4.34 eV), explains the eventual charge transfer interactions taking place within the molecule. The frontier molecular orbital diagrams are shown in Figure 4.
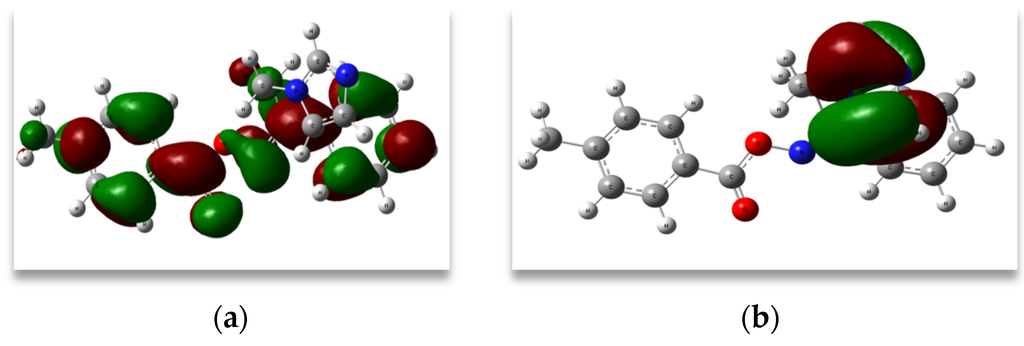
Figure 4.
(a) HOMO; (b) LUMO plots of (1E)-IPMM.
3.6. NMR Spectral Analysis
The scaled and experimental chemical shift values are presented in Table 4. The computed chemical shifts were scaled down by the linear regression method for neglecting the systematic errors [20,21,22,23,24,25]. The empirically scaling was carried out by following equation
where σ is the calculated isotropic shielding value for a particular nucleus.
The phenyl ring carbon signals are observed at 125.80, 127.20, 144.64, 129.01, 129.54, 129.61, 130.09, 131.23 and 136.91 ppm. The two signals at 133.07 and 118.73 ppm are corresponding to the imidazole ring carbon atoms. Similarly, imidazole ring proton signals are observed at 6.84, 6.94 and 7.37 ppm. The methylene groups signals were observed at 31.04 and 43.65 ppm for carbons and at 3.36, 4.21 ppm for hydrogens. Their corresponding theoretical chemical shift values appeared at 30.68 and 44.04 ppm (13C) and at 3.24 and 4.21 ppm (1H) in NMR spectra. The 13C spectrum showed a signal at 21.79 ppm for the methyl carbon, which is calculated to be 19.86 ppm. The predicted chemical shift values showed good agreement with the experimental results.

Table 4.
The predicted and experimental NMR chemical shifts of (1E)-IPMM.
| 13C | 1H | ||||
|---|---|---|---|---|---|
| Atom | δexp. | δcal. | Atom | δexp. | δcal. |
| C6 | 131.23 | 131.08 | H7 | 7.85 | 7.85 |
| C8 | 129.01 | 128.71 | H9 | 7.23 | 7.58 |
| C10 | 144.64 | 146.91 | H12 | 7.23 | 6.94 |
| C11 | 129.01 | 127.70 | H14 | 7.85 | 7.60 |
| C13 | 129.54 | 128.47 | H18 | 2.37 | 1.12 |
| C15 | 125.80 | 125.88 | H19 | 2.37 | 1.11 |
| C16 | 163.17 | 162.04 | H20 | 2.37 | 2.37 |
| C17 | 21.79 | 19.86 | H24 | 7.59 | 7.35 |
| C21 | 163.48 | 164.41 | H26 | 7.35 | 7.33 |
| C22 | 136.91 | 135.25 | H28 | 7.41 | 7.39 |
| C23 | 129.61 | 126.13 | H30 | 7.35 | 7.41 |
| C25 | 130.09 | 127.30 | H32 | 7.59 | 7.86 |
| C27 | 131.23 | 130.22 | H34 | 3.36 | 3.24 |
| C29 | 130.09 | 128.05 | H35 | 3.36 | 2.82 |
| C31 | 129.61 | 126.99 | H37 | 4.21 | 3.37 |
| C33 | 31.04 | 30.68 | H38 | 4.21 | 4.21 |
| C36 | 43.65 | 44.04 | H40 | 6.84 | 6.94 |
| C39 | 118.73 | 119.84 | H42 | 6.94 | 6.84 |
| C41 | 127.20 | 128.46 | H44 | 7.37 | 7.36 |
| C43 | 133.07 | 135.83 | – | – | – |
3.7. Molecular Docking Analysis
The structure of (1E)-IPMM was optimized based on the density functional theory using Gaussian 09 program [6]. The molecular docking was performed using AutoDock Tools-1.5.4 interfaced with the MGL Tools-1.5.4 package [26]. The antifungal target protein of Mycobacterium tuberculosis Cyp51 (PDB ID: 1EA1) in complex with the standard antifungal drug fluconazole, was selected for the present docking analysis [27]. The three-dimensional (3D) coordinates of the protein file was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) protein data bank [28], with a resolution of 2.21 Å. The protein preparation has been carried out by the following steps (i) all water molecules were removed (ii) hydrogen atoms were added to the crystal structure (iii) add Coulomb charges (iv) and previous docked inhibitor (fluconazole) was removed from the protein. The AutoGrid 4.2 [29,30] was used to create affinity grids centered on the active site with 126 × 126 × 126 grid size with a spacing of 0.42 Å. The rigid protein and flexible ligand dockings were performed by using AutoDock 4.2 with the Lamarckian genetic algorithm applying the following protocol: trials of 100 dockings, energy evaluations of 25,000,000, population size of 200, a mutation rate of 0.02, a crossover rate of 0.8, and an elitism value of 1. The docking results were evaluated by sorting the binding free energy predicted by docking confirmations. The predicted best confirmation binding energy was −7.15 kcal/mol. The amino acid ARG96 present in the active site of the target protein binds with the ligand by N–H···N hydrogen bonding. The protein-ligand interaction complex is given in Figure 5 supporting the symmetry between the observed antifungal activity of (1E)-IPMM and docking results.
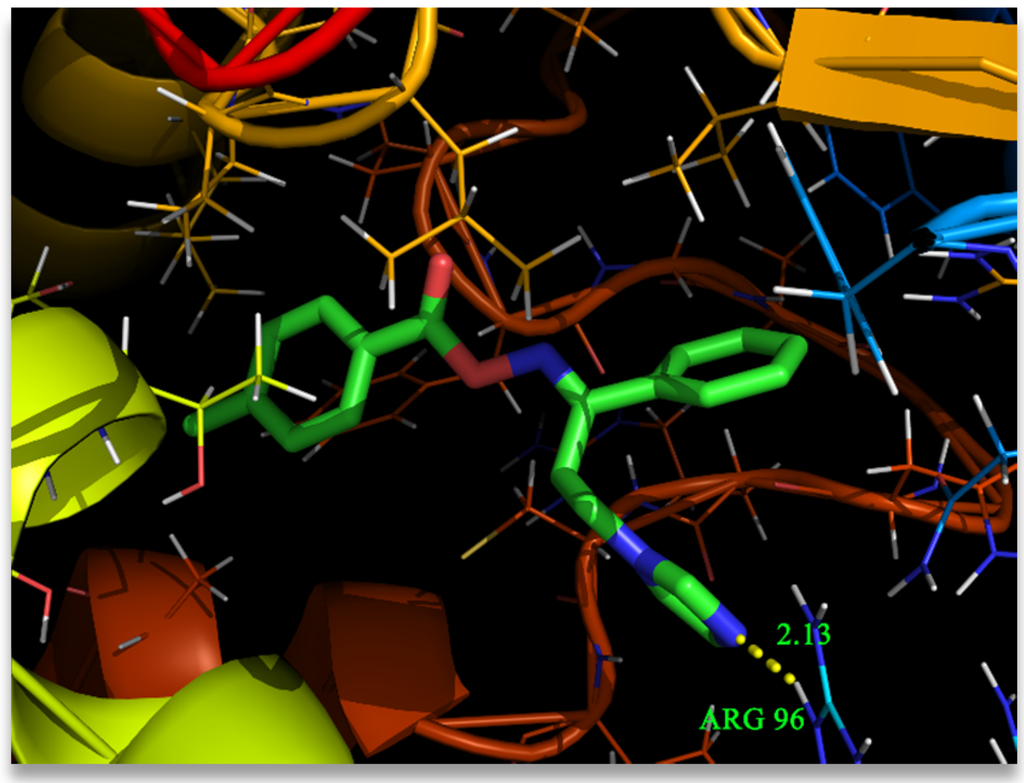
Figure 5.
Binding pose of (1E)-IPMM with amino acid residues.
4. Conclusions
All DFT calculations of (1E)-IPMM have been performed at B3LYP/6-31++G(d,p) level basis set to predict the molecular geometry, vibrational wavenumbers and orbital energy analysis. The detailed vibrational assignments were unambiguously performed on the basis of PED analysis to predict the vibrational modes. The scaled wavenumbers are in a good agreement with the experimental results. The decrease in C–H bond length reveals the presence of intra-molecular C–H···π bond interaction. The NBO analysis also confirms the C–H···π interactions. The HOMO-LUMO energy reveals the occurrence of the charge transfer interactions within the molecule. The molecular docking results indicated that the title molecule is an effective antifungal agent capable of interacting with its target protein (1EA1). Therefore, the title compound can be considered as an anti-Candida prodrug suitable for further investigations as a new antifungal candidate.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-VPP-196.
Author Contributions
All authors discussed the contents of the manuscript and contributed to its preparation. Maha S. Almutairi, Ola A. Saleh and Mohamed I. Attia carried out the synthesis and characterization of the title molecule. Devarasu Manimaran and Issac Hubert Joe performed the computational work.
Conflict of Interests
The authors have declared that there are no conflict of interests.
References
- Vanden-Bossche, H.; Dromer, F.; Improvisi, I.; Lozano-Chiu, M.; Rex, J.H.; Sanglards, D. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 1998, 36, 119–128. [Google Scholar] [PubMed]
- Odds, F.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Hamdan, J.S.; Hahn, R.C. Antifungal drugs for systemic mycosis: An overview of mechanism of action and resistance. Anti-Infect. Agents Med. Chem. 2006, 5, 403–412. [Google Scholar] [CrossRef]
- Almutairi, M.S.; Attia, M.I.; Ghabbour, H.A.; Ghoneim, S.W.; Abdel-Aziz, H.A.; Fun, H.-K. Crystal structure of ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)(4-methylphenyl)-methanone, C20H19N3O2. Z. Kristallogr. New Cryst. Struct. 2014, 229, 307–308. [Google Scholar] [CrossRef]
- Attia, M.I.; Zakaria, A.S.; Almutairi, M.S.; Ghoneim, S.W. In Vitro anti-Candida activity of certain new 3-(1H-imidazol-1-yl)propan-1-one oxime esters. Molecules 2013, 18, 12208–12221. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of Hartree-Fock, Møller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods; Gaussian Inc.: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Keresztury, G.; Chalmers, J.M.; Griffith, P.R. Raman Spectroscopy: Theory in Handbook of Vibrational Spectroscopy; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBOi, version 3.1; TCI, University of Wisconsin: Madison, WI, USA, 1998. [Google Scholar]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, version 3.1; Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin: Madison, WI, USA, 1998. [Google Scholar]
- Varsanyi, G. Vibrational Spectra of Benzene Derivatives; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Jamroz, M.H. Vibrational Energy Distribution Analysis VEDA 4. Hindawi: Warsaw, Poland, 2004. [Google Scholar]
- Socrates, G. Infrared Characteristic Group Frequencies; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: New York, NY, USA, 1999. [Google Scholar]
- Lin-Vein, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Casado, J.; Hernandez, V.; Hotta, S.; Lopez Navarrete, J.T. Vibrational spectra of charged defects in a series of α,α′-dimethyl end-capped oligothiophenes induced by chemical doping with iodine. J. Chem. Phys. 1998, 109, 10419–10429. [Google Scholar] [CrossRef]
- Gussoni, M. Infrared intensities: A new tool in chemistry. J. Mol. Struct. 1986, 141, 63–92. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; Chapman & Hall: London, UK, 1975. [Google Scholar]
- Forsyth, D.A.; Sebag, A.B. Computed 13C·NMR chemical shifts via empirically scaled GIAO shieldings and molecular mechanics geometries. Conformation and configuration from 13C shifts. J. Am. Chem. Soc. 1997, 119, 9483–9494. [Google Scholar] [CrossRef]
- Rablen, P.R.; Pearlman, S.A.; Finkbiner, J. A comparison of density functional methods for the estimation of proton chemical shifts with chemical accuracy. J. Phys. Chem. A 1999, 103, 7357–7363. [Google Scholar] [CrossRef]
- Costa, L.P.A.; de Albuquerque, C.F.; dos Santos, F.M.; de Amorim, M.B., Jr. GIAO-HDFT scaling factor for 13C·NMR chemical shifts calculation. J. Phys. Org. Chem. 2010, 23, 972–977. [Google Scholar]
- Van Eikema-Hommes, N.J.R.; Clark, T. Regression formulae for ab initio and density functional calculated chemical shifts. J. Mol. Model. 2005, 11, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Bally, T.; Rablen, P.R. Calculating accurate proton chemical shifts of organic molecules with density functional methods and modest basis sets. J. Org. Chem. 2009, 74, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Giesen, D.; Zumbulyadis, N. A hybrid quantum mechanical and empirical model for the prediction of isotropic 13C shielding constants of organic molecules. Phys. Chem. Chem. Phys. 2002, 4, 5498–5507. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Podust, L.M.; Poulos, T.L.; Waterman, M.R. Crystal structure of cytochrome P450 14alpha-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 3068–3073. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, F.C.; Koetzle, T.F.; Williams, G.J.; Meyer, E.E., Jr.; Brice, M.D.; Rodgers, J.R.; Kennard, O.; Shimanouchi, T.; Tasumi, M. The Protein Data Bank: A computer-based archival file for macromolecular structures. J. Mol. Biol. 1977, 112, 535–542. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).