Abstract
The mechanism of brain information processing unfolds within spatial and temporal domains inherently linked to the concept of space–time symmetry. Biological evolution, beginning with the prevalent molecular chirality, results in the handedness of human cognitive and psychological functions (the phenomena known as biochirality). The key element in the chain of chirality transfer from the downstream to upstream processes is the pyramidal neuron (PyrN) morphology–function paradigm (archetype). The most apparent landmark of PyrNs is the geometry of the cell soma. However, “why/how PyrN’s soma gains the shape of quasi-tetrahedral symmetry” has never been explicitly articulated. Resolving the above inquiry is only possible based on the broad-view assumption that encoding 3D space requires specific 3D geometry of the neuronal detector and corresponding network. Accordingly, our hypothesis states that if the primary function of PyrNs, at the organism level, is sensory space symmetry perception, then the pyramidal shape of soma is the best evolutionary-selected geometry to support sensory-motor coupling. The biological system’s non-equilibrium (NE) state is fundamentally linked to an asymmetric, non-racemic, steady state of molecular constituents. The chiral theory of pyramidal soma shape conceptually agrees that living systems have evolved as non-equilibrium systems that exchange energy with the environment. The molecular mechanism involved in developing PyrN’s soma is studied in detail. However, the crucial missing element—the reference to the fundamental link between molecular chirality and the function of spatial navigation—is the main obstacle to resolving the question in demand: why did PyrNs’ soma gain the shape of quasi-tetrahedral symmetry?
Justification of Subject-Matter
What is the biological significance of chirality? To answer the question, we should take into account that the intricated system of the neuro-receptors and neurotransmitters (internal molecular chirality) of living organisms, recognize and perceive all life-essential changes in the external environment, biological part of each possesses predominant, biologically friendly form (L- isoform form for amino acids) of chirality. This striking biological chiro-centricity suggests the crucial role of cell chirality and, firstly, the chirality of brain primary neuronal cells PyrNs. Accordingly, our objective is to review what is known regarding the origin, forms, and significance of PyrNs chirality.
1. Introduction
Advances in molecular biology promise a new opportunity in developing artificial intelligence (AI) devices based on biomolecular building blocks (DNA and proteins) functioning in micro- and nanoscale. To achieve this goal, the broad-view revision of new results in diverse branches of biology is in demand.
Generalizing Darwinism to the Evolution of the Universe is a challenging and attractive idea, elevating the analytical description of biomolecular, cellular, and organism levels of events at new qualitative and philosophical levels. As an integrative approach, it is necessary and beneficial to understand the common principles of biological and non-biological existence, the hierarchical organization of biological systems, and the relations between biological and artificial intelligence. From the holistic view, space–time symmetry and relativity (STSR) are indispensable forms of existence, which can neither be created nor destroyed but only transformed, suggesting that physics and biology are imminently associated branches of science. The ground of the above-mentioned association is that the most common fundamental determinants of organic and non-organic objects are space, time, symmetry, and relativity.
Below, we will focus on the spatial symmetry determinant in biology, known as biological chirality (also referring to handedness) or biochirality. The importance of chirality is appreciated in many sciences, explaining many definitions of corresponding unique geometrical concepts. When biologists justly say that chirality is the critical feature in living organisms, the essential omitted objective fact is that chirality is also the key characteristic of the inorganic world and even a fundamental attribute of space and time, known as a primary determinant of existence. Therefore, attention to symmetry’s universal role is necessary to interpret life’s phenomena adequately.
Conservation and diversion of mirror or reflection symmetry is widely recognized as a fundamental mathematical tool and a universal degree of freedom in physical and the principal concept in biology [1,2]. Accordingly, symmetry’s physical roots appearing in biological patterns are frequently addressed, and a chirality-centric view of physical and biological processes steadily gains recognition. [3,4]. It is a common agreement that animal morphogenesis is regulated by the gene regulatory networks and functional requirements [5]. “Chirality is a central feature in the evolution of biological systems” [6]. Homochirality of biomolecules (DNA, RNA, proteins, and lipids) is linked to the origin of life, i.e., to the beginning of biological evolution (Figure 1). Consequently, the entire hierarchy of biological processes (including bilaterality) can be associated with the interplay of molecular, cellular, and higher orders of biological organization, influenced by initial molecular chirality and factors related to the movement of organisms [7,8].
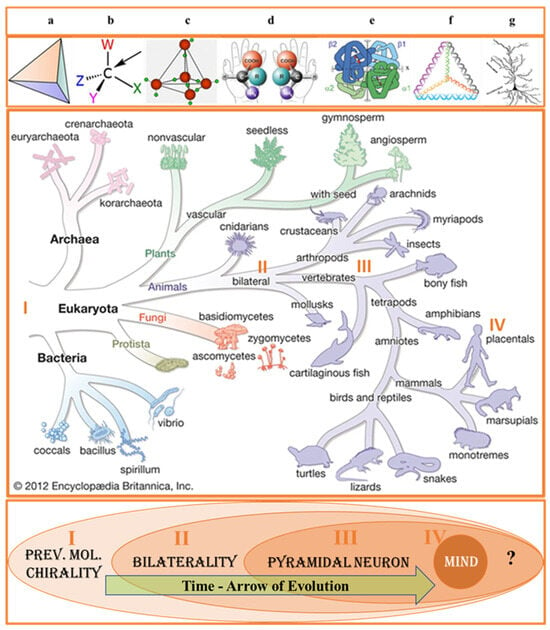
Figure 1.
Biological evolution from the biochirality perspective. A broad view of biological symmetry assumes that chirality transfer from the molecular to macromolecular and cellular levels plays a vital role in human physiology underlying perceptual and cognitive function linked with bilateral organisms’ emotional and behavioral expression. Biologists have made long-term efforts to explain the common origin of the homochiral world of DNA code and protein sequence, resulting in the emergence of the human mind. Quite unexpectedly, for many scientists, it was realized that the Ariadne thread is the concept of symmetry [8]. On the other hand, all theories ignoring the symmetry principle proved to be irrelevant. Indeed, the evolutionary history of life exhibits the chain of successive events (chain of chirality transfer) linked by the apparent common association with the notion of symmetry. The four most significant are indicated in Figure 1. Events I, II, and IV are the scientists’ long-term concerns. The appearance and evolution of PyrNs (event III) attract increasing attention but do not focus on the specific geometric shape of soma. (Top). Transfer of symmetry constraints from molecular to cellular level. (a) Tetrahedron—geometrical shape that can be transformed from chiral to achiral version. (b) Tetrahedral (sp3-hybridized) carbons with four different substituents are the principal components of biological molecules exhibiting homochirality. (c) Tetrahedral structure of water forming about 70% of cytosol. (d) L/D amino acids. (e) Tetrahedral structure of hemoglobin (Hb) expressed in neuronal and red blood cells. (f) Tetrahedral assembly of DNA. (g) Pyramidal neuron. Adopted with changes from [9,10]. PNs were discovered by the Ukrainian anatomist and histologist Vladimir A. Betz (1834–1894) [11]. (Middle). Tree of Life. Successive biologically essential evolutionary selections: I—prevalent molecular chirality, II—bilaterality, III—pyramidal neurons, and IV—human mind. Adopted from Encyclopedia Britannica Inc. 2012 with alterations. The evolution of bilaterian CNS occurs through the differentiation of cell types and network organization. A line of phylogenomic evidence suggests that some organisms (such as sponges and placozoans) may benefit from the loss and/or modification of their neural cell types. However, the mainstream of evolution demonstrates the benefit of the CNS for managing voluntary movement (events in the space–time domain). At the cellular level, the origin of CNS is characterized by the appearance of pyramidal neurons (PyrNs). However, currently, there is no systematic information concerning the differentiation of PyrNs between mammal groups (placentals, marsupials, and monotremes). (Bottom). Time-arrow of biological evolution. Learning the congruence of the molecular and cellular events helps to understand the link between voluntary movement, space perception CNS, and PyrNs’ morphology.
The ten groups of bilaterian organisms comprise two main clades: the protostomes and deuterostomes. The deuterostomes contain clades of olfactory (characterized by the origin of respiratory, sensory (olfaction), and neuronal systems insides) in Urochordata and Vertebrata.
The progress in the evolution of the locomotion, sensory system (and embryogenesis) started with olfaction in the olfactory, followed by the development of vision in both vertebrates, and bilateral vision preceded terrestrial life [11]. The end product of the bilaterian evolution is the integrated space–time chirality-oriented sensory-motor nervous system, which reached high efficiency in Vertebrata and brought advanced lateralized brain cognitive functions to humans. We want to know, “What is PyrNs’ role in the long-term evolution process, and what are their primary functions in the human brain?”.
2. Hierarchical Chain of Chirality Transfer
The stereospecific nature of living matter, beginning at the molecular level, from discriminating between the alternative enantiomers and culminating in the laterality of human cognitive functions, has long been viewed as the most striking feature challenging the curiosity of biologists, psychiatrists, and physicists [12]. The contemporary view on biochirality is based on the explicit integrative assessment of the spatial symmetry determinants persistently working in evolution and development. The association of molecular and cellular-level biological processes with mental health and illness points to the reciprocal relations between two opposite poles of the biological hierarchy, molecular biology (I) and the phenomenon of the conscious mind (V). Biological psychology (biopsychology, psychobiology, psychophysiology, neuropsychobiology) and biological psychiatry represent the branches of science dealing with this fundamental link. The molecular and cell physiology of pyramidal neurons (PyrNs) provides a natural bridge between molecular biology and higher cognitive and psychological function. At the cellular level, biological processes within CNS are grounded in the physiology and function of PyrNs [13]. In biology, many legitimate questions still need appropriate answers. But some questions have never been expressed. One such inquiry is why/how PyrNs, implicated in the spatial navigation of bilaterians, gain quasi-tetrahedral symmetry of soma. The objective reason for such silence is the combination of several factors. First, the long-time limited availability of molecular chiral discriminative tools. Second, an enormous volume of information describing the link between molecular chirality and brain laterality is dispersed in many scientific journals covering various sciences, from quantum physics and group theory to molecular biology and neurosciences. Third, the lack of a broader (generalized or philosophical) view on the subject matter (biochirality). This review is an attempt to improve the situation. In view of our hypothesis, the shape of soma is presumably associated with the tetrahedral geometry of homochiral biomolecular condensates. To my knowledge, the first attempt to approach this inquiry (examination) was made just recently [14]. In our view, the question should be resolved based on the understanding of the evolution of CNS [15].
The idea of the evolutionary link between molecular chirality (1) and brain laterality (2) immediately and inevitably catalyzes the question of the cause of PyrNs’ soma. The concept of the chain of chirality transfer is based on two complementary assumptions. First, state that every preceding step contains an internal determinant of the chirality of consecutive steps. The second assumes the existence of some fundamental driving force working on all levels of biological organization. Such fundamental force is sustained by ever-present space–time symmetry. Indeed, accumulating evidence indicates that universal space–time symmetry is manifested as chirality at the molecular, cellular, and all following levels of biological organization. The common-sense rule is that every chiral object (molecules, cells, neuronal networks, and others) is an efficient tool for discrimination of the left and right sides. Among the principal downstream factors contributing to pathways of biomolecular condensates is the geometry of chiral carbon atoms in proteinogenic AAs. Tetrahedral (sp3-hybridized) carbons with four different substituents are the principal components of biological molecules exhibiting homochirality. The chiral of carbon atom promotes the tetrahedral molecular assembly, also observed in DNA structures and the structure of “biological” water (Figure 1) [9,10,16,17,18]. Unfortunately, the geometrical definition of soma shape was not explicitly clarified. Consequently, whether the pyramidal shape of soma was evolutionarily selected or whether the current state is “an appendix of the CNS” has never been debated. Evolutionary selected biochemical homochirality is based on the chiral stereoselectivity of biosynthetic and metabolic reactions [19,20]. Chirality is the right keyword in the search for the integrity of PyrNs’ origin, structure, and functions. Such an assumption agrees that chiral asymmetry appears in nature at all levels, from elementary particles and AAs to mammals’ morphology and even galaxies’ levels [8,21,22]. The chirality of multi-dimensional elementary particles is the principal concern of string and gauge theories projecting beyond the Standard Model physics [23]. The well-known Feynman diagram reflects the space-chirality of multi-dimensional elementary particles, which is the principal concern of string and gauge theories projecting beyond the Standard Model physics [24]. The well-known Feynman diagram reflects space–time-dependent symmetry transformations in the elementary particle events. The origin of chirality at the cellular level is attributed to biomolecular homochirality [23]. Molecular homochirality and cellular chirality are the internal “forces” driving an organism’s left–right asymmetric development [23,24,25] in bilaterians. The hierarchical chain of chirality transfer from the atomic to the organism level is one of the challenging targets of contemporary biological sciences [26,27,28]. The attention to the possible all-embracing role of the symmetry determinant in biology (from the biomolecular chirality to the bilaterality of human cognitive function) has become a significant trend in cognitive neuroscience [22,29,30,31]. However, surprisingly, in this chirality-oriented stream of biological research, the question “Why/how does PyrNs gain tetrahedral geometry?” remains unarticulated, as mentioned before. In this situation, the value of questioning exceeds the value of the particular hypothesis because the first question triggers the string of the next one. The essential secondary-wave question is, “What is the mutual orientation of the corresponding PyrNs’ somas in the left and right hemispheres?” (Figure 2). Below, we introduce some relevant hypotheses based on the generalized view of biological symmetry. A broad view of biological symmetry assumes that chirality transfer from the molecular to macromolecular and cellular levels plays a vital role in physiology underlying perceptual and cognitive functions linked with the bilateral organisms’ emotional and behavioral expression [8,22,32,33]. The cellular and molecular mechanisms of psychological states are fast-developing branches of life science. From the time of Freud, Jung, and Assagioli, perceptual, cognitive, and behavioral functions have been considered the essential determinants of psychological processes [34,35,36]. Regardless of the view on the relationship between psychology’s cognitive and behavioral domains, it is commonly agreed that both exhibit a hierarchy, with the bottom referencing the basic sensory and perceptual processes and the top referencing the higher levels of cognitive and executive functioning control. From the top view, the hierarchical structure is the chain of downstream processes, including motor functions, perceptual abilities, neuronal circuits, and underlying molecular biology.
The animal mechanism of space and time perception (underlying interpretation of sensory inputs to activation of motor functions) within cellular, molecular, and cognitive levels significantly relies on the laws of space–time symmetry [8,37,38,39,40]. The perception of space, time, and symmetry are highly integrated into the complex evolutionary selected mechanism. The known examples are perception of motion and speed perception, involving spatial, temporal, and symmetry determinants of motion [41]. Fortunately, many experimental and theoretical situations allow for their study individually. Publications explicitly devoted to studying all aspects of spatial perception are the specific focus of our analysis. Unfortunately, for objective reasons, most space perception studies are conducted without attention to two essential aspects: perception of object symmetry and the hemispheric asymmetry of brain functions. Following this logic, the shape and functions of PyrNs deserve specific attention. Before considering the downstream molecular determinants, let us review fundamental upstream factors.
Space, Time, Symmetry, and Relativity
Throughout history, our notion of space and time has undergone dramatic transformations. Following the intuitive view of Euclid, Newton concluded that the external to the observer space is infinite, isotropic, uniform, perfectly penetrable, and immovable (i.e., absolute quantity), where he located the relative motion and studied its variation [42,43]. The Galilean principle of relativity formulated the first deviation from the intuitive interpretation of space and time. Later, the principle of relativity was complemented by the Noether theorem, framing the fundamental role of symmetry. All advanced physical theories directly or indirectly employ an inevitable link between four determinants of nature: space, time, symmetry, and relativity (STSR), bringing a deeper interpretation to the concepts of thermodynamics and entropy. As a consequence, the concept of STSR has gained attention in biology. Biological systems are structurally organized according to patterns repeated at each hierarchical level [44,45]. Notably and prominently, the most fundamental features, common for all hierarchical levels, are STSR and the notion of chirality, which plays a critical role as a specific form of geometric symmetry [7,45,46]. In this article, we assume that the unifying concept of STSR reflects the fundamental properties of nature [27,47,48,49,50,51] and primary determinants of life [8,47,52,53]. Now, we are prepared for an overview of the downstream molecular determinants.
3. Biological Evolution
3.1. General Trend
The new millennium in evolutionary science is characterized by fast advances in the view of the human brain–body laterality (handedness). Initially, many thought that left–right laterality (handedness) is an essential aspect of human brain organization, the basis of which is poorly understood [54]. In parallel, a complementary view was unfolding that during biological evolution, from om early bilaterians to extant humans, the brain exhibited a set of significant milestones of reorganization that enabled advanced adaptive behaviors. They are steering (taxis navigation), reinforcing (model-free reinforcement learning), simulating (model-based reinforcement learning), mentalizing (model of mind), and speaking (rhythmic semantic processing) [55]. Recently, many expressed a cautious assumption that “symmetries may have their origin in fundamental molecular asymmetries going far back in biological evolution [56]. Under the pressure of experimental evidence and looking at the roots and top of the evolutionary tree, we can bring this assumption to a more definite statement: lateralization of brain morphology and functions are the result of evolutionary selection having origin in fundamental molecular asymmetries and not less indispensable space–time symmetry of environment [8].
3.2. Molecular Chirality
Despite the long history of attention, the origin of the single chirality of the Earth’s biosystem is still referred to as a great puzzle. The first step in biological evolution was chiral selection at the molecular level. Molecular homochirality is secured by cellular functions (such as ribosomal protein synthesis). In turn, cell chirality is mediated by the prevalent chirality of the protein–enzyme complex. Molecular and cellular chirality interplay constitutes a stereospecific ground for the mixed analog–digital information procession in biological systems [57,58]. Molecular homochirality (more precisely, prevalent molecular chirality), feeding the roots of the evolutionary tree, pre-determines its branching structure and behavior of each individual sub-division (Figure 1) fall into two categories. One is searching for the possible coincidence of natural circumstances [59,60], and the second is for fundamental determinants of biological symmetry [8]. We will examine accumulating evidence in favor of indispensable causal force. Narrowing attention to the spatial symmetry at the molecular level, we can state that the majority of biological molecules (such as AAs and sugars) and macromolecules (including DNA, RNA, proteins, and lipids) exhibit the prevalent form of spatial symmetry in three domains of geometry: chirality, fractality, and topology [61,62,63,64,65]. The enormous complexity of biochirality is illustrated by the fact that an entire complex of chiral molecules modulates diverse forms of cell chirality. An additional example of such complexity is the fact that molecular compounds of chiral and achiral structures frequently demonstrate strong chiro-optical and chiro-magnetic effects [66]. The most studied biomolecules implicated in the effects of chirality are proteinogenic AAs, enzyme–substrate protein complexes, and non-proteinogenic AAs in the CNS [67,68,69,70]. The chiral non-proteinogenic AAs play an essential role in the physiology of plants, insects, and animals. An example of non-proteinogenic chiral AAs is DOPA, which is part of the normal biology of some plants and all animals. Homochiral synthesis of L-DOPA isoform in humans occurs via biosynthesis from L-tyrosine (L-Tyr) by the tyrosine hydroxylase enzyme [71]. Protein stereochemistry is represented by two classes of stereoisomers: chiral enantiomers (prevalent L-isoform) and achiral diastereomers (prevalent cis-stereo-form). The range of chirality-specific phase transitions at the molecular level includes chiral inversion and spontaneous chiral symmetry breaking in diverse states of protein folding and aggregation. Evolution branched single-cell ancestors into two main domains of life: Procaryotes and Eukarya. Structurally and functionally, eukaryotes are organized into more hierarchical levels than prokaryotes (bacteria and archaea) [72]. Protein chirality is strongly associated with the chirality of membrane phospholipids. The main component of biological membranes glycerophospholipids are glycerol-based phospholipids that have differential chirality: D-glycerol for bacteria and eukaryotes and L-L-glycerol for archaea [71]. At the cellular level, we restrict our attention to eukaryotes’ evolution, leading to the emergence of all neuronal cell types, including PyrNs (Figure 2). At the molecular level, we primarily focused on the contribution of protein handedness to the shape of PyrNs. The prevalent molecular chirality of organisms at the protein level occurs through the fine-balanced interaction of L-(major) and D-(minor) isoforms. Up-regulation of D-AAs is implicated in organism aging [68,69,73,74], psychiatric disorders [75], and cancers [76,77]. The most known adverse impacts are attributed to the up-regulation of three AAs: D-aspartic acid (D-Asp), D-serine (D-Ser), and D-alanine (D-Ala). All of them (and, potentially, by D-glutamate) are agonists for NMDA (N-methyl-D-aspartate) receptors of PyrNs [78,79,80,81,82,83]. The involvement of D-AAs in the physiology of PyrNs suggests their role as biomarkers in the spectrum of neurodegenerative and psychiatric disorders [75]. Continuous spontaneous and induced assembly of homochiral proteins from simple to complex arrangements includes the tetrahedral complexes [84] (well-known in the hemoglobin conformational dynamics) [85,86]. Notably, the tetrahedral geometry of molecular compounds is not accidental but is the consequence of the symmetry of atomic electron orbitals. But how tetrahedral molecular complexes can result in the pyramidal shape of soma remains unknown. We will return to this question later in the text.
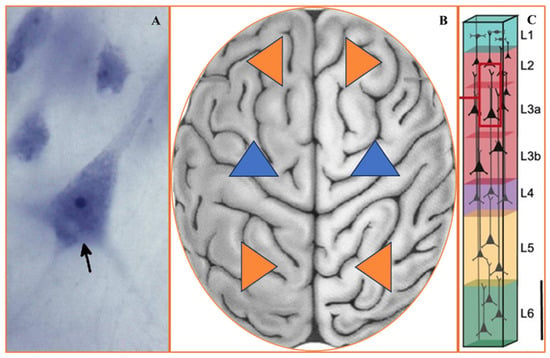
Figure 2.
(A–C) Pyramidal neurons morphology and orientation. (A) Pyramidal neurons (arrow) were identified by their large size, triangular shape, and the presence of a pially oriented apical dendrite opposite one or more basilar dendrites. The scale bar represents 10 μm. Adopted from [87,88]. (B) Arbitrary variants of PyrNs orientation in the bilateral brain may provide different spatial navigation information processing strategies. Most sensorimotor cortical neurons had typical vertically oriented apical dendrites that extended towards the pial surface [89,90]. (C) Orientation of PyrNs in column [91].
3.2.1. Link of Physiological and Psychological Functions
Before closely considering PyrNs’ morphology, let us analyze their contribution to upstream biological processes. The cellular ground of association between brain physiology and psychology is the function of PyrNs. This means that PyrNs provide an opportunity for the basis of sensory processes (including visual, auditory, gustatory, olfactory, and cutaneous systems) to participate in the higher cognitive functions (such as emotion, learning, and memory) [92,93]. Indeed, populations of PyrNs were observed in low-level sensory cortices, including primary somatosensory, visual, and auditory cortex, with PyrNs located in high-level areas such as the prefrontal cortex (PFC). Such a conceptual view allows us to trace a link between chirality-dependent molecular events in PyrNs and their role in perceptual, cognitive, and behavioral aspects of psychological functions (PFs). Object perception or object recognition is the process of meaningful interpretation of the sensory input underlying our ability to act in the world. Psychophysical and physiological evidence suggests that object recognition relies on perceptual constancy, referring to the fact conservation of perceived geometrical characteristics of objects during relative motion of subject and object. Perceptual constancy is the invariance under spatial transformations and is closely associated with the mathematical definition of symmetries. Therefore, it is not accidental that the CNS of bilateral animals possess the specific neuronal mechanism of symmetry/dissymmetry perception [94,95,96]. Indeed, the mechanisms of sensory space–time perception and space–symmetry perception (characterized as perceptual constancy) are linked to the relativity principle and space–time symmetry. All forms of mathematical formalism (of Noether’s and all others) describing mechanical movement (space–time changes) are based on the particular variant (classical or quantum) of the relativity principle). This way, mathematical logic provides the explanation (understanding) of the relation between the objective physical world and its subjective sensory and mental representations. In other words, the relativity principle is the primary participant and designer of our sensory and cognitive (mental) representations [96]. In agreement with motion-related relativity, the mechanism of space–symmetry perception can be characterized as the frame of reference-dependent. Accordingly, sensory perception is a relative modality in general. The orientation in 3D space (also said attitude), based on the sensory representation system coupled with the motor control system, requires existence at three degrees of freedom (3-D-space) [97,98]. In humans, the mechanism of spatial information processing includes (a) two hierarchical channels of transformation (sensorimotor and cognitive) [98,99], (b) interaction of sensory (modality-specific), haptic, and kinaesthetic proprioception, and (c) utilizing the interaction of egocentric (intrinsic or attached to the body) or allocentric (extrinsic to the body) [97,100]. Prevalent molecular chirality plays a pivotal role in human physiology. It can be illustrated by the regulation of intra-body dynamics of D-AAs. It is well known that D-Ser is involved in biochemical processes, physiology, and morphology of the main organs of the body, including kidneys, liver, gut, urine, stomal, lung, and brain [13,20,101]. The dependence of CNS on D/L-AAs metabolism allows us to trace the interplay between physiology and complex perceptual, cognitive, emotional, and psychological functioning [32,34,100,101,102,103,104,105]. Analyzing the types of personalities, Jung differentiated them based on complex cognitive functions, including thinking, filing, sensation, intuition, and aesthetical judgment [29]. Expressions like psychological functions (PFs) or faculties, abilities, agents, states, conditions, and types, are widely used in psychological sciences and, to a lesser degree, in clinical practice.
3.2.2. Bilaterians: Symmetry–Function Interplay
Bilateria is the largest clade of animals characterized by bilateral symmetry during embryonic development. Bilaterians include insects, fishes, amphibians, reptiles, birds, mammals, and most crustaceans. Bilateral symmetry (compared to other symmetry types) provides significant adaptive advantages for the efficiency of locomotion in three-dimensional environmental space. Although the intuitive understanding of mine elements of the phenomenon have been commonly shared for decades, the scientific formulation of this hypothesis begins only in the present [5,106,107].
A frequently circulating statement in the biological literature is “The human body and nervous system consist of seemingly symmetric left and right halves [105]. The word seemingly is critical because, in the language of geometry, two parts of the bilateral brain are asymmetrical (breaking of mirror symmetry)—the property named chirality, which is a key notion in the discussion of the morphology and function of bilateral organisms [108]. The evolutionary origin of CNS is a long-lasting fundamental question in biology. Genetic studies reveal that the Cambrian explosion, characterized by the full range of body plans across bilaterians, is linked to 157 bilaterian-specific genes, including the entire Nodal pathway (a key regulator of mesoderm development) and left–right axis specification of body plan and nervous system development [109].
Analysis of reviews devoted to the evolution of CNS (published from 2003 to 2023) reveals that most of them are concentrated on the bilateral organism without any reference to the molecular chirality and morphology of PyrNs. Only a few of them are focused on the morphology of PyrNs without attention to PyrNs’ soma shape, molecular chirality, and bilaterality of animal organisms—the situation explaining the motivation and objectives of our chirality-centric view on biological evolution (Figure 3).
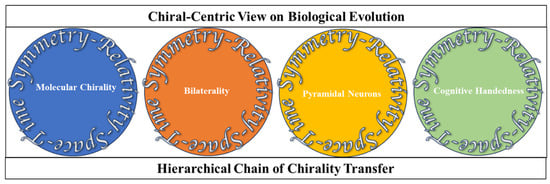
Figure 3.
The critical elements in the chain of chirality transfer from the molecular to the cognitive levels.
From an evolutionary perspective, space–time memory, sensory-motor integration, and cognitive performance are dramatically increased in bilateral organisms. In the human brain, where these functions reach the highest level, the essential enhancements come from the division of labor between the left and right parts of the body and the two brain hemispheres. The first bilateral organisms originate in invertebrates. Invertebrates, characterized by more diverse molecular homochirality, differentiation of all 3-D body axes is promoted to more advanced forms, signifying progress in space perception, voluntary movement, and navigation. In humans, the conjunction of cognitive and psychological functions allows projecting all aspects of molecular biology and space perception of the areas of psychology and psychopathology [110,111]. Such a view brings us to the understanding that the bilateral CNS of humans unavoidably exhibits bilateral patterns of physiological, cognitive, emotional, and behavioral functions underlying the psychological state of individuals (in health and disease conditions). It means that every individual has a window for the spontaneous and intended inputs influencing the state of bilateral CNS. This is practically exploited in bilateral/unilateral activation of the sensory system and corresponding cognitive functions in the healing of post-traumatic stress disorders [112] and mediating fear conditions [113]. The symmetry in biology is extended to the balanced arrangement of body parts around the 3-D axis. The Bilateria or bilaterians are animals revealing bilateral symmetry (chirality) from the embryonic state.
Neuroimaging studies reveal hemispheric asymmetry of association and limbic fiber tracts in utero [114,115]. The body plan of Bilateria has right and left sides that are mirror images of each other. The evolutionary origin of biochirality points to several major contributing factors. Three of them, relevant to the topic of our discussion, are prevalent molecular chirality, the necessity of space–time orientation of the moving body with the possibility to distinguish left and right (we point to the left/right because it is related to left–right mirror symmetry at the molecular level), a sensory system capable of space–time localization of objects representing food or social partner. From a biochirality view, it is essential to note that plants and animals, representing immobile and mobile organisms, exhibit different evolution pathways [116,117], which, in our view, are deferentially driven by the determinants of spatial symmetry. The focus of our consideration is specifically the evolution of mobile animal creatures.
According to fossil evidence, bilateral body symmetry in animals took place about 500 million years ago. In the course of evolution, the bilateral body inevitably leads to the bilateral CNS. This fact convinces many scientists that the laterality of brain functions cannot be accidental [118]. Symmetry in CNS is the balanced and co-related spatial arrangement of molecular, cellular, and anatomical components, providing the opportunity for the laterality of cognitive functions. The laterality of cognitive functions experiences the “symmetry pressure” from two sides: from the internal determinants and behavioral–environmental determinants. One is strongly associated with the genetic mechanism, and another evidently with epigenetic factors [119]. Epigenetics represents the network of molecular interfaces mediating gene–environment interactions [120]. In the language of symmetry, any epigenetic mechanism is the factor (symmetry changing factor) breaking genetically imposed homochirality of ribosomal protein synthesis [121]. Exploring human brain lateralization with attention to molecular chirality [31], cell chirality [122], genetics, and non-genetic factors in association with the underlying physical laws concerning space–time symmetry confirms that the origin of relative chiral homogeneity of biological molecules is somehow connected to the origin and evolution of life [21,30,113]. For moving bilateral organisms, persistent asymmetry in the activation of the sensory system (as the vision in the birds) becomes the environmental factor of the development of perception, cognition, and action, and, consequently, the object of intuitive attraction (attention). In humans, this attraction takes various shapes (attention, attraction, memory, addiction), from pragmatic skills of spatial orientation to aesthetic feeling. At the cellular level, molecular chirality is known as the factor promoting neuronal proliferation [123]. Space–time perception is the most influential force driving the direction of individual organism development and the directionality of biological evolution [22,124]. Both ontogeny and phylogeny can be traced at the molecular, cellular, and organism levels. In the case of humans, the interplay of prevalent molecular chirality and the sensory-motor functions is what drives evolution. Hence, the sensory-motor system’s mechanism supports higher cognitive abilities, consciousness, and psychological state. The human brain is the integrative bilaterally asymmetric machinery of space–time perception of moving organisms accompanied by executive function. In the CNS, the link of molecular chirality to the organism’s function at the cell level is mediated by multiple subtypes of neurons with unique morphologies, electrical properties, and molecular identities [25,125].
3.3. Cell Chirality
3.3.1. Cell Evolution
Prokaryotic and eukaryotic cells are descended from a single primordial ancestor but have a specificity at morphological and molecular levels. At the morphological level, eukaryotes contain a nucleus separating genetic material from the cytoplasm and cytoplasmic organelles. At the molecular level, prokaryotic and eukaryotes share two major classes of informational macromolecules: nucleic acids and proteins. After splitting animal–plant–fungi–protist pathways, animal cells of bilaterians undergo several steps of additional modifications. Bilateral organisms acquire signaling proteins necessary for neuronal proliferation, formation synapse, and CNS, allowing efficient environmental navigation and movement. In this sense, the human CNS is the final product of evolution. The tuning of spatial navigation occurs through the development of internal factors (including molecular diversity) and the appearance of sophisticated sensory systems that allow communication with the environment.
These two constantly interacting factors are the primary determinants of specific neuronal morphology, differing from the shape–functions links of other cell types, such as red blood cells.
3.3.2. Pyramidal Neurons
PyrNs within bilateral organisms are studied in reptiles, fish, birds, and mammals (Figure 1) [126]. PyrNs gain their name due to the easily recognizable geometrical shape of the soma. PyrNs’ soma shape is sharply distinct from other neuronal (Purkinje and granular cells) and non-neuronal (microglia, astrocytes, epithelial, or red blood cell) cell types. Based on this fact, it is reasonable to assume that the specificity of PyrNs’ functions accounts for this difference. The term PyrNs refers to all major classes of excitatory multipolar glutamatergic cell types sharing common (pyramidal-like) soma shapes despite the different degrees of deviation from the perfect geometrical form [127]. Even in the publications explicitly devoted to cell-kind-dependent chirality and cell morphology [122,123,124,125,126,127,128], the shape of PyrNs’ soma is out of discussion. In the most detailed description of the PyrNs’ soma shape, it is usually characterized as a “teardrop or rounded pyramid” [129], extremely elongated rod-shaped” [124], and most frequently pyramid-shaped [130]. The base geometry of the pyramid (triangular or square) is never experimentally explored or theoretically predicted. One of the best experimental images of PyrNs shows the pyramidal shape of soma (Figure 2A) [87,131]. However, confirmation of the tetrahedral geometry requires additional goal-oriented efforts. In such a situation, it is relevant to say that PyrNs’ soma has relative similarity with the pyramid’s geometry in general or tetrahedron, in particular. There are many arguments that the genetic chirality-centric architecture of the structural left–right asymmetry of the bilateral human brain is evolutionarily preserved for the optimum function of sensory perception of the spatial environment [132]. However, the tetrahedral shape of PyrNs may be induced by evolutionary tendency secondary to the sensory perception of the spatial environment. At the cellular level, space–time perception occurs through the activity of PyrNs in close collaboration with other cell types and first with the astrocytes. Astrocytes are gatekeepers for maintaining PyrNs’ excitability by glutamate biosynthesis, uptake, and release [88,133], synthesis of lactate (taken by neurons for energy production [134]), and as the primary source of D-Ser [135]. The disruption of astrocyte functions triggers cross-talk of many neurodegenerative pathways sharing a common feature—prevalent molecular chirality, which is vulnerable to spontaneous racemization. The primary functions of PyrNs include the evaluation of the distance, direction, and left–right discrimination of movement. In agreement with this view, the spatial arrangement of the PyrNs firing was found to be affected by environmental geometry [136]. Notably, aging-related cognitive deficits are attributable to the reduced activity of PyrNs [134]. Left–right discrimination of brain functions is well represented in the behavioral study [137,138]. This knowledge is supported by new results showing brain asymmetry at the molecular and cellular levels, but additional studies are required. PyrNs are the primary excitatory multipolar cell type abundant in the brain cortex [136], hippocampus [139,140], and amygdala [141]. Several PyrN types, characterized by functional division of labor, contribute to the brain’s systems for spatial navigation. Currently, the most studied is an interaction of cortical grid cells with the hippocampal place cell. Notably, grid cell fields comprise a directionally oriented, topographically organized, periodic two-dimensional triangular internal map of the external environment [142,143]. This model of neuronal space (direction, distance, symmetry) perception suggests that the mutual orientation of grid cells in a 2D map could presumably be functionally essential. However, the corresponding experimental evidence is currently unavailable.
The heterogeneity of the PyrN family is defined by their distinct axonal projections, dendritic arborization, and types of receptors. It was found that neurites, originating from the membrane protrusions of the neuronal soma, are the precursors of axons and dendrites (apical and basal). For PyrNs, specialization of neurites occurs at different times of neuronal proliferation. Apical dendrite and axonal initial segment come first, and basal dendrites, finalizing the pyramidal shape of soma, are formed later [12]. However, how the neuronal soma develops its pyramidal morphology, which directs the Proper neurite orientation, was poorly understood for a long time. Examination of all traditional graphical representations of the PyrNs community suggests that most (if not all) studies assume coherent orientation of soma in the plane orthogonal to apical dendrites axis [91]. However, such an assumption requires experimental verification.
The cortical circuit network predominantly comprises pyramidal-to-pyramidal neuron connections, yet their assembly during embryonic development has yet to be entirely understood.
PyrNs of the cortex are distributed in the sensory, motor, association, and executive areas and found in all cortical layers except layer I [11,144,145]. Each PyrN receives input from thousands of excitatory synapses segregated onto dendritic branches. It has been previously proposed that sophisticated neuronal circuits associated with non-linear properties of dendrites enable cortical neurons to recognize multiple in sequence patterns and robust sequence memory [146]. Dysfunctional PyrN circuitry has been associated with perception, cognition, and psychological conditions abnormalities. Glutamatergic signaling of PyrNs occurs through neurotransmitters AAs L-glutamate (L-Glu) [147] and D-Ser [148] in close interaction with the complex of catecholaminergic systems {with corresponding neurotransmitters: L-dopamine, L-norepinephrine (noradrenaline), and L-epinephrine (adrenaline)} [149]. Despite the involvement of neuronal circuits of different natures, the most significant contribution to the morphological asymmetry of brain hemispheres and left–right differentiation of neural pathways is attributed to PyrNs.
The fact that PyrNs are the most populated neuronal type in the human cerebral cortex and hippocampus suggests their primary role in processing space–time information utilized in sensory-motor functions. PyrN signaling is necessary for normal development and essential functions of mature organisms [150]. At the same time, the distortion of neuronal geometry and formation of aberrant synapses are associated with pathological conditions [151], including impairment of visual perception [152] and mental retardation [153]. The bilateral cortex and hippocampus, containing the majority of PyrNs, are studied in brain regions involved in a wide range of hemisphere-specific functions, including spatial coding, navigation, spatial memory, decision-making [152,153,154,155,156], and intelligence [157]. The evolutionary selected system for space–time information processing, including the morphology and spatial orientation of PyrNs, is the fundamental feature underlying the function of CNS. Experimentally observed hemispheric asymmetry of synaptic morphology of PyrNs explains well-known functional laterality of human perceptual and cognitive functions [158,159,160]. The morphology of PyrNs concerning the function was the focus of long-term attention in neuroscience. The major studied structural features were dendritic arborization, synaptic connectivity, and axonal network [120,160,161,162,163]. Apical and basal segments of the dendritic tree, complemented by the relative orientation of presynaptic and postsynaptic neurons, were carefully studied [17,25,136,162,163,164,165,166,167,168,169]. Two dendritic arbors have distinct morphology and orientation and are involved in different synaptic circuits [135]. The dendritic orientation of PyrNs is sublayer-specific and exhibits dorsal-ventral and front-back differentiation [170]. The experimental parameters characterizing the cell body include soma size, spatial distributions, the density of soma, and pyramidal somatic integrative zones. The shape and spatial orientation of pyramidal soma have had little attention, partly due to the void of reliable experimental control. In bilateral organisms, beginning from C elegans, CNS contains PyrNs. During neurogenesis from the ventricular zone (VZ), before adopting pyramidal morphology, neurons pass through several intermediate stages (multipolar, bipolar) [171,172]. Presumably, many molecular correlates contribute to the pyramidal shape of soma, but all cell morphology alterations are supported/assisted by the molecular dynamics of homochiral enzyme–substrate complexes. A commonly accepted axiom is that molecular chirality drives cell chirality [173].
Experimental evidence suggests that such a chirality transfer occurs with the participation of diverse cytoskeleton-based, long-lived, and highly dynamic structures. Indeed, it was shown that the interaction of the different families of cytoskeleton filaments (septin [174], actin [175,176], microtubules [177,178], spectrin [173,177], and neurofilaments (NF) [179]) provides fundamental cell morphogenetic mechanisms, including the shape and spatial orientation of PyrNs’ soma [176]. It was shown that NF (and other intermediate filament proteins) contain in their N-terminal domains the motifs that bind unassembled tubulin. Peptides containing such motifs inhibit microtubules’ in vitro polymerization, leading to altered cell shapes [179]. This fact suggests that NF-microtubule interaction can contribute to the shape of PyrN’ soma.
Since 2014, spectrin has been recognized as a major component of the neuronal membrane skeleton participating in synaptic transmission [180]. However, until recently, there was no experimental evidence or theoretical model suggesting the direct involvement of any cytoskeleton components in the PyrN’ soma morphology. Notably, after publishing the preprint of our review, we found one article providing convincing arguments in favor of the contribution of the spectrin cytoskeleton network [180] in neuronal soma shape. The prominent candidates for attention are microtubule and actin cytoskeleton active in neuronal synapses [181,182]. Actin filament network is involved in vital neuronal processes associated with various memory functions in different organisms, from invertebrates to mammals. The cellular processes, mediated by its activity, include cellular motility, division, intracellular transport, synaptic plasticity, and morphogenesis [183]. Actin and actin-binding proteins (α-actinin and synaptopodin) are present in the typical cisternal organelle of an axon initial segment (AIS) in subpopulation PyrNs [184]. In agreement with our generalized function-based hypothesis, the recent publication shows that developing a pyramidally-shaped soma is linked to septin functions [185]. So, molecular mechanisms involved in the neurogenesis and the development of pyramidal neurons soma are on the way to being clarified in detail. The homochirality of actin–myosin cytoskeletons allows the cells to develop polarity and left–right asymmetry [173]. However, the bidirectional impact of prevalent molecular chirality (internal determinant) and bilaterality of CNS (window to the external epigenetic factors) on the pyramidality of PyrNs’ soma has never been considered. Notable that dendritic arborization exhibits cortical layer-dependent orientation preference towards the anterior orientation [161]. However, the spatial orientation of pyramidal soma in two brain hemispheres and their relation to space–time information processing have yet to be experimentally studied or theoretically discussed.
Based on the lateralization of perceptual and cognitive functions, we can expect differential bilateral asymmetry in the morphology and orientation of PyrNs. Indeed, currently, the hemispheric difference is experimentally observed in the number/volume [186] and synaptic organization [187]. Asymmetric hemispheric allocation of NMDA receptor subunits in hippocampal PyrNs complements the whole picture [135,137]. The fact that PyrNs of the healthy human brain have a significantly greater density and larger size and are more spherical in shape on the left than on the right side points to the meaningful link between two kinds of biological events [188]. Notable that bilateral asymmetry of brain activity indicates a state of the CNS system concerning mood and anxiety. For example, studies of brain EEG associated with PyrNs firing suggest that high levels of beta in the right hemisphere are associated with anxiety symptoms. In contrast, high levels of alpha in the left hemisphere indicate depressive features [189,190]. Studying PyrNs’ functions in biological information processing is necessary for designing a strategy [121,191].
3.4. From Molecular to Cell Chirality
We consider the evolution of life in terms of the sequence of transformation of molecular chirality into cell chirality and bilaterality of organisms [24,27,192,193,194]. Our hypothesis highlights the formal consequences of three co-existent events: molecular chirality, PyNs’ shape, and bilateral body–brain morphology [195]. Indeed, episodic memory, allowing mental navigation in space and time, is based on the hemisphere asymmetrical activity of hippocampal PyrNs [155]. At the same time, from a geometrical standpoint, PyN soma (with four non-equivalent vertexes) represents a highly asymmetric (chiral) tetragonal structure. The anatomical symmetry and asymmetry of the PyrNs’ structure impose fundamental information processing capabilities. The essential point is that two mirror images of PyrNs (two seemingly identical PyrNs) located in the cortex or hippocampus are not superimposable (i.e., not identical) (see Figure 2B). Furthermore, the chirality of PyrNs provides a formal opportunity for hemisphere-specific information processing contributed by correlative protein and lipid chirality [7,89,90]. Currently, no data confirm or negate any hemispheric asymmetry (amount, content, or chirality) of lipids in animal brains. Examination of all traditional graphical representations of the PyrNs community suggests that most (if not all) studies assume the coherent orientation of soma (within the brain hemisphere) in the plane orthogonal to the apical dendrites axis (Figure 2C) [91]. However, such an assumption requires experimental verification.
3.4.1. Self-Assembly of Biomolecules
The idea of molecular chirality contribution to the soma-shape of PyrNs is based on the fact that organic and non-organic molecular nanoscale complexes can spontaneously adopt various shapes, including square-pyramidal [196], triangular-prism [197,198], octahedral, icosahedral, and tetrahedral [199,200,201,202]. Indeed, after Le Bel and Van’t Hoff showed that the substituents of a tetravalent carbon center occupy the vertices of a tetrahedron, numerous experimental results illustrated that chiral covalent stereogenic units of molecular structures are responsible for the axial, planar, helical, and tetrahedral motifs in biological polymers [203,204]. Accumulating evidence shows the ability of biomolecules to self-assemble into the range of structures (from nanoscale to microscale) of distinct geometrical symmetry [205,206]. The mechanisms of self-assembly were studied for DNA [207], nucleic acids [208], proteins [209], phospholipids [210], and their combinations. The most common DNA nanostructures are tetrahedral DNA nanostructures (TDN). In the biological environment (cytosol), TDN exhibits maximum stability. The unique spatial structure of TDN allows it to penetrate cell membranes in abundance and regulate essential cellular processes, including proliferation, migration, and differentiation. Modifying TDN vertices, tetrahedral arms, or DNA tetrahedral cages by bioactive ligands enables TDN to be used as a nanocarrier for targeted therapies, molecular diagnosis, biosensing, antibacterial treatment, antitumor strategies, and tissue regeneration [207,210]. Peptides and proteins can also self-organize into highly ordered supramolecular architectures, such as nanofibrils, nanobelts, nanotubes, nanowires, and vesicles [211]. All facts mentioned above suggest the possibility of the natural occurrence of tetrahedral molecular aggregate in living cells as the molecular determinants of cell morphology and functions. A known example is the ferritin protein cages found in nearly all life forms. The most general structures show octahedral and tetrahedral symmetry [211]. Human ferritin cage has an outer diameter of ~12 nm and an inner cavity of 7–8 nm.
3.4.2. Molecular–Cellular Co-Evolution
Many physical and biological objects, including elementary particles (such as electrons and photons), molecular structures, and cell aggregates, have an intrinsic degree of freedom—chirality [203].
Evolutionary selected molecular chirality provides all available resources for the efficient mechanism of chirality transfers across all levels of biological organization [203]. Notably, the common symmetry principles guide the external and internal determinants of cell morphology, assembly, and functions of intracellular molecules. Understanding this fundamental commonality is necessary for neuroscience-inspired AI [212,213,214]. Protein assemblies adopting tetrahedral symmetry create shell-like architectures [215]. Such structures serve as enclosures for viral genomes and potentially can serve as internal determinants of cell shape. In addition to ferritins [208,216], well-known examples of tetrahedral protein assembly are small heat shock proteins (sHSPs) [217] and globular hemoglobin (Hb) [218,219,220,221,222]. Hb is a highly conserved globular protein in all life forms and functionally tied to aerobic organisms utilizing oxygen from the atmosphere and delivering it to cells. The expression of mitochondrial Hb (Hba-a2 and Hbb) in neurons (including nigral dopaminergic neurons, striatal γ-aminobutyric acid GABA-ergic neurons, and cortical PyrNs) was experimentally observed [220,221,222,223]. Along with natural (organic) molecules, many artificial metal-organic tetrahedral (filled and porous) structures synthesized recently exhibit a chiral degree of freedom [224]. Porous molecular cages are successfully used for intracellular drug delivery [223]. Observation of amyloid beta peptides, which are prone to tetrahedral coordination of metal ions (including Cu, Zn, and Fe), suggests an active role of tetrahedral protein structures in cell physiology [225].
It is reasonable to assume that the shape of the soma can adapt to the demands of the cytoskeleton’s tetrahedral mesh-like gel, undergoing a chain of structural phase transitions [209,222,223]. In neuronal cells, the assembly of cytoskeleton proteins can be considered an internal determinant of soma’s shape. Cytoskeleton-based molecular (CMC) cages, as a form of membrane-bound and membrane-less organelles, are a well-known phenomenon in the cell physiology of intracellular segregation. CMC surrounds and protects various intracellular compartments, including the nucleus [226] and mitochondria [227], intracytoplasmic bacteria [228], and lipid droplets [229].
The diverse combination of cytoskeletal-based cages participates in intracellular physiology, including actin-based [226,229,230], vimentin-based [231], septins-based [231,232], and microtubules-based [229] complexes. There is no information about spectrin molecular cages. In many cell types (including PyrN), the liquid–liquid phase separation (LLPS) of biomolecular condensates (proteins and lipids assemblies) is involved in diverse functions, including an asymmetric cell division, the establishment of neuronal stem cell (NSC) polarity, neuronal proliferation, synaptic transmission and, likely, soma morphology of PyrNs. It became evident that sophisticated biophysical and biochemical processes govern cell-environment (interface) chirality sensation in a living system. Human embryonic stem cells (hESCs), differentiating into heart, gut, and brain tissues, show intrinsic [233] and extrinsic [234,235] cell chirality induced by the molecular (peptide–protein) chirality of intracellular and extracellular constituents. Accumulated experimental evidence related to different cell types suggests that the pyramidal shape of neuronal soma is a direct result of such bidirectional impact. The size scale range for protein–lipid tetrahedral complexes can be from several nanometers to several microns [220,235,236,237,238,239,240,241,242,243]. Despite such a size being smaller than the size of PyrNs’ soma, these experimental facts point to a principal opportunity for the molecular structure to form the corners of pyramidal soma.
From a formal geometrical view, it is possible to compose a 3-D tetrahedral structure of unlimited size from the small tetrahedral units [239]. Notably, the chirality of elementary tetrahedron can be transferred to a larger-scale structure. Such tetrahedral units are known for biological and non-biological molecules. Presently, three types of biological tetrahedral structures containing proteins, DNA, and a mixture of both are widely explored [241] (tetrahedral structures for phospholipids are the next target for exploration). Protein-based tetrahedral structures comprise helical, beta sheets, and other components [244]. Therefore, theoretically, all studied protein-based tetrahedral structures can be assembled in three-dimensional cell-size complexes, supporting the shape of PyrNs.
When the base geometry of the pyramid (triangular or square) is never experimentally characterized or theoretically predicted, it is relevant to say that PyrNs’ soma is similar to the pyramid’s geometry in general or tetrahedron, in particular. However, additional experimental verification of such pathways is required. The co-appearance of all the above-mentioned spatial arrangements of PyrNs in the visual cortex suggests that the corresponding primary function is the involvement in the space–time orientation of moving organisms. In neuronal cells, the assembly of cytoskeleton proteins can be considered a primary internal determinant of soma’s shape.
Successful advances in understanding the development of neuronal morphology should be based on the attention to co-evolution at the molecular and cellular levels. Currently available data suggest that three types of vertebrates (cartilaginous fish, bony fish, and tetrapods) have an immediate relation to the evolutionary origin of PyrNs, which can be traced from simple, “extraverted” neurons in the amphibian pallium via pyramid-like neurons in the reptilian cortex to the fully developed neo-cortical elements designated by Cajal as “psychic cells” [245,246]. In both reptilian dorsal ventricular ridge (DVR) and mammalian amygdala, pyramidal neurons lack a preferred orientation, thus differing from cortical pyramidal neurons, which are oriented perpendicular to the surface [247,248,249].
4. Conclusions
The significance of spatial (i.e., geometrical) symmetry determinants in biology is supported by the fact that group theory is routinely used to describe the symmetries and conformational behavior of various systems ranging from the quantum mechanics of atoms, protein folding, neuronal signaling, and brain functions levels to organism–environment interaction [250]. The waves of new scientific evidence bring the research community to the conclusion that there is no cell type, tissue, organ, or system of organism indifferent to the physiological balance of molecular chirality [27,250,251].
In a broad sense, the “mystery” of PyrNs is on the way to being solved. As an integrating frame of reference, PyrNs’ hypothesis/theory will help guide future studies. What remains is the need for complex, goal-oriented experimental advances, focusing on the complex of stereospecific effects illuminating the link between molecular chirality (such as of L-/D-cytoskeleton dynamics in dendritic spines or chirality of mGluR7 receptors/ligands complex), the shape of PyrNs, and laterality of sensory perception. We have introduced readers to the spectrum of facts and thoughts, the fundamental link of which is frequently hidden from the diverse research audience. Now, it is time for your own judgment regarding the hypothesis on the consideration and for a new experimental design. Let us point attention to two areas of science where PyrNs’ hypothesis is in high demand. Until recently, one of the notable differences between biological and artificial intelligence systems was that bilaterians consist primarily of chiral molecules governing neuronal functions. Indeed, in the animal kingdom, the cognitive decision-making process is based on the same basic cellular elements of CNS—neurons as a unit integrating all advantages of prevalent molecular chirality to advance intelligence. Decades of molecular assembly-based nanotechnology linked with the chiral light–matter interaction dramatically changed the situation [252,253]. Evolutionary transitions between humans and AI fuel hope of symbiotic entities in the future and become a matter of fact [254].
In the prolonged period, the left–right asymmetry of the brain has been studied chiefly through psychological examination and behavioral study, leaving its molecular and synaptic aspects of PyrNs largely unaddressed. In 2008, Shinohara and colleagues showed that “hippocampal CA1 pyramidal cell synapses differ in size, shape, and glutamate receptor expression depending on the laterality of presynaptic origin. CA1 synapses receiving neuronal input from the right CA3 PyrNs are larger and have more perforated PSD and a GluR1 expression level twice as high as those receiving input from the left CA3” [255]. After the first convincing evidence, many great studies were conducted to link synaptic hemispheric asymmetry to the function of PyrNs. For all of them, the knowledge of hemisphere-specific soma orientation could be beneficial [138,254,255,256,257,258,259,260,261,262,263,264,265,266,267].
5. Closing Remark
We are only beginning to understand the enormous complexity of the link between molecular chirality and the laterality of cognitive functions. But progress is evident. The hypothesis of pyramidal soma shape is in conceptual agreement with the fact that living systems have evolved as non-equilibrium systems consuming environmental energy to maintain dissipating states of asymmetric chiral configurations and functions of organisms [266]. Recent developments show new details of neuronal proliferation. The choroid plexus (CP) (a group of specialized cells in the cerebral ventricles) is one of the first structures to show lateralization in human brain development. Corresponding inherent asymmetries in CSF production and circulation may impact the distribution of neurons and resident immune cells between brain hemispheres. The potential consequences associated with the pyramidal shape of neuronal soma and hemispheric asymmetry of neuronal proliferation responsible for asymmetry of neuronal connectivity and brain functional laterality have yet to be investigated [267,268,269].
Author Contributions
The main contribution was made by V.V.D. and N.V.D.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The author gratefully thanks Pamela Butler, Stephen Ginsberg, Csaba Vadasz, and Henry Sershen for valuable suggestions and corrections. Thanks to Brian Hayes for permission to include the rotating tetrahedron fragment in our graphic abstract.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Amino acids (AAs). Artificial intelligence (AI). Biological intelligence (BI). Cerebrospinal fluid (CSF). Central nervous system (CNS). Electroencephalogram (EEG). Human embryonic stem cells (hESCs). Liquid–liquid phase separation (LLPS). Neurofilaments (NFs). Neuronal stem cells (NSC). Pyramidal neurons (PyNs). Psychological functions (PFs). Space–time symmetry and relativity (STSR). D-aspartic acid (D-Asp). D-serine (D-Ser). D-alanine (D-Ala). L-glutamate (L-Glu). D-proline (D-Pro). D-tyrosine (D-Tyr).
References
- Michael, G.; John, S.; Witten, E. Superstring Theory. Vol. 2: Loop Amplitudes, Anomalies and Phenomenology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Ma, Y.; Shi, L.; Yue, H.; Gao, G. Recognition at chiral interfaces: From molecules to cells. Colloids Surf. B Biointerfaces 2020, 195, 111268. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Bowerman, B. Symmetry Breaking in Biology. Cold Spring Harb. Perspect. Biol. 2010, 2, a003475. [Google Scholar] [CrossRef]
- Bormashenko, E. Fibonacci Sequences, Symmetry and Order in Biological Patterns, Their Sources, Information Origin and the Landauer Principle. Biophysica 2022, 2, 292–307. [Google Scholar] [CrossRef]
- Hollo, G. A new paradigm for animal symmetry. Interface Focus 2015, 5, 20150032. [Google Scholar] [CrossRef]
- Lee, C.; Weber, J.M.; Rodriguez, L.E.; Sheppard, R.Y.; Barge, L.M.; Berger, E.L.; Burton, A. Chirality in Organic and Mineral Systems: A Review of Reactivity and Alteration Processes Relevant to Prebiotic Chemistry and Life Detection Missions. Symmetry 2022, 14, 460. [Google Scholar] [CrossRef]
- Xin, Z.; Cai, Y.; Dang, L.T.; Burke, H.M.S.; Revote, J.; Charitakis, N.; Bienroth, D.; Nim, H.T.; Li, Y.-F.; Ramialison, M. MonaGO: A novel gene ontology enrichment analysis visualisation system. BMC Bioinform. 2022, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Dyakin, V.V. Fundamental Cause of Bio-Chirality: Space-Time Symmetry—Concept Review. Symmetry 2023, 15, 79. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, M.; Kuang, X.; Li, Y.; Hu, S. A simplified morphological classification scheme for pyramidal cells in six layers of primary somatosensory cortex of juvenile rats. IBRO Rep. 2018, 5, 74–90. [Google Scholar] [CrossRef]
- Luine, V.; Frankfurt, M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 2013, 239, 34–45. [Google Scholar] [CrossRef]
- Vigouroux, R.J.; Duroure, K.; Vougny, J.; Albadri, S.; Kozulin, P.; Herrera, E.; Nguyen-Ba-Charvet, K.; Braasch, I.; Suárez, R.; Del Bene, F.; et al. Bilateral visual projections exist in non-teleost bony fish and predate the emergence of tetrapods. Science 2021, 372, 150–156. [Google Scholar] [CrossRef]
- Palladino, P. Stereochemistry and the Nature of Life: Mechanist, Vitalist, and Evolutionary Perspectives. JSTOR 1990, 81, 44–67. [Google Scholar] [CrossRef][Green Version]
- Kushchayev, S.V.; Moskalenko, V.F.; Wiener, P.C.; Tsymbaliuk, V.I.; Cherkasov, V.G.; Dzyavulska, I.V.; Kovalchuk, O.I.; Sonntag, V.K.H.; Spetzler, R.F.; Preul, M.C. The discovery of the pyramidal neurons: Vladimir Betz and a new era of neuroscience. Brain J. Neurol. 2012, 135, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Radler, M.R.; Liu, X.; Peng, M.; Doyle, B.; Toyo-Oka, K.; Spiliotis, E.T. Pyramidal neuron morphogenesis requires a septin network that stabilizes filopodia and suppresses lamellipodia during neurite initiation. Curr. Biol. 2023, 33, 434–448.e8. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Meyer, K.A.; Santpere, G.; Gulden, F.O.; Sestan, N. Evolution of the Human Nervous System Function, Structure, and Development. Cell 2017, 170, 226–247. [Google Scholar] [CrossRef] [PubMed]
- Dahanayake, J.N.; Mitchell-Koch, K.R. Entropy connects water structure and dynamics in protein hydration layer. Phys. Chem. Chem. Phys. 2018, 20, 14765. [Google Scholar] [CrossRef] [PubMed]
- Marvan, T.; Polák, M.; Bachmann, T.; Phillips, W.A. Apical amplification—A cellular mechanism of conscious perception? Neurosci. Conscious. 2021, 2021, niab036. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Buldyrev, S.V.; Eugene, H. A tetrahedral entropy for water. Proc. Natl. Aacd. Sci. USA 2009, 106, 22130–22134. [Google Scholar] [CrossRef]
- Mason, S.F. The development of concepts of chiral discrimination. Chirality 1989, 1, 183–191. [Google Scholar] [CrossRef]
- Bastings, J.J.A.J.; van Eijk, H.M.; Damink, S.W.O.; Sander, S.; Rensen, S.S. D-amino Acids in Health and Disease: A Focus on Cancer. Nutrients 2019, 11, 2205. [Google Scholar] [CrossRef]
- Kondepudi, D. Chiral Asymmetry in Nature. In Chiral Analysis, 2nd ed.; Advances in Spectroscopy, Chromatography and Emerging Methods; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 1; pp. 3–28. [Google Scholar] [CrossRef]
- Cristadoro, G.; Degli Esposti, M.; Altmann, E.G. The common origin of symmetry and structure in genetic sequences. Sci. Rep. 2018, 8, 15817. [Google Scholar] [CrossRef]
- Book by Ibanez, L.E.; Uranga, A.M. String Theory and Particle Physics: An Introduction to String Phenomenology; Cambridge University Press: Cambridge, UK, 2012; 688p. [Google Scholar]
- Inaki, M.; Liu, J.; Matsuno, K. Cell chirality: Its origin and roles in left–right asymmetric development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150403. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M. Creating chirality. Nat. Chem. Biol. 2008, 4, 330. [Google Scholar] [CrossRef]
- Takaoka, K.; Yamamoto, M.; Hamada, H. Origin of body axes in the mouse embryo. Curr. Opin. Genet. Dev. 2007, 17, 44–350. [Google Scholar] [CrossRef] [PubMed]
- Dyakin, V.V.; Lucas, J.; Dyakina-Fagnano, N.V.; Posner, E.P.; Vadasz, C. The Chain of Chirality Transfer as Determinant of Brain Functional Laterality. Breaking the Chirality Silence: Search for New Generation of Biomarkers; Relevance to Neurodegenerative Diseases, Cognitive Psychology, and Nutrition Science. Neurol. Neurosci. Res. 2017, 1, 2. [Google Scholar] [CrossRef]
- Duan, P.; Cao, H.; Zhang, L.; Liu, M. Gelation induced supramolecular chirality: Chirality transfer, amplification and application. Soft Matter 2014, 10, 5428. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G. The evolutionary psychology of left and right: Costs and benefits of lateralization. Dev. Psychobiol. 2006, 48, 418–427. [Google Scholar] [CrossRef]
- Francks, C. Exploring human brain lateralization with molecular genetics and genomics. Ann. N. Y. Acad. Sci. 2015, 1359, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stacho, M.; Manahan-Vaughan, D. Mechanistic flexibility of the retrosplenial cortex enables its contribution to spatial cognition. Trends Neurosci. 2022, 45, P284–P296. [Google Scholar] [CrossRef]
- Lee, T.-W.; Dolan, R.J.; Critchley, H.D. Controlling Emotional Expression: Behavioral and Neural Correlates of Nonimitative Emotional Responses. Cereb. Cortex 2008, 18, 104–113. [Google Scholar] [CrossRef]
- Yuan, J.; Lu, X.; Zhang, S.; Zheng, F.; Deng, Q.; Han, L.; Lu, Q. Molecular Chirality and Morphological Structural Chirality of Exogenous Chirality-Induced Liquid Crystalline Block Copolymers. Macromolecules 2022, 55, 1566–1575. [Google Scholar] [CrossRef]
- Jung, C.G. Psychological Types (The Collected Works of C. G. Jung, Vol. 6) (Bollingen Series XX); Part of the Jung’s Collected Works (#6) Series and Dzieła (#2) Series; Princeton University Press: Princeton, NJ, USA, 1976. [Google Scholar]
- Assagioli, A. Dynamic Psychology and Psychosynthesis; Psychosynthesis Research Foundation, Inc.: New York, NY, USA, 1958. [Google Scholar]
- Myers, S. The five functions of psychological type. Anal. Psychol. 2016, 61, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.S.; Jirsa, V.K. Perspective. Symmetry Breaking in Space-Time Hierarchies Shapes Brain Dynamics and Behavior. Neuron 2017, 94, 1010–1026. [Google Scholar] [CrossRef] [PubMed]
- Jirsa, V.; Sheheitli, H. Entropy, free energy, symmetry and dynamics in the brain. J. Phys. Complex. 2022, 3, 015007. [Google Scholar] [CrossRef]
- Dyakin, V.V.; Uversky, V.N. Arrow of Time, Entropy, and Protein Folding: Holistic View on Biochirality. Int. J. Mol. Sci. 2022, 23, 3687. [Google Scholar] [CrossRef] [PubMed]
- Iohnston, I.G.; Dingle, K.; Greenbury, S.F.; Camargo, C.Q.; Doye, J.P.K.; Ahnert, S.E.; Louis, A.A. Symmetry and simplicity spontaneously emerge from the algorithmic nature of evolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2113883119. [Google Scholar] [CrossRef] [PubMed]
- Sharman, R.J.; Gheorghiu, E. Speed tuning properties of mirror symmetry detection mechanisms. Sci. Rep. 2019, 9, 3431. [Google Scholar] [CrossRef] [PubMed]
- Jammer, M. Concepts of Space: The history of Theories of Space in Physics, 3rd ed.; Dover: New York, NY, USA, 1993. [Google Scholar]
- Capecchi, D. Development of the Concept of Space up to Newton. Encyclopedia 2022, 2, 1528–1544. [Google Scholar] [CrossRef]
- Nederbr, H. Hierarchical Organization of Biological Systems and the Structure of Adaptation in Evolution and Tumorigenesis. J. Theor. Biol. 1997, 184, 149–156. [Google Scholar] [CrossRef]
- Eronen, M.I. Levels of Organization in Biology; Stanford Encyclopedia of Philosophy: Stanford, CA, USA, 2023; Available online: https://plato.stanford.edu/entries/levels-org-biology/ (accessed on 22 January 2023).
- Blackmond, D.G. The Origin of Biological Homochirality. Cold Spring Harb. Perspect. Biol. 2010, 2, a002147. [Google Scholar] [CrossRef]
- Ocklenburg, M.; Ocklenburg, S.; Mundorf, A. Symmetry and asymmetry in biological structures. Proc. Natl. Acad. Sci. USA 2022, 119, e2204881119. [Google Scholar] [CrossRef]
- Tasson, J.D. What Do We Know About Lorentz Invariance? Rep. Prog. Phys. 2014, 77, 062901. [Google Scholar] [CrossRef] [PubMed]
- Comte, C. Symmetry, relativity and quantum mechanics. Nuov Cim. B 1996, 111, 937–956. [Google Scholar] [CrossRef]
- Field, J.N. Space-Time Exchange Invariance: Special Relativity as a Symmetry Principle. Am. J. Phys. 2001, 69, 569. [Google Scholar] [CrossRef]
- Ajaltouni, Z.J. Symmetry and relativity: From classical mechanics to modern particle physics. Nat. Sci. 2014, 6, 43343. [Google Scholar] [CrossRef]
- Auffray, C.; Nottale, L. Scale relativity theory and integrative systems biology: 1: Founding principles and scale laws. Progress Biophys. Mol. Biol. 2008, 97, 79–114. [Google Scholar] [CrossRef]
- Noble, R.; Tasaki, K.; Noble, P.J.; Noble, D. Biological Relativity Requires Circular Causality but Not Symmetry of Causation: So, Where, What and When Are the Boundaries? Front. Physiol. Sec. Integr. Physiol. 2019, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- de Kovel, C.G.F.; Lisgo, S.N.; Fisher, S.E.; Francks, C. Subtle left-right asymmetry of gene expression profiles in embryonic and foetal human brains. Sci. Rep. 2018, 8, 12606. [Google Scholar] [CrossRef]
- Bennett, M.S. Five Breakthroughs: A First Approximation of Brain Evolution from Early Bilaterians to Humans. Front. Neuroanat. 2021, 15, 693346. [Google Scholar] [CrossRef]
- Corballis, M.C. Bilaterally Symmetrical: To Be or Not to Be? Symmetry 2020, 12, 326. [Google Scholar] [CrossRef]
- Muskhelishvili, G.; Travers, A. Integration of syntactic and semantic properties of the DNA code reveals chromosomes as thermodynamic machines converting energy into information. Cell. Mol. Life Sci. 2013, 70, 4555–4567. [Google Scholar] [CrossRef]
- Chatterjee, S.; Yadav, S. The Coevolution of Biomolecules and Prebiotic Information Systems in the Origin of Life: A Visualization Model for Assembling the First Gene. Life 2022, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Devínsky, F. Chirality and the Origin of Life. Symmetry 2021, 13, 2277. [Google Scholar] [CrossRef]
- Piñeros, W.D.; Tlusty, T. Spontaneous chiral symmetry breaking in a random driven chemical system. Nat. Commun. 2022, 13, 2244. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Chiral Architectures from Macromolecular Building Blocks. Chem. Rev. 2001, 101, 4039–4070. [Google Scholar] [CrossRef] [PubMed]
- Todoroff, N.; Kunze, J.; Schreuder, H.; Hessler, G.; Baringhaus, K.-H.; Schneider, G. Fractal Dimensions of Macromolecular Structures. Mol. Inform. 2014, 33, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Panagiotou, E. The protein folding rate and the geometry and topology of the native state. Sci. Rep. 2022, 12, 6384. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Mandal, S.; Danielsen, M.B.; Brown, A.; Hu, C.; Christensen, N.J.; Kulakova, A.V.; Song, S.; Brown, T.; Jensen, K.J.; et al. Chirality transmission in macromolecular domains. Nat. Common. 2022, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Choi, H.; Shahzad, Z.M.; Ki, H.; Lee, J.; Chae, H.; Kim, Y.H. Supramolecular assembly of protein building blocks: From folding to function. Nano Converg. 2022, 9, 4. [Google Scholar] [CrossRef]
- MacKenzie, L.E.; Stachelek, P. The twists and turns of chiral chemistry. Nat. Chem. 2021, 13, 521–522. [Google Scholar] [CrossRef]
- Dhanavade, M.J.; Sonawane, K.D. Amyloid beta peptide-degrading microbial enzymes and its implication in drug design. 3 Biotech 2020, 10, 247. [Google Scholar] [CrossRef]
- Reetz, M.T.; Garcia-Borràs, M. The Unexplored Importance of Fleeting Chiral Intermediates in Enzyme-Catalyzed Reactions. J. Am. Chem. Soc. 2021, 143, 14939–14950. [Google Scholar] [CrossRef] [PubMed]
- Dyakin, V.V.; Dyakina-Fagnano, N.V.; Mcintire, L.B.; Uversky, V.N. Fundamental Clock of Biological Aging: Convergence of Molecular, Neurodegenerative, Cognitive and Psychiatric Pathways: Non-Equilibrium Thermodynamics Meet Psychology. Int. J. Mol. Sci. 2022, 23, 285. [Google Scholar] [CrossRef]
- Dyakin, V.V.; Wisniewski, T.M.; Lajtha, A. Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration. Symmetry 2020, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Stansley, B.L.; Yamamoto, B.K. L-dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology 2013, 67, 243–251. [Google Scholar] [CrossRef] [PubMed]
- van Hooff, J.J.E. Towards unraveling the origins of eukaryotic nuclear genome organization. Trends Cell Biol. 2023, 33, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.; Driessen, A.J.M. Archaeal phospholipids: Structural properties and biosynthesis. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Takata, T.; Fujii, N.; Aki, K.; Sakaue, H. D-Amino Acid Residues in Proteins Related to Aging and Age-Related Diseases and a New Analysis of the Isomers in Proteins. In D-Amino Acid; Yoshimura, T., Nishikawa, T., Homma, H., Eds.; D-Amino Acids Springer: Tokyo, Japan, 2016; pp. 241–245. [Google Scholar] [CrossRef]
- Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Omar Cauli, Academic Editor and Soraya L. Valles, Academic Editor D-Amino Acids as a Biomarker in Schizophrenia. Diseases 2022, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Endicott, M.; Jones, M.; Hull, J. Amino acid metabolism as a therapeutic target in cancer: A review. Amino Acids. 2021, 53, 1169–1179. [Google Scholar] [CrossRef]
- Murtas, G.; Pollegioni, L. D-Amino Acids and Cancer: Friends or Foes? Int. J. Mol. Sci. 2023, 24, 3274. [Google Scholar] [CrossRef]
- Wolosker, H.; Balu, D.T. D-Serine as the gatekeeper of NMDA receptor activity: Implications for the pharmacologic management of anxiety disorders. Transl. Psychiatry 2020, 10, 184. [Google Scholar] [CrossRef]
- Li, Y.; Han, H.; Yin, J.; Li, T.; Yina, Y. Role of D-aspartate on biosynthesis, racemization, and potential functions: A mini-review. Anim. Nutr. 2018, 4, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Katane, M.; Miyamoto, T.; Sekine, M.; Sakai-Kato, K.; Homma, H. D-Serine and D-Alanine Regulate Adaptive Foraging Behavior in Caenorhabditis elegans via the NMDA Receptor. J. Neurosci. 2020, 40, 7531–7544. [Google Scholar] [CrossRef] [PubMed]
- Kera, Y.; Aoyama, H.; Matsumura, H.; Hisae Nagasaki, H.; Yamada, R. Presence of free D-glutamate and D-aspartate in rat tissues. Biochim. Biophys. Acta (BBA) 1995, 1243, 282–286. [Google Scholar] [CrossRef]
- Mangas, A.; Coveñas, R.; Bodet, D.; Geffard, M.; Aguilar, L.A.; Yajeya, J. Immunocytochemical visualization of d-glutamate in the rat brain. Neuroscience 2007, 144, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yang, H.T.; Lane, H.Y. d-glutamate, D-serine, and d-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 2019, 185, 172760. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hou, C.; Bai, Y.; Wang, R.; Liu, J. Protein Assembly: Versatile Approaches to Construct Highly Ordered Nanostructures. Chem. Rev. (Am. Chem. Soc.) 2016, 116, 13571–13632. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.; Vitagliano, L.; di Prisco, G.; Zagari, A.; Mazzarella, L. The crystal structure of a tetrameric hemoglobin in a partial hemichrome state. Proc. Natl. Acad. Sci. USA 2002, 99, 9801–9806. [Google Scholar] [CrossRef]
- Ha, C.-E.; Bhagavan, N.V. Hemoglobin Chapter in Essentials of Medical Biochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780124166974. [Google Scholar]
- Goldstein, E.B. Crosstalk between psychophysics and physiology in the study of perception. In Blackwell Handbook of Perception; Goldstein, E.B., Ed.; Blackwell Publishing: Oxford, UK, 2001; pp. 1–23. [Google Scholar]
- Xu, X.; Hanganu-Opatz, I.L.; Bieler, M. Cross-Talk of Low-Level Sensory and High-Level Cognitive Processing: Development, Mechanisms, and Relevance for Cross-Modal Abilities of the Brain. Front. Neurorobot. 2020, 14, 7. [Google Scholar] [CrossRef]
- Logothetis, N.K.; Pauls, J. Psychophysical and Physiological Evidence for Viewer-centered Object Representations in the Primate. Cereb. Cortex 1995, 3, 270–288. [Google Scholar] [CrossRef]
- Mascalzoni, E.; Osorio, D.; Regolinm, L.; Vallortigara, G. Symmetry perception by poultry chicks and its implications for three-dimensional object recognition. Proc. Biol. Sci. 2012, 279, 841–846. [Google Scholar] [CrossRef]
- Pizlo, Z.; de Barros, J.A. The Concept of Symmetry and the Theory of Perception. Front. Comput. Neurosci. 2021, 15, 681162. [Google Scholar] [CrossRef] [PubMed]
- Fabien, C. Design of a Bio-Inspired 3D Orientation Coordinate System and Application in Robotised Tele-Sonography. In Robot Arms; Goto, S., Ed.; 5 Princes Gate Court; IntecOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Craighero, L. The Role of the Sensorimotor System in Cognitive Functions. Brain Sci. 2022, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Paillard, J. Cognitive versus sensorimotor encoding of spatial information. In Cognitive Processes and Spatial Orientation in Animal and Man, Vol. II, Neurophysiology and Developmental Aspects; Ellen, P., Thinus-Blanc, C., Eds.; Martinus Nijhoff: Dordrecht, The Netherlands, 1987; Volume 37, ISBN 9-02473-448-7. [Google Scholar] [CrossRef]
- Fernandez-Baizan, C.; Nuñez, P.; Arias, J.L.; Mendez, M. Egocentric and allocentric spatial memory in typically developed children: Is spatial memory associated with visuospatial skills, behavior, and cortisol? Brain Behav. 2020, 10, e01532. [Google Scholar] [CrossRef] [PubMed]
- Nakade, Y.; Iwata, Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R.; Miyake, T.; Sakai, N.; Kitajima, S.; Toyama, T.; et al. Gut microbiota-derived D-serine protects against acute kidney injury. J. Clin. Investig. Insight 2018, 3, e97957. [Google Scholar] [CrossRef] [PubMed]
- Freud, S. An Outline of Psychoanalysis, 45th ed.; Vintage: London, UK, 1940; Volume 23. [Google Scholar]
- Wada, K.; Yamamoto, M.; Nakashima, K. Psychological function in aging. Nihon Rinsho 2013, 71, 1713–1719. [Google Scholar]
- Bottaccioli, A.G.; Bologna, M.; Bottaccioli, F. Psychic Life-Biological Molecule Bidirectional Relationship: Pathways, Mechanisms, and Consequences for Medical and Psychological Sciences—A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3932. [Google Scholar] [CrossRef] [PubMed]
- Corballis, M.C.; Beale, I.L. Bilateral symmetry and behavior. Psychol. Rev. 1970, 77, 451–464. [Google Scholar] [CrossRef]
- Savin, D.N.; Tseng, S.-C.; Morton, S.M. Bilateral Adaptation During Locomotion Following a Unilaterally Applied Resistance to Swing in Nondisabled Adult. JPN J. Neirophysiol. 2010, 104I, 3600–3611. [Google Scholar] [CrossRef]
- Holló, G.; Novák, M. The manoeuvrability hypothesis to explain the maintenance of bilateral symmetry in animal evolution. Biol. Direct. 2012, 7, 22. [Google Scholar] [CrossRef]
- Alqadah, A.; Hsieh, Y.-W.; Morrissey, Z.D.; Chuang, C.-F. Asymmetric development of the nervous system. Dev. Dyn. 2018, 247, 124–137. [Google Scholar] [CrossRef]
- Heger, P.; Zheng, W.; Rottmann, A.; Panfilio, K.A.; Wiehe, T. The genetic factors of bilaterian evolution. eLife 2020, 9, e45530. [Google Scholar] [CrossRef] [PubMed]
- Tamada, A.; Igarashi, M. Revealing chiral cell motility by 3D Riesz transform-differential interference contrast microscopy and computational kinematic analysis. Nat. Commun. 2017, 8, 2194. [Google Scholar] [CrossRef] [PubMed]
- Delvenne, J.-F.; Castronovo, J.; Demeyere, N.; Umphreys, G.W. Bilateral Field Advantage in Visual Enumeration. PLoS ONE 2011, 6, e17743. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Toichi, M. The Role of Alternating Bilateral Stimulation in Establishing Positive Cognition in EMDR. Therapy: A Multi-Channel Near-Infrared Spectroscopy Study. PLoS ONE 2016, 11, e0162735. [Google Scholar] [CrossRef] [PubMed]
- Boukezzi, S.; Silva, C.; Nazarian, B.; Rousseau, P.-F.; Guedj, E.; Valenzuela-Moguillansky, C. Bilateral Alternating Auditory Stimulations Facilitate Fear Extinction and Retrieval. Front. Psychol. Sec. Psychol. Clin. Set. 2017, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Kasprian, G.; Langs, G.; Brugger, P.C.; Bittner, M.; Weber, M.; Arantes, M.; Prayer, D. The prenatal origin of hemispheric asymmetry: An in-utero neuroimaging study. Cereb. Cortex 2011, 21, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Mitter, C.; Prayer, D.; Brugger, P.C.; Weber, M.; Kaspria, G. In Vivo Tractography of Fetal Association Fibers. PLoS ONE 2015, 10, e011953. [Google Scholar] [CrossRef]
- Nakamura, M.; Hashimoto, T. Mechanistic Insights into Plant Chiral Growth. Symmetry 2020, 12, 2056. [Google Scholar] [CrossRef]
- Zagórska-Marek, B.; Sokołowska, K.; Turzańska, M. Chiral events in developing gametophores of Physcomitrella patens and other moss species are driven by an unknown, universal direction-sensing mechanism. Am. J. Bot. 2018, 105, 1986–1994. [Google Scholar] [CrossRef]
- Than, K. Symmetry in Nature: Fundamental Fact or Human Bias. Live Science. 2005. Available online: https://www.livescience.com/4002-symmetry-nature-fundamental-fact-human-bias.html (accessed on 22 January 2023).
- Gunturkun, O.; Ocklenburg, S. Ontogenesis of Lateralization. Neuron 2017, 94, 249–263. [Google Scholar] [CrossRef]
- Mehler, M.F. Epigenetic Principles and Mechanisms Underlying Nervous System Functions in Health and Disease. Prog. Neurobiol. 2008, 86, 305–341. [Google Scholar] [CrossRef] [PubMed]
- Zion, E.; Chen, X. Breaking Symmetry: The Asymmetries in Epigenetic Inheritance. Biochem 2021, 43, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Q.; Chin, A.S.; Worley, K.E.; Ray, P. Cell chirality: Emergence of asymmetry from cell culture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150413. [Google Scholar] [CrossRef]
- Sun, N.; Dou, X.; Tang, Z.; Zhang, D.; Ni, N.; Wang, J.; Gao, H.; Ju, Y.; Dai, X.; Zhao, C.; et al. Bio-inspired chiral self-assemblies promoted neuronal differentiation of retinal progenitor cells through activation of metabolic pathway. Bioact. Mater. 2021, 6, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Madar, A.D.; Sheffield, M.E.J. Distinct place cell dynamics in CA1 and CA3 encode experience in new environments. Nat. Commun. 2021, 12, 2977. [Google Scholar] [CrossRef]
- Graham, H.K.; Duan, X. Molecular mechanisms regulating synaptic specificity and retinal circuit formation. Wiley Interdiscip. Rev. Dev. Biol. 2021, 10, e379. [Google Scholar] [CrossRef]
- Striedter, G.F. Special Issue: Cortical Evolution. Evolution of the hippocampus in reptiles and birds. JCN (Res. Syst. Neurosci.) 2016, 524, 496–517. [Google Scholar] [CrossRef]
- Matho, K.S.; Huilgol, D.; Galbavy, W.; He, M.; Kim, G.; An, X.; Lu, J.; Wu, P.; Di Bella, D.J.; Shetty, A.S.; et al. Genetic dissection of the glutamatergic neuron system in cerebral cortex. Nature 2021, 598, 182–187. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, L.Q. Cell Chirality as A Novel Measure for Cytotoxicity. Adv. Biol. 2022, 6, e2101088. [Google Scholar] [CrossRef]
- Bekkers, J.M. Pyramidal neurons. Curr. Biol. Curr. Biol. 2011, 21, R975. [Google Scholar] [CrossRef]
- Banovac, I.; Sedmak, D.; Džaja, D.; Jalšovec, D.; Milošević, N.J.; Rašin, M.R.; Petanjek, Z. Somato-dendritic morphology and axon origin site specify von Economo neurons as a subclass of modified pyramidal neurons in the human anterior cingulate cortex. J. Anat. 2019, 235, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Johns, P. Neurons and glial cells. In Clinical Neuroscience; Elsevier: St George’s University of London, London, UK, 2014; Chapter 5; pp. 61–69. [Google Scholar] [CrossRef]
- Barger, N.; Sheley, M.F.; Schumann, C.M. Stereological study of pyramidal neurons in the human superior temporal gyrus from childhood to adulthood. J. Comp. Neurol. 2015, 523, 1054–1072. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Schijven, D.; Carrion-Castillo, A.; Joliot, M.; Mazoyer, B.; Fisher, S.E.; Crivello, F.; Francks, C. The genetic architecture of structural left-right asymmetry of the human brain. Nat. Hum. Behav. 2021, 5, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Ottersen, O.P.; Zhang, N.; Walberg, F. Metabolic compartmentation of glutamate and glutamine: Morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience 1992, 46, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Sanghai, N.; Tranmer, G.K. Biochemical and Molecular Pathways in Neurodegenerative Diseases: An Integrated View. Cell 2023, 12, 2318. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Li, L.; Moss, J.; Petrelli, F.; Cassé, F.; Gebara, E.; Lopatar, J.; Pfrieger, F.W.; Bezzi, P.; Bischofberger, J.; et al. Synaptic Integration of Adult-Born Hippocampal Neurons Is Locally Controlled by Astrocytes. Neuron 2015, 88, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Krupic, J.; Bauza, M.; Burton, S.; Barry, C.; O’Keefe, J. Grid cell symmetry is shaped by environmental geometry. Nature 2015, 518, 232–235. [Google Scholar] [CrossRef]
- Oh, M.M.; Simkin, D.; Disterhoft, J.F. Intrinsic Hippocampal Excitability Changes of Opposite Signs and Different Origins in CA1 and CA3 Pyramidal Neurons Underlie Aging-Related Cognitive Deficits. Front. Syst. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef]
- Constant, M.; Mellet, E. The Impact of Handedness, Sex, and Cognitive Abilities on Left–Right Discrimination: A Behavioral Study. Front. Psychol. 2018, 9, 405. [Google Scholar] [CrossRef]
- Merrick, C.M.; Dixon, T.C.; Breska, A.; Lin, J.; Chang, E.F.; King-Stephens, D.; Laxer, K.D.; Weber, P.B.; Carmena, J.; Knight, R.T.; et al. Left hemisphere dominance for bilateral kinematic encoding in the human brain. eLife 2022, 11, e69977. [Google Scholar] [CrossRef]
- Jiang, S.; Guan, Y.; Chen, S.; Jia, X.; Ni, H.; Zhang, Y.; Han, Y.; Peng, X.; Zhou, C.; Li, A.; et al. Anatomically revealed morphological patterns of pyramidal neurons in layer 5 of the motor cortex. Sci. Rep. 2020, 10, 7916. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Shinohara, Y.; Kato, Y.; Sugiyama, H.; Shigemoto, R.; Ito, I. Asymmetrical allocation of NMDA receptor ε2 subunits in hippocampal sircuitry. Science 2003, 300, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Hafting, T.; Fyhn, M.; Molden, S.; Moser, M.-B.; Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Monsalve-Mercado, C.L. The effect of boundaries on grid cell patterns. Phys. Rev. Res. 2020, 2, 043137. [Google Scholar] [CrossRef]
- Galakhova, A.A.; Hunt, A.S.; Wilbers, R.; de Kock, C.P.J.; Mansvelder, H.D.; Goriounova, N.A.; Goriounova, N.A. Evolution of cortical neurons supporting human cognition. Trends Cognit. Sci. 2022, 26, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Hirase, H.; Watanabe, M.; Itakura, M.; Takahashi, M.; Shigemoto, R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc. Natl. Aacd. Sci. USA 2008, 105, 19498–19503. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.F.; Mascagni, F.; McDonald, A.J. Pyramidal Cells of the Rat Basolateral Amygdala. Synaptology and Innervation by Parvalbumin-immunoreactive Interneurons. J. Comp. Neurol. 2006, 494, 635–650. [Google Scholar] [CrossRef]
- Lorente de Nó, R.L. Cerebral cortex: Architecture, intracortical connections and motor projections. In Physiology of the Nervous System, 3rd ed.; Fulton, J.F., Ed.; Oxford University Press: Oxford, UK, 1949; pp. 288–330. [Google Scholar]
- Chan, C.H.; Godinho, L.N.; Thomaidou, D.; Tan, S.S.; Gulisano, M.; Parnavelas, J.G. Emx1 is a Marker for Pyramidal Neurons of the Cerebral Cortex. Cereb. Cortex 2001, 11, 1191–1198. [Google Scholar] [CrossRef]
- Hawkins, J.; Ahmad, S. Hypothesis and theory article. Why Neurons Have Thousands of Synapses, a Theory of Sequence Memory in Neocortex. Front. Neural Circuits 2016, 10, 23. [Google Scholar] [CrossRef]
- Jia, W.; Hu, C.; Wang, Y.; Liu, Y.; Wang, L.; Zhang, S.; Zhu, Q.; Gu, Y.; Zhang, P.; Ma, J.; et al. Identification of Single-Molecule Catecholamine Enantiomers Using a Programmable Nanopore. ACS Nano 2022, 16, 6615–6624. [Google Scholar] [CrossRef] [PubMed]
- Parnavelas, J.G.; Dinopoulos, A.; Davies, S.W. The central visual pathways. In Handbook of Chemical Neuroanatomy, Vol. 7. Integrated Systems of the CNS, Part II; Björklund, A., Hökfelt, T., Swanson, L.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 1–164. [Google Scholar]
- Dallérac, G.; Li, X.; Lecouflet, P.; Morisot, N.; Sacchi, S.; Asselot, R.; Pham, T.H.; Potier, B.; Watson, D.J.G.; Schmidt, S.; et al. Dopaminergic neuromodulation of prefrontal cortex activity requires the NMDA receptor coagonist D-serine. Proc. Natl. Acad. Sci. USA 2021, 118, e2023750118. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Krupic, J. Do hippocampal pyramidal cells respond to nonspatial stimuli? Physiol. Rev. 2021, 101, 1427–1456. [Google Scholar] [CrossRef] [PubMed]
- Purpura, D.P.; Suzuki, K. Distortion of neuronal geometry and formation of aberrant synapses in neuronal storage disease. Brain Res. 1976, 116, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Adámek, P.; Langová, V.; Horáček, J. Early-stage visual perception impairment in schizophrenia, bottom-up and back again. Schizophrenia 2022, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Granato, A.; De Giorgio, A. Alterations of neocortical pyramidal neurons: Turning points in the genesis of mental retardation. Front. Pediatr. 2014, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Rennó-Costa, C.; Adriano Tort, A.B.L. Place and Grid Cells in a Loop: Implications for Memory Function and Spatial Coding. J. Neurosci. 2017, 37, 8062–8076. [Google Scholar] [CrossRef] [PubMed]
- Kitanishi, T.; Ito, H.T.; Hayashi, Y.; Shinohara, Y.; Mizuseki, K. Network mechanisms of hippocampal laterality, place coding, and goal-directed navigation. J. Physiol. Sci. 2017, 67, 247–258. [Google Scholar] [CrossRef]
- Zhong, S.; He, Y.; Shu, H.; Gaolang Gong, G. Developmental Changes in Topological Asymmetry Between Hemispheric Brain White Matter Networks from Adolescence to Young Adulthood. Cereb. Cortex 2017, 7, 2560–2570. [Google Scholar] [CrossRef]
- Goriounova, N.A.; Heyer, D.B.; Wilbers, R.; Verhoog, M.B.; Giugliano, M.; Christophe Verbist, C.; Obermayer, J.; Kerkhofs, A.; Smeding, H.; Verberne, M.; et al. Large and fast human pyramidal neurons associated with intelligence. Elife 2018, 18, e41714. [Google Scholar] [CrossRef]
- Shipton, O.A.; El-Gaby, M.; Apergis-Schoute, J.; Deisseroth, K.; Bannerman, D.M.; Paulsen, O.; Kohl, M.M. Left–right dissociation of hippocampal memory processes in mice. Proc. Natl. Aacd. Sci. USA 2014, 111, 15238–15243. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.J.; Ruela, C.; Madeira, M.D. Dendritic right/left asymmetries in the neurons of the human hippocampal formation: A quantitative Golgi study. Arq. Neuropsiquiatr. 2007, 65, 1105–1113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spruston, N. Pyramidal neurons: Dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008, 9, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kurashima, R.; Gokan, H.; Inoue, N.; Ito, I.; Watanabe, S. Left−Right Asymmetry Defect in the Hippocampal Circuitry Impairs Spatial Learning and Working Memory in iv Mice. PLoS ONE 2010, 5, e15468. [Google Scholar] [CrossRef] [PubMed]
- Ukai, H.; Kawahara, A.; Hirayama, K.; Isao, I. ItPirB regulates asymmetries in hippocampal circuitry. PLoS ONE 2017, 12, e179377. [Google Scholar] [CrossRef] [PubMed]
- Luengo-Sanchez, S.; Bielza, C.; Benavides-Piccione, R.; Fernaud-Espinosa, I.; DeFelipe, J.; Larrañaga, P. A univocal definition of the neuronal soma morphology using Gaussian mixture models. Front. Neuroanat. 2015, 9, 137. [Google Scholar] [CrossRef]
- Rockland, K.S. Pyramidal Neurons: Looking for the origins of axons. Evol. Biol. Neurosci. 2022, 11, e79839. [Google Scholar] [CrossRef]
- Abrous, D.N.; Koehl, M.; Lemoine, M.A. Baldwin interpretation of adult hippocampal neurogenesis: From functional relevance to physiopathology. Mol. Psychiatry 2022, 27, 383–402. [Google Scholar] [CrossRef]
- Leguey, I.; Benavides-Piccione, R.; Rojo, C.; Larrañaga, P.; Bielza, C.; DeFelipe, J. Patterns of Dendritic Basal Field Orientation of Pyramidal Neurons in the Rat Somatosensory Cortex. eNeuro 2018, 5, ENEURO.0142-18.2018. [Google Scholar] [CrossRef]
- Weiler, S.; Nilo, D.G.; Bonhoeffer, T.; Hübener, M.; Rose, T.; Scheuss, V. Orientation and direction tuning align with dendritic morphology and spatial connectivity in mouse visual cortex. Curr. Biol. 2020, 32, 1743–1753.e7. [Google Scholar] [CrossRef]
- Wahle, P.; Sobierajski, E.; Gasterstädt, I.; Lehmann, N.; Weber, S.; Lübke, H.R.; Engelhardt, M.; Distler, C.; Meyer, G. Neocortical pyramidal neurons with axons emerging from dendrites are frequent in non-primates, but rare in monkey and human. Elife 2022, 11, e76101. [Google Scholar] [CrossRef] [PubMed]
- Musall, S.; Sun, X.R.; Mohan, H.; An, X.; Gluf, S.; Li, S.-J.; Drewes, R.; Cravo, E.; Lenzi, I.; Yin, C.; et al. Pyramidal cell types drive functionally distinct cortical activity patterns during decision-making. Nat. Neurosci. 2023, 26, 495–505. [Google Scholar] [CrossRef]
- Park, J.; Papoutsi, A.; Ash, R.T.; Poirazi, P.; Smirnakis, S.M.; Ghani, N. Contribution of apical and basal dendrites to orientation encoding in mouse V1 L2/3 pyramidal neurons. Nat. Commun. 2019, 10, 5372. [Google Scholar] [CrossRef] [PubMed]
- Elaine, M.; Pinheiro, E.M.; Xie, Z.; Norovich, A.L.; Vidaki, M.; Tsai, L.-H.; Gertler, F.B. Lpd depletion reveals that SRF specifies radial versus tangential migration of pyramidal neurons. Nat. Cell Biol. 2011, 13, 989–995. [Google Scholar] [CrossRef]
- Hobert, O. Development of left/right asymmetry in the Caenorhabditis elegans nervous system: From zygote to postmitotic neuron. Genesis 2014, 52, 528–543. [Google Scholar] [CrossRef]
- Tee, Y.; Shemesh, T.; Thiagarajan, V.; Hariadi, R.F.; Anderson, K.L.; Page, C.; Volkmann, N.; Hanein, D.; Sivaramakrishnan, S.; Kozlov, M.M.; et al. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat. Cell Biol. 2015, 17, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.; Boubakar, L.; Valérie Castellani, V. Septin functions during neuro-development, a yeast perspective. Curr. Opin. Neurobiol. 2019, 57, 102–109. [Google Scholar] [CrossRef]
- Inaki, M.; Sasamura, T.; Matsuno, K. Cell Chirality Drives Left-Right Asymmetric Morphogenesis. Front. Cell Dev. Biol. 2018, 6, 34. [Google Scholar] [CrossRef]
- Konietzny, A.; Bär, J.; Mikhaylova, M. Dendritic Actin Cytoskeleton: Structure, Functions, and Regulations. Front. Cell. Neurosci. Sec. Cell. Neurophysiol. 2017, 11, 147. [Google Scholar] [CrossRef]
- Machnicka, B.; Czogalla, A.; Hryniewicz-Jankowska, A.; Bogusławska, D.M.; Grochowalska, R.; Heger, E.; Sikorski, A.F. Spectrins: A structural platform for stabilization and activation of membrane channels, receptors and transporters. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 620–634. [Google Scholar] [CrossRef]
- Lorenzo, D.N.; Edwards, R.J.; Slavutsky, A.L. Spectrins: Molecular organizers and targets of neurological disorders. Nat. Rev. Neurosci. 2023, 24, 195–212. [Google Scholar] [CrossRef]
- Satir, P. Chirality of the cytoskeleton in the origins of cellular asymmetry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150408. [Google Scholar] [CrossRef] [PubMed]
- Parato, J.; Bartolini, F. The microtubule cytoskeleton at the synapse. Neurosci. Lett. 2021, 753, 135850. [Google Scholar] [CrossRef]
- Lamprecht, R. The actin cytoskeleton in memory formation. Progress. Neurobiol. 2014, 117, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ponce, D.; Blázquez-Llorca, L.; De Felipe, J.; Garrido, J.J.; Muñoz, A. Colocalization of α-actinin and Synaptopodin in the Pyramidal Cell Axon Initial Segment. Cereb. Cortex 2012, 22, 1648–1661. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Gupton, S.L. Cytoskeleton: Septin wreaths regulate actin in neuritogenesis. Curr. Biol. 2023, 33, R98–R100. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, A.; Berges, R.; Frank, R.; Robert, P.; Peterson, A.C.; Eyer, J. Neurofilaments Bind Tubulin and Modulate Its Polymerization. J. Neurosci. 2009, 29, 11043–11054. [Google Scholar] [CrossRef]
- Uylings, H.B.; Jacobsen, A.M.; Zilles, K.; Amunts, K. Left-right asymmetry in volume and number of neurons in adult Broca’s area. Cortex 2006, 42, 652–658. [Google Scholar] [CrossRef]
- El-Gaby, M.; Reeve, H.M.; Lopes-dos-Santos, V.; Campo-Urriza, N.; Perestenko, P.V.; Morley, A.; Strickland, L.A.M.; Lukács, I.P.; Paulsen, O.; Dupret, D. An Emergent Neural Coactivity Code for Dynamic Memory. Nat. Neurosci. 2021, 24, 694–704. [Google Scholar] [CrossRef]
- Cullen, T.J.; Walker, M.A.; Eastwood, S.L.; Esiri, M.M.; Harrison, P.J.; Crow, T.J. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br. J. Psychiatry 2006, 188, 26–31. [Google Scholar] [CrossRef]
- Souta, R. An Introductory Perspective on the Emerging Application of qEEG. In Introduction to Quantitative EEG and Neurofeedback. Advsanced Theory and Applications, 2nd ed.; Budzynski, T.H., Washington, P., Budzynski, H.K., Washington, P., Evans, J.R., Abarbanel, A., Eds.; Academic Press: New York, NY, USA, 2009. [Google Scholar]
- Beniaguev, D.; Segev, I.; London, M. Single cortical neurons as deep artificial neural networks. Neuron 2021, 109, 2727–2739.e3. [Google Scholar] [CrossRef] [PubMed]
- Toxvaerd, S. The emergence of the bilateral symmetry in animals: A review and a new hypothesis. Symmetry 2021, 13, 261. [Google Scholar] [CrossRef]
- Matsuo, K.; Tamura, R.; Hotta, K.; Okada, M.; Takeuchi, A.; Wu, Y.; Hashimoto, K.; Takano, H.; Momose, A.; Nishino, A. Bilaterally Asymmetric Helical Myofibrils in Ascidian Tadpole Larvae. Front. Cell Dev. Biol. 2021, 9, 800455. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.A.; Giomi, L. Theory of cellular homochirality and trait evolution in flocking systems. arXiv 2023, arXiv:2307.14360. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Cao, T.T.; Fan, H.W.; Zhang, M.-Z.; Duan, H.-K.; Li, J.; Zhang, X.-J.; Li, Q.; Wang, P.; Chen, T. The increased in vivo firing of pyramidal cells but not interneurons in the anterior cingulate cortex after neuropathic pain. Mol. Brain. 2022, 15, 12. [Google Scholar] [CrossRef]
- Koga, K. Early evolution of membrane lipids: How did the lipid divide occur? J. Mol. Evol. 2011, 72, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.-X.; Jain, N.; Preuss, T.M.; Kaas, J.K. Inverted pyramidal neurons in chimpanzee sensorimotor cortex are revealed by immunostaining with monoclonal antibody SMI-32. Somatosens. Mot. Res. 1999, 16, 49–56. [Google Scholar] [CrossRef]
- Steger, R.; Ramos, R.L.; Cao, R.; Yang, Q.; Chen, C.-C.; Dominici, J.; Brumberg, J.C. Physiology and morphology of inverted pyramidal neurons in the rodent neocortex. Neuroscience 2013, 17, 165–179. [Google Scholar] [CrossRef]
- Martin, H.S.; Podolsky, K.A.; Devaraj, N.K. Probing the role of chirality in phospholipid membranes. ChemBioChem 2021, 22, 3148–3315. [Google Scholar] [CrossRef]
- Kong, X.-Z.; Postema, M.C.; Guadalupe, T.; de Kovel, C.; Boedhoe, P.C.W.; Hoogman, M.; Mathias, S.R.; van Rooij, D.; Schijven, D.; Glahn, D.C.; et al. Mapping brain asymmety in health and disease through the ENIGMA consortium. Hum. Brain Map. 2022, 43, 167–181. [Google Scholar] [CrossRef]
- Kay, J.W.; Phillips, W.A. Contextual Modulation in Mammalian Neocortex is Asymmetric. Symmetry 2020, 12, 815. [Google Scholar] [CrossRef]
- Tripathi, S.; Dey, A.; Shanmugam, M.; Narayanan, R.S.; Chandrsekhar, V. Cobalt (II) Complexes as Single-Ion Magnets. In Organometallic Magnets; Topics in Organometallic Chemistry Series; Springer: Cham, Switzerland, 2019; Volume 64. [Google Scholar] [CrossRef]
- Kagamiyama, H.; Hayashi, H. Crystallographic Structures. Branched-Chain Amino Acids, Part B. In Methods in Enzymoligy; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 2004; ISBN 978-0-13-039913-7. [Google Scholar]
- Gradišar, H.; Božič, S.; Doles, T.; Vengust, D.; Hafner-Bratkovič, I.; Mertelj, A.; Webb, B.; Šali, A.; Klavžar, S.; Jerala, R. Design of a single-chain polypeptide tetrahedron assembled from coiled-coil segments. Nat. Chem. Biol. 2013, 9, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Le Bel, J.A. Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc. Chim. Fr. 1874, 22, 337. [Google Scholar]
- Mun, J.; Kim, M.; Yang, Y.; Badloe, T.; Ni, J.; Chen, Y.; Qiu, C.; Rho, J. Electromagnetic chirality: From fundamentals to nontraditional chiroptical phenomena. Light. Sci. Appl. 2020, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.J. Use of chiral cell shape to ensure highly directional swimming in trypanosomes. PLoS Comput. Biol. 2017, 13, e1005353. [Google Scholar] [CrossRef] [PubMed]
- Van‘t Hoff, J.H. A suggestion looking to the extension into space of the structural formulas at present used in chemistry and a note upon the relation between the optical activity and the chemical constitution of organic compounds. Arch. Neérl. Sci. Exactes. Nat. 1874, 9, 445–454. Available online: https://www.chemteam.info/Chem-History/Van’t-Hoff-1874.html (accessed on 12 July 2023).
- Xie, N.; Liu, S.; Yang, X.; He, X.; Huang, J.; Wang, K. DNA tetrahedron nanostructures for biological applications: Biosensors and drug delivery. Analyst 2017, 18, 3322–3332. [Google Scholar] [CrossRef]
- Mathur, D.; Rogers, K.E.; Díaz, S.A.; Muroski, M.E.; Klein, W.P.; Nag, O.K.; Lee, K.; Field, L.D.; Delehanty, J.B.; Medin, I.L. Determining the Cytosolic Stability of Small DNA Nanostructures in Cellula. Nano Lett. 2022, 22, 5037–5045. [Google Scholar] [CrossRef]
- Aumiller, W.A., Jr.; Uchida, M.; Douglas, T. Protein cage assembly across multiple length scales. Chem. Soc. Rev. 2018, 47, 3433–3469. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Hong, S.; Jiang, W.; Ding, Q.; Lin, K.; Zhao, C.; Wang, X. The Current Progress of Tetrahedral DNA Nanostructure for Antibacterial Application and Bone Tissue Regeneration. Int. J. Nanomed. 2023, 18, 3761–3780. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Mao, K.; Chen, S.; Zhu, H. Chirality Effects in Peptide Assembly Structures. Front. Bioeng. Biothechnol. 2021, 9, 703004. [Google Scholar] [CrossRef] [PubMed]
- Hassabis, D.; Kumaran, D.; Summerfield, C.; Botvinick, M. Neuroscience-Inspired Artificial Intelligence. Neuron 2017, 95, P245–P258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, M.; Zhou, L.; Wang, X.; Jia, J. The Application of Artificial Intelligence in Brain-Computer Interface and Neural System Rehabilitation. Front. Neurosci. 2022, 16, 1024316. [Google Scholar] [CrossRef] [PubMed]
- Surianarayanan, C.; Lawrence, J.J.; Chelliah, P.R.; Prakash, E.; Hewage, C. Convergence of Artificial Intelligence and Neuroscience towards the Diagnosis of Neurological Disorders—A Scoping Review. Sensors 2023, 23, 3062. [Google Scholar] [CrossRef] [PubMed]
- Strychalski, W. 3D Computational Modeling of Bleb Initiation Dynamics. Front. Phys. Sec. Biophys. 2021, 9, 775465. [Google Scholar] [CrossRef]
- Johnson, E.; Cascio, D.; Sawaya, M.R.; Gingery, M.; Schröder, I. Crystal Structures of a Tetrahedral Open Pore Ferritin from the Hyperthermophilic Archaeon Archaeoglobus fulgidus. Structure 2005, 13, 637–648. [Google Scholar] [CrossRef]
- Zhang, K.; Ezemaduka, A.; Wang, Z.; Hu, H.; Shi, X.; Liu, C.; Lu, X.; Fu, X.; Chang, Z.; Yin, C. A Novel Mechanism for Small Heat Shock Proteins to Function as Molecular Chaperones. Sci. Rep. 2015, 5, 8811. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, H.-S. Foldectures: 3D Molecular Architectures from Self-Assembly of Peptide Foldamers. Acc. Chem. Res. 2017, 50, 832–841. [Google Scholar] [CrossRef]
- Sasaki, E.; Böhringer, D.; van de Waterbeemd, M.; Leibundgut, M.; Zschoche, R. Structure and assembly of scalable porous protein cages. Nat. Commun. 2017, 8, 14663. [Google Scholar] [CrossRef]
- Quaye, I.K. Extracellular hemoglobin: The case of a friend turned foe. Front. Physiol. 2015, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.W. Retrospective on statistical mechanical models for hemoglobin allostery editors-pick. J. Chem. Phys. 2022, 157, 184104. [Google Scholar] [CrossRef]
- Richter, F.; Meurers, B.H.; Zhu, C.; Medvedeva, V.P.; Chesselet, M.-F. Neurons Express Hemoglobin α- and β-Chains in Rat and Human Brains. J. Comp. Neurol. 2009, 15, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ronson, T.K.; Mosquera, J.; Martinez, A.; Guy, L.; Nitschke, J.R. Anion Binding in Water Drives Structural Adaptation in an Azaphosphatrane-Functionalized FeII4L4 Tetrahedron. J. Am. Chem. Soc. 2017, 139, 6574–6577. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Li, Q.; Mogilner, A.; Bershadsky, A.D.; Shivashankar, G.V. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. Proc. Natl. Acad. Sci. USA 2015, 112, E2595–E2601. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, A.J.; Kishi-Itakura, C.; Masters, T.A.; Rorbach, J.E.; Grice, G.L.; Kendrick-Jones, J.; James, A.; Nathan, J.A.; Minczuk, M. Myosin VI-Dependent Actin Cages Encapsulate Parkin-Positive Damaged Mitochondria. Dev. Cell. 2018, 26, 484–499.e6. [Google Scholar] [CrossRef]
- Mostowy, S.; Bonazzi, M.; Hamon, M.A.; Tham, T.N.; Mallet, A.; Lelek, M.; Gouin, E.; Demangel, C.; Brosch, R.; Zimmer, C.; et al. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe 2010, 8, 433–444. [Google Scholar] [CrossRef]
- Heid, H.; Rickelt, S.; Zimbelmann, R.; Winter, S.; Schumacher, H.; Dörflinger, Y.; Kuhn, C.; Franke, W.W. On the formation of lipid droplets in human adipocytes: The organization of the perilipin-vimentin cortex. PLoS ONE 2014, 9, e90386. [Google Scholar] [CrossRef]
- Tessarz, P.; Schwarz, M.; Mogk, A.; Bukau, B. The yeast AAA+ chaperone Hsp104 is part of a network that links the actin cytoskeleton with the inheritance of damaged proteins. Mol. Cell Biol. 2009, 29, 3738–3745. [Google Scholar] [CrossRef]
- Franke, W.W.; Hergt, M.; Grund, C. Rearrangement of the vimentin cytoskeleton during adipose conversion: Formation of an intermediate filament cage around lipid globules. Cell 1987, 49, 131–141. [Google Scholar] [CrossRef]
- Biliński, S.M.; Jaglarz, M.K. Organization and possible functions of microtubule cytoskeleton in hymenopteran nurse cells. Cell Motil. Cytoskeleton. 1999, 43, 213–220. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, P.; Liu, X.; Liu, T.; Yan, Y.; Yan, J.; Zhong, J.; Sun, G.; He, D. Tumor-Penetrating Peptide-Modified DNA Tetrahedron for Targeting Drug Delivery. Biochemistry 2016, 55, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, T.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wu, S.; Chu, L.T.; Kwong, H.K.; Hartanto, H.; Huang, Y.; Lam, M.L.; Lam, R.H.W.; Chen, T.-H. Early Committed Clockwise Cell Chirality Upregulates Adipogenic Differentiation of Mesenchymal Stem Cells. Adv. Biosyst. 2020, 10, e2000161. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Hu, Y.; Cao, B.; Peng, R.; Ding, J. Effects of surface molecular chirality on adhesion and differentiation of stem cells. Biomaterials 2013, 34, 9001–9009. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yoshitomi, T.; Yoshimoto, K. Analysis of Chirality Effects on Stem Cell Fate Using Three-dimensional Fibrous Peptide Hydrogels. ACS Appl. Bio. Mater. 2018, 1, 538–543. [Google Scholar] [CrossRef]
- Walser, M.; Svensson, J.; Karlsson, M.R.; Åberg, M.; Kuhn, H.G.; Isgaard, J.; Åberg, N.D. Growth Hormone and Neuronal Hemoglobin in the Brain—Roles in Neuroprotection and Neurodegenerative Diseases. Front. Endocrinol. 2021, 11, 606089. [Google Scholar] [CrossRef]
- Zheng, R.; Yan, Y.; Pu, J.; Zhang, B. Physiological and Pathological Functions of Neuronal Hemoglobin: A Key Underappreciated Protein in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 9088. [Google Scholar] [CrossRef]
- Flynn, K.S. The cytoskeleton and neurite initiation. Bioarchitecture 2013, 3, 86–109. [Google Scholar] [CrossRef]
- Indelicato, G.; Wahome, N.; Müller, P.R.S.A.; Nieh, M.-P.; Burkhard, P.; Twarock, R. Principles Governing the Self-Assembly of Coiled-Coil Protein Nanoparticles. Biophys. J. 2016, 110, P646–P660. [Google Scholar] [CrossRef]
- Heberle, F.A.; Feigenson, G.W. Phase Separation in Lipid Membranes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004630. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cai, Q.; Feng, Z.; Zhang, M. Liquid-Liquid Phase Separation in Neuronal Development and Synaptic Signaling. Dev. Cell 2020, 55, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Wonka, P.; Gervautz, M. Ray Tracing of Nonlinear Fractals. J. WSCG 1998, 6, 424–431. Available online: https://wscg.zcu.cz/wscg1998/wscg98.htm (accessed on 13 January 2022).
- Nieuwenhuys, R. The neocortex. Anat. Embryol. 1994, 190, 307–337. [Google Scholar] [CrossRef] [PubMed]
- Pabic, P.L.; Dranow, D.B.; Hoyle, D.J.; Schilling, T.F. Zebrafish endochondral growth zones as they relate to human bone size, shape and disease. Front. Endocrinol. 2022, 13, 1060187. [Google Scholar] [CrossRef] [PubMed]
- Hall, E. The amygdala of the cat: A Golgi study. Z. Zellforsch. Mikrosk. Anat. 1972, 134, 439–458. [Google Scholar] [CrossRef]
- DeMarco, E.; Tesmer, A.L.; Hech, B.; Kawakami, K.; Robles, E. Pyramidal Neurons of the Zebrafish Tectum Receive Highly Convergent Input From Torus Longitudinalis. Front. Neuroanat. 2021, 15, 636683. [Google Scholar] [CrossRef]
- Suryanarayana, S.M. On the Evolutionary Origin of the Vertebrate Cortex; Department of Neuroscience Karolinska Institutet: Srickholm, Sweden, 2019. [Google Scholar]
- Taylor, L.L.K.; Riddell, I.A.; Smulders, M.M.J. Self-Assembly of Functional Discrete Three-Dimensional Architectures in Water. Angew. Chem. 2019, 58, 1280–1307. [Google Scholar] [CrossRef]
- Chirikjian, G.S. Group theory and biomolecular conformation: I. Mathematical and computational models. J. Phys. Condens. Matter. 2010, 22, 323103. [Google Scholar] [CrossRef]
- Dyakin, V.V.; Posner, E.V. Molecular Chirality as an Integral Biomarker of Lactation, Nutrition and Cognitive Development Express-Review. Biomed. Sci. Res. 2020, 8, AJBSR.2020.08.001337. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Armstrong, D.W.; Wolosker, H.; Zheng, Y. Detection and analysis of chiral molecules as disease biomarkers. Nat. Rev. Chem. 2023, 7, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Richards, G.J.; Ishihara, S.; Izawa, H.; Jonathan, P.; Hill, J.P. Intelligent Chiral Sensing Based on Supramolecular and Interfacial Concepts. Sensors 2010, 10, 6796–6820. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Liu, R.; Tu, Y.; Li, Y.; Cheng, J.; He, T.; Zhu, X. Autonomous discovery of optically active chiral inorganic perovskite nanocrystals through an intelligent cloud lab. Nat. Commun. 2020, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
- Rainey, P.B. Major evolutionary transitions in individuality between humans and AI. Philos. Trans. R. Soc. B 2023, 378. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, N.; Amar, M.; Baux, G.; Fossier, P. Homeostatic control of the excitation–inhibition balance in cortical layer 5 pyramidal neurons. Eur. J. Neurosci. 2006, 24, 3507–3518. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.C.; Horwood, N.; Isaacs, S.L.; Mann, D.M.A. The effect of age and Alzheimer’s disease on pyramidal neuron density in the individual fields of the hippocampal formation. Acta Neuropathol. 1992, 83, 510–517. [Google Scholar] [CrossRef]
- El-Gaby, M.; Shipton, O.A.; Paulsen, O. Synaptic Plasticity and Memory: New Insights from Hippocampal Left–Right Asymmetries. Neuroscientist 2014, 21, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, A.; Kosaki, Y.; Ito, I.; Watanabe, S. Mice lacking hippocampal left-right asymmetry show non-spatial learning deficits. Behav. Brain Res. 2018, 336, 156–165. [Google Scholar] [CrossRef]
- Bussière, T.; Giannakopoulos, P.; Bouras, C.; Perl, D.P.; Morrison, J.H.; Patrick, R.; Hof, P.R. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: Stereologic analysis of prefrontal cortex area 9. J. Compap. Neurol. 2003, 463, 281–302. [Google Scholar] [CrossRef]
- Cholvin, T.; Bartos, M. Hemisphere-specific spatial representation by hippocampal granule cells. Nat. Commun. 2022, 13, 6227. [Google Scholar] [CrossRef]
- Wang, J.; Ma, S.; Yu, P.; He, X. Evolution of Human Brain Left–Right Asymmetry: Old Genes with New Functions. Mol. Biol. Evol. 2023, 40, msad181. [Google Scholar] [CrossRef] [PubMed]
- Cutia, C.A.; Leverton, L.K.; Christian-Hinman, C.A. Sex and Estrous Cycle Stage Shape Left-Right Asymmetry in Chronic Hippocampal Seizures in Mice. eNeuro 2023, 10, ENEURO.0041-23.2023. [Google Scholar] [CrossRef]
- Shiptona, O.A.; Tanga, C.S.; Paulsena, O.; Vargas-Caballero, M. Differential vulnerability of hippocampal CA3-CA1 synapses to Ab. Acta Neuropathol. Commun. 2022, 10, 45. [Google Scholar] [CrossRef]
- Hanane, U.; Ben-Moshe, A.; Diamant, H.; Markovich, G. Edited by Lia Addadi, Weizmann Institute of Science, Rehovot, Israel. Spontaneous and directed symmetry breaking in the formation of chiral nanocrystals. Proc. Natl. Aacd. Sci. USA 2019, 116, 11159–11164. [Google Scholar] [CrossRef]
- Higginbotham, H.R.; Gleeson, J.G. The centrosome in neuronal development. Trends Neurosci. 2007, 30, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, A.; Fornasiero, E.F.; Dotti, C.G. Cadherins as regulators of neuronal polarity. Cell Adh. Migr. 2015, 9, 175–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lubben, N.; Ensink, E.; Coetzee, G.A.; Labrie, V. The enigma and implications of brain hemispheric asymmetry in neurodegenerative diseases. Brain Commun. 2021, 3, fcab211. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).