Abstract
Plasma medicine is a field of research that focuses on the sterilization of bacteria, wounds and cancer treatment, tissue regeneration and other biomedical applications using plasma. Dielectric barrier discharge microplasma was used for biomedical applications such as sterilization of bacteria and skin treatment for transdermal drug delivery. In this study, we investigated the feasibility of using microplasma for improving blood coagulation parameters. Blood samples collected from one dog and one cat were treated with microplasma, and the blood coagulation effect of this treatment was compared with the effect achieved by treating the blood with air flow only. The microplasma electrodes were energized using a negative pulse voltage power supply and environmental air was used as discharge gas. The microplasma treatment produced clear coagulation effects that increased proportionally with treatment time, discharge voltage and frequency. In contrast, the treatment with air flow only had no coagulation effects after the same treatment time as for the microplasma treatment. The observed blood coagulation effects induced by microplasma treatment could be attributed to the reactive oxygen and nitrogen species generated by microplasma. The blood sample subjected to microplasma treatment had a slight temperature increase (≈4 °C) confirming the nonthermal operation. In conclusion, this study shows promising results that suggest the potential of using microplasma treatment as a tool for improving blood coagulation parameters. Furthermore, microplasma’s suitability for portability and integration indicates the potential for developing a compact microplasma device tailored for use by first responders in cases of bleeding.
1. Introduction
Dielectric barrier discharge (DBD) microplasma gained attention in the last decade due to its actual and potential applications combined with economic and ecologic advantages over other plasma technologies, or more conventional approaches in various application fields such as air treatment [1], induced flow [2], surface treatment of polymers [3], sterilization of bacteria or transdermal drug delivery [4,5]. Discharges that have dimensions within a range of micrometers to a few millimeters are known as microplasmas. This type of microplasma, generated at atmospheric pressure, has the advantage of not requiring costly vacuum enclosures. Moreover, microplasma has the advantage of operating at low temperatures with highly energetic electrons, cold positive ions and neutrals within a small volume. This promotes the formation of a reactive environment with excited species, radicals, charged particles and photons that requires low energy consumption. The small electrode and reactor size used for microplasma generation allows the device to be portable or easily integrated with other systems. Microplasmas exhibit power densities several orders of magnitude higher than those of conventional atmospheric pressure nonthermal plasmas. The microplasma’s small dimensions, steep spatial gradients and high surface-to-volume ratios contribute to its non-equilibrium nature, even at high power densities [6].
DBD microplasma developed for specific applications requires relatively low discharge voltages (i.e., below 5 kV) to be generated [6,7], and the discharge voltage could be reduced even more in the case of using discharge gases such as argon. This is another advantage over other types of plasma which are generated at tens of kilovolts. In this study, a surface DBD microplasma electrodes system was used. The microplasma electrode consists of a high voltage electrode covered with a dielectric layer on the back side, and a dielectric layer in between the high voltage electrode and grounded electrode. Microplasma is generated at the surface of the grounded electrode. This type of electrode can be scaled to various surfaces and can be bent to various shapes since it is flexible. Although the electrode can have various sizes, the discharge voltage remains the same, thus there is no need for additional insulation on larger surfaces.
One possible application of DBD microplasma could be enhancement of hemostasis and thus alleviation of bleeding. Thus, development of a tool to mitigate bleeding, particularly in the prehospital environment, would be most welcomed.
An important step of hemostasis is blood coagulation, which occurs immediately after the vascular endothelium is damaged to prevent blood loss [8]. As the blood has a natural tendency to coagulate, this is the main mechanism to counteract injuries. In the case of modern surgical procedures where bleeding occurs there are attempts to control blood coagulation rates by, e.g., decreasing the duration of surgeries or increasing the blood coagulation rates after surgery for wound healing. In the case of accident injuries, the measures to stop bleeding have to be taken immediately. The tools available for bleeding suppression are electric cauterization, various medications, thermal coagulation acceleration and others. As most of these methods have unwanted and deleterious side effects such as the cauterization of healthy tissue, a method for bleeding suppression that is safe and rapid is needed. As studies showed [8,9,10,11,12,13,14,15,16], one such method could be treatment with nonthermal plasma. As a potential tool for controlling blood coagulation and promoting wound healing, nonthermal plasma offers the advantage of precisely regulating coagulation rates at targeted sites while minimizing side effects. Nonthermal plasma mainly affects blood proteins, making the coagulation process selective and largely free of thermal effects compared to thermal coagulation, which acts on the whole blood composition and surrounding tissues [9]. As shown by Yan et al. [8] plasma can lead to rapid blood coagulation, which is necessary in, e.g., traffic accident injuries scenarios. In this regard, plasma can rapidly and effectively promote blood coagulation by forming a transparent white membrane on the surface of blood, primarily composed of aggregated platelets. Moreover, after the blood coagulation process induced by plasma, the blood coagulation layer is thicker and denser [8]. Based on such observations, Yan et al. [8] concluded that nonthermal plasma is a promising tool for stanching bleeding during, e.g., surgical operations.
Nonthermal plasma as a new tool for the control of blood coagulation also has the capability to promote wound healing [10]. The treatment of wounds using nonthermal plasma provides, in addition to faster healing, sterilization of the wound.
After accidents, a response to address an injury with a tool that provides a short coagulation time could be the difference between life and death. As shown above, nonthermal plasma has exactly this property, which could make a nonthermal plasma device ideal for treating injuries by first responders. Although there is already research carried out in this field, the majority of nonthermal plasma devices used for plasma medicine applications rely on high-voltage systems that are costly and less practical to operate. For example, Cheng et al. [10] and Nomura et al. [11] showed good results concerning blood coagulation and wound healing using nonthermal plasma jet type devices, which were also used by others [12,13,14,15]. However, such plasma devices have relatively high discharge voltages of about 9 kV and use discharge gases from gas cylinders, which makes them unfeasible as portable devices. Furthermore, our previous work [5] demonstrated that plasma jet irradiation can cause damage to target samples, including etching effects and the formation of small pores. Alternatively, a floating electrode DBD system was proposed by Friedman et al. [16], but this system is also energized at about 15 kV.
In conclusion, in order to make a nonthermal plasma tool suitable for first responders, the device must be portable and thus capable of being powered by batteries, this later aspect also points to the need of low discharge voltages. As explained above, such advantages could be provided by microplasma devices for blood coagulation and wound healing. In this study, as a first step towards the development of nonthermal plasma tools for such applications, we have investigated the coagulation effect of microplasma treatment on blood samples collected from one dog and one cat.
2. Materials and Methods
2.1. Experimental Setup
Figure 1a presents a schematic image of the experimental setup designed for blood coagulation experiments using microplasma treatment. As shown in Figure 1b, the microplasma electrode is mounted on a support structure that includes electrical connections and a gas inlet to supply gas flow to the electrode. During the experiments, the electrode was positioned 1.5 to 2 mm above the surface of blood droplets.
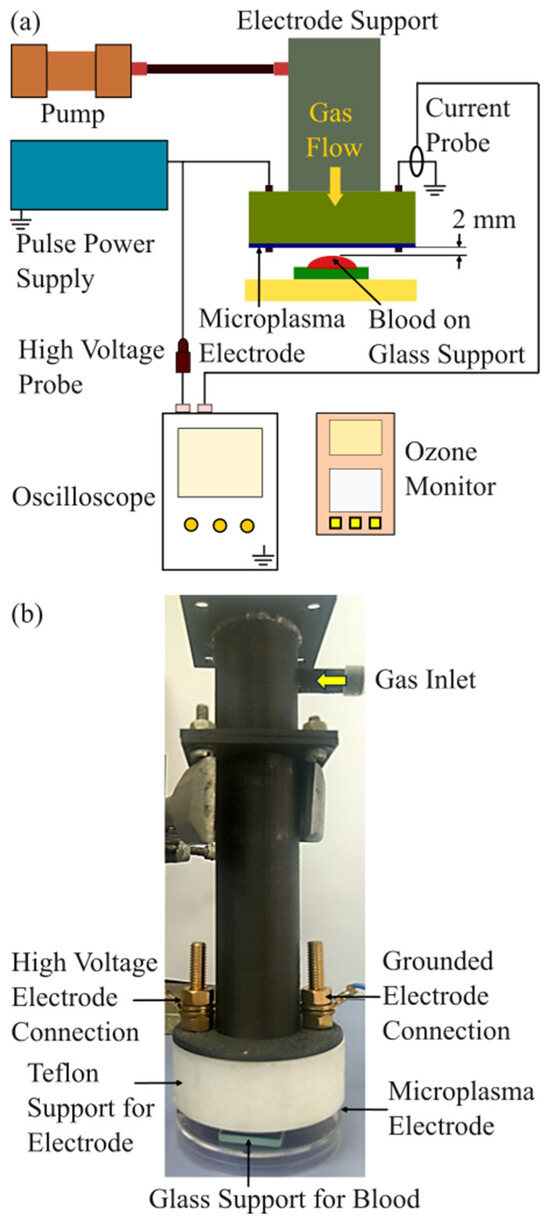
Figure 1.
Experimental setup. (a) Schematic image of the experimental setup; (b) image of the microplasma electrode support system with the glass support for the blood droplet.
Air was supplied using a miniature pump (Mitsumi, Tokyo, Japan, R14-1604), which operates within a voltage range of 4.5–12 V, making it suitable for battery-powered and portable systems. Ambient air with a relative humidity (RH) of 35–55% was used, and the flow rate was maintained at 0.6 L/min. An ozone monitor (NORM, Changzhou, China, ADKS-1 O3 Ozon) was positioned 2 mm below the microplasma electrode to measure ozone concentration. The discharge voltage was gradually increased from −3.0 kV to −3.4 kV in 0.2 kV increments for blood coagulation experiments. Ozone concentration measurements were performed for discharge voltages ranging from −1.4 kV to −3.4 kV, also in 0.2 kV increments, at the same air flow rate of 0.6 L/min.
A custom-built, two-stage Marx Generator equipped with MOSFET switches (IXYS Corporation, Milpitas, CA, USA, IXTH1N250) was developed in the laboratory to serve as the pulsed power supply for the microplasma electrodes. The generator produces negative voltage pulses with peak amplitudes of up to −4 kV. In the blood coagulation experiments, discharge voltages were set at −3.0 kV, −3.2 kV and −3.4 kV, with pulse frequencies of 700 Hz and 825 Hz. A pulse waveform with a pulse width of 800 ns which is shorter than previously reported in [4] was employed to improve energy efficiency.
The discharge voltage and current were measured using a high-voltage probe (Tek-tronix, Beaverton, OR, USA, Tek P5100) and a current probe (Tektronix A622), respectively. Both probes were connected to a digital oscilloscope (Tektronix MSO 4104).
A light microscope (Olympus, Hamburg, Germany, Olympus BX51) coupled with a CCD camera was used to analyze the blood samples.
A professional thermal camera (Teledyne FLIR, Wilsonville, OR, USA, FLIR T540) was used for temperature measurements.
2.2. Microplasma Electrode
The microplasma electrode was facing the blood droplet, which was placed on a glass lamella support. Microplasma was generated at atmospheric pressure using a surface DBD electrode. The setup included a 25 µm thick dielectric layer placed between two grid-shaped wires: the high-voltage electrode (1.2 mm width) and the grounded electrode (0.2 mm width), as illustrated in Figure 2a,b. A 50 µm thick dielectric backing layer covered the high-voltage side.
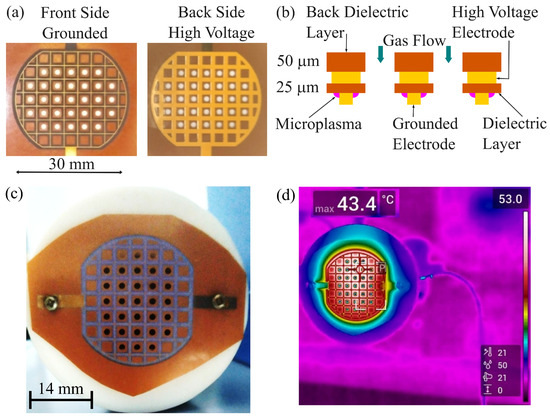
Figure 2.
Microplasma electrode system. (a) Image of the microplasma electrode system with grounded front side electrode and high voltage back side electrode; (b) schematic image of the cross-section of the microplasma electrode system; (c) image of the microplasma electrode during discharge in air at −3.4 kV and 825 Hz; (d) thermal image of the microplasma electrode during discharge in air at −3.4 kV and 825 Hz.
The electrode, 30 mm in diameter, featured a grid pattern with 3.5 mm spacing. It also contained 31 perforated holes, each 2 mm in diameter (Ø), allowing gas flow and facilitating the delivery of reactive species produced by the microplasma to a specific target. As shown in Figure 2b,c, the microplasma discharge was observed near the grounded electrode grid.
The symmetric electrode pattern allows straightforward scaling to different surface areas. Although the electrode surface area is modified, the discharge voltage remains the same, only the discharge power will modify, thus allowing us to keep the same electrical insulation level.
The output of the Marx generator energized the electrodes at a negative pulse voltage with pulse width of 800 ns and a pulse rising time of 160 ns, as shown in Figure 3. The spikes convoluted in the current waveform at the rising time of discharge voltage appeared due to microplasma generation. The microplasma power is obtained by multiplying the voltage V and current I waveforms. The power was calculated by integrating the power waveform in time and divided with the period corresponding to the frequency of 700 Hz and 825 Hz. The calculated discharge power is shown in Table 1.
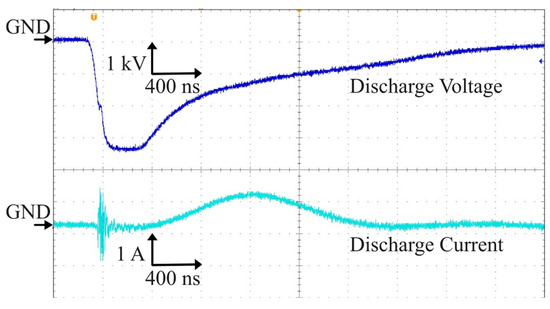
Figure 3.
Waveforms of the discharge voltage and corresponding discharge current.

Table 1.
Microplasma power at various discharge voltages and frequencies.
2.3. Ozone Generation
The concentrations of ozone generated by the microplasma increased with discharge voltage at a gas flow rate of 0.6 L/min, as shown in Figure 4. At the discharge voltage of −1.4 kV, no ozone was measured. At −2.6 kV and above, ozone readings reached the instrument’s upper detection limit (100 ppm), implying higher actual levels. Thus, we can assume that the values of ozone concentration at −3 kV, −3.2 kV and −3.4 kV were more than 100 ppm. Pulse frequency was 825 Hz.
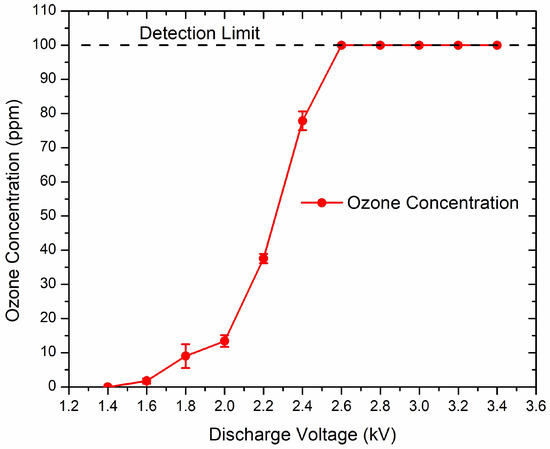
Figure 4.
Ozone concentration increases with discharge voltage. Pulse frequency was 825 Hz.
2.4. Blood Sample Preparation
Blood samples from a dog and a cat were obtained from the Hematology Laboratory of the University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania. The blood was stored in a refrigerator until the experiments were carried out. The blood samples were collected in EDTA (ethylenediaminetetraacetic acid) tubes for preventing blood clotting. A volume of 20 µL blood was dispersed with a pipette on a glass lamella support placed at the distance of 1.5 ÷ 2 mm below the microplasma electrode and the microplasma was applied. Based on the observed blood coagulation effect, the microplasma treatment parameters consisting of treatment time, discharge voltage and pulse frequency were modified. For each experimental condition, at least two measurements were carried out. The blood sample was photographed before and after each experimental point. After treatment, we gently scratched the surface with a pipette tip to verify film formation. A rough estimation of the coagulation layer thickness was carried out using the obtained images. The blood samples were obtained on EDTA anticoagulant, which does not allow prothrombin time (PT) and activated partial thromboplastin time (aPTT) determination. As the available blood volume was limited, we focused on showing the relative acceleration of surface coagulation by microplasma treatment, leaving full coagulation panel characterization for future studies.
3. Results
Using the same type of electrode as that described above, we previously achieved sterilization of Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus using environmental air as discharge gas at a flow rate of 0.6 L/min and discharge voltages below −2 kV [4]. Based on those previous results [4], here we carried out the microplasma blood treatment experiments, starting with the same discharge voltages. As at those voltages we did not observe any coagulation effect, we increased the discharge voltage above −2 kV, and the first visible coagulation effect was achieved at −3.2 kV, 700 Hz, 0.6 L/min air flow after 5 min of treatment (Figure 5). In a control experiment, samples of blood were also subjected to treatment using only the air flow at 0.6 L/min for 5 min without plasma generation. After 5 min of microplasma treatment, a coagulated layer with a thickness estimated at around 150–200 µm was formed on the surface of the blood droplet. As illustrated in Figure 5 and Figure 6, no coagulated layer was formed on the blood droplet during the treatment with air flow only.

Figure 5.
Dog blood samples: (first row) before and after blood treatment with air at 0.6 L/min for 5 min and (second row) before and after microplasma treatment for 5 min at −3.2 kV, 700 Hz, air flow at 0.6 L/min.
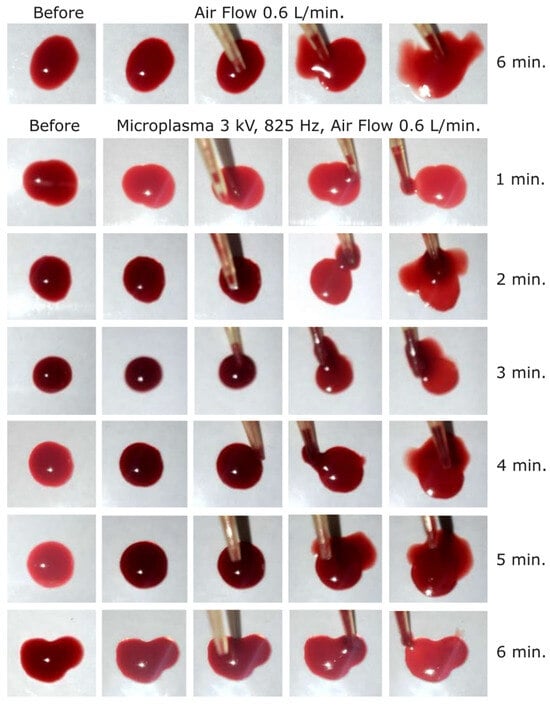
Figure 6.
Dog blood samples: (first row) before and after blood treatment with air at 0.6 L/min for 6 min and (2 to 7 row) before and after microplasma treatment for 1 to 6 min at −3 kV, 825 Hz, air flow at 0.6 L/min.
In order to increase the microplasma on time, and thus the coagulation effect, we in-creased the frequency to 825 Hz. Using that frequency, we started at −3 kV and carried out experiments for one minute to 6 min with one-minute incremental steps. As shown in Figure 6, a relatively weak coagulation effect could be observed after 5 min of treatment. The coagulated layer thickness was estimated at around 150–200 µm after 5 and 6 min of microplasma treatment. In contrast, no effect was observed even after 6 min of treatment with air flow only (Figure 6).
After that first set of experiments, the discharge voltage was increased to −3.2 kV at 825 Hz frequency, and a visible coagulation effect occurred after 3 min of treatment, as shown in Figure 7. After 6 min of microplasma treatment, the layer from the surface of the blood droplet was completely coagulated and could even be peeled off with the tip of the pipette. The coagulated layer thickness after 3 min of microplasma treatment was estimated at around 150–200 µm and increased to about 500 µm after 6 min of treatment. Two different color intensities were also visible, i.e., one of the peeled-off coagulated layer (dark red), and one of the non-coagulated blood (light red) (Figure 7).
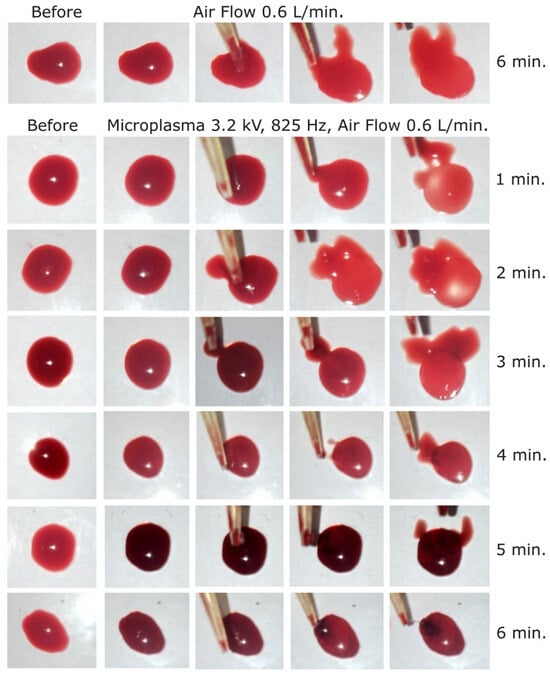
Figure 7.
Dog blood samples: (first row) before and after blood treatment with air at 0.6 L/min for 6 min and (2 to 7 row) before and after microplasma treatment for 1 to 6 min at −3.2 kV, 825 Hz, air flow at 0.6 L/min.
In the next experimental step, the discharge voltage was further increased to −3.4 kV, frequency 825 Hz, in order to investigate if the blood coagulation effect is also stronger at this voltage. As shown in Figure 8, a coagulation layer was visible at the surface of the blood droplet after 2 min of treatment at this voltage. The coagulated layer thickness after 2 min of microplasma treatment could be estimated at <200 µm and increased to about 800 µm after 6 min of treatment. As a discharge voltage of −3.4 kV is close to the microplasma electrode breakdown voltage, no further increase was carried out above this voltage.
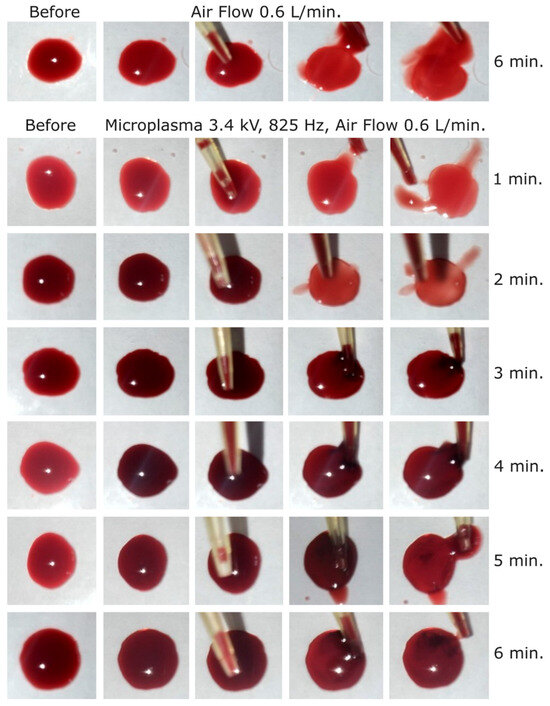
Figure 8.
Dog blood samples: (first row) before and after blood treatment with air at 0.6 L/min for 6 min and (2 to 7 row) before and after microplasma treatment for 1 to 6 min at −3.4 kV, 825 Hz, air flow at 0.6 L/min.
Although the experiments were carried out close to the breakdown limit of the electrode, the discharge was still stable. There are no risks or inconsistent discharge that could affect the safety or efficacy of the treatment process. The safety of this type of microplasma electrode is assured by the fact that the microplasma is generated at the surface of the grounded grid part of the electrode. The grid part of the electrode which is energized by high voltage is on the back side and is electrically insulated, thus there is no risk of electrical shock.
In order to investigate in more depth, the coagulation effects induced using −3.4 kV, 825 Hz and for 6 min of treatment, cat blood samples subjected to that treatment were analyzed through microscopy. Samples of non-treated blood, blood treated for 6 min with air flow rate of 0.6 L/min, and blood treated with microplasma at −3.4 kV, 825 Hz and at an air flow rate of 0.6 L/min for 6 min, were prepared by spreading the blood with glass lamellas. During the spreading process, only a thin layer of blood remained on the glass lamella and was analyzed by microscopy (samples are shown in Figure 9). The non-treated blood sample and the blood samples treated with air flow showed almost the same color at 1× magnification, and at 10× and 40× magnification similar patterns of light color spots. The blood sample treated with microplasma showed a darker color compared with the other two samples at 1× magnification, and also different patterns at 10× and 40× magnifications, where larger light color spots can be observed. Most probably, those larger spots appeared due to the fact that the treated blood became more solid and stuck to the glass, and was therefore less susceptible to be uniformly spread over the glass lamella.
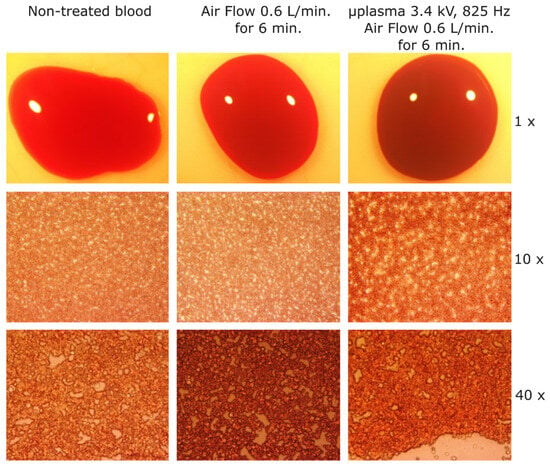
Figure 9.
Microscope images of cat blood samples. (left) Non-treated (reference) blood sample; (center) after blood treatment with air at 0.6 L/min for 6 min; and (right) blood sample after microplasma treatment for 6 min at −3.4 kV, 825 Hz, air flow at 0.6 L/min.
In order to also investigate the thermal effect of microplasma, one cat blood sample was subjected to microplasma treatment for 6 min. Microplasma was generated at −3.4 kV, 825 Hz and the air flow rate was set at 0.6 L/min. The blood sample of 20 µL was spread using a pipette on a glass lamella that was then covered with an electrical insulation tape and placed at a distance of 2 mm under the microplasma electrode. The glass lamella was covered with the electrical insulation tape to reduce the reflections for making possible thermal camera measurements. Thermal images of the blood sample before and after microplasma treatment are shown in Figure 10. The blood samples are the oval shapes in the center of Figure 10a,b. The sample temperature rose from 21.2 °C to 25.6 °C after 6 min of treatment, as shown in Figure 10.
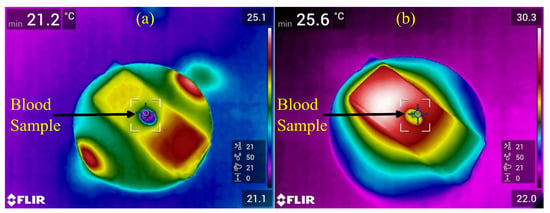
Figure 10.
Thermal images of cat blood sample. (a) Thermal image of cat blood sample before microplasma treatment and (b) thermal image of cat blood sample after 6 min of microplasma treatment at −3.4 kV, 825 Hz and an air flow rate of 0.6 L/min.
4. Discussions
4.1. Discussions of the Obtained Results
This study shows that treatment of blood samples with microplasma discharges in atmospheric air activates blood coagulation processes which lead to the formation of complete coagulated layers on the surface of the blood droplets subjected to the treatment (Figure 6, Figure 7 and Figure 8) and of specific coagulation structures in the blood (Figure 9). As discussed in the following, those blood coagulation effects of the microplasma treatment are specific and proportional with the voltage, frequency and action time of the delivered discharge pulses.
The microplasma discharge in air generates reactive oxygen and nitrogen species (RONS). Excited states N2(C3Πu), N2+(B2Σu+), N2(A3Σu), O(3p5P), O(3p3P), OH(A2Σ) were produced in microplasma through the reactions which are initiated by the electron impact dissociation of N2, O2 and H2O (water vapors in room air), as shown in reactions (1) to (12). Also, the reactive products are forming furthermore other RONS [15,17]:
Ozone (O3) is produced by the three-body reaction of O2 and O [18]:
where M is a third collision partner: O2, O3 or O.
O + O2 + M → O3 + M
In comparison with using argon or other discharge gases, the use of environmental air as discharge gas for microplasma treatment of bleedings is a much more practical solution since air is available freely, does not need gas cylinders for being stored and is thus more economical. The air flow rate value of 0.6 L/min was chosen considering our previously reported research [4]. We also reported in [2] that the air containing active species is flown to the treatment target by the electrohydrodynamic (EHD)-induced flow.
Although in the present study the emission spectra of the microplasma discharge were not measured, the light emission from microplasma can be observed in Figure 2c. As this light emission indicates the presence of RONS, in the following we discuss the implications of the RONS in the blood coagulation process based on related references. In order to determine the influence of such active species on the blood, we consider here the research reported in [19]. According to Sakiyama et al. [19], the lifetimes of reactive neutral species such as O and OH are approximately 0.1 ms, while that of N is around 1 ms. Given a 2 mm distance between the electrode and the blood sample, the presence of electrode holes and an air flow rate of 0.6 L/min, the air carrying these active species is estimated to reach the blood after a minimum of 5 ms. Therefore, it can be concluded that the coagulation process is primarily driven by the presence of long-lived active species. However, as indicated in [19], the density of reactive species may influence the interpretation of the results. Reactive oxygen species (e.g., superoxide and hydrogen peroxide) and reactive nitrogen species (e.g., nitric oxide) are known for influencing blood coagulation by several mechanisms that can accelerate clot formation. These mechanisms include platelet activation, endothelial dysfunction, tissue faction expression and fibrin formation [20,21,22]. Ozone could also play a role in the coagulation process. The coagulation effect induced by microplasma treatment may thus result from multiple mechanisms, including the activation of coagulation factors, stimulation of platelet aggregation and enhancement of fibrin polymerization [15].
The energy consumption for the experimental point −3.4 kV, 825 Hz and 6 min of treatment time was 466 J. Standard hemostatic methods—thermal, electro-, and chemical cautery—often damage surrounding tissue. Moreover, the electrocautery devices have typical power values of up to 400 W for surgical units. Consequently, as our microplasma device has a power of only 1.2 W, we can conclude that, besides the fact that microplasma treatment is a safer method as it does provoke tissue injures, this method is also more energy efficient. From this perspective, although only 20 µL of blood was treated in this study with microplasma, the blood droplet was about 8 mm in diameter, which indicates quite a low energy consumption relative to the targeted area. In fact, the energy consumption would be the same in the case of treating a bleeding area equal to the microplasma discharge area of the electrode. Considering the dimensions of the electrode that we used in this study (see Section 2), a large bleeding area corresponding to the 30 mm electrode diameter could be treated with this electrode in the case of real wounds without increasing energy consumption. Moreover, due to the scalability of this type of microplasma electrode, larger electrode surface sizes can be produced and used for generating the same discharge voltage with little power increments. Concerning the coagulation speed, we strongly believe that a microplasma device could also be improved from this perspective, as the present basic findings set the foundation for future studies aimed at improving the microplasma devices for providing, e.g., stronger coagulation effects in shorter time.
The thermal effect of microplasma on the blood and the supporting or surrounding tissues is minor since microplasma is a type of nonthermal plasma. In this regard, the temperature of the rat skin placed at a distance of 3 mm from the microplasma electrode surface and treated with microplasma for 3 min was reported previously [5] to be below 40 °C. The thermal image of the microplasma electrode that we used indicated a maximum temperature of 43.4 °C during discharge in air at −3.4 kV and 825 Hz (Figure 2d). Meanwhile, our thermal image experiment with blood treated with microplasma showed that the temperature of the blood sample positioned at 2 mm from the electrode increased from 21.2 °C before microplasma treatment to 25.6 °C after 6 min of treatment (Figure 10). In our opinion, that temperature increase of 4.4 °C had minor effects on blood coagulation activation observed in our study, which is consistent with the results of other studies. Thus, Mindukshev et al. [23] showed that a temperature increase from 20 °C to 25 °C has little effect on clot formation in human blood and also that the spontaneous clot formation time at the temperature of 25 °C is 14.47 min and at 41 °C is 9.51 min.
If we assume that OH was generated by microplasma, this means that UV light was also emitted by excited OH with peaks at 306.4, 307.8 and 308.9 nm [3]. In addition, as we previously reported in [3], nitrogen species N2 SPS with peaks emitted in the UV range at 313.6 and 315.9 nm are generated by microplasma. Thus, some influence of UV light in the blood coagulation process that we induced in this study could also be considered.
To summarize, the blood coagulation was carried out in this study using microplasma discharge in atmospheric air. The coagulation effect could be clearly observed starting at the discharge voltage of −3.2 kV and at least 3 min of treatment time with the blood sample placed at a distance of 2 mm from the microplasma electrode. The strongest coagulation effect was observed at −3.4 kV, 825 Hz and 6 min of treatment time. In contrast, the blood samples did not coagulate at all even after 6 min of treatment using air flow only, which indicates that the above-mentioned results were specifically related to microplasma treatment. While the blood coagulation effects induced by microplasma treatment in this study were most probably due to the action of RONS, the level of those effects increased with the increase in discharge voltage, frequency and treatment time. These results represent preliminary evidence that suggests the potential of using microplasma treatment as a technology for a faster, more economical and safer enhancement of coagulation when this is necessary, as this procedure requires low discharge voltage and power and is achievable at room temperature. In addition, due to its low discharge voltage and power consumption, microplasma treatment also represents a promising option as a portable coagulation tool for use by first responders.
4.2. Limitations/Future Work
One limitation of this study is the lack of platelet and fibrin assays to illustrate the physiological mechanisms supporting the observed effects. Another limitation of this study is the reduced number of blood samples available for the tests. We propose solving those limitations as future research directions. In addition, since the observed effects were likely related to RONS-mediated platelet activation and fibrin formation, experimental validation of D-dimer, fibrinogen changes, hemolysis assay, platelet markers, and also of the blood clots’ strength and durability, blood cell integrity and potential cytotoxic effects should be carried out in future studies. The clots’ mechanical integrity was described only qualitatively, thus in future studies, quantitative testing will also be carried out. As we here used small residual EDTA blood samples, PT/aPTT values could not be determined in this study. Future experiments with more appropriate models (e.g., on pig blood) may allow complete hemostasis characterization, including PT and aPTT characterization under controlled conditions.
In such future studies, we also suggest the use of emission spectroscopy methods to experimentally determine the presence of active species generated by microplasma at various distances from the electrode. In addition, experiments carried out with blood samples located at a distance of more than 2 mm from the plasma electrode should be performed in order to determine the efficacy of microplasma treatment for blood coagulation at various distances from the blood samples or wounds.
Last but not least, as the final goal of such studies is to use microplasma for blood coagulation in a prehospital environment in cases of bleeding, future work must be carried out with in vivo experiments.
5. Conclusions
This study showed that microplasma at −3.4 kV, 825 Hz, and 0.6 L/min for 6 min produced a strong blood coagulation effect which is specific as no effect was notable due exclusively to the air flow. The minimum conditions for coagulation were discharge voltage −3.2 kV, pulse frequency 700 Hz and 3 min of treatment time, and an increase in the coagulation effect was observed with the increase in discharge voltage, frequency and treatment time. The blood sample temperature increased by 4.4 °C after 6 min of microplasma treatment generated at −3.4 kV, 825 Hz and 0.6 L/min air flow rate. These preliminary results may open new directions for research and, in the future, potential clinical applications.
However, a substantial follow-up is necessary in vitro to evaluate the mechanistic bases of the observed coagulation effects and in vivo to investigate if similar effects could occur in the case of hemorrhages.
Author Contributions
M.G.B.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing—original draft, review and editing. A.D.S.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing—original draft, review and editing. C.S.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing—original draft, review and editing. S.C.T.: Data curation, Formal analysis, Investigation. V.S.: Data curation, Formal analysis, Investigation. K.S.: Conceptualization, Investigation, Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerul Educatiei si Cercetarii, Romania: “Nucleu” Program within the National Plan for Research, Development and Innovation 2022–2027, project PN 23 24 02 01.
Informed Consent Statement
The blood was obtained from the Hematology Laboratory, coordinated by Professor Bogdan Sevastre at the University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania. The Laboratory has a hospital section that treats animals and all the animal owners when they are bringing the animals to hospital are signing an agreement in which is stated that they agree that the blood can be used for scientific purposes.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the Hematology Laboratory, coordinated by Bogdan Sevastre at the University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania, for kindly providing the biological samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shimizu, K.; Blajan, M.; Kuwabara, T. Removal of indoor air contaminant by atmospheric microplasma. IEEE Trans. Ind. Appl. 2011, 47, 2351–2358. [Google Scholar] [CrossRef]
- Blajan, M.; Nonaka, D.; Kristof, J.; Shimizu, K. Study of Induced EHD flow by microplasma vortex Generator. IEEE Trans. Plasma Sci. 2019, 47, 5345–5354. [Google Scholar] [CrossRef]
- Blajan, M.; Umeda, A.; Muramatsu, S.; Shimizu, K. Emission spectroscopy of pulsed powered microplasma for surface treatment of PEN film. IEEE Trans. Ind. Appl. 2011, 47, 1100–1108. [Google Scholar] [CrossRef]
- Blajan, M.G.; Ciorita, A.; Surducan, E.; Surducan, V.; Shimizu, K. Biological decontamination by microplasma. Appl. Sci. 2025, 15, 2527. [Google Scholar] [CrossRef]
- Shimizu, K.; Hayashida, K.; Blajan, M. Novel method to improve transdermal drug delivery by atmospheric microplasma irradiation. Biointerphases 2015, 10, 029517. [Google Scholar] [CrossRef]
- Iza, F.; Kim, G.J.; Lee, S.M.; Lee, J.K.; Walsh, J.L.; Zhang, Y.T.; Kong, M.G. Microplasmas: Sources, particle kinetics, and biomedical applications. Plasma Process. Polym. 2008, 5, 322–344. [Google Scholar] [CrossRef]
- Gershman, S.; Harreguy, M.B.; Yatom, S.; Raitses, Y.; Efthimion, P.; Haspel, G. A low power flexible dielectric barrier discharge disinfects surfaces and improves the action of hydrogen peroxide. Sci. Rep. 2021, 11, 4626. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Jin, Q.; Zheng, C.; Deng, G.; Yin, S.; Liu, Z. Pulsed cold plasma-induced blood coagulation and its pilot application in stanching bleeding during rat hepatectomy. Plasma Sci. Technol. 2018, 20, 044005. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Cheng, K.; Lin, Z.; Cheng, Y.; Chiu, H.; Yeh, N.; Wu, T.; Wu, J. Wound healing in Streptozotocin-Induced diabetic rats using Atmospheric-Pressure argon plasma jet. Sci. Rep. 2018, 8, 12214. [Google Scholar] [CrossRef]
- Nomura, Y.; Takamatsu, T.; Kawano, H.; Miyahara, H.; Okino, A.; Yoshida, M.; Azuma, T. Investigation of blood coagulation effect of nonthermal multigas plasma jet in vitro and in vivo. J. Surg. Res. 2017, 219, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ikehara, S.; Takei, H.; Akimoto, Y.; Sakakita, H.; Ishikawa, K.; Ueda, M.; Ikeda, J.; Yamagishi, M.; Kim, J.; et al. Red blood cell coagulation induced by low-temperature plasma treatment. Arch. Biochem. Biophys. 2016, 605, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; von Woedtke, T.; Partecke, L.; van der Linde, J. Platelets are key in cold physical plasma-facilitated blood coagulation in mice. Clin. Plasma Med. 2017, 7–8, 58–65. [Google Scholar] [CrossRef]
- Rad, Z.S.; Davani, F.A.; Etaati, G. Determination of proper treatment time for in vivo blood coagulation and wound healing application by non-thermal helium plasma jet. Australas. Phys. Eng. Sci. Med. 2018, 41, 905–917. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Zheng, H.; Zhang, B.; Li, Y.; Zhang, Y.; Xu, Z.; Xu, A.; Jin, S.; Fang, Z.; et al. Low-temperature plasma efficiently promotes blood coagulation with less thermal injury in porcine models. Sci. Rep. 2025, 15, 31596. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Peddinghaus, M.; Balasubramanian, M.; Ayan, H.; Fridman, A.; Gutsol, A.; Brooks, A. Blood coagulation and living tissue sterilization by Floating-Electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Polito, J.; Quesada, M.J.H.; Stapelmann, K.; Kushner, M.J. Reaction mechanism for atmospheric pressure plasma treatment of cysteine in solution. J. Phys. D Appl. Phys. 2023, 56, 395205. [Google Scholar] [CrossRef]
- Eliasson, B.; Hirth, M.; Kogelschatz, U. Ozone synthesis from oxygen in dielectric barrier discharges. J. Phys. D Appl. Phys. 1987, 20, 1421–1437. [Google Scholar] [CrossRef]
- Sakiyama, Y.; Graves, D.B.; Chang, H.; Shimizu, T.; Morfill, G.E. Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. J. Phys. D Appl. Phys. 2012, 45, 425201. [Google Scholar] [CrossRef]
- Trostchansky, A.; Moore-Carrasco, R.; Fuentes, E. Oxidative pathways of arachidonic acid as targets for regulation of platelet activation. Prostaglandins Other Lipid Mediat. 2019, 145, 106382. [Google Scholar] [CrossRef]
- Yu, J.; Duan, W.; Zhang, J.; Hao, M.; Li, J.; Zhao, R.; Wu, W.; Sua, H.H.; Jun, H.K.; Liu, Y.; et al. Superhydrophobic ROS biocatalytic metal coatings for the rapid healing of diabetic wounds. Mater. Today Bio 2025, 32, 101840. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Bychkova, A.V.; Shchegolikhin, A.N.; Leonova, V.B.; Kostanova, E.A.; Biryukova, M.I.; Sultimova, N.B.; Konstantinova, M.L. Fibrin self-assembly is adapted to oxidation. Free Radic. Biol. Med. 2016, 95, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mindukshev, I.; Fock, E.; Dobrylko, I.; Sudnitsyna, J.; Gambaryan, S.; Panteleev, M.A. Platelet Hemostasis Reactions at Different Temperatures Correlate with Intracellular Calcium Concentration. Int. J. Mol. Sci. 2022, 23, 10667. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).