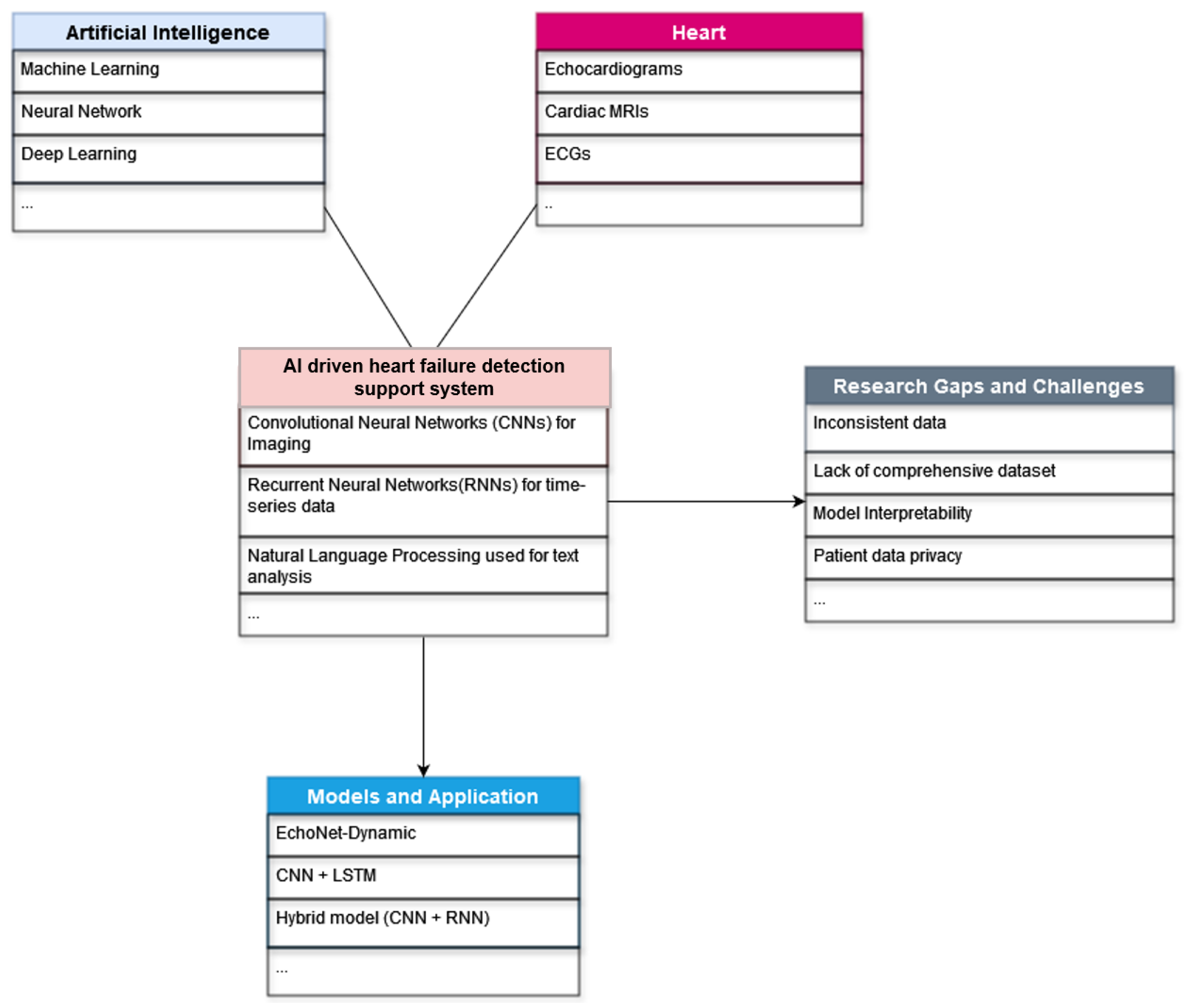
AI-Driven Technology in Heart Failure Detection and Diagnosis: A Review of the Advancement in Personalized Healthcare
Abstract
1. Introduction
- Synthesize current research on AI-driven methodologies for HF detection and diagnosis;
- Evaluate the advantages and limitations of both established and emerging techniques;
- Identify gaps in existing studies, particularly in risk prediction, anomaly detection, and personalization;
- Propose future directions that could enhance diagnostic precision, interpretability, and clinical adoption.
2. Related Work
2.1. Model Architectures: Symmetry and Asymmetry
2.2. Overview of AI Applications in Heart Failure Detection
- Diagnostic Tools: AI-driven models have been applied to analyze ECG data, medical imaging, and EHRs, providing highly accurate detection and classification of HF. For instance, studies have demonstrated the efficacy of CNNs in processing echocardiograms and X-ray images, while RNNs have been integrated to capture temporal dependencies in ECG signals.
- Risk Stratification and Personalized Medicine: Several studies emphasize the use of AI for risk prediction, offering the potential to tailor treatments to individual patients. However, as noted by Hong et al. [19], previous reviews have primarily overlooked this aspect, pointing to a significant research gap.
- Advanced Modeling Approaches: The evolution from symmetric to asymmetric model designs and generative modeling techniques has improved diagnostic precision, though data quality, interpretability, and model bias persist.
3. Methodology
3.1. Search Strategy, Data Gathering and Extraction
- Peer-reviewed articles published in English;
- Studies published between 2014 and 2025 to ensure contemporary relevance;
- Research involving human subjects, focusing on heart failure and using AI, ML, or deep learning;
- Articles addressing epidemiology, risk factors, pathophysiology, preventive strategies, and technological advances in heart failure.
- Non-English publications;
- Studies involving animal models without direct human data;
- Reviews, editorials, and opinion pieces, unless they provided substantial insights or were foundational papers;
- Studies not focusing on the application of AI, ML, or deep learning in heart failure.
3.2. Selection of Study Criteria
3.3. Methodological Insights on Deep Learning Model in Cardiology
3.3.1. Implementation of the EchoNet-Dynamic Experiment
3.3.2. Multi-Input Neural Network Model
3.3.3. Machine Learning and End-to-End Deep Learning
3.3.4. CNN-Based Classifier
3.3.5. Deep Learning Framework for Feature Rearrangement
3.3.6. Modified VGG16-Based Model
3.3.7. CNN and RNN-Based Fusion Model
3.3.8. Deep Learning Model with Residual Blocks
3.3.9. CNN Based on Contextual Data
3.3.10. 3D CNN with Frame-Level Segmentation
3.3.11. Deep Learning Algorithm on ECG
3.4. Impact of Symmetry and Asymmetry in Model Design on Heart Failure Detection
3.5. Methodological Insights on Symmetry in Computer Vision Models
3.6. Reinforcement Learning in Cardiology
3.7. Natural Language Processing in Cardiology

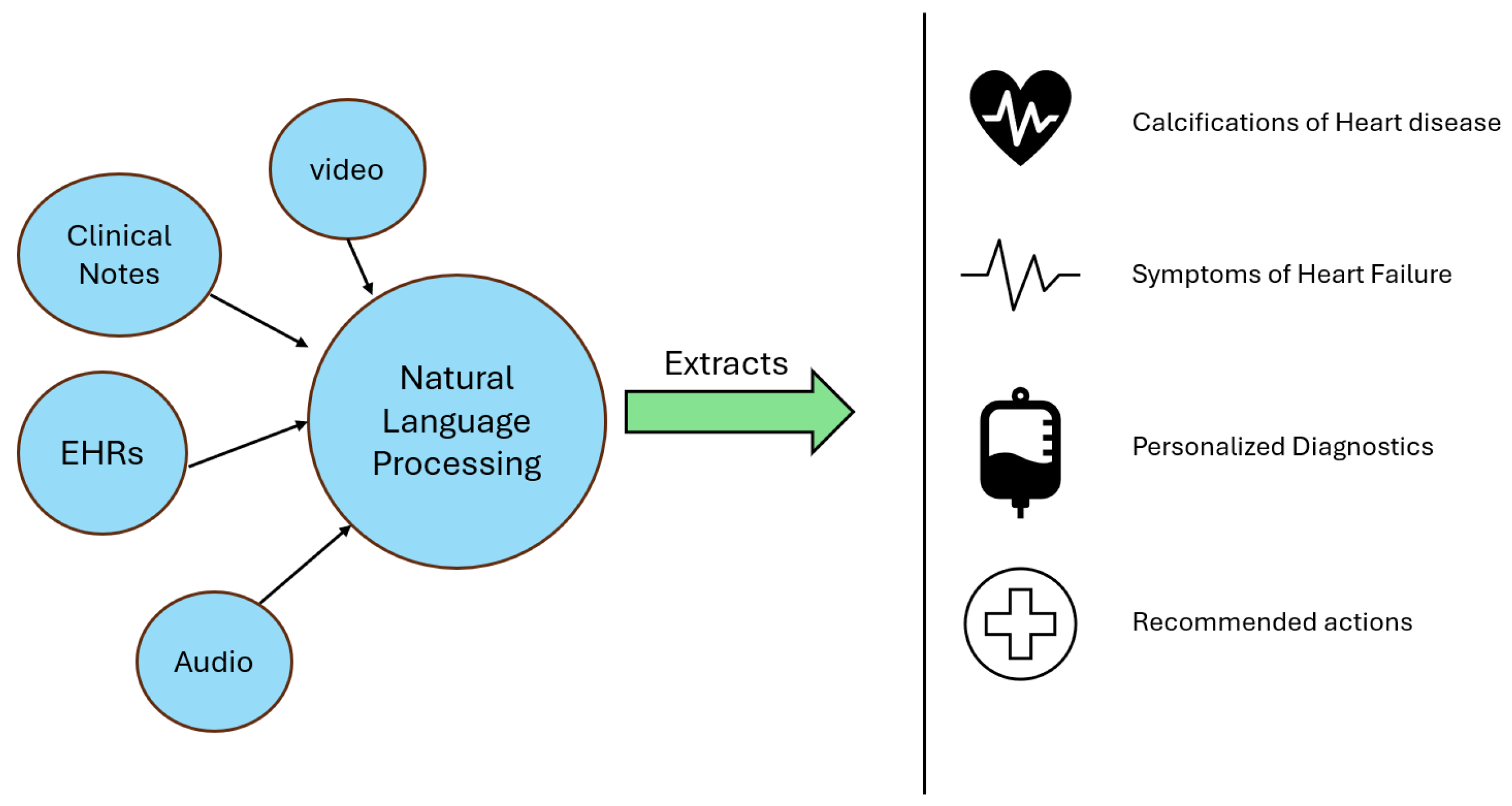
4. Results
5. Discussion
6. Conclusions
- Enhance Explainability: Integrate generative models with explainability frameworks (e.g., SHAP values) to demystify model decisions and build clinician trust.
- Develop Lightweight Architectures: Focus on creating computationally efficient models that can be deployed in resource-constrained environments.
- Leverage Hybrid Approaches: Combine transformers with VAEs or integrate multi-modal data fusion techniques to capture complex interactions among diverse clinical data sources.
- Improve Dataset Diversity: Expand and diversify training datasets to cover various demographics, clinical conditions, and imaging protocols, thereby enhancing model generalization.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogunniyi, M.; Commodore-Mensah, Y.; Ferdinand, K. Race, ethnicity, hypertension, and heart disease: JACC focus seminar 1/9. J. Am. Coll. Cardiol. 2021, 78, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Sapna, F.; Raveena, F.; Chandio, M.; Bai, K.; Sayyar, M.; Varrassi, G.; Khatri, M.; Kumar, S.; Mohamad, T. Advancements in heart failure management: A comprehensive narrative review of emerging therapies. Cureus 2023, 15, e46486. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.; Esenwa, C.; Elkind, M. Stroke risk factors, genetics, and prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.; Francis, G.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.; Ramasubbu, K.; et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country, 2000–2019. 2020. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 5 March 2025).
- Savarese, G.; Becher, P.; Lund, L.; Seferovic, P.; Rosano, G.; Coats, A. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.; Mosterd, A.; Hoes, A. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Ponikowski, P.; Anker, S.; AlHabib, K.; Cowie, M.; Force, T.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef]
- Dattani, S.; Samborska, V.; Ritchie, H.; Roser, M. Cardiovascular Diseases. 2023. Available online: https://ourworldindata.org/cardiovascular-diseases (accessed on 5 March 2025).
- Nazar, W.; Nazar, K.; Daniłowicz-Szymanowicz, L. Machine Learning and Deep Learning Methods for Fast and Accurate Assessment of Transthoracic Echocardiogram Image Quality. Life 2024, 14, 761. [Google Scholar] [CrossRef]
- Sandau, K.E.; Funk, M.; Auerbach, A.; Barsness, G.; Blum, K.; Cvach, M.; Lampert, R.; May, J.; McDaniel, G.; Perez, M.; et al. Update to practice standards for electrocardiographic monitoring in hospital settings: A scientific statement from the American Heart Association. Circulation 2017, 136, e273–e344. [Google Scholar] [CrossRef]
- Sharma, S.; Guleria, K. A systematic literature review on deep learning approaches for pneumonia detection using chest X-ray images. Multimed. Tools Appl. 2024, 83, 24101–24151. [Google Scholar] [CrossRef]
- Dayarathna, S.; Islam, K.T.; Uribe, S.; Yang, G.; Hayat, M.; Chen, Z. Deep learning based synthesis of MRI, CT and PET: Review and analysis. Med. Image Anal. 2024, 92, 103046. [Google Scholar] [CrossRef]
- Counseller, Q.; Aboelkassem, Y. Recent technologies in cardiac imaging. Front. Med. Technol. 2023, 4, 984492. [Google Scholar] [CrossRef]
- Moons, K.; Altman, D.; Reitsma, J.; Ioannidis, J.; Macaskill, P.; Steyerberg, E.; Vickers, A.; Ransohoff, D.; Collins, G. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed]
- Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Freire, R.B.; Fernández, A.E.; Sobrino, J.L.B.; Pérez, C.F.; Somoza, F.J.E.; Miguel, C.M.; Vilacosta, I. In-hospital mortality and readmissions for heart failure in Spain. A Study of Index Episodes and 30-Day and 1-year Cardiac Readmissions. Rev. Esp. Cardiol. 2019, 72, 998–1004. [Google Scholar] [CrossRef]
- Fischer, C.; Steyerberg, E.W.; Fonarow, G.C.; Ganiats, T.G.; Lingsma, H.F. A systematic review and meta-analysis on the association between quality of hospital care and readmission rates in patients with heart failure. Am. Heart J. 2015, 170, 1005–1017. [Google Scholar] [CrossRef]
- Hong, S.; Zhou, Y.; Shang, J.; Xiao, C.; Sun, J. Opportunities and challenges of deep learning methods for electrocardiogram data: A systematic review. Comput. Biol. Med. 2020, 122, 103801. [Google Scholar] [CrossRef]
- Madani, A.; Ong, J.R.; Tibrewal, A.; Mofrad, M.R. Deep echocardiography: Data-efficient supervised and semi-supervised deep learning towards automated diagnosis of cardiac disease. NPJ Digit. Med. 2018, 1, 59. [Google Scholar] [CrossRef]
- Liu, D.; Lu, S.; Zhang, L.; Liu, Y. Anomaly detection in chest X-rays based on dual-attention mechanism and multi-scale feature fusion. Symmetry 2023, 15, 668. [Google Scholar] [CrossRef]
- Jin, B.; Che, C.; Liu, Z.; Zhang, S.; Yin, X.; Wei, X. Predicting the risk of heart failure with EHR sequential data modeling. IEEE Access 2018, 6, 9256–9261. [Google Scholar] [CrossRef]
- Schreibmann, E.; Dhabaan, A.; Elder, E.; Fox, T. Patient-specific quality assurance method for VMAT treatment delivery. Med. Phys. 2009, 36, 4530–4535. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Désir, C.; Bernard, S.; Petitjean, C.; Heutte, L. A random forest based approach for one class classification in medical imaging. In Proceedings of the Machine Learning in Medical Imaging: Third International Workshop, MLMI 2012, Held in Conjunction with MICCAI 2012, Nice, France, 1 October 2012; Revised Selected Papers 3. Springer: Berlin/Heidelberg, Germany, 2012; pp. 250–257. [Google Scholar] [CrossRef]
- Geweid, G.; Abdallah, M. A new automatic identification method of heart failure using improved support vector machine based on duality optimization technique. IEEE Access 2019, 7, 149595–149611. [Google Scholar] [CrossRef]
- Yongcharoenchaiyasit, K.; Arwatchananukul, S.; Temdee, P.; Prasad, R. Gradient Boosting Based Model for Elderly Heart Failure, Aortic Stenosis, and Dementia Classification. IEEE Access 2023, 11, 48677–48696. [Google Scholar] [CrossRef]
- Yasmin, F.; Shah, S.; Naeem, A.; Shujauddin, S.; Jabeen, A.; Kazmi, S.; Siddiqui, S.; Kumar, P.; Salman, S.; Hassan, S.; et al. Artificial intelligence in the diagnosis and detection of heart failure: The past, present, and future. Rev. Cardiovasc. Med. 2021, 22, 1095–1113. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Alom, M.K.; Karim, M.E. ALZENET: Deep Learning-Based Early Prediction of Alzheimer’s Disease through Magnetic Resonance Imaging Analysis. Telemat. Inform. Rep. 2025, 17, 100189. [Google Scholar] [CrossRef]
- Sahu, H.P.; Kashyap, R. FINE_DENSEIGANET: Automatic medical image classification in chest CT scan using Hybrid Deep Learning Framework. Int. J. Image Graph. 2025, 25, 2550004. [Google Scholar] [CrossRef]
- Borzooei, S.; Briganti, G.; Golparian, M.; Lechien, J.R.; Tarokhian, A. Machine learning for risk stratification of thyroid cancer patients: A 15-year cohort study. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 2095–2104. [Google Scholar] [CrossRef]
- Du, W.; Bi, W.; Liu, Y.; Zhu, Z.; Tai, Y.; Luo, E. Machine learning-based decision support system for orthognathic diagnosis and treatment planning. BMC Oral Health 2024, 24, 286. [Google Scholar] [CrossRef]
- Pedreschi, D.; Giannotti, F.; Guidotti, R.; Monreale, A.; Ruggieri, S.; Turini, F. Meaningful explanations of black box AI decision systems. In Proceedings of the AAAI Conference on Artificial Intelligence 2019, Honolulu, HI, USA, 27 January–7 February 2019; Volume 33, pp. 9780–9784. [Google Scholar] [CrossRef]
- Kaissis, G.; Makowski, M.; Rückert, D.; Braren, R. Secure, privacy-preserving and federated machine learning in medical imaging. Nat. Mach. Intell. 2020, 2, 305–311. [Google Scholar] [CrossRef]
- Ouyang, D.; He, B.; Ghorbani, A.; Yuan, N.; Ebinger, J.; Langlotz, C.; Heidenreich, P.; Harrington, R.; Liang, D.; Ashley, E.; et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 2020, 580, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Li, S.; Wei, D.; Guo, L.; Chen, H. Automatic detection of congestive heart failure based on a hybrid deep learning algorithm in the internet of medical things. IEEE Internet Things J. 2020, 8, 12550–12558. [Google Scholar] [CrossRef]
- Kanani, P.; Padole, M. ECG heartbeat arrhythmia classification using time-series augmented signals and deep learning approach. Procedia Comput. Sci. 2020, 171, 524–531. [Google Scholar] [CrossRef]
- Huang, S.H.; Chuang, B.L.; Lin, Y.H.; Hung, C.S.; Ma, H.P. A Congestive Heart Failure Detection System via Multi-Input Deep Learning Networks. In Proceedings of the 2019 IEEE Global Communications Conference (GLOBECOM), Honolulu, HI, USA, 9–13 December 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Gjoreski, M.; Gradišek, A.; Budna, B.; Gams, M.; Poglajen, G. Machine Learning and End-to-End Deep Learning for the Detection of Chronic Heart Failure From Heart Sounds. IEEE Access 2020, 8, 20313–20324. [Google Scholar] [CrossRef]
- Nirschl, J.; Janowczyk, A.; Peyster, E.; Frank, R.; Margulies, K.; Feldman, M.; Madabhushi, A. A deep-learning classifier identifies patients with clinical heart failure using whole-slide images of H&E tissue. PLoS ONE 2018, 13, e0192726. [Google Scholar] [CrossRef]
- Nirschl, J.J.; Janowczyk, A.; Peyster, E.G.; Frank, R.; Margulies, K.B.; Feldman, M.D.; Madabhushi, A. Deep learning tissue segmentation in cardiac histopathology images. In Deep Learning for Medical Image Analysis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 179–195. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Li, D.; Yin, Y.; Zhang, J. Feature rearrangement based deep learning system for predicting heart failure mortality. Comput. Methods Programs Biomed. 2020, 191, 105383. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kodera, S.; Shinohara, H.; Ieki, H.; Yamaguchi, T.; Higashikuni, Y.; Kiyosue, A.; Ito, K.; Ando, J.; Takimoto, E.; et al. Diagnosing heart failure from chest X-ray images using deep learning. Int. Heart J. 2020, 61, 781–786. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Lu, L.; Lu, Z.; Bagheri, M.; Summers, R. Chestx-ray8: Hospital-scale chest x-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 2097–2106. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar] [CrossRef]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Fei-Fei, L. Imagenet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 248–255. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Bae, J.; Li, H.; Johnston, J.; Sanger, T. Predicting heart failure readmission from clinical notes using deep learning. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 12–21 November 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2642–2648. [Google Scholar] [CrossRef]
- Duffy, G.; Cheng, P.; Yuan, N.; He, B.; Kwan, A.; Shun-Shin, M.; Alexander, K.; Ebinger, J.; Lungren, M.; Rader, F.; et al. High-throughput precision phenotyping of left ventricular hypertrophy with cardiovascular deep learning. JAMA Cardiol. 2022, 7, 386–395. [Google Scholar] [CrossRef]
- Narang, A.; Bae, R.; Hong, H.; Thomas, Y.; Surette, S.; Cadieu, C.; Chaudhry, A.; Martin, R.; McCarthy, P.; Rubenson, D.; et al. Utility of a deep-learning algorithm to guide novices to acquire echocardiograms for limited diagnostic use. JAMA Cardiol. 2021, 6, 624–632. [Google Scholar] [CrossRef]
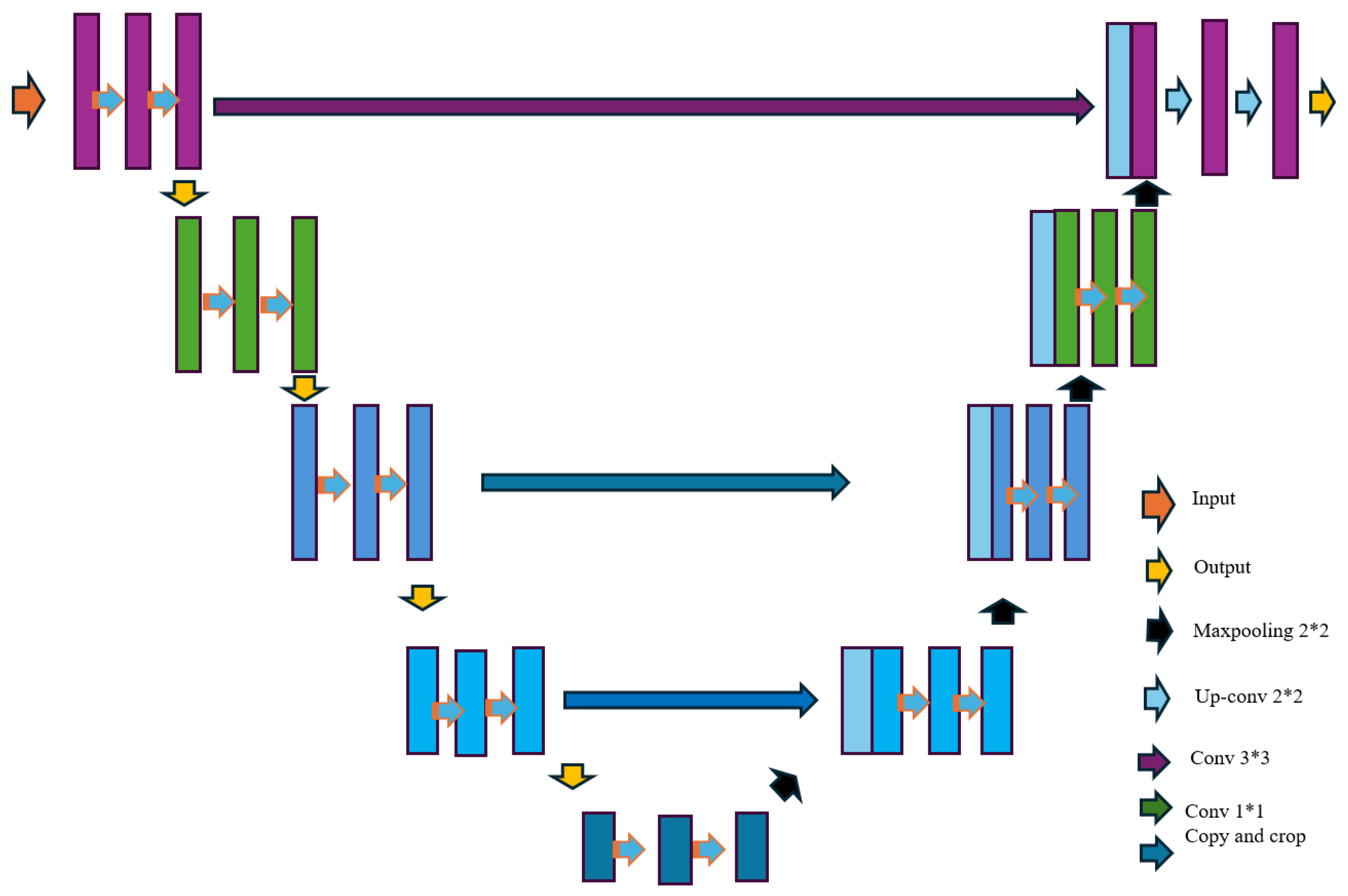
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the Medical Image Computing and Computer Assisted Intervention (MICCAI) 2015: 18th International Conference, Munich, Germany, 5–9 October 2015; Proceedings, Part III 18. Springer: Berlin/Heidelberg, Germany, 2015; pp. 234–241. [Google Scholar] [CrossRef]
- Valehi, A.; Razi, A.; Chen, J. Smart heart monitoring: Early prediction of heart problems through predictive analysis of ECG signals. IEEE Access 2019, 7, 457–465. [Google Scholar] [CrossRef]
- McCracken, C.; Szabo, L.; Abdulelah, Z.A.; Condurache, D.G.; Vago, H.; Nichols, T.E.; Petersen, S.E.; Neubauer, S.; Raisi-Estabragh, Z. Ventricular volume asymmetry as a novel imaging biomarker for disease discrimination and outcome prediction. Eur. Heart J. 2024, 4, e059. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; García-Hermoso, A. Effects of exercise training on Fetuin-a in obese, type 2 diabetes and cardiovascular disease in adults and elderly: A systematic review and Meta-analysis. Lipids Health Dis. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Kasmaee, A.M.M.; Ataei, A.; Moravvej, S.V.; Alizadehsani, R.; Gorriz, J.M.; Zhang, Y.D.; Tan, R.S.; Acharya, U.R. ELRL-MD: A deep learning approach for myocarditis diagnosis using cardiac magnetic resonance images with ensemble and reinforcement learning integration. Physiol. Meas. 2024, 45, 055011. [Google Scholar] [CrossRef]
- Adejumo, P.; Thangaraj, P.M.; Dhingra, L.S.; Aminorroaya, A.; Zhou, X.; Brandt, C.; Xu, H.; Krumholz, H.M.; Khera, R. Natural language processing of clinical documentation to assess functional status in patients with heart failure. JAMA Netw. Open 2024, 7, e2443925. [Google Scholar] [CrossRef]
- Nargesi, A.A.; Adejumo, P.; Dhingra, L.S.; Rosand, B.; Hengartner, A.; Coppi, A.; Benigeri, S.; Sen, S.; Ahmad, T.; Nadkarni, G.N.; et al. Automated identification of heart failure with reduced ejection fraction using deep learning-based natural language processing. Heart Fail. 2025, 13, 75–87. [Google Scholar] [CrossRef]
- Sutton, R.S.; Barto, A.G. Reinforcement Learning: An introduction, 1st ed.; MIT Press: Cambridge, MA, USA, 1998; Volume 1. [Google Scholar]
- Li, D.; Li, X.; Zhao, J.; Bai, X. Automatic staging model of heart failure based on deep learning. Biomed. Signal Process. Control 2019, 52, 77–83. [Google Scholar] [CrossRef]
- Sridevi, S.; Murugesan, S.; Sakthivel, V. Computer-aided decision support system for symmetry-based prenatal congenital heart defects. Adv. Mach. Vis. Syst. 2021, 2, 45–60. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Tong, W.; Shrestha, K. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur. Heart J. 2008, 29, 2506–2513. [Google Scholar] [CrossRef]
- Sarker, I.H. Deep learning: A comprehensive overview on techniques, taxonomy, applications and research directions. SN Comput. Sci. 2021, 2, 420. [Google Scholar] [CrossRef]
- Ragab, A.; El Koujok, M.; Ghezzaz, H.; Amazouz, M.; Ouali, M.S.; Yacout, S. Deep understanding in industrial processes by complementing human expertise with interpretable patterns of machine learning. Expert Syst. Appl. 2019, 122, 388–405. [Google Scholar] [CrossRef]
- Munappy, A.; Bosch, J.; Olsson, H.; Arpteg, A.; Brinne, B. Data management for production quality deep learning models: Challenges and solutions. J. Syst. Softw. 2022, 191, 111359. [Google Scholar] [CrossRef]
- Casella, B.; Riviera, W.; Aldinucci, M.; Menegaz, G. MiFL: Multi-Input Neural Networks in Federated Learning. Authorea Preprints 2023. [CrossRef]
- De Bois, M.; El Yacoubi, M.; Ammi, M. Enhancing the interpretability of deep models in healthcare through attention: Application to glucose forecasting for diabetic people. Int. J. Pattern Recognit. Artif. Intell. 2021, 35, 2160006. [Google Scholar] [CrossRef]
- Snyderman, R. Personalized health care: From theory to practice. Biotechnol. J. 2012, 7, 973–979. [Google Scholar] [CrossRef]
- Chintala, S. AI-Driven Personalised Treatment Plans: The Future of Precision Medicine. Mach. Intell. Res. 2023, 17, 9718–9728. [Google Scholar]
- Morrison, T.; Pathmanathan, P.; Adwan, M.; Margerrison, E. Advancing regulatory science with computational modeling for medical devices at the FDA’s Office of Science and Engineering Laboratories. Front. Med. 2018, 5, 241. [Google Scholar] [CrossRef]
- Krois, J.; Garcia Cantu, A.; Chaurasia, A.; Patil, R.; Chaudhari, P.K.; Gaudin, R.; Gehrung, S.; Schwendicke, F. Generalizability of deep learning models for dental image analysis. Sci. Rep. 2021, 11, 6102. [Google Scholar] [CrossRef]
- Chen, C.; Bai, W.; Davies, R.; Bhuva, A.; Manisty, C.; Augusto, J.; Moon, J.; Aung, N.; Lee, A.; Sanghvi, M.; et al. Improving the generalizability of convolutional neural network-based segmentation on CMR images. Front. Cardiovasc. Med. 2020, 7, 105. [Google Scholar] [CrossRef]
- Miranda, E.; Adiarto, S.; Bhatti, F.; Zakiyyah, A.; Aryuni, M.; Bernando, C. Understanding Arteriosclerotic Heart Disease Patients Using Electronic Health Records: A Machine Learning and Shapley Additive Explanations Approach. Healthc. Inform. Res. 2023, 29, 228. [Google Scholar] [CrossRef]
- Assegie, T.A. Evaluation of Local Interpretable Model-Agnostic Explanation and Shapley Additive Explanation for Chronic Heart Disease Detection. Proc. Eng. Technol. Innov. 2023, 23, 48–59. [Google Scholar] [CrossRef]
- Hedeker, D.; Gibbons, R. Longitudinal Data Analysis, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 336. [Google Scholar]
- Yacouby, R.; Axman, D. Probabilistic extension of precision, recall, and f1 score for more thorough evaluation of classification models. In Proceedings of the First Workshop on Evaluation and Comparison of NLP Systems, Online, 20 November 2020; pp. 79–91. [Google Scholar] [CrossRef]
- Ratner, B. Statistical and Machine-Learning Data Mining: Techniques for Better Predictive Modeling and Analysis of Big Data, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017; p. 324. [Google Scholar]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef]
- Krittanawong, C.; Zhang, H.; Wang, Z.; Aydar, M.; Kitai, T. Artificial intelligence in precision cardiovascular medicine. J. Am. Coll. Cardiol. 2017, 69, 2657–2664. [Google Scholar] [CrossRef]
- de Bakker, M. Circulating Biomarkers for Dynamic Cardiovascular Risk Assessment: A Precision Medicine Approach. Ph.D. Thesis, Erasmus University Rotterdam, Rotterdam, The Netherlands, 2024. [Google Scholar]
- Lotan, E.; Tschider, C.; Sodickson, D.; Caplan, A.; Bruno, M.; Zhang, B.; Lui, Y. Medical imaging and privacy in the era of artificial intelligence: Myth, fallacy, and the future. J. Am. Coll. Radiol. 2020, 17, 1159–1162. [Google Scholar] [CrossRef]
- Kelly, C.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]



| Word | Count |
|---|---|
| Heart | 691 |
| Failure | 540 |
| Learning | 481 |
| Deep | 452 |
| Data | 427 |
| Features | 365 |
| CHF | 332 |
| CNN | 305 |
| Patients | 286 |
| Neural | 273 |
| Aspect | Symmetric Models | Asymmetric Models |
|---|---|---|
| Architecture | Uniform, structured (e.g., U-Net) | Specialized, task-specific |
| Strengths | Efficient processing, handles balanced data | Captures rare conditions, robust for imbalanced datasets |
| Weaknesses | May overlook subtle anomalies or rare cases | Computationally complex, less generalized |
| Applications | Standard echocardiographic and structured data analysis | Multimodal data integration, anomaly detection in imbalanced datasets |
| Authors | Model/Strategy | Outcome | Data | Accuracy |
|---|---|---|---|---|
| Li et al. [58] | CNN + RNN | Predict cardiovascular disease risk | Medical records | Accuracy of 97.6%, with a sensitivity of 96.3% and a specificity of 97.4% |
| Gjoresk et al. [39] | Machine learning (ML) and end-to-end deep learning (DL) | Diagnose chronic heart failure | Heart sound recordings | Accuracy = 93.2% |
| Narang et al. [49] | CNN | Guide novice echocardiography users | Echocardiography videos | Diagnostic quality: 98.8% of cases for LV size and function and pericardial effusion, and 92.5% of cases for RV size% |
| Duffy et al. [48] | 3D CNN | Measure LV dimensions | Echocardiogram videos | = 0.96, MAEs = 1.7 mm (95% CI, 1.6–1.8 mm) IVS thickness, 3.8 mm (95% CI, 3.5–4.0 mm) LVID, 1.8 mm (95% CI, 1.7–2.0 mm) LVPW thickness |
| Liu et al. [47] | CNN + LSTM | Predict heart failure | Clinical and imaging data | F1 score of 0.756 on general readmission prediction and 0.733 on 30-day readmission prediction |
| Huang et al. [38] | CNN | Detection system of congestive heart failure (CHF) | Electronic health records | Accuracy: 86.74%, Sensitivity: 88.03%, Specificity: 85.45% |
| Wang et al. [42] | Ensemble model | Early detection of heart failure | Clinical data | (22.28–38.41)% on F1 score and (76.15–88.81)% AUC across different methods |
| Pratik Kanani et al. [37] | 6 residual blocks with 1D convolution | Improve classification accuracy of ECG signals | MIT-BIH Arrhythmia Dataset | F1 score: 0.98 (with augmentation) |
| Ning et al. [36] | Hybrid model (CNN + RNN) | Improve CHF detection from ECG signals | RR interval sequences from ECG signals | Accuracy: 99.93%, Sensitivity: 99.85%, Specificity: 100%, AUC close to 1 |
| Matsumoto et al. [43] | Modified VGG16 | Detect heart failure from X-ray images | CHestX-ray8 database | Accuracy: 82%, Sensitivity: 75%, Specificity: 94.4% |
| David Ouyang et al. [35] | EchoNet-Dynamic. | Accurate segmentation, ejection fraction prediction, heart failure classification. | 10,030 echocardiogram videos from Stanford Medicine and external datasets | Segmentation dice coefficient of 0.92, MAE of 4.1% (internal), 6.0% (external), AUC of 0.97 (internal), 0.96 (external) |
| Authors | Name of the Paper | Dataset | Comments |
|---|---|---|---|
| Li et al. [58] | Automatic staging model of heart failure based on deep learning | Medical records | Limited by the quality and completeness of medical records, potential overfitting due to high accuracy reported |
| Gjoresk et al. [39] | Machine Learning and End-to-End Deep Learning for the Detection of Chronic Heart Failure From Heart Sounds | Heart sound recordings | Small sample size, potential lack of generalizability across diverse populations |
| Narang et al. [49] | Utility of a deep-learning algorithm to guide novices to acquire echocardiograms for limited diagnostic use | Echocardiography videos | Dependency on video quality, may require high computational resources for real-time guidance |
| Duffy et al. [48] | High-throughput precision phenotyping of left ventricular hypertrophy with cardiovascular deep learning | Echocardiogram videos | Limited external validation, performance may vary with different imaging protocols |
| Liu et al. [47] | Predicting heart failure readmission from clinical notes using deep learning | Clinical and imaging data | Complexity of integrating multi-modal data, possible overfitting due to high accuracy metrics |
| Huang et al. [38] | A Congestive Heart Failure Detection System via Multi-input Deep Learning Networks | Electronic Health Records | Potential bias in EHR data, need for longitudinal validation to ensure model robustness |
| Wang et al. [42] | Feature rearrangement-based deep learning system for predicting heart failure mortality | Clinical data | High variability in F1 scores, may require large and diverse datasets for better generalizability |
| Pratik Kanani et al. [37] | ECG heartbeat arrhythmia classification using time-series augmented signals and deep learning approach | MIT-BIH Arrhythmia Dataset | Data augmentation may not reflect real-world scenarios, potential overfitting due to high-performance metrics |
| Ning et al. [36] | Automatic detection of congestive heart failure based on a hybrid deep learning algorithm in the Internet of Medical Things | RR interval sequences from ECG signals | High AUC may suggest overfitting, limited by the quality of ECG signal preprocessing |
| Matsumoto et al. [43] | Diagnosing Heart Failure from Chest X-Ray Images Using Deep Learning | CHestX-ray8 database | Needs a bigger dataset, images with ambiguous radiolucency were prone to being misdiagnosed by the model, did not differentiate between cardiac and non-cardiac diseases |
| David Ouyang et al. [35] | Video-based AI for Beat-to-Beat Assessment of Cardiac Function | 10,030 echocardiogram videos from Stanford Medicine and external datasets | The model demonstrated strong generalizability and robust performance across different datasets, highlighting its clinical potential |
| Authors | Name of the Paper | Dataset | Comments |
|---|---|---|---|
| Arash Madani et al. [20] | Deep Echocardiography: Data-efficient supervised and semi-supervised deep learning | Echocardiography datasets from hospitals | Symmetric models for cardiac diagnosis; limited for rare conditions. |
| Dandan Liu et al. [21] | Anomaly detection in chest X-rays based on dual-attention mechanism | Chest X-ray datasets | Asymmetric models with attention mechanisms for subtle anomalies. |
| Sridevi et al. [59] | Computer-aided decision support system for prenatal congenital heart defects | Prenatal ultrasound datasets | Explored symmetry for congenital heart defect detection. |
| Valehi et al. [51] | Smart heart monitoring: Early prediction of heart problems | ECG signal datasets | Focused on asymmetry in ECG signal analysis for early detection. |
| McCracken et al. [52] | Ventricular volume asymmetry as a novel imaging biomarker | Cardiac imaging datasets | Demonstrated ventricular asymmetry as a predictive biomarker. |
| Tang et al. [60] | Differential effects of arginine methylation on diastolic dysfunction | Clinical biomarker datasets | Analyzed metabolic asymmetry for heart failure progression. |
| Ramírez-Vélez et al. [53] | Effects of exercise training on Fetuin-A in cardiovascular disease | Exercise intervention datasets | Meta-analysis on biomarker asymmetries in cardiovascular research. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udoy, I.A.; Hassan, O. AI-Driven Technology in Heart Failure Detection and Diagnosis: A Review of the Advancement in Personalized Healthcare. Symmetry 2025, 17, 469. https://doi.org/10.3390/sym17030469
Udoy IA, Hassan O. AI-Driven Technology in Heart Failure Detection and Diagnosis: A Review of the Advancement in Personalized Healthcare. Symmetry. 2025; 17(3):469. https://doi.org/10.3390/sym17030469
Chicago/Turabian StyleUdoy, Ikteder Akhand, and Omiya Hassan. 2025. "AI-Driven Technology in Heart Failure Detection and Diagnosis: A Review of the Advancement in Personalized Healthcare" Symmetry 17, no. 3: 469. https://doi.org/10.3390/sym17030469
APA StyleUdoy, I. A., & Hassan, O. (2025). AI-Driven Technology in Heart Failure Detection and Diagnosis: A Review of the Advancement in Personalized Healthcare. Symmetry, 17(3), 469. https://doi.org/10.3390/sym17030469