Process Optimization and Performance Characterization of Preparing 4A Molecular Sieves from Coal Gangue
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents/Chemicals
2.2. Preparation of Molecular Sieves
2.3. Performance Indicators of Molecular Sieves
2.3.1. Calcium Ion Adsorption Capacity
2.3.2. Loss on Ignition
2.3.3. Static Water Adsorption Capacity
2.3.4. pH Test
2.4. Batch Adsorption Experiment
2.5. Physical and Chemical Measurements
3. Results and Discussion
3.1. Influence of Purity of Raw Materials in Pretreatment Process
3.2. Influence of Synergistic Process of Calcination and Alkali Fusion on Activation of Raw Materials
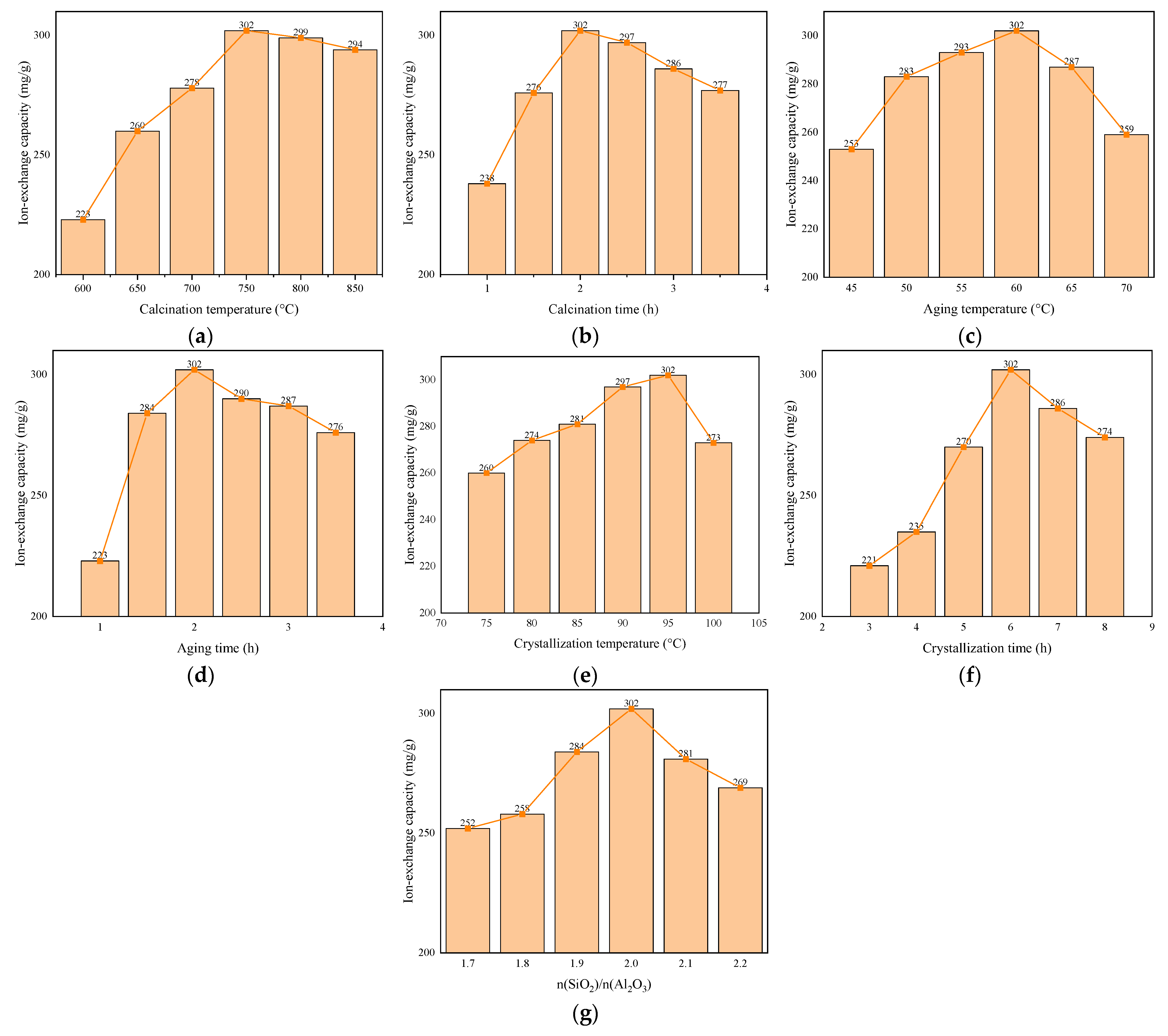
3.3. Effect of Preparation Conditions on Performance of 4A Molecular Sieves
3.4. Performance Test
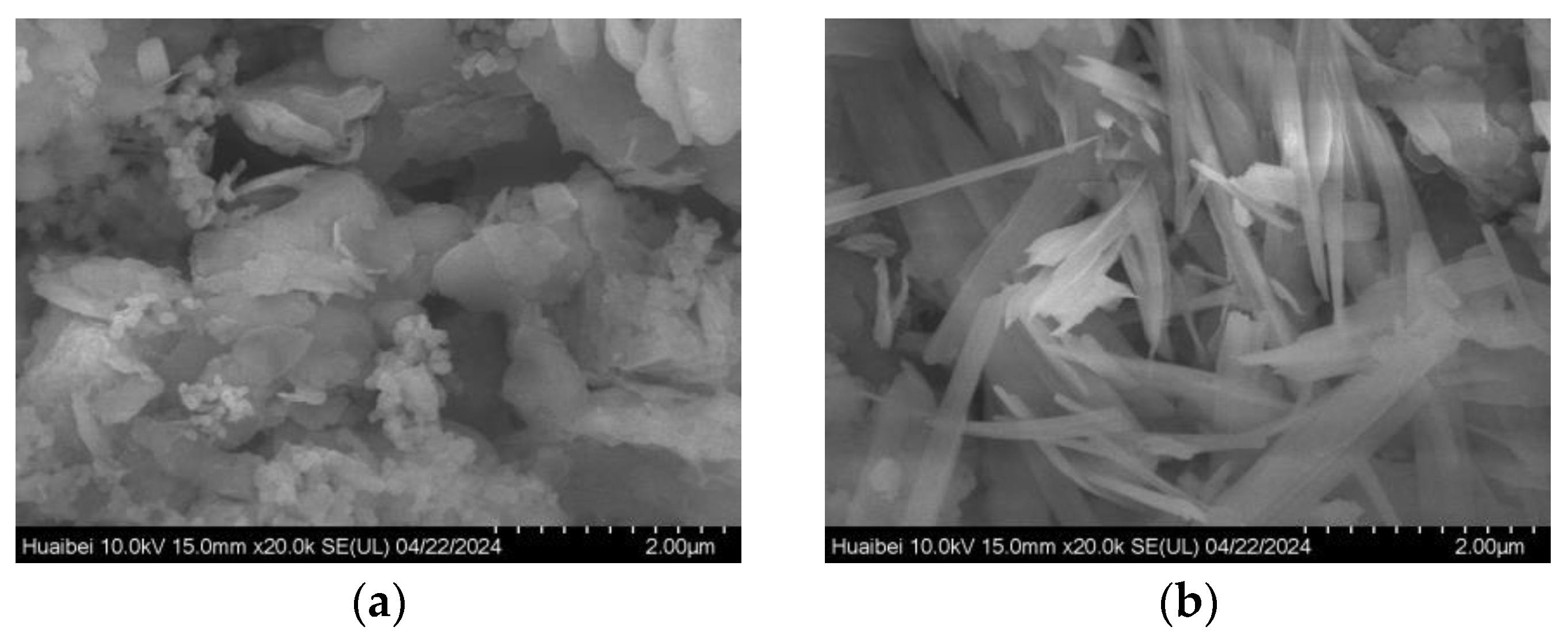
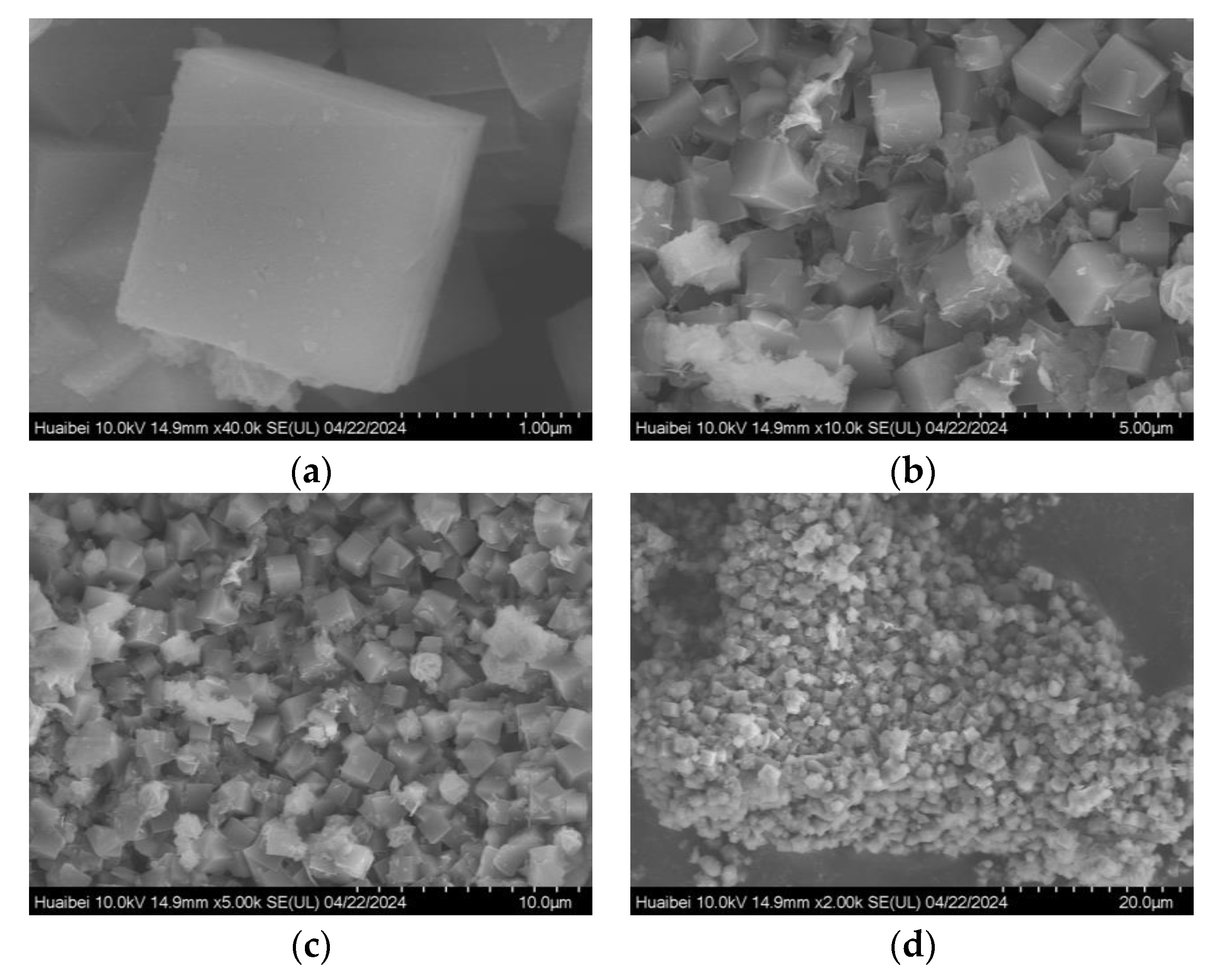
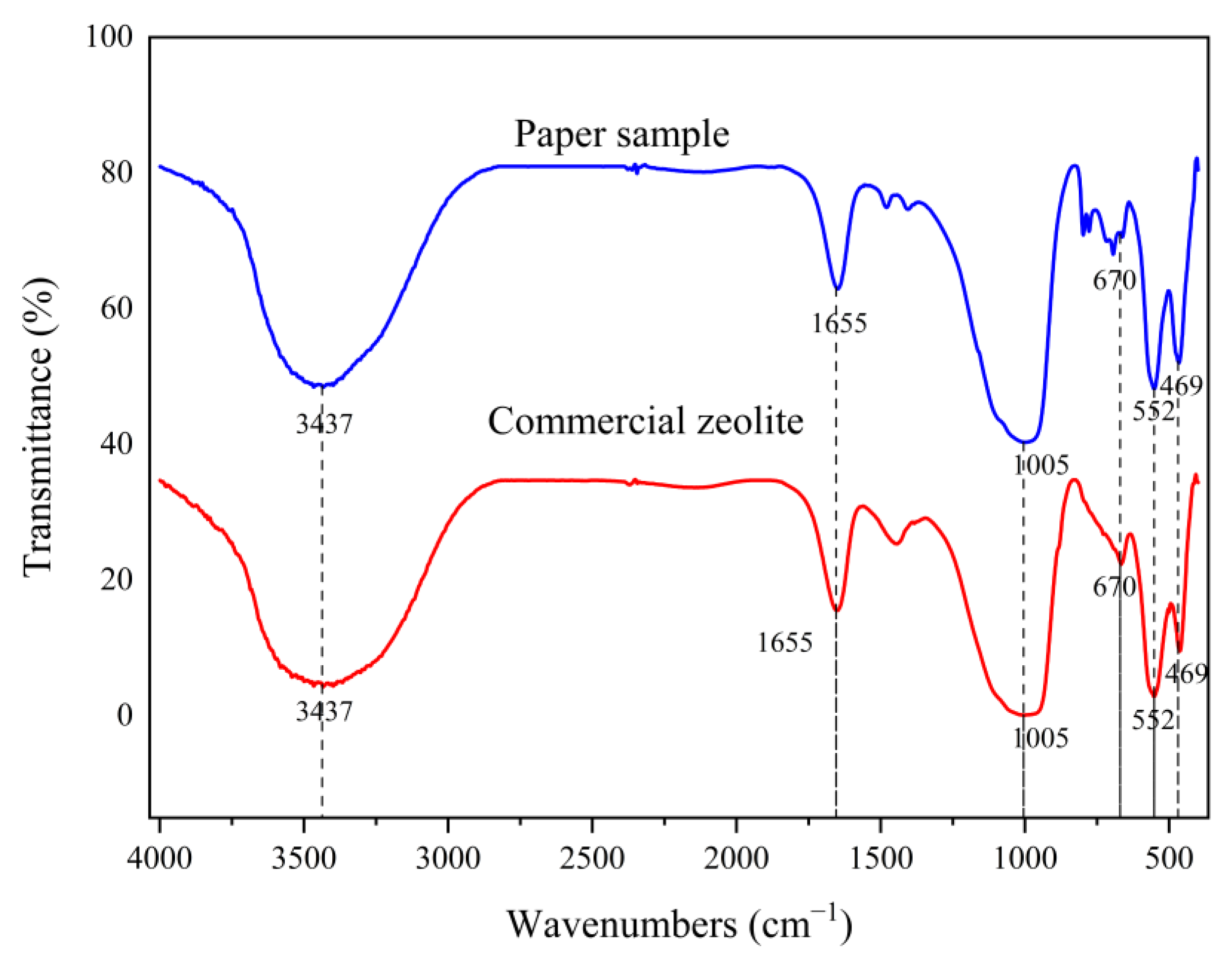
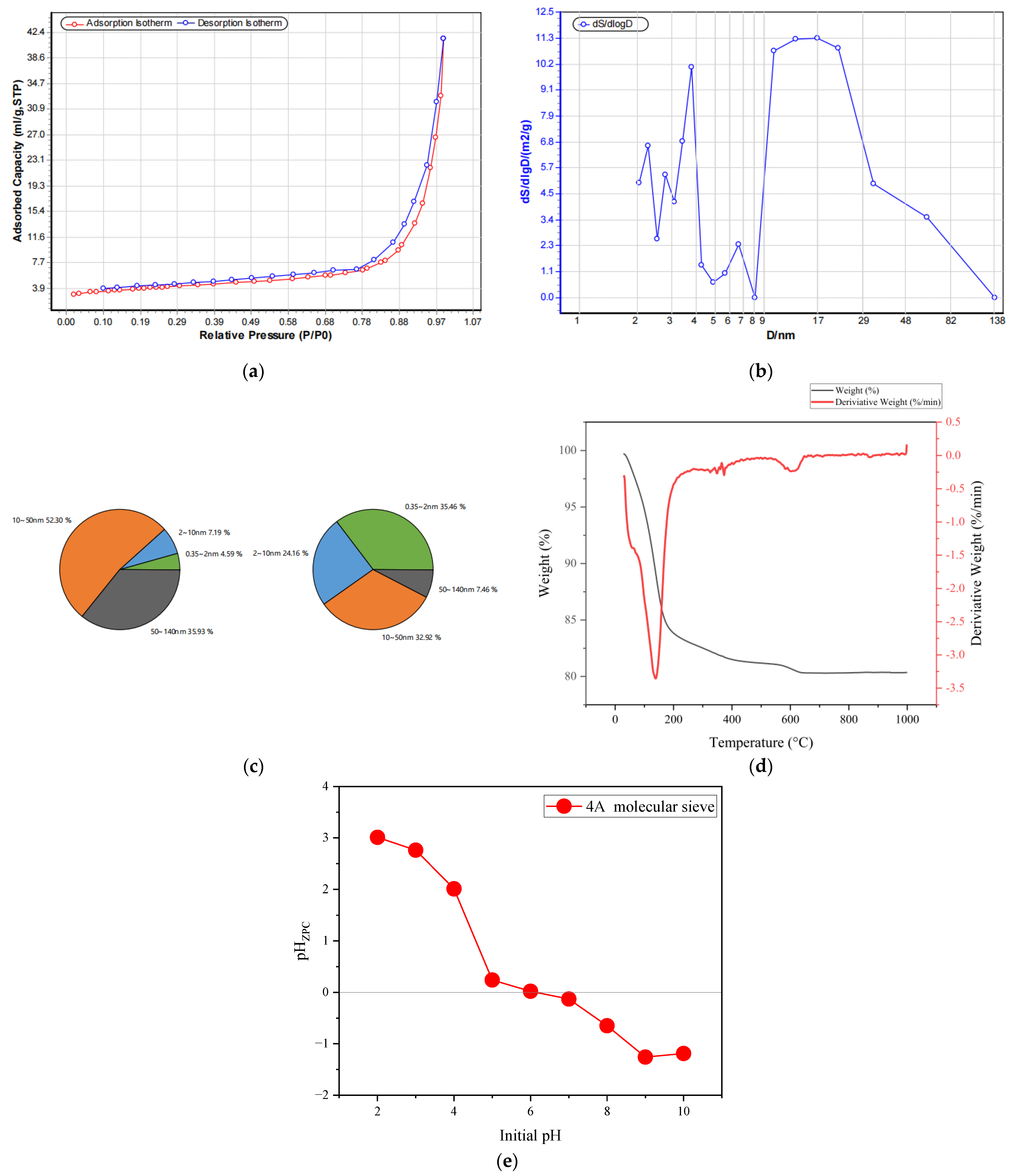
3.5. Sample Characterization
3.6. Verification of Adsorption Performance of Molecular Sieves
4. Conclusions
- (1)
- Optimization of Pretreatment Process: Through low-temperature oxidation (350 °C, 1 h) combined with HCl acid leaching (6 mol/L, 70 °C, 2 h), the Fe2O3 content in coal gangue was reduced from 4.7 wt% to 0.15 wt%. This achievement significantly improves raw material purity and provides highly reactive silicon and aluminum sources for subsequent synthesis.
- (2)
- Synergistic Regulation of Calcination and Alkali Fusion: The XRD peaks of kaolinite disappeared, and TG-DTG analysis fully confirmed that kaolinite was transformed into amorphous metakaolin after calcination at 750 °C for 2 h. A Na2CO3-to-coal gangue mass ratio of 1:1.3 achieved maximum silicon and aluminum dissolution rates (SiO2: 92.3%; Al2O3: 88.1%). The alkali fusion products (NaAlSiO4 and Na2SiO3) serve as highly reactive precursors for hydrothermal crystallization.
- (3)
- Optimization of Hydrothermal Crystallization Conditions: Synergistic aging at 60 °C for 2 h and crystallization at 95 °C for 6 h effectively regulated the cubic crystal morphology, suppressing impurity phase formation. The products exhibit a relative crystallinity of 84.8% and inhibited a calcium ion adsorption capacity of 302 mg/g, meeting the industry standard (QB/T 1768-2003) [33], and demonstrated excellent Cu2+ removal efficiency (90.57% at 40 °C). The pH of zero point charge (pHZPC) of the 4A molecular sieve is 6.13.
- (4)
- The optimized 4A molecular sieve demonstrates Cu2+ adsorption behavior consistent with the Langmuir monolayer model (qmax = 209.2 mg/g, R2= 0.997), suggesting that the adsorption mechanism primarily involves uniform monolayer adsorption on the surface without intermolecular interactions. Kinetically, the adsorption followed a pseudo-second-order model (R2 = 0.999), indicating that the adsorption of Cu2+ on zeolite is primarily controlled by chemisorption rather than physisorption. The adsorption process can be divided into two stages: an initial rapid phase with a smaller diffusion boundary layer, followed by a slower phase with increased resistance as equilibrium approaches.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Gao, X.; Zhu, S.; Liang, H. Polycyclic aromatic hydrocarbons (PAHs) in coal preparation plant products: A contributor to environmental pollution. Sci. Total Environ. 2024, 906, 167887. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, X.; Li, Q.; Hu, Z. Environmental impact assessment of acidic coal gangue leaching solution on groundwater: A coal gangue pile in Shanxi, China. Environ. Geochem. Health 2024, 46, 120. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Li, L.; Li, W.; Ma, K.; Dong, W.; Bai, C.; Zhou, J.L. Enhanced mechanical properties and durability of coal gangue reinforced cement-soil mixture for foundation treatments. J. Clean. Prod. 2019, 231, 468–482. [Google Scholar] [CrossRef]
- Tan, W.-F.; Wang, L.-A.; Huang, C. Environmental effects of coal gangue and its utilization. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3716–3721. [Google Scholar] [CrossRef]
- Li, L.; Huang, Q.; Zuo, X.; Wu, J.; Wei, B.; He, Y.; Zhang, W.; Zhang, J. Study on the slurry diffusion law of fluidized filling gangue in the caving goaf of thick coal seam fully mechanized caving mining. Energies 2022, 15, 8164. [Google Scholar] [CrossRef]
- Lv, W.; Guo, K.; Wu, Y.; Tan, Y.; Ding, K.; Li, B. Compression characteristics of local filling gangue in steeply dipping coal seam. Energy Explor. Exploit. 2022, 40, 1131–1150. [Google Scholar] [CrossRef]
- Zhu, X.; Gong, W.; Li, W.; Bai, X.; Zhang, C. Reclamation of waste coal gangue activated by Stenotrophomonas maltophilia for mine soil improvement: Solubilizing behavior of bacteria on nutrient elements. J. Environ. Manag. 2022, 320, 115865. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine–A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef]
- Moshoeshoe, M.; Nadiye-Tabbiruka, M.S.; Obuseng, V. A review of the chemistry, structure, properties and applications of zeolites. Am. J. Mater. Sci 2017, 7, 196–221. [Google Scholar]
- Lin, S.; Jiang, X.; Zhao, Y.; Yan, J. Zeolite greenly synthesized from fly ash and its resource utilization: A review. Sci. Total Environ. 2022, 851, 158182. [Google Scholar] [CrossRef]
- Djioko, F.H.K.; Fotsop, C.G.; Youbi, G.K.; Nwanonenyi, S.C.; Oguzie, E.E.; Madu, C.A. Efficient removal of pharmaceutical contaminant in wastewater using low-cost zeolite 4A derived from kaolin: Experimental and theoretical studies. Mater. Chem. Phys. 2024, 315, 128994. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Yaodong, L.; Cuiping, Y.; Liyuan, H.; Liang, M. Synthesis of 4A zeolite from red mud and mechanism of adsorption removal of Cu2+ and Mn2+ from water. Environ. Prot. Chem. Ind. 2023, 43, 813–820. [Google Scholar]
- Ali, I.O.; Hassan, A.M.; Shaaban, S.M.; Soliman, K.S.J.S.; Technology, P. Synthesis and characterization of ZSM-5 zeolite from rice husk ash and their adsorption of Pb2+ onto unmodified and surfactant-modified zeolite. Sep. Purif. Technol. 2011, 83, 38–44. [Google Scholar]
- Leyva-Ramos, R.; Jacobo-Azuara, A.; Diaz-Flores, P.; Guerrero-Coronado, R.; Mendoza-Barron, J.; Berber-Mendoza, M.J.C.; Physicochemical, S.A.; Aspects, E. Adsorption of chromium (VI) from an aqueous solution on a surfactant-modified zeolite. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 35–41. [Google Scholar] [CrossRef]
- Yusof, A.M.; Malek, N.A.N.N. Removal of Cr (VI) and As (V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef]
- Zeng, Y.; Woo, H.; Lee, G.; Park, J.J.M.; Materials, M. Adsorption of Cr (VI) on hexadecylpyridinium bromide (HDPB) modified natural zeolites. Microporous Mesoporous Mater. 2010, 130, 83–91. [Google Scholar] [CrossRef]
- Omisanya, N.; Folayan, C.; Aku, S.; Adefila, S. Synthesis and characterisation of zeolite a for adsorption refrigeration application. Adv. Appl. Sci. Res. 2012, 3, 3746–3754. [Google Scholar]
- Chang, H.-L.; Shih, W.-H. Synthesis of zeolites A and X from fly ashes and their ion-exchange behavior with cobalt ions. Ind. Eng. Chem. Res. 2000, 39, 4185–4191. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P. Investigation on the synthesis of zeolite NaX from Kerala kaolin. J. Porous Mater. 1999, 6, 283–297. [Google Scholar] [CrossRef]
- Alkan, M.; Hopa, Ç.; Yilmaz, Z.; Güler, H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater. 2005, 86, 176–184. [Google Scholar] [CrossRef]
- Ganji, F.; Mohammadi, K.; Roozbehani, B. Efficient synthesis of 4A zeolite based-NiCo2O4 (NiCo2O4@ 4A) nanocomposite by using hydrothermal method. J. Solid State Chem. 2020, 282, 121111. [Google Scholar] [CrossRef]
- Jin, Y.; Li, L.; Liu, Z.; Zhu, S.; Wang, D. Synthesis and characterization of low-cost zeolite NaA from coal gangue by hydrothermal method. Adv. Powder Technol. 2021, 32, 791–801. [Google Scholar] [CrossRef]
- Ge, Q.; Moeen, M.; Tian, Q.; Xu, J.; Feng, K. Highly effective removal of Pb2+ in aqueous solution by Na-X zeolite derived from coal gangue. Environ. Sci. Pollut. Res. 2020, 27, 7398–7408. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. A and X-type zeolites synthesised from kaolinite at low temperature. Appl. Clay Sci. 2013, 80, 162–168. [Google Scholar] [CrossRef]
- García-Díaz, I.; López, F.A.; Alguacil, F.J. Carbon nanofibers: A new adsorbent for copper removal from wastewater. Metals 2018, 8, 914. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8. [Google Scholar] [CrossRef]
- Xie, W.-M.; Zhou, F.-P.; Bi, X.-L.; Chen, D.-D.; Li, J.; Sun, S.-Y.; Liu, J.-Y.; Chen, X.-Q. Accelerated crystallization of magnetic 4A-zeolite synthesized from red mud for application in removal of mixed heavy metal ions. J. Hazard. Mater. 2018, 358, 441–449. [Google Scholar] [CrossRef]
- Iqbal, A.; Sattar, H.; Haider, R.; Munir, S. Synthesis and characterization of pure phase zeolite 4A from coal fly ash. J. Clean. Prod. 2019, 219, 258–267. [Google Scholar] [CrossRef]
- Zhao, S.; Xiao, Y.; Ma, Q.; Yang, Z.; Wang, J.; Fan, X. Study on adsorption of Cu (II) on 4A zeolite synthesized by aluminum extraction residue by fly ash. Inorg. Chem. Ind. 2024, 56, 127–134. [Google Scholar]
- Liu, X.; Wang, R. Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite. J. Hazard. Mater. 2017, 326, 157–164. [Google Scholar] [CrossRef] [PubMed]
- QB/T 1768-2003; 4A Zeolite for Detergents. Industry Standard—Light Industry. China Light Industry Press: Beijing, China, 2003; 17p.
- Wu, D.; Sui, Y.; He, S.; Wang, X.; Li, C.; Kong, H. Removal of trivalent chromium from aqueous solution by zeolite synthesized from coal fly ash. J. Hazard. Mater. 2008, 155, 415–423. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Eren, E. Removal of basic dye by modified Unye bentonite, Turkey. J. Hazard. Mater. 2009, 162, 1355–1363. [Google Scholar] [CrossRef]
- Lu, X.; Shi, D.; Chen, J. Sorption of Cu2+ and Co2+ using zeolite synthesized from coal gangue: Isotherm and kinetic studies. Environ. Earth Sci. 2017, 76, 591. [Google Scholar] [CrossRef]
- Domínguez-Vargas, J.R.; Gonzalez, T.; Palo, P.; Cuerda-Correa, E.M. Removal of carbamazepine, naproxen, and trimethoprim from water by amberlite XAD-7: A kinetic study. CLEAN-Soil Air Water 2013, 41, 1052–1061. [Google Scholar] [CrossRef]
- Kong, D.; Li, L.; Fan, J.; Jie, Y.; Li, Z. Preparation of P type molecular sieves from gangue of high iron and high silica content. Bull. Chin. Ceram. Soc 2013, 32, 1052–1056. [Google Scholar]
- Li, H.; Li, M.; Zheng, F.; Wang, J.; Chen, L.; Hu, P.; Zhen, Q.; Bashir, S.; Liu, J.L. Efficient removal of water pollutants by hierarchical porous zeolite-activated carbon prepared from coal gangue and bamboo. J. Clean. Prod. 2021, 325, 129322. [Google Scholar] [CrossRef]
- Kang, C.; Qiao, J.; Yang, S.; Peng, C.; Fu, Y.; Liu, B.; Liu, J.; Aleksandrova, T.; Duan, C. Research progress on activation extraction of valuable metals in coal gangue. CIESC J. 2023, 74, 2783–2799. [Google Scholar]
- Xiong, Y.; Lu, G.; Wang, Y.; Sun, Q. Synthesis and influence factors study of 4A molecular sieve via halloysite. J. Electron. Mater. 2019, 48, 7756–7761. [Google Scholar] [CrossRef]
- Fei, E.; Zhang, X.; Su, L.; Liu, B.; Li, B.; Li, W. Analysis of calcination activation modified coal gangue and its acid activation mechanism. J. Build. Eng. 2024, 95, 109916. [Google Scholar]
- Hu, Y.; Han, X.; Sun, Z.; Jin, P.; Li, K.; Wang, F.; Gong, J. Study on the reactivity activation of coal gangue for efficient utilization. Materials 2023, 16, 6321. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lin, Q.; Yang, T.; Bao, Y.C.; Liu, J. Preparation and characterization of ZSM-5 molecular sieve using coal gangue as a raw material via solvent-free method: Adsorption performance tests for heavy metal ions and methylene blue. Chemosphere 2023, 341, 139741. [Google Scholar] [CrossRef]
- Kong, D.; Jiang, R. Preparation of NaA zeolite from high iron and quartz contents coal gangue by acid leaching—alkali melting activation and hydrothermal synthesis. Crystals 2021, 11, 1198. [Google Scholar] [CrossRef]
- Qian, T.; Li, J. Synthesis of Na-A zeolite from coal gangue with the in-situ crystallization technique. Adv. Powder Technol. 2015, 26, 98–104. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Li, Z. Synthesis of a loess-based 4A molecular sieve and its application performance in detergents. Inorganica Chim. Acta 2024, 559, 121798. [Google Scholar] [CrossRef]
- Bahraminia, S.; Anbia, M. Carbon dioxide methanation using Ni catalysts supported on low-cost Lynde Type A zeolite synthesized from pretreated Iranian coal gangue. Int. J. Hydrogren Energy 2024, 83, 842–855. [Google Scholar] [CrossRef]
- Qiang, L. Effect of calcination temperature on the activity of coal gangue and mechanism study. Non-Met. Mines 2020, 43, 100–102+106. [Google Scholar]
- Li, Y.; Zhu, Y.-T.; Guo, M.; Zhang, M. Synthesis of 4A molecular sieves from titanium-containing electric furnace molten slag and their adsorption properties. Chin. J. Eng. 2014, 36, 1656–1665. [Google Scholar]
- Zheng, Y.; Zhou, J.; Ma, Z.; Weng, X.; Cheng, L.; Tang, G. Preparation of a high-silicon ZSM-5 molecular sieve using only coal gangue as the silicon and aluminum sources. Materials 2023, 16, 4338. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zheng, Y.; Wang, W. Synthesis of 4A zeolite from kaolinite-type pyrite flotation tailings (KPFT). Minerals 2018, 8, 338. [Google Scholar] [CrossRef]
- Ou, Y.; Peng, L.; Li, C.; Zhao, H.; Su, P.; Gao, P. Preparation of 4A molecular sieve using aluminum ash and microsilica powder. Mater. Sci. Technol. 2023, 31, 45–52. [Google Scholar]
- Djamel, N.; Samira, A. Mechanism of Cu2+ ions uptake process by synthetic NaA zeolite from aqueous solution: Characterization, Kinetic, intra-crystalline diffusion and thermodynamic studies. J. Mol. Liq. 2021, 323, 114642. [Google Scholar] [CrossRef]
- Nibou, D.; Mekatel, H.; Amokrane, S.; Barkat, M.; Trari, M. Adsorption of Zn2+ ions onto NaA and NaX zeolites: Kinetic, equilibrium and thermodynamic studies. J. Hazard. Mater. 2010, 173, 637–646. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.-J.; Cao, J.-X. Preparation of 4 A Molecular Sieve from Acid Insoluble Ash Residue. Wuhan Ligong Daxue Xuebao (J. Wuhan Univ. Technol.) 2012, 34, 31–35. [Google Scholar]
- Biaokun, B.; Yue, M.; Shuping, C. Adsorption Performance and Pore Structure of 4A Molecular Sieve. Bull. Chin. Ceram. Soc. 2020, 39, 3367–3372. [Google Scholar]
- Huang, Z.; Cai, Y.; Fan, X.; Ning, K.; Yu, X.; Zheng, S.; Chen, H.; Xie, Y. Synthesis of 4A zeolite molecular sieves by modifying fly ash with water treatment residue to remove ammonia nitrogen from water. Sustainability 2024, 16, 5683. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Lyu, X.; ElDeeb, A.B. Synthesized zeolite based on Egyptian boiler ash residue and kaolin for the effective removal of heavy metal ions from industrial wastewater. Nanomaterial 2023, 13, 1091. [Google Scholar] [CrossRef]
- Lyu, F.; Niu, S.; Wang, L.; Liu, R.; Sun, W.; He, D. Efficient removal of Pb (II) ions from aqueous solution by modified red mud. J. Hazard. Mater. 2021, 406, 124678. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qian, X.; Yuan, P.; Bai, H.; Miki, T.; Men, F.; Li, H.; Nagasaka, T. Green synthesis of zeolite 4A using fly ash fused with synergism of NaOH and Na2CO3. J. Clean. Prod. 2019, 212, 250–260. [Google Scholar] [CrossRef]
- Yao, G.; Zhang, X.; Sun, Z.; Zheng, S. High adsorption selectivity of zeolite X in the binary ionic system of Cu (II) and Zn (II). J. Porous Mater. 2019, 26, 1197–1207. [Google Scholar] [CrossRef]
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, J.; Wang, L.; Yang, G.; Zhang, X. Adsorption characteristics of Pb2+ onto wine lees-derived biochar. Bull. Environ. Contam. Toxicol. 2016, 97, 294–299. [Google Scholar] [CrossRef]
- Silva, A.R.; Cavallini, G.S.; de Mello Brandão, H.; Oliveira, L.F.C.; Souza, N.L.G.D. Experimental design of polymer synthesis applied to the removal of Cd2+ ions from water via adsorption. Discov. Water 2024, 4, 44. [Google Scholar] [CrossRef]
- Yan, J.; Zuo, X.; Yang, S.; Chen, R.; Cai, T.; Ding, D. Evaluation of potassium ferrate activated biochar for the simultaneous adsorption of copper and sulfadiazine: Competitive versus synergistic. J. Hazard. Mater. 2022, 424, 127435. [Google Scholar] [CrossRef]
- Zhu, T.; Zhu, T.; Gao, J.; Zhang, L.; Zhang, W. Enhanced adsorption of fluoride by cerium immobilized cross-linked chitosan composite. J. Fluor. Chem. 2017, 194, 80–88. [Google Scholar] [CrossRef]



| Ingredient | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | Else | Heat Loss |
|---|---|---|---|---|---|---|---|---|
| CGS-I | 59.9% | 24.6% | 4.7% | 0.94% | 8.9% | 0.96% | 2.9% | 11.9% |
| After acid leaching 1 | 73.8% | 21.4% | 0.15% | 0.87% | 0.06% | 0.33% | 3.22% | 18.5% |
| CGS-II | 55.22% | 28.55% | 4.45% | 0.95% | 8.42% | 0.81% | 1.6% | 12.7% |
| After acid leaching 2 | 69.57% | 26.3% | 0.21% | 0.69% | 0.05% | 0.23% | 2.95% | 20.03% |
| Level | FactorA | FactorB (°C) | FactorC (°C) |
|---|---|---|---|
| 1 | 1.9 | 700 | 55 |
| 2 | 2.0 | 750 | 60 |
| 3 | 2.1 | 800 | 65 |
| Exp. No | A | B | C | Ion-Exchange Capacity |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 272 |
| 2 | 1 | 2 | 2 | 284 |
| 3 | 1 | 3 | 3 | 253 |
| 4 | 2 | 1 | 2 | 278 |
| 5 | 2 | 2 | 3 | 287 |
| 6 | 2 | 3 | 1 | 261 |
| 7 | 3 | 1 | 3 | 268 |
| 8 | 3 | 2 | 1 | 276 |
| 9 | 3 | 3 | 2 | 243 |
| K1 | 809 | 818 | 809 | |
| K2 | 826 | 847 | 805 | |
| K3 | 787 | 757 | 808 | |
| k1 | 269.7 | 272.7 | 269.7 | |
| k2 | 275.3 | 282.3 | 268.3 | |
| k3 | 262.3 | 252.3 | 269.3 | |
| R | 13 | 30 | 1.4 |
| Indicator | Papered Sample | Commercial Molecular Sieve | Qualification Criteria |
|---|---|---|---|
| Calcium ion adsorption capacity | 302 | 306 | ≥295 |
| Loss on ignition (%) | 21 | 22 | ≤22 |
| Static saturated water adsorption capacity | 22 | 23 | ≥20 |
| pH | 10.8 | 9.4 | ≤11.3 |
| Specific Surface Area/(m2·g−1) | Specific Surface Area of Micropores/(m2·g−1) | Pore Volume/(m2·g−1) | Pore Volume of Micropores/(m2·g−1) | Average Pore Size/nm | Specific Surface Area/(m2·g−1) | Specific Surface Area of Micropores/(m2·g−1) |
|---|---|---|---|---|---|---|
| 13.8965 | 5.5163 | 0.0580 | 0.0023 | 16.4969 | 13.8965 | 5.5163 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| T (°C) | qm(mg·g−1) | KL(L·g−1) | R2 | KF(L·g−1) | n | R2 |
| 20 | 125.4705 | 0.0132 | 0.99361 | 30.2144 | 4.8740 | 0.87037 |
| 25 | 183.1502 | 0.0074 | 0.98871 | 19.2879 | 3.1329 | 0.92729 |
| 30 | 178.8909 | 0.0100 | 0.98226 | 26.2548 | 3.5833 | 0.93169 |
| 35 | 193.4236 | 0.0104 | 0.98428 | 26.3519 | 3.4098 | 0.90901 |
| 40 | 209.2050 | 0.0170 | 0.99693 | 40.4433 | 4.0358 | 0.86316 |
| D-R | Temkin | |||||
| T (°C) | E(KJ·mol−1) | qm(mg·g−1) | R2 | KT | bT | R2 |
| 20 | 0.020126 | 111.7165 | 0.88316 | 0.4005 | 118.5467 | 0.8744 |
| 25 | 0.018287 | 143.9518 | 0.95354 | 0.0862 | 66.5522 | 0.9523 |
| 30 | 0.025678 | 146.9643 | 0.86509 | 0.1542 | 73.6090 | 0.9337 |
| 35 | 0.026419 | 160.1971 | 0.90576 | 0.1474 | 67.9058 | 0.9271 |
| 40 | 0.038337 | 184.9704 | 0.94186 | 0.3883 | 73.1632 | 0.9172 |
| Temp. (K) | Kd | ΔG (KJ·mol−1) | ΔH (KJ·mol−1) | ΔS (kJ·mol−1·K−1) |
|---|---|---|---|---|
| 293.15 | 0.559 | 1.39 | 38.96 ± 4.47 | 0.1277 ± 0.0148 |
| 298.15 | 0.6595 | 1.03 | ||
| 303.15 | 0.943 | 0.148 | ||
| 308.15 | 1.034 | −0.085 | ||
| 313.15 | 1.601 | −1.22 |
| Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | |||||
|---|---|---|---|---|---|---|
| T (°C) | K1 (min−1) | qm (mg·g−1) | R2 | K2 (g·min−1mg−1) | qm (mg·g−1) | R2 |
| 20 | 0.0465 | 60.3 | 0.6354 | 0.108 | 84.03 | 0.99946 |
| 25 | 0.0417 | 42.5 | 0.6328 | 0.001 | 91.74 | 0.9985 |
| 30 | 0.0483 | 68.1 | 0.6854 | 0.00189 | 95.24 | 0.99866 |
| 35 | 0.0382 | 66.7 | 0.6541 | 0.00202 | 92.59 | 0.9973 |
| 40 | 0.0385 | 95.6 | 0.8762 | 0.00271 | 96.15 | 0.9985 |
| The first stage of the intra-particle diffusion model | The second stage of the intra-particle diffusion model | |||||
| T (°C) | K1d | C | R2 | K2d | C | R2 |
| 20 | 7.53 | 13.6941 | 0.9999 | 2.83 | 46.6134 | 0.8673 |
| 25 | 8.16 | 30.5929 | 0.9068 | 1.13 | 70.7917 | 0.8166 |
| 30 | 11.52 | 21.3446 | 0.9444 | 0.84 | 81.0261 | 0.8087 |
| 35 | 11.16 | 25.2030 | 0.9465 | 0.61 | 84.1140 | 0.9143 |
| 40 | 8.72 | 39.2659 | 0.9659 | 0.61 | 88.6871 | 0.7443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Zhu, L.; Ma, T.; Liang, X.; Sun, N.; Liu, F. Process Optimization and Performance Characterization of Preparing 4A Molecular Sieves from Coal Gangue. Symmetry 2025, 17, 603. https://doi.org/10.3390/sym17040603
Zhang D, Zhu L, Ma T, Liang X, Sun N, Liu F. Process Optimization and Performance Characterization of Preparing 4A Molecular Sieves from Coal Gangue. Symmetry. 2025; 17(4):603. https://doi.org/10.3390/sym17040603
Chicago/Turabian StyleZhang, Dongpeng, Laiyang Zhu, Tiantian Ma, Xiwen Liang, Nie Sun, and Fei Liu. 2025. "Process Optimization and Performance Characterization of Preparing 4A Molecular Sieves from Coal Gangue" Symmetry 17, no. 4: 603. https://doi.org/10.3390/sym17040603
APA StyleZhang, D., Zhu, L., Ma, T., Liang, X., Sun, N., & Liu, F. (2025). Process Optimization and Performance Characterization of Preparing 4A Molecular Sieves from Coal Gangue. Symmetry, 17(4), 603. https://doi.org/10.3390/sym17040603





