Individual Differences in Vertebrate Behavioural Lateralisation: The Role of Genes and Environment
Abstract
1. Introduction
2. A Brief Summary of the Knowledge on the Basis of Individual Differences in Lateralisation in Humans
2.1. Human Studies on the Genetic Basis of Individual Differences
2.2. Plasticity as Possible Source of Phenotypic Variation in Humans
2.3. Limits of Research in Humans
3. Animal Models to Study Lateralisation
3.1. Lateralisation in Non-Human Vertebrates
3.2. Practical Advantages of Animal Models
3.3. Animal Models and the Study of Evolution of Lateralisation
4. Genetic Contribution to Individual Differences in Non-Human Vertebrates
4.1. The Mouse Paw Preference Model
4.2. Heritability of Lateralisation in Primates
4.3. The Genetic of Laterality in Poecilid Fish
4.3.1. Brachyraphis episcopi
4.3.2. Girardinus falcatus
4.3.3. Gambusia hubbsi
4.3.4. Methodological Issue in Research on Poeciliid Fish
4.4. Genes, Brain Asymmetries, and Behavioural Lateralisation in the Zebrafish
4.5. Morphological Asymmetries and Behaviour
4.5.1. Asymmetry in Mouth Opening
4.5.2. Asymmetry in Male Intromittent Organs
4.5.3. Behavioural and Morphological Asymmetries in Flatfish
5. Phenotypic Plasticity of Lateralisation in Non-Human Vertebrates
5.1. Light-Induced Lateralisation Plasticity in Birds
5.2. Light-Induced Plasticity in Other Vertebrates
5.3. Predator-Induced Lateralisation Plasticity
5.4. Hormonal Effects on Lateralisation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boorman, C.J.; Shimeld, S.M. The evolution of left–right asymmetry in chordates. Bioessays 2002, 24, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Manuel, M. Early evolution of symmetry and polarity in metazoan body plans. Comptes Rendus Biol. 2009, 332, 184–209. [Google Scholar] [CrossRef] [PubMed]
- Namigai, E.K.; Kenny, N.J.; Shimeld, S.M. Right across the tree of life: The evolution of left–right asymmetry in the Bilateria. Genesis 2014, 52, 458–470. [Google Scholar] [PubMed]
- Fritsch, G.T.; Hitzig, E. Uber die elektrische Erregbarkeit des Grosshirns. Arch. Anat. Physiol. 1870, 37, 300–332. [Google Scholar]
- Harris, L.J. The discovery of cerebral specialization. A Hist. Neuropsychol. 2019, 44, 1–14. [Google Scholar]
- Wickens, A.P. A History of the Brain: From Stone Age Surgery to Modern Neuroscience; Psychology Press: Hove, UK, 2014. [Google Scholar]
- O’Regan, L.; Serrien, D.J. Individual differences and hemispheric asymmetries for language and spatial attention. Front. Hum. Neurosci. 2018, 12, 380. [Google Scholar]
- Packheiser, J.; Schmitz, J.; Arning, L.; Beste, C.; Güntürkün, O.; Ocklenburg, S. A large-scale estimate on the relationship between language and motor lateralization. Sci. Rep. 2020, 10, 13027. [Google Scholar]
- Mandal, M.K.; Bulman-Fleming, M.B.; Tiwari, G. (Eds.) Side Bias: A Neuropsychological Perspective; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Marzoli, D.; Tommasi, L. Side biases in humans (Homo sapiens): Three ecological studies on hemispheric asymmetries. Naturwissenschaften 2009, 96, 1099–1106. [Google Scholar]
- Bourne, V.J. Examining the relationship between degree of handedness and degree of cerebral lateralization for processing facial emotion. Neuropsychology 2008, 22, 350. [Google Scholar] [CrossRef]
- Costanzo, E.Y.; Villarreal, M.; Drucaroff, L.J.; Ortiz-Villafañe, M.; Castro, M.N.; Goldschmidt, M.; Wainsztein, A.E.; Ladrón-de-Guevara, M.L.; Romero, C.; Brusco, L.I.; et al. Hemispheric specialization in affective responses, cerebral dominance for language, and handedness: Lateralization of emotion, language, and dexterity. Behav. Brain Res. 2015, 288, 11–19. [Google Scholar]
- Groen, M.A.; Whitehouse, A.J.; Badcock, N.A.; Bishop, D.V. Associations between handedness and cerebral lateralisation for language: A comparison of three measures in children. PLoS ONE 2013, 8, e64876. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Hamed, E.; Said, A.; Basahi, J. Handedness and language cerebral lateralization. Eur. J. Appl. Physiol. 2002, 87, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Papousek, I.; Schulter, G. Quantitative assessment of five behavioural laterality measures: Distributions of scores and intercorrelations among right-handers. Laterality Asymmetries Body Brain Cogn. 1999, 4, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Eitler, K.; Bibok, A.; Telkes, G. Situs inversus totalis: A clinical review. Int. J. Gen. Med. 2022, 15, 2437–2449. [Google Scholar] [CrossRef]
- Corballis, M.C.; Badzakova-Trajkov, G.; Häberling, I.S. Right hand, left brain: Genetic and evolutionary bases of cerebral asymmetries for language and manual action. Wiley Interdiscip. Rev. Cogn. Sci. 2012, 3, 1–17. [Google Scholar] [CrossRef]
- McManus, I.C. Right Hand, Left Hand: The origins of Asymmetry in Brains, Bodies, Atoms, and Cultures; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Güntürkün, O.; Ströckens, F.; Ocklenburg, S. Brain lateralization: A comparative perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [CrossRef]
- Palmer, A.R. Symmetry breaking and the evolution of development. Science 2004, 306, 828–833. [Google Scholar] [CrossRef]
- Apostolou, M. Sexual selection under parental choice: The role of parents in the evolution of human mating. Evol. Hum. Behav. 2007, 28, 403–409. [Google Scholar] [CrossRef]
- Fehr, E.; Fischbacher, U. The nature of human altruism. Nature 2003, 425, 785–791. [Google Scholar] [CrossRef]
- Puts, D.A. Beauty and the beast: Mechanisms of sexual selection in humans. Evol. Hum. Behav. 2010, 31, 157–175. [Google Scholar] [CrossRef]
- Rosenberg, K.R. The evolution of human infancy: Why it helps to be helpless. Annu. Rev. Anthropol. 2021, 50, 423–440. [Google Scholar] [CrossRef]
- Güntürkün, O.; Ocklenburg, S. Ontogenesis of lateralization. Neuron 2017, 94, 249–263. [Google Scholar] [PubMed]
- Manns, M. It is not just in the genes. Symmetry 2021, 13, 1815. [Google Scholar] [CrossRef]
- Baldwin, J.M. Origin of right or left handedness. Science 1890, 404, 247–248. [Google Scholar] [CrossRef][Green Version]
- Jordan, H.E. The inheritance of left-handedness. J. Hered. 1911, 2, 19–29. [Google Scholar]
- Kellogg, G.M. The physiology of right-and left-handedness. J. Am. Med. Assoc. 1898, 30, 356–358. [Google Scholar]
- Duboc, V.; Dufourcq, P.; Blader, P.; Roussigné, M. Asymmetry of the brain: Development and implications. Annu. Rev. Genet. 2015, 49, 647–672. [Google Scholar] [CrossRef]
- Francks, C. The genetic bases of brain lateralization. In Human Language: From Genes and Brain to Behavior; MIT Press: Cambridge, MA, USA, 2019; pp. 595–608. [Google Scholar]
- McManus, C. Cerebral polymorphisms for lateralisation: Modelling the genetic and phenotypic architectures of multiple functional modules. Symmetry 2022, 14, 814. [Google Scholar] [CrossRef]
- Schmitz, J.; Metz, G.A.; Güntürkün, O.; Ocklenburg, S. Beyond the genome—Towards an epigenetic understanding of handedness ontogenesis. Prog. Neurobiol. 2017, 159, 69–89. [Google Scholar]
- Zhang, H.T.; Hiiragi, T. Symmetry breaking in the mammalian embryo. Annu. Rev. Cell Dev. Biol. 2018, 34, 405–426. [Google Scholar]
- Flöel, A.; Buyx, A.; Breitenstein, C.; Lohmann, H.; Knecht, S. Hemispheric lateralization of spatial attention in right-and left-hemispheric language dominance. Behav. Brain Res. 2005, 158, 269–275. [Google Scholar] [PubMed]
- Sha, Z.; Pepe, A.; Schijven, D.; Carrión-Castillo, A.; Roe, J.M.; Westerhausen, R.; Joliot, M.; Fisher, S.E.; Crivello, F.; Francks, C. Handedness and its genetic influences are associated with structural asymmetries of the cerebral cortex in 31,864 individuals. Proc. Natl. Acad. Sci. USA 2021, 118, e2113095118. [Google Scholar] [CrossRef] [PubMed]
- Hepper, P.G. The developmental origins of laterality: Fetal handedness. Dev. Psychobiol. 2013, 55, 588–595. [Google Scholar] [PubMed]
- Bryden, M.P. Speech lateralization in families: A preliminary study using dichotic listening. Brain Lang. 1975, 2, 201–211. [Google Scholar] [CrossRef]
- Reiss, M.; Reiss, G. Ocular dominance: Some family data. Laterality Asymmetries Body Brain Cogn. 1997, 2, 7–16. [Google Scholar]
- Risch, N.; Pringle, G. Segregation analysis of human hand preference. Behav. Genet. 1985, 15, 385–400. [Google Scholar]
- Somers, M.; Ophoff, R.A.; Aukes, M.F.; Cantor, R.M.; Boks, M.P.; Dauwan, M.; de Visser, K.L.; Kahn, R.S.; Sommer, I.E. Linkage analysis in a Dutch population isolate shows no major gene for left-handedness or atypical language lateralization. J. Neurosci. 2015, 35, 8730–8736. [Google Scholar] [CrossRef]
- Medland, S.E.; Duffy, D.L.; Wright, M.J.; Geffen, G.M.; Martin, N.G. Handedness in twins: Joint analysis of data from 35 samples. Twin Res. Hum. Genet. 2006, 9, 46–53. [Google Scholar] [CrossRef]
- Sicotte, N.L.; Woods, R.P.; Mazziotta, J.C. Handedness in twins: A meta-analysis. Laterality Asymmetries Body Brain Cogn. 1999, 4, 265–286. [Google Scholar]
- Vuoksimaa, E.; Koskenvuo, M.; Rose, R.J.; Kaprio, J. Origins of handedness: A nationwide study of 30 161 adults. Neuropsychologia 2009, 47, 1294–1301. [Google Scholar]
- Bourrat, P.; Lu, Q.; Jablonka, E. Why the missing heritability might not be in the DNA. BioEssays 2017, 39, 1700067. [Google Scholar]
- Mayhew, A.; Meyre, D. Assessing the heritability of complex traits in humans: Methodological challenges and opportunities. Curr. Genom. 2017, 18, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.J.; Turkheimer, E. Three legs of the missing heritability problem. Stud. Hist. Philos. Sci. 2022, 93, 183–191. [Google Scholar] [PubMed]
- Polderman, T.J.; Benyamin, B.; De Leeuw, C.A.; Sullivan, P.F.; Van Bochoven, A.; Visscher, P.M.; Posthuma, D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 2015, 47, 702–709. [Google Scholar] [CrossRef]
- Schaafsma, S.M.; Riedstra, B.J.; Pfannkuche, K.A.; Bouma, A.; Groothuis TG, G. Epigenesis of behavioural lateralization in humans and other animals. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 915–927. [Google Scholar] [CrossRef]
- Bishop, D.V.; Bates, T.C. Heritability of language laterality assessed by functional transcranial Doppler ultrasound: A twin study. Wellcome Open Res. 2019, 4, 161. [Google Scholar]
- Paracchini, S.; Scerri, T. Genetics of human handedness and laterality. Lateralized Brain Functions: Methods in Human and Non-Human Species; Humana Press: Totowa, NJ, USA, 2017; pp. 523–552. [Google Scholar]
- Eichler, E.E.; Flint, J.; Gibson, G.; Kong, A.; Leal, S.M.; Moore, J.H.; Nadeau, J.H. Missing heritability and strategies for finding the underlying causes of complex disease. Nat. Rev. Genet. 2010, 11, 446–450. [Google Scholar]
- Manolio, T.A.; Collins, F.S.; Cox, N.J.; Goldstein, D.B.; Hindorff, L.A.; Hunter, D.J.; McCharty, M.I.; Ramos, E.M.; Cardon, L.R.; Chakravarti, A.; et al. Finding the missing heritability of complex diseases. Nature 2009, 461, 747–753. [Google Scholar]
- Provins, K.A. Handedness and speech: A critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psychol. Rev. 1997, 104, 554. [Google Scholar]
- Obel, C.; Hedegaard, M.; Brink, T.; Secher, N.J.; Olsen, J. Psychological factors in pregnancy and mixed-handedness in the offspring. Dev. Med. Child Neurol. 2003, 45, 557–561. [Google Scholar]
- Coren, S. Family patterns in handedness: Evidence for indirect inheritance mediated by birth stress. Behav. Genet. 1995, 25, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Levander, M.; Schalling, D.; Levander, S.E. Birth stress, handedness and cognitive performance. Cortex 1989, 25, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.E.; Johnston, D.W.; Shields, M.A. Adverse birth factors predict cognitive ability, but not hand preference. Neuropsychology 2012, 26, 578. [Google Scholar] [CrossRef]
- Pfannkuche, K.A.; Bouma, A.; Groothuis, T.G. Does testosterone affect lateralization of brain and behaviour? A meta-analysis in humans and other animal species. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 929–942. [Google Scholar] [CrossRef] [PubMed]
- De Kovel, C.G.; Carrión-Castillo, A.; Francks, C. A large-scale population study of early life factors influencing left-handedness. Sci. Rep. 2019, 9, 584. [Google Scholar] [CrossRef]
- Donnot, J.; Vauclair, J. Infant holding preferences in maternity hospitals: Testing the hypothesis of the lateralized perception of emotions. Dev. Neuropsychol. 2007, 32, 881–890. [Google Scholar] [CrossRef]
- Vervloed, M.P.; Hendriks, A.W.; van den Eijnde, E. The effects of mothers’ past infant-holding preferences on their adult children’s face processing lateralisation. Brain Cogn. 2011, 75, 248–254. [Google Scholar] [CrossRef]
- Gerrits, R. Variability in hemispheric functional segregation phenotypes: A review and general mechanistic model. Neuropsychol. Rev. 2024, 34, 27–40. [Google Scholar] [CrossRef]
- Young, G. Multi-factorial Causality in Laterality. In Causality and Development; Springer: Berlin, Germany, 2019. [Google Scholar]
- Bisazza, A.; Brown, C. Lateralization of cognitive functions in fish. In Fish Cognition and Behavior; Brown, C., Laland, K., Kraus, J., Eds.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bisazza, A.; Rogers, L.J.; Vallortigara, G. The origins of cerebral asymmetry: A review of evidence of behavioural and brain lateralization in fishes, reptiles and amphibians. Neurosci. Biobehav. Rev. 1998, 22, 411–426. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J.; Bisazza, A. Possible evolutionary origins of cognitive brain lateralization. Brain Res. Rev. 1999, 30, 164–175. [Google Scholar] [CrossRef]
- Wiper, M.L. Evolutionary and mechanistic drivers of laterality: A review and new synthesis. Laterality Asymmetries Body Brain Cogn. 2017, 22, 740–770. [Google Scholar] [CrossRef] [PubMed]
- Corballis, M.C. The evolution and genetics of cerebral asymmetry. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B.; Lochy, A. Is human face recognition lateralized to the right hemisphere due to neural competition with left-lateralized visual word recognition? A critical review. Brain Struct. Funct. 2022, 227, 599–629. [Google Scholar] [CrossRef] [PubMed]
- Franklin, W.E., III; Lima, S.L. Laterality in avian vigilance: Do sparrows have a favourite eye? Anim. Behav. 2001, 62, 879–885. [Google Scholar] [CrossRef]
- Krakauer, A.H.; Blundell, M.A.; Scanlan, T.N.; Wechsler, M.S.; McCloskey, E.A.; Jennifer, H.Y.; Patricelli, G.L. Successfully mating male sage-grouse show greater laterality in courtship and aggressive interactions. Anim. Behav. 2016, 111, 261–267. [Google Scholar] [CrossRef]
- Rogers, L.J. Evolution of hemispheric specialization: Advantages and disadvantages. Brain Lang. 2000, 73, 236–253. [Google Scholar] [CrossRef]
- Rogers, L.J. Advantages and disadvantages of lateralization. In Comparative Vertebrate Lateralization; Cambridge University Press: Cambridge, UK, 2002; pp. 126–153. [Google Scholar]
- Vallortigara, G.; Rogers, L. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef]
- Rogers, L.J.; Zucca, P.; Vallortigara, G. Advantages of having a lateralized brain. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, S420–S422. [Google Scholar] [CrossRef]
- Dadda, M.; Bisazza, A. Lateralized female topminnows can forage and attend to a harassing male simultaneously. Behav. Ecol. 2006, 17, 358–363. [Google Scholar] [CrossRef]
- Bisazza, A.; Dadda, M. Enhanced schooling performance in lateralized fishes. Proc. R. Soc. B Biol. Sci. 2005, 272, 1677–1681. [Google Scholar] [CrossRef]
- Gatto, E.; Agrillo, C.; Brown, C.; Dadda, M. Individual differences in numerical skills are influenced by brain lateralization in guppies (Poecilia reticulata). Intelligence 2019, 74, 12–17. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Montalbano, G.; Dadda, M.; Bertolucci, C. Lateralization correlates with individual differences in inhibitory control in zebrafish. Biol. Lett. 2020, 16, 20200296. [Google Scholar] [CrossRef] [PubMed]
- Magat, M.; Brown, C. Laterality enhances cognition in Australian parrots. Proc. R. Soc. B Biol. Sci. 2009, 276, 4155–4162. [Google Scholar] [CrossRef] [PubMed]
- McGrew, W.C.; Marchant, L.F. Laterality of hand use pays off in foraging success for wild chimpanzees. Primates 1999, 40, 509–513. [Google Scholar] [CrossRef]
- Dadda, M.; Zandona, E.; Agrillo, C.; Bisazza, A. The costs of hemispheric specialization in a fish. Proc. R. Soc. B Biol. Sci. 2009, 276, 4399–4407. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J.; Bisazza, A.; Lippolis, G.; Robins, A. Complementary right and left hemifield use for predatory and agonistic behaviour in toads. NeuroReport 1998, 9, 3341–3344. [Google Scholar] [CrossRef]
- Lippolis, G.; Bisazza, A.; Rogers, L.J.; Vallortigara, G. Lateralisation of predator avoidance responses in three species of toads. Laterality Asymmetries Body Brain Cogn. 2002, 7, 163–183. [Google Scholar] [CrossRef]
- Whiteside, M.A.; Bess, M.M.; Frasnelli, E.; Beardsworth, C.E.; Langley, E.J.; van Horik, J.O.; Madden, J.R. Low survival of strongly footed pheasants may explain constraints on lateralization. Sci. Rep. 2018, 8, 13791. [Google Scholar] [CrossRef]
- Bisazza, A.; Cantalupo, C.; Capocchiano, M.; Vallortigara, G. Population lateralisation and social behaviour: A study with 16 species of fish. Laterality Asymmetries Body Brain Cogn. 2000, 5, 269–284. [Google Scholar] [CrossRef]
- Mitchell, A.; Booth, D.J.; Nagelkerken, I. Ocean warming and acidification degrade shoaling performance and lateralization of novel tropical–temperate fish shoals. Glob. Change Biol. 2022, 28, 1388–1401. [Google Scholar] [CrossRef]
- Billiard, S.; Faurie, C.; Raymond, M. Maintenance of handedness polymorphism in humans: A frequency-dependent selection model. J. Theor. Biol. 2005, 235, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Munoz, E.; Rato, C.; Jorge, F.; Carretero, M.A. Lateralization in escape behaviour at different hierarchical levels in a Gecko: Tarentola angustimentalis from Eastern Canary Islands. PLoS ONE 2013, 8, e78329. [Google Scholar] [PubMed]
- Ghirlanda, S.; Frasnelli, E.; Vallortigara, G. Intraspecific competition and coordination in the evolution of lateralization. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 861–866. [Google Scholar]
- Faurie, C.; Raymond, M.; Uomini, N. Origins, development, and persistence of laterality in humans. In Laterality in Sports; Academic Press: Cambridge, MA, USA, 2016; pp. 11–30. [Google Scholar]
- Ghirlanda, S.; Vallortigara, G. The evolution of brain lateralization: A game-theoretical analysis of population structure. Proc. R. Soc. London. Ser. B Biol. Sci. 2004, 271, 853–857. [Google Scholar]
- Hopkins, W.D. Heritability of hand preference in chimpanzees (Pan troglodytes): Evidence from a partial interspecies cross-fostering study. J. Comp. Psychol. 1999, 113, 307. [Google Scholar] [CrossRef]
- Berg, M.L.; Micallef, S.A.; Eastwood, J.R.; Ribot, R.F.; Bennett, A.T. Spatial and temporal patterns of lateralization in a parrot species complex. Evol. Ecol. 2020, 34, 789–802. [Google Scholar]
- Brown, C.; Gardner, C.; Braithwaite, V.A. Population variation in lateralized eye use in the poeciliid Brachyraphis episcopi. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, S455–S457. [Google Scholar] [CrossRef]
- García-Muñoz, E.; Gomes, V.; Carretero, M.A. Lateralization in refuge selection in Podarcis hispanica at different hierarchical levels. Behav. Ecol. 2012, 23, 955–959. [Google Scholar]
- Hulthén, K.; Heinen-Kay, J.L.; Schmidt, D.A.; Langerhans, R.B. Predation shapes behavioral lateralization: Insights from an adaptive radiation of livebearing fish. Behav. Ecol. 2021, 32, 1321–1329. [Google Scholar]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar]
- Pigliucci, M. Phenotypic Plasticity: Beyond Nature and Nurture; JHU Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Sultan, S.E. Evolutionary implications of phenotypic plasticity in plants. In Evolutionary Biology; Springer: Boston, MA, USA, 1987; Volume 21, pp. 127–178. [Google Scholar]
- West-Eberhard, M.J. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 1989, 20, 249–278. [Google Scholar]
- Romano, M.; Parolini, M.; Caprioli, M.; Spiezio, C.; Rubolini, D.; Saino, N. Individual and population-level sex-dependent lateralization in yellow-legged gull (Larus michahellis) chicks. Behav. Process. 2015, 115, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Western, J.; Braithwaite, V.A. The influence of early experience on, and inheritance of, cerebral lateralization. Anim. Behav. 2007, 74, 231–238. [Google Scholar] [CrossRef]
- Bisazza, A.; Facchin, L.; Vallortigara, G. Heritability of lateralization in fish: Concordance of right–left asymmetry between parents and offspring. Neuropsychologia 2000, 38, 907–912. [Google Scholar] [CrossRef]
- Bisazza, A.; Dadda, M.; Facchin, L.; Vigo, F. Artificial selection on laterality in the teleost fish Girardinus falcatus. Behav. Brain Res. 2007, 178, 29–38. [Google Scholar] [CrossRef]
- Barth, K.A.; Miklosi, A.; Watkins, J.; Bianco, I.H.; Wilson, S.W.; Andrew, R.J. Fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 2005, 15, 844–850. [Google Scholar]
- Facchin, L.; Argenton, F.; Bisazza, A. Lines of Danio rerio selected for opposite behavioural lateralization show differences in anatomical left–right asymmetries. Behav. Brain Res. 2009, 197, 157–165. [Google Scholar]
- Domenichini, A.; Dadda, M.; Facchin, L.; Bisazza, A.; Argenton, F. Isolation and genetic characterization of mother-of-snow-white, a maternal effect allele affecting laterality and lateralized behaviors in zebrafish. PLoS ONE 2011, 6, e25972. [Google Scholar]
- Westergaard, G.C.; Suomi, S.J. Lateral bias in capuchin monkeys (Cebus apella): Concordance between parents and offspring. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 1997, 31, 143–147. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Bales, S.A.; Bennett, A.J. Heritability of hand preference in chimpanzees (Pan). Int. J. Neurosci. 1994, 74, 17–26. [Google Scholar]
- Collins, R.L. On the inheritance of handedness. I. Laterality in inbred mice. J. Hered. 1968, 59, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.L. On the inheritance of handedness. II. Selection for sinistrality in mice. J. Hered. 1969, 60, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Roubertoux, P.L.; Le Roy, I.; Tordjman, S.; Cherfou, A.; Migliore-Samour, D. Analysis of quantitative trait loci for behavioral laterality in mice. Genetics 2003, 163, 1023–1030. [Google Scholar] [CrossRef]
- Collins, R.L. On the inheritance of direction and degree of asymmetry. In Cerebral Lateralization in Nonhuman Species; Glick, S.D., Ed.; Academic Press: New York, NY, USA, 1985; pp. 41–71. [Google Scholar]
- Biddle, F.G.; Coffaro, C.M.; Ziehr, J.E.; Eales, B.A. Genetic variation in paw preference (handedness) in the mouse. Genome 1993, 36, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.A.; Biddle, F.G. The SWV and C57BL/6 inbred strains exhibit the same difference in lateralization of handedness as the selected LO and HI lines. Mouse Genome 1992, 90, 90–92. [Google Scholar]
- Collins, R.L. When left-handed mice live in right-handed worlds. Science 1975, 187, 181–184. [Google Scholar] [CrossRef]
- Manns, M.; El Basbasse, Y.; Freund, N.; Ocklenburg, S. Paw preferences in mice and rats: Meta-analysis. Neurosci. Biobehav. Rev. 2021, 127, 593–606. [Google Scholar] [CrossRef]
- Hopkins, W.D. Laterality in maternal cradling and infant positional biases: Implications for the development and evolution of hand preferences in nonhuman primates. Int. J. Primatol. 2004, 25, 1243–1265. [Google Scholar] [CrossRef]
- Evans, J.P.; Pilastro, A.; Schlupp, I. (Eds.) Ecology and Evolution of Poeciliid Fishes; University of Chicago Press: Chicago, IL, USA, 2011. [Google Scholar]
- Meffe, G.K.; Snelson, F.F. An ecological overview of poeciliid fishes. In Ecology and Evolution of Livebearing Fishes (Poeciliidae). Prentice Hall: Englewood Cliffs, NJ, USA, 1989; pp. 13–31. [Google Scholar]
- Bisazza, A.; Sovrano, V.A.; Vallortigara, G. Consistency among different tasks of left–right asymmetries in lines of fish originally selected for opposite direction of lateralization in a detour task. Neuropsychologia 2001, 39, 1077–1085. [Google Scholar] [CrossRef]
- Bisazza, A.; Facchin, L.; Pignatti, R.; Vallortigara, G. Lateralization of detour behaviour in poeciliid fish: The effect of species, gender and sexual motivation. Behav. Brain Res. 1998, 91, 157–164. [Google Scholar] [CrossRef]
- Agrillo, C.; Dadda, M.; Bisazza, A. Escape behaviour elicited by a visual stimulus. A comparison between lateralised and non-lateralised female topminnows. Lateralit 2009, 14, 300–314. [Google Scholar]
- Bisazza, A.; Dadda, M.; Cantalupo, C. Further evidence for mirror-reversed laterality in lines of fish selected for leftward or rightward turning when facing a predator model. Behav. Brain Res. 2005, 156, 165–171. [Google Scholar] [PubMed]
- Dadda, M. Vantaggi Selettivi Della Lateralizzazione Cerebrale. Master Degree Thesis, University of Padova, Padova, Italy, 2001. [Google Scholar]
- Dadda, M.; Zandonà, E.; Bisazza, A. Emotional responsiveness in fish from lines artificially selected for a high or low degree of laterality. Physiol. Behav. 2007, 92, 764–772. [Google Scholar] [PubMed]
- Dadda, M.; Bisazza, A. Prenatal light exposure affects development of behavioural lateralization in a livebearing fish. Behav. Process. 2012, 91, 115–118. [Google Scholar]
- Dadda, M.; Vendramin, V.; Agrillo, C. Prenatal visual exposure to a predator influences lateralization in goldbelly topminnows. Symmetry 2020, 12, 1257. [Google Scholar] [CrossRef]
- Dionne, M. Cannibalism, food availability, and reproduction in the mosquito fish (Gambusia affinis): A laboratory experiment. Am. Nat. 1985, 126, 16–23. [Google Scholar]
- Meffe, G.K. Density-dependent cannibalism in the endangered Sonoran topminnow (Poeciliopsis occidentalis). Southwest. Nat. 1984, 29, 500–503. [Google Scholar]
- Bošković, A.; Rando, O.J. Transgenerational epigenetic inheritance. Annu. Rev. Genet. 2018, 52, 21–41. [Google Scholar]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar]
- Wang, L.; Liu, Z.; Lin, H.; Ma, D.; Tao, Q.; Liu, F. Epigenetic regulation of left–right asymmetry by DNA methylation. EMBO J. 2017, 36, 2987–2997. [Google Scholar]
- Concha, M.L.; Burdine, R.D.; Russell, C.; Schier, A.F.; Wilson, S.W. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron 2000, 28, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.E.; Liang, J.O.; Gamse, J.T. Leaning to the left: Laterality in the zebrafish forebrain. Trends Neurosci. 2003, 26, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Jesuthasan, S. Fear, anxiety, and control in the zebrafish. Dev. Neurobiol. 2012, 72, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Parhar, I.S. Role of habenula in social and reproductive behaviors in fish: Comparison with mammals. Front. Behav. Neurosci. 2022, 15, 818782. [Google Scholar] [CrossRef]
- Signore, I.A.; Palma, K.; Concha, M.L. Nodal signalling and asymmetry of the nervous system. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150401. [Google Scholar] [CrossRef]
- Dreosti, E.; Llopis, N.V.; Carl, M.; Yaksi, E.; Wilson, S.W. Left-right asymmetry is required for the habenulae to respond to both visual and olfactory stimuli. Curr. Biol. 2014, 24, 440–445. [Google Scholar] [CrossRef]
- Dadda, M.; Domenichini, A.; Piffer, L.; Argenton, F.; Bisazza, A. Early differences in epithalamic left–right asymmetry influence lateralization and personality of adult zebrafish. Behav. Brain Res. 2010, 206, 208–215. [Google Scholar] [CrossRef]
- Palmer, A.R. Animal asymmetry. Curr. Biol. 2009, 19, R473–R477. [Google Scholar] [CrossRef]
- Hori, M.; Nakajima, M.; Hata, H.; Yasugi, M.; Takahashi, S.; Nakae, M.; Yamaoka, K.; Kohda, M.; Kitamura, J.; Maehata, M.; et al. Laterality is universal among fishes but increasingly cryptic among derived groups. Zool. Sci. 2017, 34, 267–274. [Google Scholar] [CrossRef]
- Malashichev, Y.B. Asymmetries in amphibians: A review of morphology and behaviour. Laterality Asymmetries Body Brain Cogn. 2002, 7, 197–217. [Google Scholar] [CrossRef]
- Laia, R.C.; Pinto, M.P.; Menezes, V.A.; Rocha, C.F.D. Asymmetry in reptiles: What do we know so far? Springer Sci. Rev. 2015, 3, 13–26. [Google Scholar] [CrossRef]
- Matsui, S.; Takeuchi, Y.; Hori, M. Relation between morphological antisymmetry and behavioral laterality in a poeciliid fish. Zool. Sci. 2013, 30, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Hori, M.; Myint, O.; Kohda, M. Lateral bias of agonistic responses to mirror images and morphological asymmetry in the Siamese fighting fish (Betta splendens). Behav. Brain Res. 2010, 208, 106–111. [Google Scholar] [CrossRef]
- Palmer, A.R. What determines direction of asymmetry: Genes, environment or chance? Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150417. [Google Scholar]
- Schreiber, A.M. Flatfish: An asymmetric perspective on metamorphosis. Curr. Top. Dev. Biol. 2013, 103, 167–194. [Google Scholar]
- Suzuki, T.; Washio, Y.; Aritaki, M.; Fujinami, Y.; Shimizu, D.; Uji, S.; Hashimoto, H. Metamorphic pitx2 expression in the left habenula correlated with lateralization of eye-sidedness in flounder. Dev. Growth Differ. 2009, 51, 797–808. [Google Scholar]
- Seki, S.; Kohda, M.; Hori, M. Asymmetry of mouth morph of a freshwater goby, Rhinogobius flumineus. Zool. Sci. 2000, 17, 1321–1325. [Google Scholar]
- Bisazza, A.; Cantalupo, C.; Vallortigara, G. Lateral asymmetries during escape behavior in a species of teleost fish (Jenynsia lineata). Physiol. Behav. 1997, 61, 31–35. [Google Scholar]
- Torres-Dowdall, J.; Rometsch, S.J.; Aguilera, G.; Goyenola, G.; Meyer, A. Asymmetry in genitalia is in sync with lateralized mating behavior but not with the lateralization of other behaviors. Curr. Zool. 2020, 66, 71–81. [Google Scholar]
- Johnson, E.S.; Nielsen, M.E.; Johnson, J.B. Does asymmetrical gonopodium morphology predict lateralized behavior in the fish Xenophallus umbratilis? Front. Ecol. Evol. 2020, 8, 606856. [Google Scholar]
- Torres-Dowdall, J.; Rometsch, S.J.; Velasco, J.R.; Aguilera, G.; Kautt, A.F.; Goyenola, G.; Petry, A.C.; Deprá, G.C.; da Graça, W.J.; Meyer, A. Genetic assimilation and the evolution of direction of genital asymmetry in anablepid fishes. Proc. R. Soc. B 2022, 289, 20220266. [Google Scholar] [CrossRef] [PubMed]
- Torres-Dowdall, J.; Rometsch, S.J.; Kautt, A.F.; Aguilera, G.; Meyer, A. The direction of genital asymmetry is expressed stochastically in internally fertilizing anablepid fishes. Proc. R. Soc. B 2020, 287, 20200969. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.M. Asymmetric craniofacial remodeling and lateralized behavior in larval flatfish. J. Exp. Biol. 2006, 209, 610–621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashimoto, H.; Mizuta, A.; Okada, N.; Suzuki, T.; Tagawa, M.; Tabata, K.; Yokoyama, Y.; Sakaguchi, M.; Tanaka, M.; Toyohara, H. Isolation and characterization of a Japanese flounder clonal line, reversed, which exhibits reversal of metamorphic left-right asymmetry. Mech. Dev. 2002, 111, 17–24. [Google Scholar] [CrossRef]
- Kuan, Y.S.; Gamse, J.T.; Schreiber, A.M.; Halpern, M.E. Selective asymmetry in a conserved forebrain to midbrain projection. J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 669–678. [Google Scholar] [CrossRef]
- Policansky, D. Flatfishes and the inheritance of asymmetries. Behav. Brain Sci. 1982, 5, 262–265. [Google Scholar] [CrossRef]
- Bao, B. Genetic basis for eye migration in flatfish. In Flatfish Metamorphosis; Springer Nature: Singapore, 2023; pp. 249–267. [Google Scholar]
- Miner, B.G.; Sultan, S.E.; Morgan, S.G.; Padilla, D.K.; Relyea, R.A. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 2005, 20, 685–692. [Google Scholar] [CrossRef]
- Whitman, D.W.; Agrawal, A.A. What is phenotypic plasticity and why is it important. In Phenotypic Plasticity of Insects: Mechanisms and Consequences; Science Publisher: Berlin/Heidelberg, Germany, 2009; pp. 1–63. [Google Scholar]
- Black, A.R. Predator-induced phenotypic plasticity in Daphnia pulex: Life history and morphological responses to Notonecta and Chaoborus. Limnol. Oceanogr. 1993, 38, 986–996. [Google Scholar] [CrossRef]
- Lüning, J. Phenotypic plasticity of Daphnia pulex in the presence of invertebrate predators: Morphological and life history responses. Oecologia 1992, 92, 383–390. [Google Scholar] [CrossRef]
- Oda, S.; Hanazato, T.; Fujii, K. Change in phenotypic plasticity of a morphological defence in Daphnia galeata (Crustacea: Cladocera) in a selection experiment. J. Limnol. 2007, 66, 142–152. [Google Scholar] [CrossRef]
- Spitze, K. Predator-mediated plasticity of prey life history and morphology: Chaoborus americanus predation on Daphnia pulex. Am. Nat. 1992, 139, 229–247. [Google Scholar]
- Brönmark, C.; Miner, J.G. Predator-induced phenotypical change in body morphology in crucian carp. Science 1992, 258, 1348–1350. [Google Scholar]
- Brönmark, C.; Pettersson, L.B. Chemical cues from piscivores induce a change in morphology in crucian carp. Oikos 1994, 70, 396–402. [Google Scholar]
- Domenici, P.; Turesson, H.; Brodersen, J.; Brönmark, C. Predator-induced morphology enhances escape locomotion in crucian carp. Proc. R. Soc. B Biol. Sci. 2008, 275, 195–201. [Google Scholar]
- Nilsson, P.A.; Brönmark, C.; Pettersson, L.B. Benefits of a predtor-induced morphology in crucian carp. Oecologia 1995, 104, 291–296. [Google Scholar] [CrossRef]
- Gotthard, K.; Nylin, S. Adaptive plasticity and plasticity as an adaptation: A selective review of plasticity in animal morphology and life history. Oikos 1995, 74, 3–17. [Google Scholar]
- Price, T.D.; Qvarnström, A.; Irwin, D.E. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 1433–1440. [Google Scholar]
- Handelsman, C.A.; Broder, E.D.; Dalton, C.M.; Ruell, E.W.; Myrick, C.A.; Reznick, D.N.; Ghalambor, C.K. Predator-induced phenotypic plasticity in metabolism and rate of growth: Rapid adaptation to a novel environment. Integr. Comp. Biol. 2013, 53, 975–988. [Google Scholar]
- Nylin, S.; Gotthard, K. Plasticity in life-history traits. Annu. Rev. Entomol. 1998, 43, 63–83. [Google Scholar]
- Ekström, A.; Sandblom, E.; Blier, P.U.; Dupont Cyr, B.A.; Brijs, J.; Pichaud, N. Thermal sensitivity and phenotypic plasticity of cardiac mitochondrial metabolism in European perch, Perca fluviatilis. J. Exp. Biol. 2017, 220, 386–396. [Google Scholar]
- Menéndez, J.; Ruperto, E.F.; Taraborelli, P.A.; Sassi, P.L. Phenotypic plasticity in the energy metabolism of a small Andean rodent: Effect of short-term thermal acclimation and developmental conditions. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 303–315. [Google Scholar]
- Quinn, J.L.; Cresswell, W. Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behav. 2005, 142, 1377–1402. [Google Scholar]
- Tüzün, N.; Savaşçı, B.B.; Stoks, R. Seasonal time constraints shape life history, physiology and behaviour independently, and decouple a behavioural syndrome in a damselfly. Oikos 2021, 130, 274–286. [Google Scholar]
- Cauchoix, M.; Chaine, A.S.; Barragan-Jason, G. Cognition in context: Plasticity in cognitive performance in response to ongoing environmental variables. Front. Ecol. Evol. 2020, 8, 106. [Google Scholar]
- Lucon-Xiccato, T.; Montalbano, G.; Bertolucci, C. Adaptive phenotypic plasticity induces individual variability along a cognitive trade-off. Proc. R. Soc. B 2023, 290, 20230350. [Google Scholar]
- Du, W.G. Phenotypic plasticity in reproductive traits induced by food availability in a lacertid lizard, Takydromus septentrionalis. Oikos 2006, 112, 363–369. [Google Scholar]
- Noble, D.W.; Stenhouse, V.; Schwanz, L.E. Developmental temperatures and phenotypic plasticity in reptiles: A systematic review and meta-analysis. Biol. Rev. 2018, 93, 72–97. [Google Scholar]
- Härer, A.; Karagic, N.; Meyer, A.; Torres-Dowdall, J. Reverting ontogeny: Rapid phenotypic plasticity of colour vision in cichlid fish. R. Soc. Open Sci. 2019, 6, 190841. [Google Scholar]
- Wright, D.S.; Rietveld, E.; Maan, M.E. Developmental effects of environmental light on male nuptial coloration in Lake Victoria cichlid fish. PeerJ 2018, 6, e4209. [Google Scholar]
- Karlsson, K.; Eroukhmanoff, F.; Svensson, E.I. Phenotypic plasticity in response to the social environment: Effects of density and sex ratio on mating behaviour following ecotype divergence. PLoS ONE 2010, 5, e12755. [Google Scholar]
- Rodd, F.H.; Reznick, D.N.; Sokolowski, M.B. Phenotypic plasticity in the life history traits of guppies: Responses to social environment. Ecology 1997, 78, 419–433. [Google Scholar]
- Rogers, L.J.; Anson, J.M. Lateralisation of function in the chicken fore-brain. Pharmacol. Biochem. Behav. 1979, 10, 679–686. [Google Scholar] [PubMed]
- Rogers, L.J. Early experiential effects on laterality: Research on chicks has relevance to other species. Laterality Asymmetries Body Brain Cogn. 1997, 2, 199–219. [Google Scholar]
- Vallortigara, G.; Andrew, R.J. Lateralization of response by chicks to change in a model partner. Anim. Behav. 1991, 41, 187–194. [Google Scholar]
- Rogers, L.J. Light experience and asymmetry of brain function in chickens. Nature 1982, 297, 223–225. [Google Scholar]
- Casey, M.B.; Sleigh, M.J. Prenatal visual experience induces postnatal motor laterality in Japanese quail chicks (Coturnix coturnix japonica). Dev. Psychobiol. 2014, 56, 489–497. [Google Scholar]
- George, I.; Lerch, N.; Jozet-Alves, C.; Lumineau, S. Effect of embryonic light exposure on laterality and sociality in quail chicks (Coturnix coturnix japonica). Appl. Anim. Behav. Sci. 2021, 236, 105270. [Google Scholar]
- Gülbetekin, E.; Güntürkün, O.; Dural, S.; Cetinkaya, H. Visual asymmetries in Japanese quail (Coturnix japonica) retain a lifelong potential for plasticity. Behav. Neurosci. 2009, 123, 815. [Google Scholar]
- Letzner, S.; Manns, M.; Güntürkün, O. Light-dependent development of the tectorotundal projection in pigeons. Eur. J. Neurosci. 2020, 52, 3561–3571. [Google Scholar]
- Letzner, S.; Patzke, N.; Verhaal, J.; Manns, M. Shaping a lateralized brain: Asymmetrical light experience modulates access to visual interhemispheric information in pigeons. Sci. Rep. 2014, 4, 4253. [Google Scholar]
- Manns, M.; Güntürkün, O. Monocular deprivation alters the direction of functional and morphological asymmetries in the pigeon’s (Columba livia) visual system. Behav. Neurosci. 1999, 113, 1257. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.; Diekamp, B.; Güntürkün, O. Embryonic light stimulation induces different asymmetries in visuoperceptual and visuomotor pathways of pigeons. Behav. Brain Res. 2002, 134, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.; Ströckens, F. Functional and structural comparison of visual lateralization in birds–similar but still different. Front. Psychol. 2014, 5, 206. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S.; Mench, J.A. Exposing avian embryos to light affects post-hatch anti-predator fear responses. Appl. Anim. Behav. Sci. 2017, 186, 80–84. [Google Scholar] [CrossRef]
- Wichman, A.; Freire, R.; Rogers, L.J. Light exposure during incubation and social and vigilance behaviour of domestic chicks. Laterality 2009, 14, 381–394. [Google Scholar] [CrossRef]
- Zappia, J.V.; Rogers, L.J. Light experience during development affects asymmetry of forebrain function in chickens. Dev. Brain Res. 1983, 11, 93–106. [Google Scholar] [CrossRef]
- Lorenzi, E.; Mayer, U.; Rosa-Salva, O.; Morandi-Raikova, A.; Vallortigara, G. Spontaneous and light-induced lateralization of immediate early genes expression in domestic chicks. Behav. Brain Res. 2019, 368, 111905. [Google Scholar] [CrossRef]
- Wichman, A.; Rogers, L.J.; Freire, R. Visual lateralization and development of spatial and social spacing behaviour of chicks (Gallus gallus domesticus). Behav. Process. 2009, 81, 14–19. [Google Scholar] [CrossRef]
- Andrew, R.J.; Johnston, A.N.; Robins, A.; Rogers, L.J. Light experience and the development of behavioural lateralisation in chicks: II. Choice of familiar versus unfamiliar model social partner. Behav. Brain Res. 2004, 155, 67–76. [Google Scholar] [CrossRef]
- Chiandetti, C.; Vallortigara, G. Distinct effect of early and late embryonic light-stimulation on chicks’ lateralization. Neuroscience 2019, 414, 1–7. [Google Scholar] [CrossRef]
- Rogers, L.J.; Andrew, R.J.; Johnston AN, B. Light experience and the development of behavioural lateralization in chicks: III. Learning to distinguish pebbles from grains. Behav. Brain Res. 2007, 177, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chiandetti, C.; Regolin, L.; Rogers, L.J.; Vallortigara, G. Effects of light stimulation of embryos on the use of position-specific and object-specific cues in binocular and monocular domestic chicks (Gallus gallus). Behav. Brain Res. 2005, 163, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Costalunga, G.; Kobylkov, D.; Rosa-Salva, O.; Morandi-Raikova, A.; Vallortigara, G.; Mayer, U. Responses in the left and right entopallium are differently affected by light stimulation in embryo. iScience 2024, 27, 109268. [Google Scholar] [PubMed]
- Costalunga, G.; Kobylkov, D.; Rosa-Salva, O.; Vallortigara, G.; Mayer, U. Light-incubation effects on lateralisation of single unit responses in the visual Wulst of domestic chicks. Brain Struct. Funct. 2021, 227, 1–17. [Google Scholar]
- Andrew, R.J.; Osorio, D.; Budaev, S. Light during embryonic development modulates patterns of lateralization strongly and similarly in both zebrafish and chick. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 983–989. [Google Scholar]
- Rogers, L.J.; Deng, C. Light experience and lateralization of the two visual pathways in the chick. Behav. Brain Res. 1999, 98, 277–287. [Google Scholar]
- Rogers, L.J.; Rajendra, S. Modulation of the development of light-initiated asymmetry in chick thalamofugal visual projections by oestradiol. Exp. Brain Res. 1993, 93, 89–94. [Google Scholar] [CrossRef]
- Rogers, L.J.; Workman, L. Light exposure during incubation affects competitive behaviour in domestic chicks. Appl. Anim. Behav. Sci. 1989, 23, 187–198. [Google Scholar]
- Budaev, S.; Andrew, R.J. Patterns of early embryonic light exposure determine behavioural asymmetries in zebrafish: A habenular hypothesis. Behav. Brain Res. 2009, 200, 91–94. [Google Scholar] [CrossRef]
- Budaev, S.V.; Andrew, R.J. Shyness and behavioural asymmetries in larval zebrafish (Brachydanio rerio) developed in light and dark. Behaviour 2009, 146, 1037–1052. [Google Scholar]
- Berlinghieri, F.; Jansen, N.; Riedstra, B.; Brown, C.; Groothuis, T.G. The effect of light during embryonic development on laterality and exploration in Western Rainbowfish. Laterality 2024, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Davies-Colley, R.J.; Quinn, J.M. Stream lighting in five regions of North Island, New Zealand: Control by channel size and riparian vegetation. N. Z. J. Mar. Freshw. Res. 1998, 32, 591–605. [Google Scholar] [CrossRef]
- Reznick, D.; Butler, M.J., IV; Rodd, H. Life-history evolution in guppies. VII. The comparative ecology of high-and low-predation environments. Am. Nat. 2001, 157, 126–140. [Google Scholar] [CrossRef]
- Cobcroft, J.M.; Pankhurst, P.M.; Hart, P.R.; Battalene, S.C. The effects of light intensity and algae-induced turbidity on feeding behaviour of larval striped trumpeter. J. Fish Biol. 2001, 59, 1181–1197. [Google Scholar]
- Sovrano, V.A.; Bertolucci, C.; Frigato, E.; Foà, A.; Rogers, L.J. Influence of exposure in ovo to different light wavelengths on the lateralization of social response in zebrafish larvae. Physiol. Behav. 2016, 157, 258–264. [Google Scholar] [CrossRef]
- Endler, J.A. The color of light in forests and its implications. Ecol. Monogr. 1993, 63, 1–27. [Google Scholar] [CrossRef]
- DeNicola, M.; Hoagland, K.D.; Roemer, S.C. Influences of canopy cover on spectral irradiance and periphyton assemblages in a prairie stream. J. N. Am. Benthol. Soc. 1992, 11, 391–404. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Dadda, M.; Bisazza, A. Vegetation cover induces developmental plasticity of lateralization in tadpoles. Curr. Zool. 2020, 66, 393–399. [Google Scholar] [CrossRef]
- Babbitt, K.J.; Tanner, G.W. Effects of cover and predator identity on predation of Hyla squirella tadpoles. J. Herpetol. 1997, 31, 128–130. [Google Scholar] [CrossRef]
- Kopp, K.; Wachlevski, M.; Eterovick, P.C. Environmental complexity reduces tadpole predation by water bugs. Can. J. Zool. 2006, 84, 136–140. [Google Scholar] [CrossRef]
- Fujisawa, K.; Takami, T.; Shintani, H.; Sasai, N.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Seasonal variations in photoperiod affect hepatic metabolism of medaka (Oryzias latipes). FEBS Open Bio 2021, 11, 1029–1040. [Google Scholar] [PubMed]
- Masahiko, A.; Isao, H. Seasonal changes in ovarian response to photoperiods in orangered type medaka. Zool. Sci. 1989, 6, 943–950. [Google Scholar]
- Lucon-Xiccato, T.; Montalbano, G.; Frigato, E.; Loosli, F.; Foulkes, N.S.; Bertolucci, C. Medaka as a model for seasonal plasticity: Photoperiod-mediated changes in behaviour, cognition, and hormones. Horm. Behav. 2022, 145, 105244. [Google Scholar] [PubMed]
- Dadda, M.; Bisazza, A. Early visual experience influences behavioral lateralization in the guppy. Anim. Cogn. 2016, 19, 949–958. [Google Scholar] [CrossRef]
- El Hage, W.; Griebel, G.; Belzung, C. Long-term impaired memory following predatory stress in mice. Physiol. Behav. 2006, 87, 45–50. [Google Scholar]
- Ferrari, M.C. Short-term environmental variation in predation risk leads to differential performance in predation-related cognitive function. Anim. Behav. 2014, 95, 9–14. [Google Scholar]
- Ramalingam, L.; Madhaiyan, V. Effect of acute predator stress on learning and memory in a zebrafish model. Natl. J. Physiol. Pharm. Pharmacol. 2023, 13, 2304–2308. [Google Scholar]
- De Santi, A.; Bisazza, A.; Cappelletti, M.; Vallortigara, G. Prior exposure to a predator influences lateralization of cooperative predator inspection in the guppy, Poecilia reticulata. Ital. J. Zool. 2000, 67, 175–178. [Google Scholar]
- Kelley, J.L.; Magurran, A.E. Learned predator recognition and antipredator responses in fishes. Fish Fish. 2003, 4, 216–226. [Google Scholar]
- Broder, E.D.; Angeloni, L.M. Predator-induced phenotypic plasticity of laterality. Anim. Behav. 2014, 98, 125–130. [Google Scholar] [CrossRef]
- Ferrari, M.C.; McCormick, M.I.; Mitchell, M.D.; Allan, B.J.; Gonçalves, E.J.; Chivers, D.P. Daily variation in behavioural lateralization is linked to predation stress in a coral reef fish. Anim. Behav. 2017, 133, 189–193. [Google Scholar]
- Lucon-Xiccato, T.; Crane, A.L.; Ferrari, M.C.; Chivers, D.P. Exposure to predation risk reduces lateralization in fathead minnows. Can. J. Exp. Psychol. 2020, 74, 260. [Google Scholar] [PubMed]
- Panizzon, P.; Gismann, J.; Riedstra, B.; Nicolaus, M.; Brown, C.; Groothuis, T. Effects of early predation and social cues on the relationship between laterality and personality. Behav. Ecol. 2024, 35, arae012. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.C.; McCormick, M.I.; Allan, B.J.; Choi, R.B.; Ramasamy, R.A.; Chivers, D.P. The effects of background risk on behavioural lateralization in a coral reef fish. Funct. Ecol. 2015, 29, 1553–1559. [Google Scholar]
- Lucon-Xiccato, T.; Chivers, D.P.; Mitchell, M.D.; Ferrari, M.C. Prenatal exposure to predation affects predator recognition learning via lateralization plasticity. Behav. Ecol. 2016, 28, 253–259. [Google Scholar]
- Dadda, M.; Bisazza, A. Does brain asymmetry allow efficient performance of simultaneous tasks? Anim. Behav. 2006, 72, 523–529. [Google Scholar]
- Dadda, M.; Koolhaas, W.H.; Domenici, P. Behavioural asymmetry affects escape performance in a teleost fish. Biol. Lett. 2010, 6, 414–417. [Google Scholar]
- Gazzola, A.; Balestrieri, A.; Scribano, G.; Fontana, A.; Pellitteri-Rosa, D. Contextual behavioural plasticity in Italian agile frog (Rana latastei) tadpoles exposed to native and alien predator cues. J. Exp. Biol. 2021, 224, jeb240465. [Google Scholar] [CrossRef]
- Gazzola, A.; Guadin, B.; Balestrieri, A.; Pellitteri-Rosa, D. Effects of predation risk on the sensory asymmetries and defensive strategies of Bufotes balearicus tadpoles. Anim. Cogn. 2023, 26, 491–501. [Google Scholar]
- Pellitteri-Rosa, D.; Gazzola, A. Context-dependent behavioural lateralization in the European pond turtle, Emys orbicularis (Testudines, Emydidae). J. Exp. Biol. 2018, 221, jeb186775. [Google Scholar]
- Jozet-Alves, C.; Hébert, M. Embryonic exposure to predator odour modulates visual lateralization in cuttlefish. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122575. [Google Scholar] [CrossRef] [PubMed]
- Beking, T.; Geuze, R.H.; Groothuis, T.G. Investigating effects of steroid hormones on lateralization of brain and behavior. In Lateralized Brain Functions: Methods in Human and Non-Human Species; Humana: New York, NY, USA, 2017; pp. 633–666. [Google Scholar]
- Denenberg, V.H.; Fitch, R.H.; Schrott, L.M.; Cowell, P.E.; Waters, N.S. Corpus callosum: Interactive effects of infantile handling and testosterone in the rat. Behav. Neurosci. 1991, 105, 562. [Google Scholar] [CrossRef] [PubMed]
- Lauter, J.L. The EPIC model of functional asymmetries: Implications for research on laterality in the auditory and other systems. Front. Biosci. 2007, 12, 3734–3756. [Google Scholar] [CrossRef] [PubMed]
- Possenti, C.D.; Romano, A.; Caprioli, M.; Rubolini, D.; Spiezio, C.; Saino, N.; Parolini, M. Yolk testosterone affects growth and promotes individual-level consistency in behavioral lateralization of yellow-legged gull chicks. Horm. Behav. 2016, 80, 58–67. [Google Scholar] [CrossRef]
- Riedstra, B.J.; Pfannkuche, K.A.; Groothuis, A.G. Organisational and Activational Effects of Prenatal Exposure to Testosterone on Lateralisation in the Domestic Chicken (Gallus gallus domesticus). In Behavioral Lateralization in Vertebrates: Two Sides of the Same Coin; Springer: Berlin/Heidelberg, Germany, 2012; pp. 87–105. [Google Scholar]
- Henriksen, R.; Rettenbacher, S.; Groothuis, T.G. Maternal corticosterone elevation during egg formation in chickens (Gallus gallus domesticus) influences offspring traits, partly via prenatal undernutrition. Gen. Comp. Endocrinol. 2013, 191, 83–91. [Google Scholar] [CrossRef]
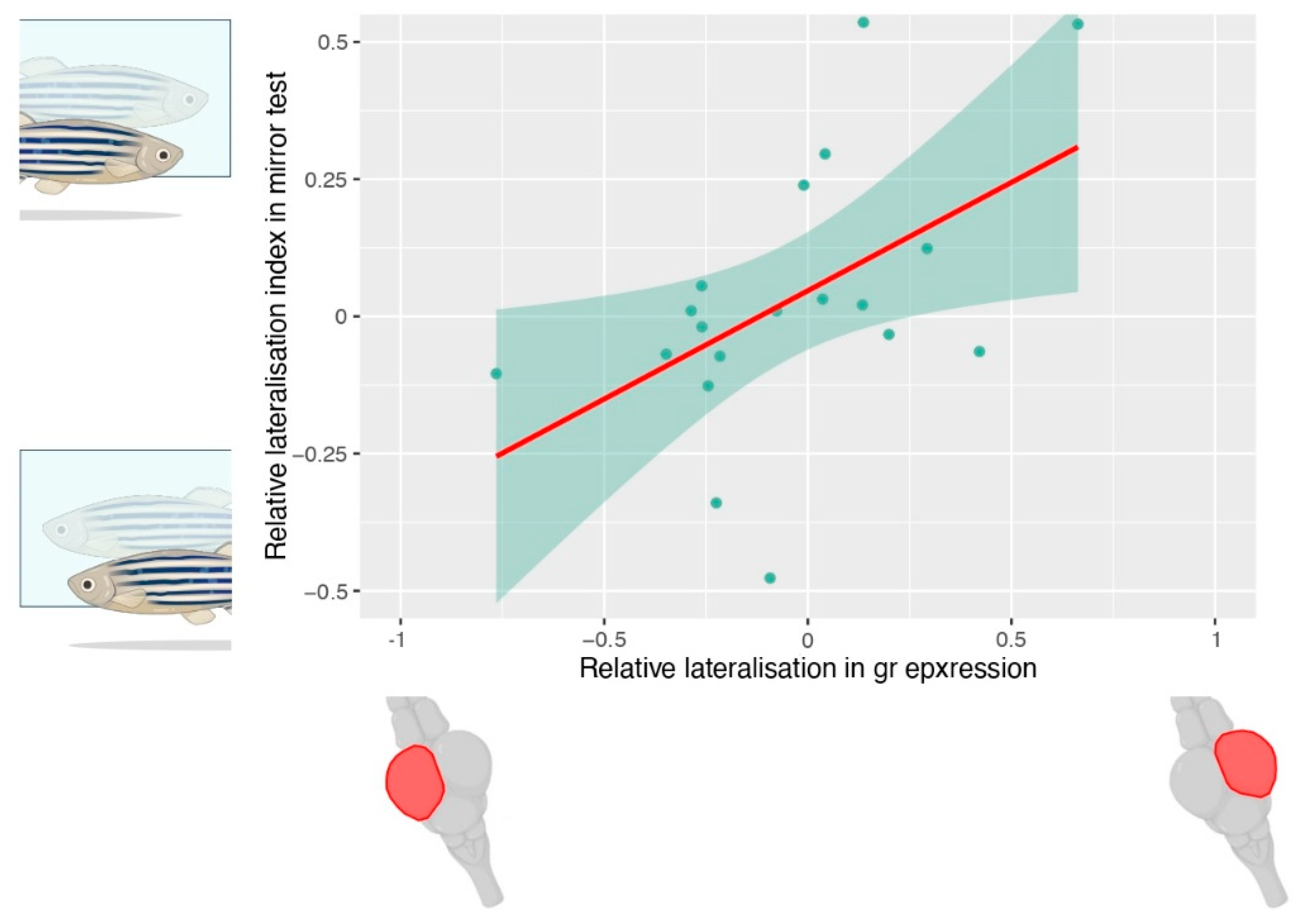
- Rovegno, E.; Frigato, E.; Dalla Valle, L.; Bertolucci, C.; Lucon-Xiccato, T. Linking stress and cognitive lateralisation: Expression of glucocorticoid-receptor affects individual differences in lateralisation in zebrafish. Anim. Cogn. 2024, 28, 21. [Google Scholar] [CrossRef]
- Besson, M.; Gache, C.; Bertucci, F.; Brooker, R.M.; Roux, N.; Jacob, H.; Cécile, B.; Anna Sovrano, V.A.; Dixson, D.L.; Lecchini, D. Exposure to agricultural pesticide impairs visual lateralization in a larval coral reef fish. Sci. Rep. 2017, 7, 9165. [Google Scholar] [CrossRef]
- Domenici, P.; Allan, B.; McCormick, M.I.; Munday, P.L. Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol. Lett. 2012, 8, 78–81. [Google Scholar] [CrossRef]
- Domenici, P.; Allan, B.J.; Watson, S.A.; McCormick, M.I.; Munday, P.L. Shifting from right to left: The combined effect of elevated CO2 and temperature on behavioural lateralization in a coral reef fish. PLoS ONE 2014, 9, e87969. [Google Scholar] [CrossRef]
- Suriyampola, P.S.; Huang, A.J.; Lopez, M.; Conroy-Ben, O.; Martins, E.P. Exposure to environmentally relevant concentrations of Bisphenol-A linked to loss of visual lateralization in adult zebrafish (Danio rerio). Aquat. Toxicol. 2024, 268, 106862. [Google Scholar] [CrossRef]
- De Russi, G.; Bertolucci, C.; Lucon-Xiccato, T. Artificial light at night impairs visual lateralisation in a fish. J. Exp. Biol. 2025, 228, JEB249272. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Barari, M. Meta-analysis and traditional systematic literature reviews—What, why, when, where, and how? Psychol. Mark. 2022, 39, 1099–1115. [Google Scholar]
- Gregory, A.T.; Denniss, A.R. An introduction to writing narrative and systematic reviews—Tasks, tips and traps for aspiring authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [PubMed]
- Pigliucci, M. Evolution of phenotypic plasticity: Where are we going now? Trends Ecol. Evol. 2005, 20, 481–486. [Google Scholar]
- Previc, F.H. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol. Rev. 1991, 98, 299. [Google Scholar]
- Ocklenburg, S.; Korte, S.M.; Peterburs, J.; Wolf, O.T.; Güntürkün, O. Stress and laterality–The comparative perspective. Physiol. Behav. 2016, 164, 321–329. [Google Scholar] [CrossRef]
- Brandler, W.M.; Morris, A.P.; Evans, D.M.; Scerri, T.S.; Kemp, J.P.; Timpson, N.J.; Pourcain, B.; Smith, G.D.; Ring, S.M.; Stein, J.; et al. Common variants in left/right asymmetry genes and pathways are associated with relative hand skill. PLoS Genet. 2013, 9, e1003751. [Google Scholar] [CrossRef]
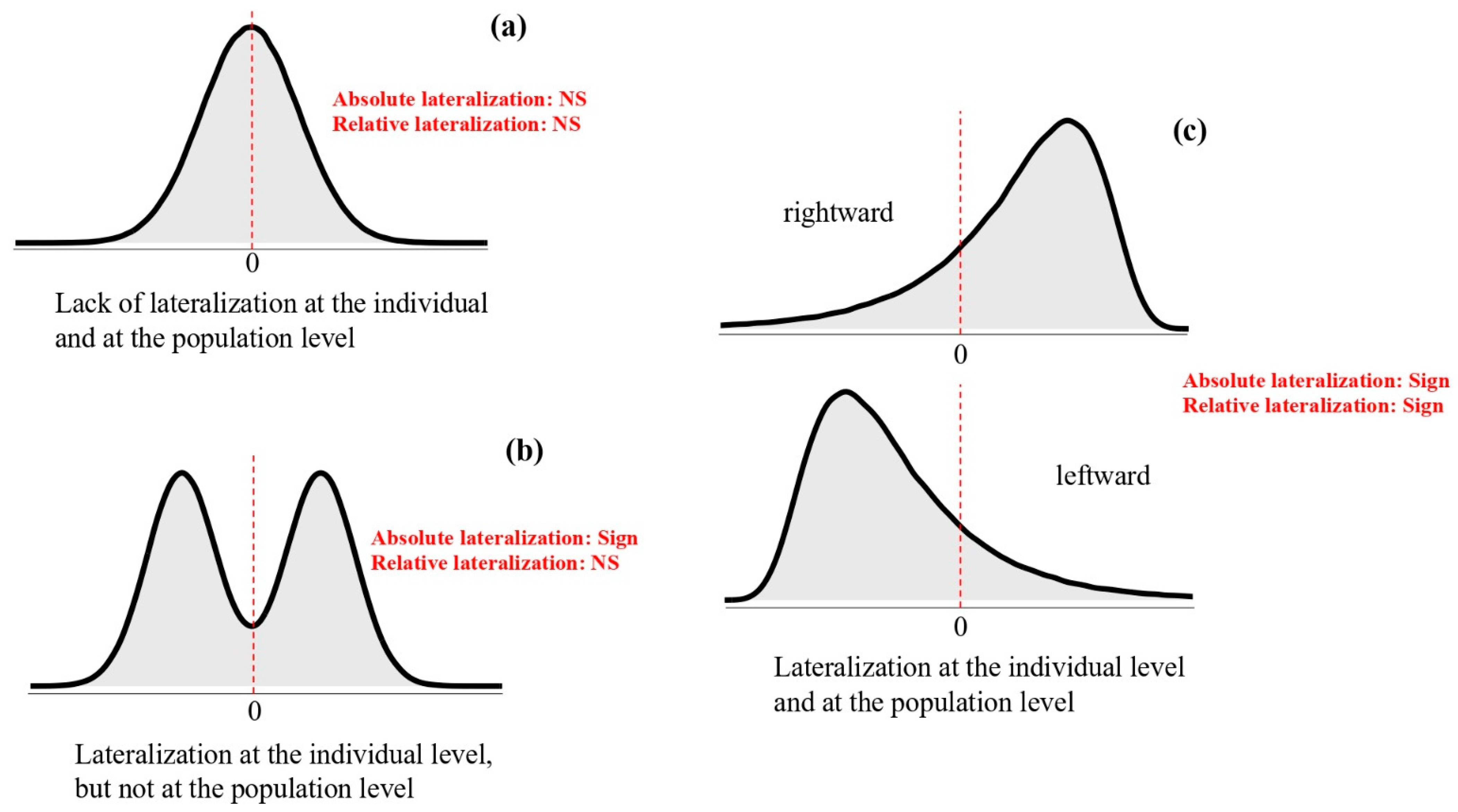
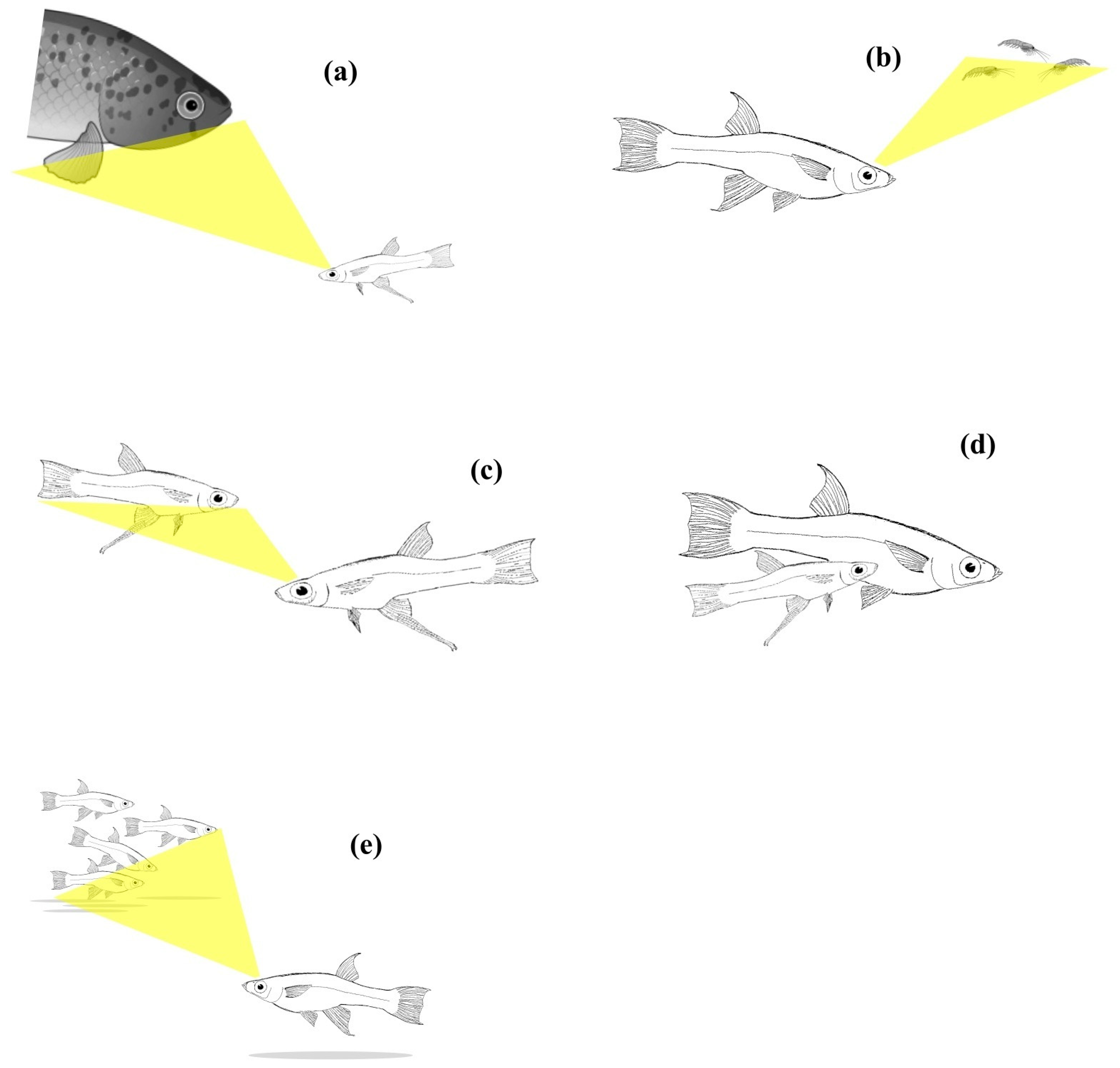
| Vertebrate Group | Species | Method | Trait Investigated | Genetic Contribution: Direction/Strength | Reference |
|---|---|---|---|---|---|
| Teleost fish | Bishop toothcarp Brachyraphis episcopi | Common garden design | Eye preference for social or unfamiliar stimuli | −/+ | [104] |
| Bahamas mosquitofish Gambusia hubbsi | Sibling concordance | Turning direction | +/+ | [98] | |
| Goldbelly topminnows Girardinus falcatus | Parent-offspring concordance | Eye preference for predator | +/+ | [105] | |
| Goldbelly topminnows Girardinus falcatus | Artificial selection | Eye preference for predator | +/+ | [106] | |
| Zebrafish Danio rerio | Study of mutants (fsi mutation) | Forebrain asymmetry, eye preference for social stimuli and food | +/+ | [107] | |
| Zebrafish Danio rerio | Motor asymmetries | −/− | [107] | ||
| Zebrafish Danio rerio | Artificial selection | Eye preference for social stimuli | +/+ | [108] | |
| Zebrafish Danio rerio | Candidate gene analysis | Forebrain asymmetry, eye preference for predator and social stimuli | +/+ | [109] | |
| Bird | Yellow-legged gull Larus michahellis | Sibling concordance | Reverting from supine posture | −/− | [103] |
| Yellow-legged gull Larus michahellis | Begging | −/− | [103] | ||
| Mammal | Capuchin monkey Cebus apella | Parent-offspring concordance | Looking bias | +/− | [110] |
| Capuchin monkey Cebus apella | Hand preference | −/− | [110] | ||
| Capuchin monkey Cebus apella | Turning bias | +/− | [110] | ||
| Chimpanzee Pan troglodytes | Parent-offspring concordance | Hand preference | +/− | [111] | |
| Chimpanzee Pan troglodytes | Parent-offspring concordance (Cross-fostering design) | Hand preference (coordinated bimanual tasks) | −/− | [111] | |
| Mouse Mus musculus | Artificial selection, | Paw preference | −/+ | [112,113] | |
| Mouse Mus musculus | QTL | Paw preference | −/+ | [114] |
| Trait Investigated | References |
|---|---|
| Antipredator response | [201,202] |
| Attack behaviour | [192,203] |
| Brain gene expression | [204] |
| Competition | [205] |
| Copulation | [192,203] |
| Fear | [201] |
| Flexibility/perseveration | [206,207,208] |
| Monocular visual learning | [209] |
| Neuronal activity | [210,211] |
| Novelty exploration | [206] |
| Refuge seeking | [206] |
| Social behaviour | [206] |
| Stimulus recognition | [206,212] |
| Tectofugal visual projections | [213] |
| Thalamofugal visual projections | [213,214] |
| Vigilance behaviour | [202] |
| Vertebrate Group | Species (Age) | Manipulation | Effect of Predation Risk | Reference |
|---|---|---|---|---|
| Teleost fish | Ambon damselfish Pomacentrus amboinensis (juveniles) | Exposure to alarm cues (9 days) | Increased lateralisation | [238] |
| Fathead minnows Pimephales promelas (adults) | Exposure to alarm cues (for either 2, 4, or 8 days) | Decreased lateralisation | [239] | |
| Goldbelly topminnows Girardinus falcatus (juveniles) | Maternal visual exposure to predator (18 times across 6 weeks) | Increased lateralisation | [130] | |
| Guppies, Poecilia reticultata (adults) | Exposure to alarm and predator cues (from the past generation) | Increased lateralisation | [237] | |
| Three-spined stickle-backs Gasterosteus aculeatus (juveniles) | Olfactory exposure to predator | Increased lateralisation | [240] | |
| Whitetail damselfish Pomacentrus chrysurus (juveniles) | Exposure to alarm cues (4 days) | Increased lateralisation | [241] | |
| Amphibian | Wood frog Lithobates sylvaticus (tadpoles) | Exposure to alarm cues as embryos (between eggs laying and hatching) | Increased lateralisation | [242] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisazza, A.; Lucon-Xiccato, T. Individual Differences in Vertebrate Behavioural Lateralisation: The Role of Genes and Environment. Symmetry 2025, 17, 527. https://doi.org/10.3390/sym17040527
Bisazza A, Lucon-Xiccato T. Individual Differences in Vertebrate Behavioural Lateralisation: The Role of Genes and Environment. Symmetry. 2025; 17(4):527. https://doi.org/10.3390/sym17040527
Chicago/Turabian StyleBisazza, Angelo, and Tyrone Lucon-Xiccato. 2025. "Individual Differences in Vertebrate Behavioural Lateralisation: The Role of Genes and Environment" Symmetry 17, no. 4: 527. https://doi.org/10.3390/sym17040527
APA StyleBisazza, A., & Lucon-Xiccato, T. (2025). Individual Differences in Vertebrate Behavioural Lateralisation: The Role of Genes and Environment. Symmetry, 17(4), 527. https://doi.org/10.3390/sym17040527






