Abstract
The asymmetric formal [3 + 3] annulation process of (E)-2-(3-phenylacryloyl)pyridine N-oxide with benzyl methyl ketone was investigated. The possibility of a stereoselective outcome was checked using salts of natural amino acids, as well as chiral bifunctional derivatives containing amino groups and thiourea or squaramide fragments as organocatalysts. Different types of organocatalysts applied led to opposite enantiomers of 2-(3-oxo-4,5-diphenyl-cyclohex-1-en-yl)pyridine 1-oxide (up to 60% ee). Spectroscopic analysis of the isolated product and analysis of the reaction course was carried out, taking into account the obtained regio- and stereoselectivity. In order to verify the postulated results, calculations of the energy of the intermediate reaction products and the final product using the Kohn–Sham Density Functional Theory (KS-DFT) were made.
1. Introduction
Heteroaromatic N-oxides appear as a structural motif in many compounds with exceptional properties [1,2]. The presence of the N+–O− bond is the reason for the special properties and diverse usefulness of aza-aromatic N-oxides. Among many derivatives, there are products with specific biological properties [3,4,5], for use as pharmaceuticals [6], and as ingredients of personal hygiene products, cosmetics, and detergents [7]; other derivatives are used as oxidants in organic reactions [8]. N-oxides obtained in the enantiomerically enriched form are successfully used as chiral ligands or organocatalysts in asymmetric synthesis [9,10,11]. Aza-aromatic N-oxides have significant synthetic value due to their unique properties resulting from the presence of the N+–O− bond in their structure [12,13,14]. In this last context, particularly interesting are heteroaromatic N-oxides having several places in their structure susceptible to transformations, preferably asymmetric ones, and allowing for significant and sterically controlled expansion of the skeleton [15,16,17,18,19]. The use of tandem reactions as a synthetic tool gives the possibility of obtaining complex products quickly and relatively easily in a one-pot experiment. The use of organocatalytic procedures allows these processes to be performed not only effectively (high yield and stereoselectivity), but also in a more environmentally friendly way [20]. Although several elegant organocatalytic tandem reactions have already been reported in the literature [21,22,23], the construction of cyclic molecules with a few stereogenic centers in a cascade manner remains attractive and desirable.
Here we present studies on the selectivity in the catalytic formal [3 + 3] annulation reaction of (E)-2-(3-phenylacryloyl)pyridine N-oxide when an unsymmetrical ketone is used. The first step of the sequential transformation (Michael addition) can discriminate between the formation of one of the isomers of substituted cyclohexenone, as shown in Scheme 1.
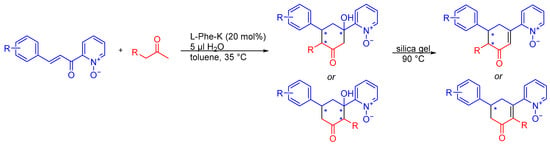
Scheme 1.
Michael/aldol/dehydration sequence reactions of (E)-2-(3-phenylacryloyl)pyridine N-oxide (in blue) with an unsymmetrical ketone (in red)—possible regio- and stereoisomeric products. Possible stereogenic centers marked with an asterisk.
When choosing (E)-2-(3-phenylacryloyl)pyridine N-oxide as a substrate, we were guided by its potential in terms of reactive sites and the possibility of employing it in various transformations. At the same time, due to the presence of carbonyl and N-oxide oxygens close to each other, this pattern may interact with hydrogen bond donors, which may be an advantage when using catalysts with this type of interaction. Although the versatility of this substrate seems to be significant, so far, relatively little attention has been paid to its use in cascade reactions, which are a reasonably desirable tool in the hands of synthetic chemists. The first asymmetric reactions involving 2-enoylpyridine N-oxide were described in 2014 and 2015. It was an asymmetric direct vinylogous Michael addition, catalyzed respectively by difunctional thioureas [24], and bifunctional amine-squaramide [25], which in both cases were derivatives of cinchona alkaloids. The first cascade reaction, reported by Wang group, was the formal [3 + 3] annulation of 2-enoyl pyridine N-oxides, which involved Michael addition in the first stage followed by the intramolecular aldol reaction or aldol condensation [26]. L-phenylalanine potassium was used as an organocatalyst for the reaction with acetone, giving enantiomerically enriched (up to 94% ee) cyclohexenones with various aromatic substituents at the chiral carbon atom. The Li group then presented phosphine-catalyzed [4 + 1]-annulation of 2-enoylpyridine N-oxides, resulting in a series of optically active 2,3-dihydrobenzofurans with high diastereomeric ratio and excellent enantiomeric purity (up to 99%) [27].
2. Materials and Methods
2.1. General
Solvents were distilled, and other reagents were used as received. Reactions were monitored by thin-layer chromatography (TLC) on silica gel 60 F-254 precoated plates (Merck, Darmstadt, Germany), and spots were visualized with a UV lamp. Products were purified by standard column chromatography on silica gel 60 (230–400 mesh) (Merck). Optical rotations at 578 nm were measured using an Optical Activity Ltd. (Huntington, UK) Model AA-5 automatic polarimeter. Melting points were determined using a Boëtius hotstage apparatus (PHMK VEB Analytic, Dresden, Germany). 1H and 13C NMR (400 MHz and 100 MHz, respectively) spectra were recorded in CDCl3 on Jeol 400 MHz (Japan) and Bruker Avance II 600 MHz instruments (Karlsruhe, Germany). High-resolution mass spectra (HRMS) were recorded using electrospray ionization mode on the Waters LCT Premier XE TOF spectrometer (Waters Corporation, Milford, MA, USA). HPLC analysis was performed on SHIMADZU NEXERA X2 apparatus (Chiral Technologies INC. West Chester, PA, USA) using CHIRALPAK IA-3 column (4.6 mm × 25 cm) without a guard column. 1HNMR and 13C NMR spectra of all obtained compounds are shown in Supplementary Materials (Figures S1–S3).
2.2. Preparation of (E)-2-(3-Phenylacryloyl)Pyridine N-Oxide
The first step of the synthesis was performed according to the literature procedure [28]: to a solution of 2-acetylpyridine (1.2 g, 10 mmol, 1 eq) in dichloromethane (100 mL), m-CPBA (70%, 2.6 g, 1.5 eq) was added portion wise at 0 °C. After complete addition, the reaction was allowed to reach room temperature and was stirred for 72 h. The concentrated solution was washed with aqueous sat. NaHCO3 (20 mL), the water phase was extracted with chloroform (3 × 15 mL), and the combined organic layers were dried with MgSO4. The solvent was evaporated giving the crude product which was purified by column chromatography on silica gel with eluent CH2Cl2:MeOH (10:1, v:v).
2-acetylpyridine cetylpyridine N-oxide, yield: 0.634 g (48%); yellowish liquid. 1H NMR (400 MHz, CDCl3): δ = 2.79 (s, 3H, CH3), 7.26–7.32 (m, 1H, Py), 7.32–7.38 (m, 1H, Py), 7.68 (dd, J = 7.8, 2.3 Hz, 1H, Py), 8.17–8.21 (m, 1H, Py); spectroscopic data in agreement with literature data [28].
The second step was based on the report of the Pedro group [29]: to a solution of 2-acetylpyridine N-oxide (0.580 g, 4.2 mmol, 1.0 eq) and benzaldehyde (0.85 mL, 8.4 mmol, 2.0 eq) in MeOH (21 mL) an aqueous solution of KOH (1 M, 0.85 mL) was added at 0 °C. The mixture was stirred for 24 h at 0 °C and then it was quenched with a solution of HCl (2 M, 0.41 mL). After addition of water (40 mL) the solution was extracted with CH2Cl2 (3 × 30 mL). The combined organic extracts were dried with Na2SO4. The solvent was evaporated giving the crude product which was purified by column chromatography on silica gel with eluent CH2Cl2:MeOH (10:1, v:v).
(E)-2-(3-phenylacryloyl)pyridine N-oxide, yield: 0.770 g (81%), yellow-orange solid; mp. 104–105 °C (lit.29 104–105 °C). 1H NMR (400 MHz, CDCl3): δ = 7.32–7.42 (m, 5H, Ar), 7.59–7.66 (m, 2H, Py), 7.66–7.74 (m, 2H, Py, = CH), 7.76–7.84 (m, 1H, = CH), 8.27 (dd, J = 6.1, 0.9 Hz, 1H, Py); spectroscopic data in agreement with literature data [29].
2.3. General Procedure of Catalytic Formal [3 + 3] Annulation
The catalytic formal [3 + 3] annulation of (E)-2-(3-phenylacryloyl)pyridine N-oxide with benzyl methyl ketone was based on the literature procedure [26] using different catalysts. Thus (E)-2-(3-phenylacryloyl)pyridine N-oxide (67.5 mg, 0.30 mmol, 1.0 eq) and catalyst (0.06 mmol, 20% mol) was placed in a sealed tube, and a toluene as the solvent was added (1 mL). Then, a solution of benzyl methyl ketone (48 mg, 0.36 mmol, 1.2 eq) in toluene (0.2 mL) and water (5 μL) was added. The resulting mixture was stirred at 35 °C for 6 days. Then, silica gel (0.540 g) was added and stirring was continued at 90 °C for 24 h. After that, the reaction mixture was purified by column chromatography on silica gel with eluent AcOEt: MeOH (5:1, v:v).
2-(3-oxo-4,5-diphenyl-cyclohex-1-en-yl)pyridine 1-oxide (III), yield: 41 mg (40%) in experiment with catalyst B, brown, gummy liquid; [α]D20 = −12.4 (c 1.0, CHCl3) for 60% ee. 1H NMR (400 MHz, CDCl3): δ = 3.21 (dd, J = 18.0, 4.3 Hz, 1H, CH2), 3.34 (ddd, J = 18.0, 11.1, 2.8 Hz, 1H, CH2), 3.75 (ddd, J = 12.5, 11.1, 4.3 Hz, 1H, CH), 3.96 (d, J = 12.5 Hz, 1H, CH), 6.49 (d, J = 2.8 Hz, 1H, =CH), 6.95–7.00 (m, 2H, Py), 7.03–7.19 (m, 8H, Ar), 7.26–7.37 (m, 3H, Py, Ar), 8.21–8.25 (m, 1H, Py); 13C NMR (100 MHz, CDCl3): δ = 35.6 (C6), 48.4 (C5), 60.1 (C4), 126.1, 126.2, 126.2, 126.8, 126.9, 127.7, 128.3, 128.4, 129.4, 130.4, 137.7, 140.6, 141.6, 148.8, 153.8, 199.1 (C3); HRMS calculated for C23H19NO2 [M + H]+ 342.1494, found 342.1488; HPLC (Chiralpak IA, n-hexane:i-PrOH = 8:2, 1.0 mL/min, λ = 231 nm), t = 46.5 min (major), t = 57.6 min (minor).
2-(5-oxo-3,4-diphenyl-hexanoyl)pyridine 1-oxide (I), yield: 13 mg (12%) in experiment with the catalyst B, dark blue gummy liquid; 1H NMR (400 MHz, CDCl3): δ = 1.82 (s, 3 H, CH3), 3.08 (dd, J = 16.8, 4.1 Hz, 1H, CH2), 3.55 (dd, J = 16.8, 10.4 Hz, 1H, CH2), 4.05–4.16 (m, 2H, 2xCH), 6.89 (dd, J = 7.8, 2.0 Hz, 1H, Py), 6.97–7.05 (m, 2H, Py), 7.10–7.20 (m, 5H, Ar), 7.28–7.41 (m, 5H, Ar), 8.04 (d, J = 6.4 Hz, 1H, Py); 13C NMR (100 MHz, CDCl3): δ = 30.5 (C6), 43.7 (C3), 46.9 (C4), 65.3 (C2), 125.4, 126.4, 126.8, 127.4, 128.3, 128.4, 129.0, 129.1, 136.1, 140.0, 142.3, 146.8, 196.8 (C1), 206.7 (C5); HRMS calculated for C23H21NO3 [M + H]+ 360.1600, found 360.1610.
2-(1-hydroxy-3-oxo-4,5-diphenyl-cyclohexyl)pyridine 1-oxide (V), yield: 11 mg (10%) in experiment with the catalyst B, yellow, gummy liquid; 1H NMR (400 MHz, CDCl3): δ = 1.66 (br s, 1H, OH), 2.04 (d, J = 12.4 Hz, 1H, CH2), 2.07 (d, J = 12.4 Hz, 1H, CH2), 2.81 (dd, J = 12.8, 3.7 Hz, 1H, CH2), 3.99 (td, J = 10.2, 3.7 Hz, 1H, CH), 5.23 (dd, J = 12.8, 10.2 Hz, 1H, CH2), 5.64 (d, J = 10.2 Hz, 1H, CH), 6.68 (dd, J = 7.9, 1.8 Hz, 1H), 6.72–6.78 (m, 1H), 6.89–6.95 (m, 1 H), 7.00–7.04 (m, 1H), 7.10 (t, J = 7.5 Hz, 2H), 7.20–7.23 (m, 2 H), 7.30–7.33 (m, 2 H), 7.53–7.56 (m, 2 H), 7.74–7.81 (m, 2H), 8.17 (dd, J = 6.4, 0.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ = 41.5 (C6), 41.6 (C5), 56.5 (C4), 76.2 (C2), 95.8 (C1), 123.7, 124.8, 125.3, 126.9, 127.2, 127.9, 128.1, 128.3, 128.3, 128.4, 128.5, 139.6, 140.0, 141.7, 146.4, 150.7, 198.9 (C3); HRMS calculated for C23H21NO3 [M + H]+ 360.1600, found 360.1590.
2.4. Computational Details
To propose a plausible molecular mechanism of the discussed Michael addition and aldol condensation reactions KS-DFT calculations for the main compounds shown in Scheme 2 (I, II, III, IV) were performed. The relative values of Gibbs free energies are shown in Supplementary Materials (Table S1). The stationary points were located at the ωB97XD/SCRF (toluene)/def2-TZVPP level and the corresponding Gibbs energies were computed assuming def2-TZVPP basis set.
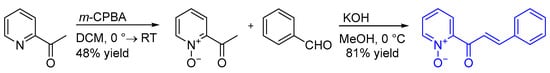
Scheme 2.
Preparation of 2-enoylpyridine N-oxide—substrate used in the annulation procedure.
3. Results
The (E)-2-(3-phenylacryloyl)pyridine N-oxide was prepared in two steps, following the literature procedure [28,29], as shown in Scheme 2.
Using the N-oxide as a substrate for stereoselective transformation involves not only the searching for an appropriate, effective catalyst [17]. An equally important factor was the choice of the second substrate for the reaction. Encouraged by Wang’s results [26], we decided to try to expand his concept to include unsymmetrical ketones. The introduction of this seemingly small modification may result in the formation of various products as shown in Scheme 1, making the regioselectivity of the reaction an important factor to analyze, especially at its first stage. Thus, we chose benzyl methyl ketone (BMK) as a model to monitor the course of the reaction using different catalysts (Table 1). At first, we implemented similar reaction conditions as in the cited work [26], applying BMK in some excess (1.2 eq) and freshly prepared L-phenylalanine potassium salt (L-Phe-K) as organocatalyst (20 mol%). The product of expected annulation was observed in the post-reaction mixture next to the unreacted substrates, but in very low yield (15%). Similar results were obtained with the use of the racemic form D/L-Phe-K salt (entry 7); moreover the use of BMK in greater excess (1.5 eq, entry 8) did not improve the result. Instead of toluene, other solvents were also tested in the reaction. Since the dehydration step requires a rather elevated temperature, it was decided to use 1,2-dichloroethane (1,2-DCE) and 1,4-dioxane. The summarized results are shown in Table 1.

Table 1.
Results of the catalyzed formal [3 + 3] annulation of (E)-2-(3-phenylacryloyl)pyridine N-oxide with benzyl methyl ketone a.
Taking into account the reaction mechanism proposed by the authors of reference [26], including the interaction of the counterion and enamine activation, we selected a few more catalysts that could be used in our experiments. In addition to L-Phe-K, salts of amino acids such as L-proline and L-tert-leucine were tested. Organocatalysts derived from L-proline were previously successfully used in enantioselective transformations involving enones, also in [3 + 3]-annulation [20,30,31,32]. Unfortunately, attempts to use other amino acid salts as organocatalysts did not bring a significant improvement in results compared to the L-Phe-K application (15%, 18% ee, Table 1, entry 1). Contrary to expectations, a decrease in yield and ee was observed when L-Pro-K was used (12% yield, 8% ee, entry 2). The application of L-tert-leucine salt (L-tert-Leu-K) resulted in a significant in ee up to 40% (entry 3), however, the yield obtained was very unsatisfactory (16% yield). Also, changing the counterion of the L-phenylalanine salt and using L-Phe-Na as a catalyst resulted in a low reaction yield (10%) and a racemic product (entry 6) was obtained. For the L-Phe-K salt, changing the solvent to 1,2-dichloroethane resulted in an approximately two-fold decrease in yield and enantioselectivity (Table 1, entry 5), while in 1,4-dioxane the reaction did not occur at all (entry 4). When the procedure did work, in all cases, after a sequence of cascade reactions and chromatographic purification, the same product was obtained. The components were identified based on a comparative analysis of 1H NMR spectra by studying the reaction mechanism.
By analogy to those effectively employed in chalcone [33] or enoyl-pyridine N-oxide transformations [20,24], some well investigated bifunctional organocatalysts, based on the structure of thiourea with a chiral fragment derived from quinine or (1R, 2R)-diaminocyclohexane (DACH) (A, B, respectively, Figure 1), and amine-squaramide with a quinine or mefloquine motif (C and D, respectively, Figure 1), were also investigated.
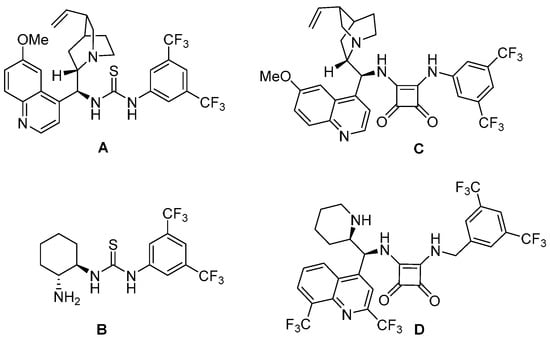
Figure 1.
Structures of hydrogen-donor catalysts tested in the formal [3 + 3] annulation reaction; (A,B)—thiourea derivatives, (C,D)—amine-squaramide derivatives.
In the case of the second type of catalysts, the counterion interaction occurring in amino acid salts was replaced by the interaction associated with the hydrogen bond formation. All compounds shown in Figure 1 were tested in the reaction, but the only catalyst effective in this reaction was the thiourea catalyst B. Moreover, it also provided the highest yield and enantiomeric excess of the obtained product (40% yield, 60% ee, entry 10 in Table 1). The isolated diastereomeric product showed optical rotation ([α]D20 = ̶ 12.4 (c 1.0, CHCl3)). A repeated reaction provided the same results. In the case of other catalysts in this series, solely the recovered substrate (BMK) was isolated from the post-reaction mixture (77% with catalyst A, 80% with C, and 50% with D). In no case did the analysis of the 1H NMR spectra reveal even traces of the product (the spectra were complicated, and definitely contained signals that may have come from catalysts).
4. Discussion
In Scheme 3 the possible variants of the reaction course, ultimately leading to various possible products are depicted. After the first stage of the reaction, in which Michael addition occurs, the formation of two products (I and II) is potentially possible. However, taking into account the stability of the carbanions being formed, the formation of product I seems more likely. Moreover, when the reaction was stopped at an early stage, the product I of the Michael reaction was identified in the post-reaction mixture (Figure S1). The 1H NMR spectrum showed a characteristic singlet (1.82 ppm, integrated as 3 hydrogen atoms), derived from the acetyl group. There should be no such signal when product II is formed. Analyzing both products in terms of subsequent intramolecular aldol condensation, two further possible paths should be considered for each of them. In the case of product I, formed in Michael addition, the subsequent reaction leads to the formation of product III.
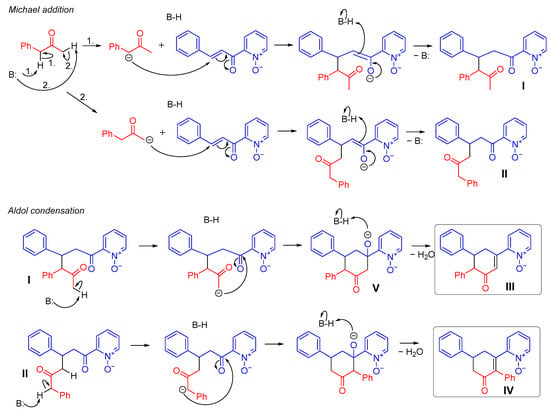
Scheme 3.
Analysis of the formation of the product in the formal [3 + 3] annulation reaction of 2-enoyl pyridine N-oxide and benzyl methyl ketone.
Analogous consideration of the possible transformation of product II leads to the final formation of compound IV. The formation of products II and IV seem rational, although compound III seems to be a slightly more likely reaction product due to the preferential formation of compound I during the Michael addition. These considerations seemed to be reflected in the 1H NMR spectra. Figure 2 shows the crucial fragment of the 1H NMR spectrum of the isolated final product. Analysis of signals and respective measured coupling constants allows for an easy identification of its structure. It can be concluded that the first stage, (i.e., Michael addition), is completely regioselective, taking into account the structure of the ketone and the carbon atom with which it is attached to the β carbon of the enone system. This reaction also proceeds completely diastereoselectively, leading finally to a cyclic product with phenyl substituents in the trans position (equatorial–equatorial). Compound III was identified based on the characteristic peak at 6.49 ppm, originating from the hydrogen of the =CH group. The signal with a similar shift was also found in cyclohex-2-enones described in the previous work [26]. For analogous products obtained in the reaction of chalcone with acetone or 2-butanone, a similar signal appeared at 6.52 or 6.54 ppm, respectively [32].
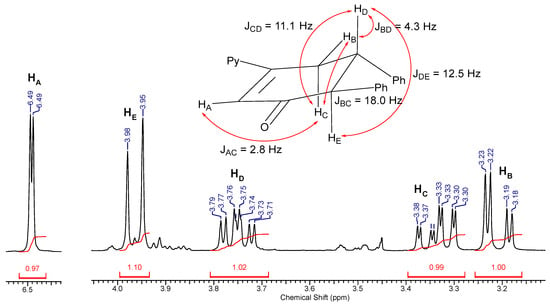
Figure 2.
Identification of product III by 1H NMR analysis.
An in-depth analysis of the 1H NMR spectrum (Figure 2) in terms of multiplets and coupling constants in the alicyclic region confirmed our predictions. From the one side, the received doublet from the proton marked as HE has the coupling constant JDE = 12.5 Hz (compared to the 13.4 Hz described for the trans product obtained from chalcone [32]). Such a high value might be observed only for vicinal protons in axial positions. On the other hand, the doublet observed from the proton marked as HA would not occur in the previously considered compound IV. Results of the performed KS-DFT calculations were in agreement with these findings. The large difference in the value of the free energy between compounds I and II (13.2 kcal/mol) suggests that the formation of compound II (and in consequence compound IV) is unlikely. There is also a relatively large difference in value between compounds III and IV (2.3 kcal/mol). Moreover, in the experiment that ended prior to conducting dehydration, we isolated a hydroxy compound (V) with multiplet pattern in 1H NMR corresponding to the aldol structure (see Figure S2). The transition state proposed for Michael addition seems to justify well our final result (see Figure 3b).
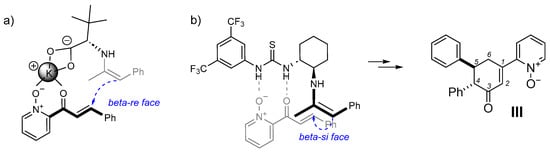
Figure 3.
Postulated stereochemical model of the transition state responsible for the stereocontrolled course of the reaction: (a) for amino acid salts applied as catalysts (according to lit. [26]), (b) for a catalyst with a thiourea fragment (this work).
The obtained product III has two phenyl groups at adjacent carbon atoms in the resulting cyclohex-2-enone on opposite sides of the formed ring (trans isomer: (R, R) or (S, S)). We did not observe the formation of a cis stereoisomer with two spatially large phenyl groups located in close proximity. Moreover, the molecular calculations support our conclusion. According to calculations in KS-DFT, both trans isomers have the same free energies of optimized molecular geometries, which is lower than the free energies of cis isomers ((R, S) or (S, R), see Table S1). There are probably steric barriers preventing the formation of cis isomers. Additionally, there are interactions between functional groups and catalysts that may prevent the formulation of cis isomers and support the formulation of trans isomers. Compared to the reaction catalyzed by amino acid salts, the product obtained was the opposite enantiomer when thiourea catalyst B was used (Figures S7 and S10). The absolute configuration of the main isomer was not determined because the product neither formed crystals suitable for X-ray analysis nor was obtained in a sufficiently enantiomerically enriched form. Using Wang’s experience, we can only speculate that the major enantiomer has the absolute configuration (4S, 5S), since in the case of the described reaction with acetone, the newly formed stereogenic center has the R configuration (see Figure 3a) [26]. The attack of the nucleophile on carbon β in the Michael reaction takes place from different sides: re in the transition state postulated by Wang, and si in the case of the thiourea catalyst we used. The lack of activity in the reaction of quinine-containing catalysts with thiourea or square-amide motifs (A and C respectively) can be explained by the presence in their structure of the tertiary nitrogen atom, which is the alkaline center, but cannot take part in enamine activation. Surprisingly, the catalyst D with a mefloquine fragment, i.e., having a secondary (analogous to proline) nitrogen atom in its structure, was also inactive in this reaction.
We have also conducted experiments with butanone as a carbonyl substrate in the presence of racemic Phe-K salt or compounds B or D applied as organocatalysts. The reaction progress was not observed using amino acid salt, and only traces of the expected product might have been found in the mass spectrum after the reaction was catalyzed by B or D. Moreover, the measured 1H NMR spectra were complex, and we identified mainly butanone and/or applied organocatalysts in the post-reaction mixture, instead of the desired product.
The obtained results show that the catalyst effective in the formal reaction [3 + 3] annulation should have a primary amino group apart from the hydrogen bond-forming moiety. Similar requirements for catalysts have been observed in analogous transformations of chalcones [34,35]. The preliminary research results are promising due to the regio- and diastereoselective course of the reaction, leading to a compound with a complex structure, having two stereogenic centers.
5. Conclusions
The obtained results confirm the possibility of using BMK for the [3 + 3] annulation reaction of (E)-2-(3-phenylacryloyl)pyridine N-oxide, although the obtained yields were not high. It was possible to achieve the formation of a trisubstituted cyclohexenone with two stereogenic centers in a regio- and diastereoselective manner. Salts of amino acids (L-Phe-K, L-tert-Leu-K) and a thiourea derivative of chiral diaminocyclohexane were used as organocatalysts. Depending on the type of catalyst applied, opposite enantiomers of the trans-diphenylsubstituted product were obtained ((R, R) or (S, S)) up to 60% ee. A thorough analysis of 1H NMR spectra, supported by the results of the performed KS-DFT calculations, allowed us to identify the structure of the product being formed and propose a transition state responsible for the stereoselectivity of the process. Although an enamine activation mechanism is postulated in the reaction, catalysts containing a secondary amine fragment did not work. To date, no such comparative experiments have been presented regarding the use of the presented types of organocatalysts in the annulation reaction. The results obtained may in the future help in selecting the appropriate catalyst to obtain the desired product stereochemistry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sym16010104/s1, Figure S1: 1H and 13C NMR spectra of 2-(5-oxo-3,4-diphenyl-hexanoyl)pyridine 1-oxide—I; Figure S2: 1H and 13C NMR spectra of 2-(1-hydroxy-3-oxo-4,5-diphenyl-cyclohexyl)pyridine 1-oxide—V; Figure S3: 1H and 13C NMR spectra of 2-(3-oxo-4,5-diphenyl-cyclohex-1-en-yl)pyridine 1-oxide—III; Figure S4: HPLC chromatogram of the product obtained with catalyst D/L-Phe-K (racemate); Figure S5: HPLC chromatogram of the product obtained with catalyst L-Phe-K (18% ee); Figure S6: HPLC chromatogram of the product obtained with catalyst L-Pro-K (8% ee); Figure S7: HPLC chromatogram of the product obtained with catalyst L-tert-Leu-K (40% ee); Figure S8: HPLC chromatogram of the product obtained with catalyst L-Phe-K (in 1,2-dichloroethane, 3% ee); Figure S9: HPLC chromatogram of the product obtained with catalyst L-Phe-Na (3% ee); Figure S10: HPLC chromatogram of the product obtained with thiourea catalyst B (60% ee), [α]D20 = −12.4 (c 1.0, CHCl3); Figure S11: 3D structures of optimized geometries of diastereomers of compound III; Table S1: Values of relative free energies between main compounds.
Author Contributions
Conceptualization, R.S. and Z.W.; methodology, R.S.; software, Z.W.; validation, R.S. and Z.W.; formal analysis, R.S.; investigation, Z.W.; resources, R.S. and Z.W.; data curation, Z.W.; writing—original draft preparation, R.S. and Z.W.; writing—review and editing, R.S.; visualization, R.S. and Z.W. supervision, R.S.; project administration, R.S.; funding acquisition, R.S.; KS-DFT calculations and interpretations J.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support given by the Polish Ministry of Science and Higher Education through subvention activity for the Faculty of Chemistry at Wrocław University of Science and Technology and Computational grant from Wrocław Centre of Networking and Supercomputing (WCSS) is also gratefully acknowledged.
Data Availability Statement
The data are available on request from the corresponding author.
Acknowledgments
The authors thank Przemysław Boratyński and Rafał Kowalczyk from WUSiT for lending hydrogen-donor compounds to be tested as catalysts (https://doi.org/10.1021/acs.joc.3c01791, accessed on 9 December 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Youssif, S. Recent trends in the chemistry of pyridine N-oxides. Arkivoc 2001, 1, 242–268. [Google Scholar] [CrossRef]
- Gonzalez-Bello, C.; Castedo, L. Six-Membered Heterocycles: Pyridines in Modern Heterocyclic Chemistry; Alvarez-Builla, J., Vaquero, J.J., Barluenga, J., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 1431–1525. [Google Scholar]
- Ishuichi, K.; Kobota, T.; Ishiyama, H.; Hayashi, S.; Shibata, T.; Mori, K.; Obara, Y.; Nakahata, N.; Kobayashi, J. Lyconadins D and E, and complanadine E, new Lycopodium alkaloids from Lycopodium complanatum. Bioorg. Med. Chem. 2011, 19, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fu, Y.; Xiong, J.; Li, M.; Ma, G.-L.; Yang, G.-X.; Wei, B.-G.; Zhao, Y.; Zhang, H.-Y.; Hu, J.-F. Casuarinines A–J, Lycodine-Type Alkaloids from Lycopodiastrum casuarinoides. J. Nat. Prod. 2013, 76, 1475–1484. [Google Scholar] [CrossRef]
- Li, D.; Wu, P.; Sun, N.; Lu, Y.-J.; Wong, W.-L.; Fang, Z.; Zhang, K. The Diversity of Heterocyclic N-oxide Molecules: Highlights on their Potential in Organic Synthesis, Catalysis and Drug Applications. Curr. Org. Chem. 2019, 23, 616–627. [Google Scholar] [CrossRef]
- Larionov, O.V.; Stephens, D.; Mfuh, A.M.; Arman, H.D.; Naumova, A.S.; Chavez, G.; Skender, B. Insights into the mechanistic and synthetic aspects of the Mo/P-catalyzed oxidation of N-heterocycles. Org. Biomol. Chem. 2014, 12, 3026–3036. [Google Scholar] [CrossRef]
- Limnios, D.; Kokotos, C.G. 2,2,2-Trifluoroacetophenone as an Organocatalyst for the Oxidation of Tertiary Amines and Azines to N-Oxides. Chem. Eur. J. 2014, 20, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Hauptmann, R.; Pospech, J. Heteroarene N-Oxides as Oxygen Source in Organic Reactions. Eur. J. Org. Chem. 2018, 38, 5237–5252. [Google Scholar] [CrossRef]
- Chelucci, G.; Murineddu, G.; Pinna, G.A. Chiral pyridine N-oxides: Useful ligands for asymmetric catalysis. Tetrahedron Asymmetry 2004, 15, 1373–1389. [Google Scholar] [CrossRef]
- Koukal, P.; Ulč, J.; Nečas, D.; Kotora, M. Pyridine N-Oxides and Derivatives Thereof in Organocatalysis. In Heterocyclic N-Oxides. Topics in Heterocyclic Chemistry; Larionov, O., Ed.; Springer: Cham, Switzerland, 2017; Volume 53, pp. 29–58. [Google Scholar]
- Wrzeszcz, Z.; Siedlecka, R. Heteroaromatic N-Oxides in Asymmetric Catalysis: A Review. Molecules 2020, 25, 330. [Google Scholar] [CrossRef]
- Habib, I.; Singha, K.; Hossain, M. Recent Progress on Pyridine N-Oxide in Organic Transformations: A Review. ChemistrySelect 2023, 8, e202204099. [Google Scholar] [CrossRef]
- Anugu, N.; Thunga, S.; Poshala, S.; Kokatla, H.P. N-Oxide-Induced Ugi Reaction: A Rapid Access to Quinoline-C2-amino Amides via Deoxygenative C(sp2)-H Functionalization. J. Org. Chem. 2022, 87, 10435–10440. [Google Scholar] [CrossRef] [PubMed]
- Váňa, J.; Roithová, J.; Kotora, M.; Beran, P.; Rulíšek, L.; Kočovský, P. Proton Affnities of Organocatalysts Derived from Pyridine N-oxide. Croat. Chem. Acta 2014, 87, 349–356. [Google Scholar] [CrossRef]
- Ray, S.; Singh, P.; Molleti, N.; Singh, V. Enantioselective synthesis of coumarin derivatives by PYBOX-DIPH-Zn (II) complex catalyzed Michael reaction. J. Org. Chem. 2012, 77, 8802–8808. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Rout, S.; Singh, V. Enantioselective synthesis of 3, 4-dihydropyran derivatives via a Michael addition reaction catalysed by chiral pybox–diph–Zn (II) complex. Org. Biomol. Chem. 2013, 11, 2412–2416. [Google Scholar] [CrossRef]
- Desimoni, G.; Faita, G.; Quadrelli, P. Enantioselectively-Catalyzed Reactions with (E)-2-Alkenoyl-pyridines, Their N-Oxides, and the Corresponding Chalcones. Chem. Rev. 2014, 114, 6081–6129. [Google Scholar] [CrossRef]
- Kutasevich, A.V.; Perevalov, V.P.; Mityanov, V.S. Recent Progress in Non-Catalytic C–H Functionalization of Heterocyclic N-Oxides. Eur. J. Org. Chem. 2021, 2021, 357–373. [Google Scholar] [CrossRef]
- Wang, D.; Désaubry, L.; Li, G.; Huang, M.; Zheng, S. Recent Advances in the Synthesis of C2-Functionalized Pyridines and Quinolines Using N-Oxide Chemistry. Adv. Synth. Catal. 2021, 363, 2–39. [Google Scholar] [CrossRef]
- Wang, N.; Wu, Z.; Wang, J.; Ullah, N.; Lu, Y. Recent applications of asymmetric organocatalytic annulation reactions in natural product synthesis. Chem. Soc. Rev. 2021, 50, 9766–9793. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, C.; Zhao, G. Asymmetric Robinson-Type Annulation Reaction between β-Ketoamides and α,β-Unsaturated Ketones. J. Org. Chem. 2015, 80, 3798–3805. [Google Scholar] [CrossRef]
- Feng, J.; Liu, B. Formal carbo [3 + 3] annulation and its application in organic synthesis. Tetrahedron Lett. 2015, 56, 1474–1485. [Google Scholar] [CrossRef][Green Version]
- Inokoishi, Y.; Sasakura, N.; Nakano, K.; Ichikawa, Y.; Kotsuki, H. A New Powerful Strategy for the Organocatalytic Asymmetric Construction of a Quaternary Carbon Stereogenic Center. Org. Lett. 2010, 12, 1616–1619. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Ray, S.K.; Unhale, R.A.; Singh, V.K. Asymmetric Direct Vinylogous Michael Addition to 2-Enoylpyridine N-Oxides Catalyzed by Bifunctional Thio-Urea. Org. Lett. 2014, 16, 5568–5571. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Wu, Z.-J.; Huang, X.-Q.; Yue, D.-F.; You, Y.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Diastereo-and enantioselective direct vinylogous Michael addition of γ-substituted butenolides to 2-enoylpyridines catalyzed by chiral bifunctional amine-squaramides. Chem. Commun. 2015, 51, 15835–15838. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, S.; Li, L.; Wang, Y.; Zha, Z.; Wang, Z. L-Phenylalanine Potassium Catalyzed Asymmetric Formal [3 + 3] Annulation of 2-enoyl-Pyridine N-Oxides with Acetone. Org. Chem. Front. 2018, 5, 376–379. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, X.; Liu, C.; Li, W.; Li, P. Enantioselective Construction of Pyridine N-Oxides Featuring 2,3-Dihydrofuran Motifs via Phosphine-Catalyzed [4 + 1]-Annulation of 2-Enoylpyridine N-Oxides with Morita-Baylis-Hillman Carbonates. Org. Lett. 2019, 21, 152–155. [Google Scholar] [CrossRef]
- Zhong, J.; Long, Y.; Yan, X. Rhodium-Catalyzed Pyridine N-Oxide Assisted Suzuki–Miyaura Coupling Reaction via C(O)–C Bond Activation. Org. Lett. 2019, 21, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Barroso, S.; Blay, G.; Pedro, J.R. 2-Alkenoyl Pyridine N -Oxides, Highly Efficient Dienophiles for the Enantioselective Cu(II)-Bis(oxazoline) Catalyzed Diels-Alder Reaction. Org. Lett. 2007, 9, 1983–1986. [Google Scholar] [CrossRef]
- Cao, C.-L.; Sun, X.-L.; Kang, Y.-B.; Tang, Y. Annulation for the Direct Construction of Bicyclic Skeletons with Four Stereogenic Centers. Org. Lett. 2007, 9, 4151–4154. [Google Scholar] [CrossRef]
- Cao, C.-L.; Zhou, Y.-Y.; Zhou, J.; Sun, X.-L.; Tang, Y.; Li, Y.-X.; Li, G.-Y.; Sun, J. An Organocatalytic Asymmetric Tandem Reaction for the Construction of Bicyclic Skeletons. Chem. Euro. J 2009, 15, 11384–11389. [Google Scholar] [CrossRef] [PubMed]
- Wagh, S.J.; Dhage, G.R. Primary-Secondary Diamine Catalyzed Enantioselective Synthesis of Substituted Cyclohex-2-enones by Cascade Michael–Aldol–Dehydration of Ketones with Chalcones. Synlett 2017, 28, 1353–1357. [Google Scholar] [CrossRef][Green Version]
- Xie, J.-K.; Wang, Y.; Lin, J.-B.; Ren, X.-R.; Xu, P.-F. Direct Noncovalent Activation of α, β-Unsaturated Aldehydes for the Stereodivergent Synthesis of Substituted Cyclohexenes. Chem. Eur. J. 2017, 23, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Luo, Y.; Xue, J.; He, Y.; Guan, Z. Highly enantioselective Michael-aldol-dehydration reaction for the synthesis of chiral 3,5-diaryl-cyclohexenones catalyzed by primary amine. Tetrahedron 2017, 73, 1114–1119. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Chai, Z.; Wang, H.-F.; Chen, X.-K.; Hai-Feng Cui, H.-F.; Zheng, C.-W.; Xiao, H.; Li, P.; Zhao, G. Chiral Primary–Secondary Diamines Catalyzed Michael–Aldol–Dehydration Reaction between Benzoylacetates and α,β-Unsaturated Ketones: Highly Enantioselective Synthesis of Functionalized Chiral Cyclohexenones. Chem. Eur. J. 2009, 15, 13295–13298. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).