Systematic Study of Vibrational Spectra of Octahedral Rhenium Clusters {Re6S8-xBrx}Bry (x = 0, 1, 2, 3, 4) with Mixed Sulfur/Bromine Inner Ligands
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
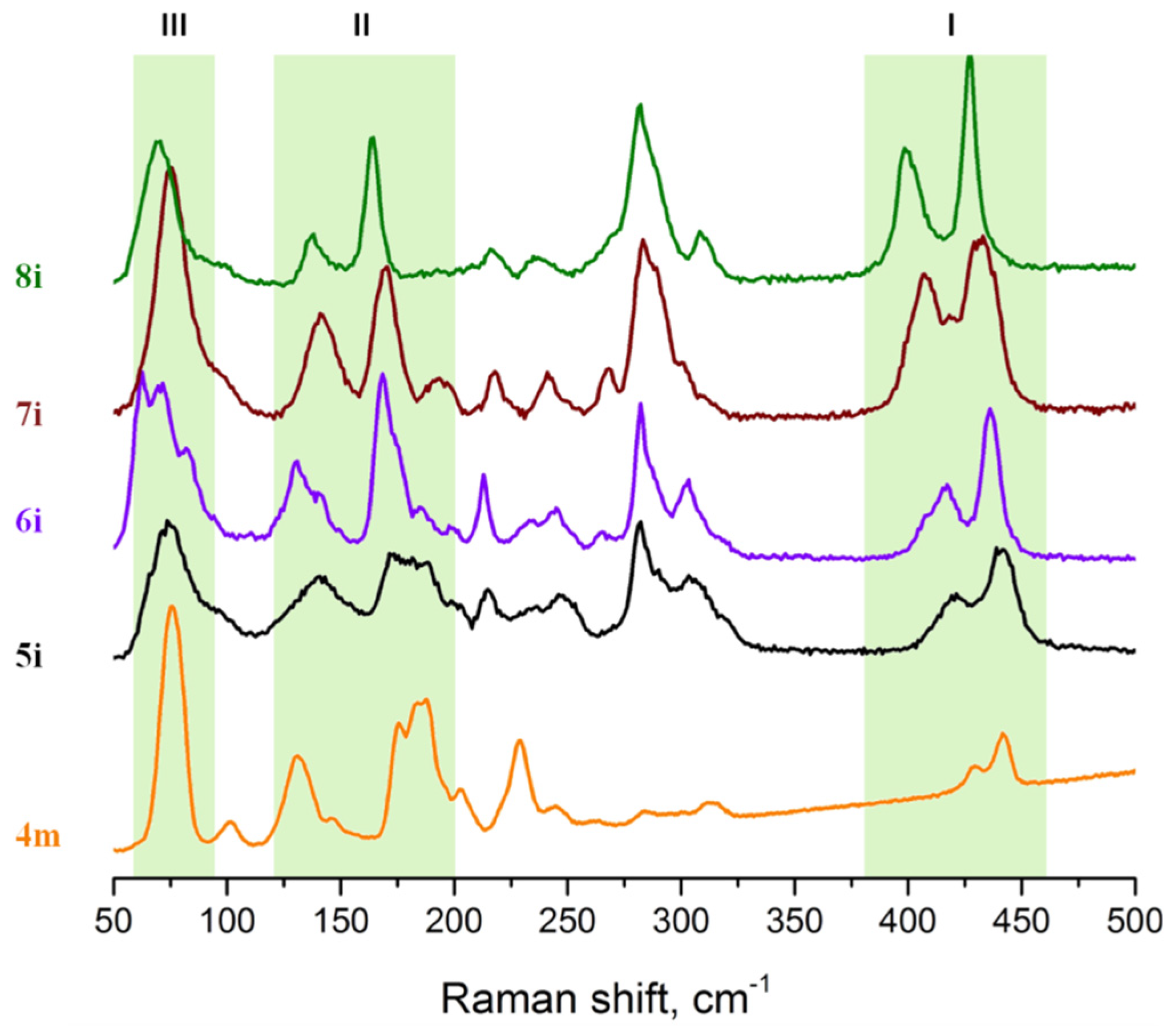
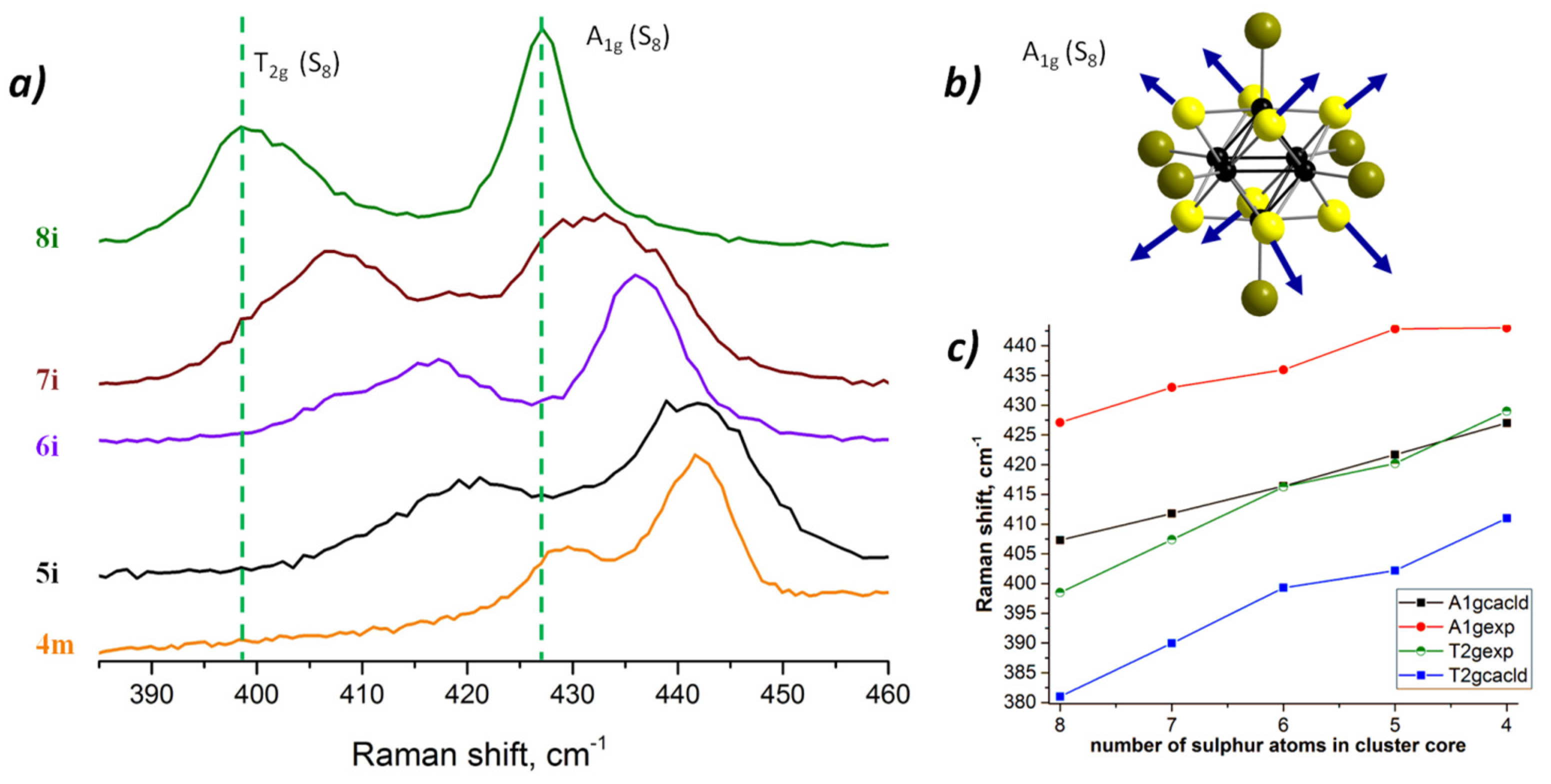
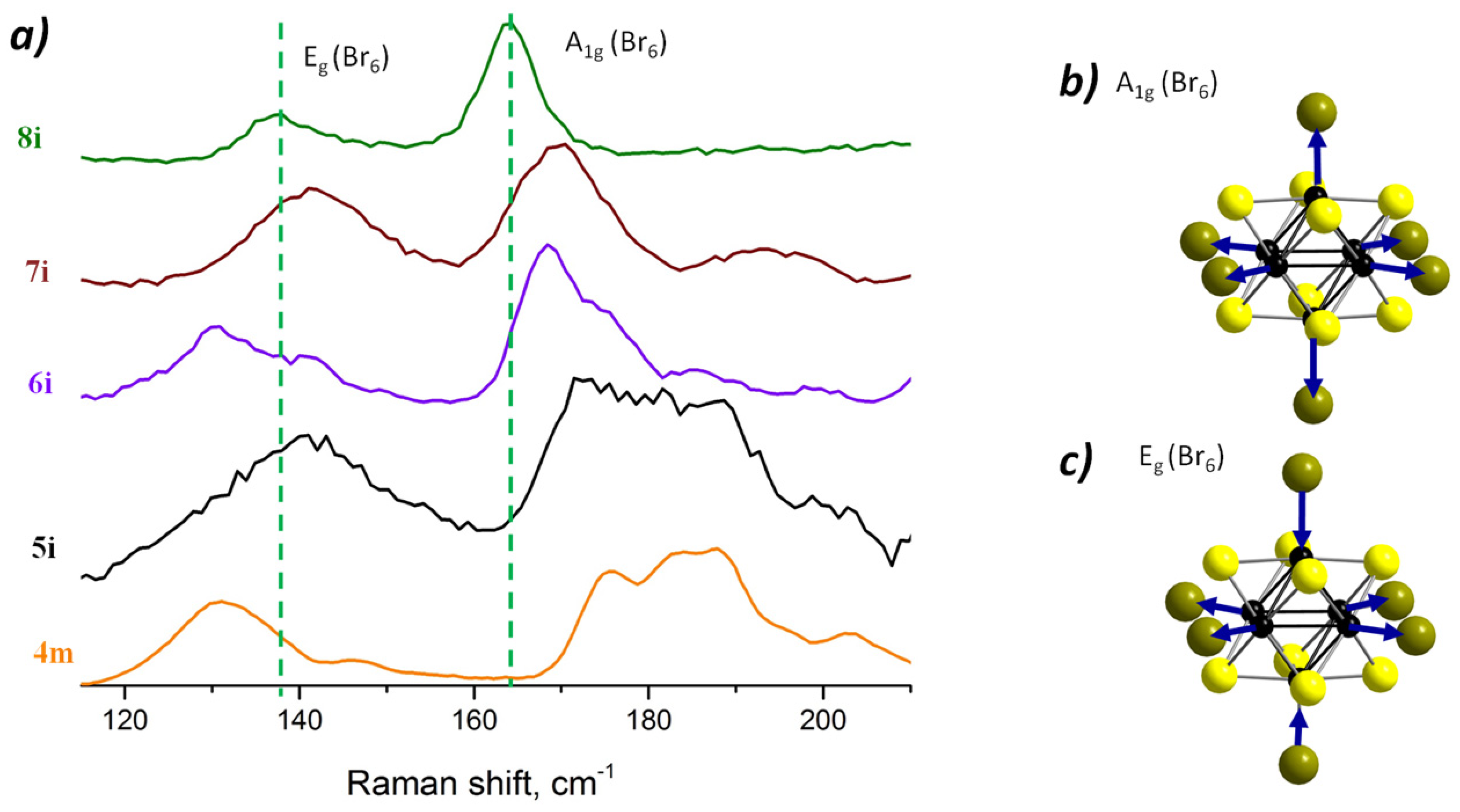
3.1. Raman Spectroscopy
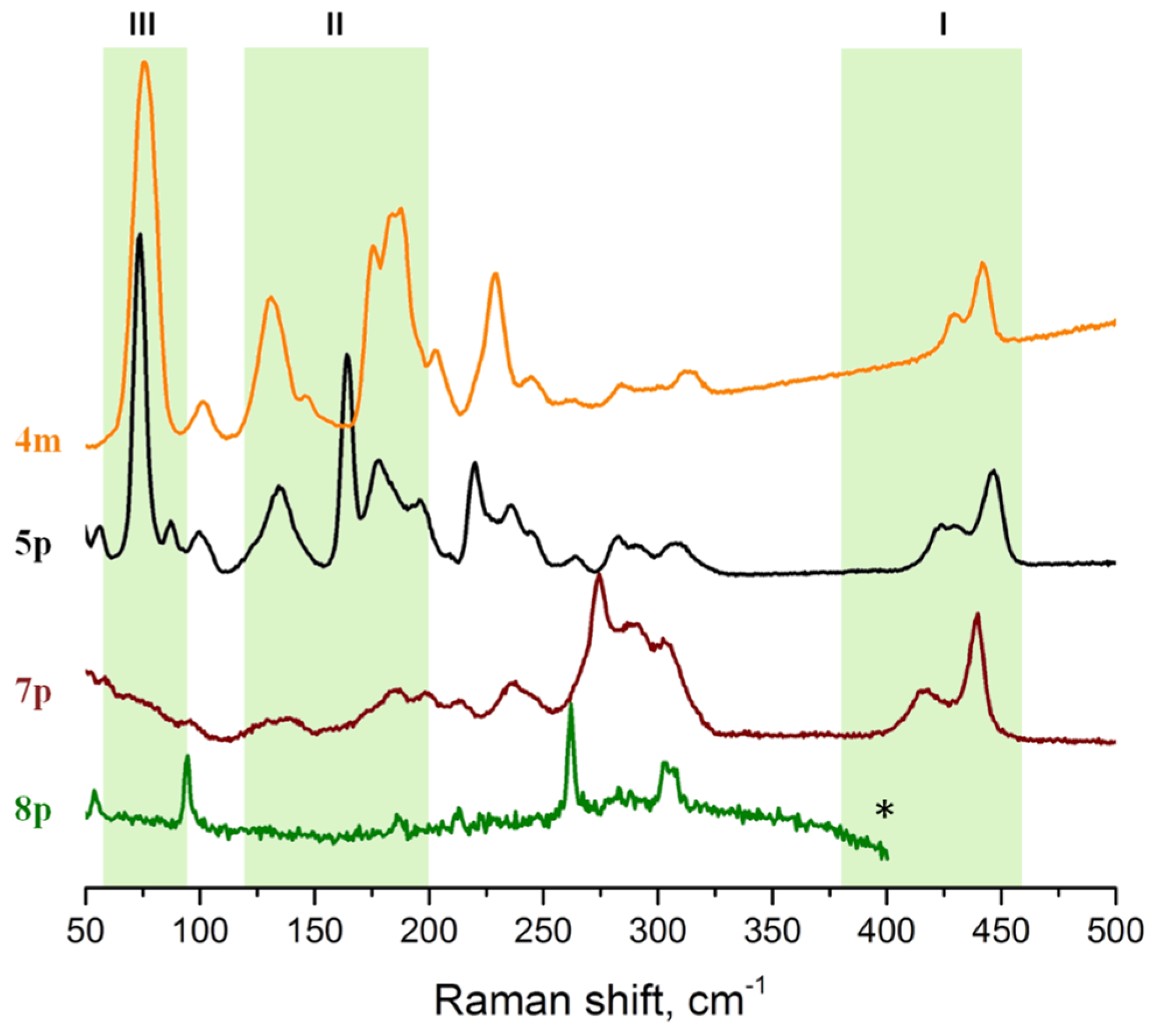
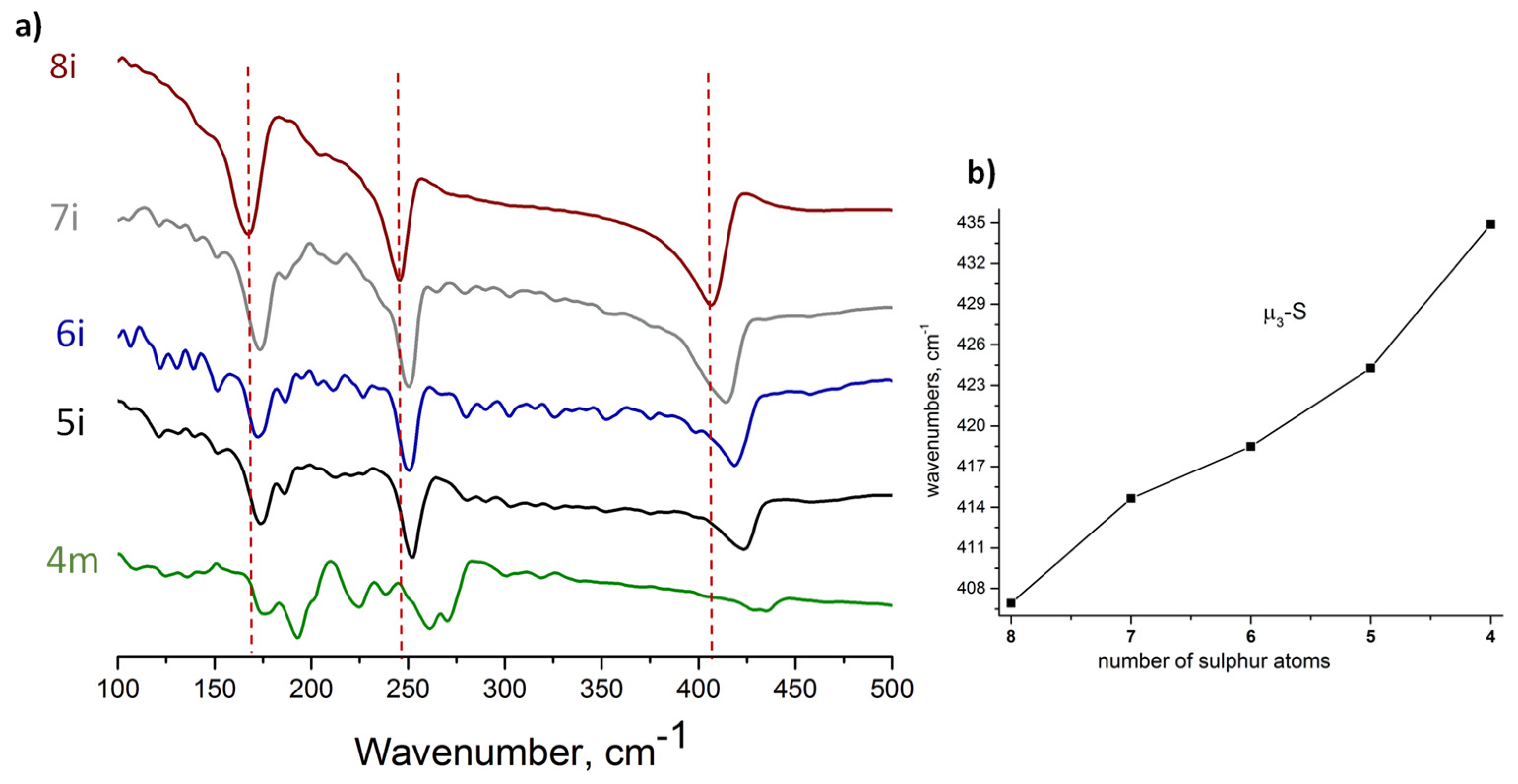
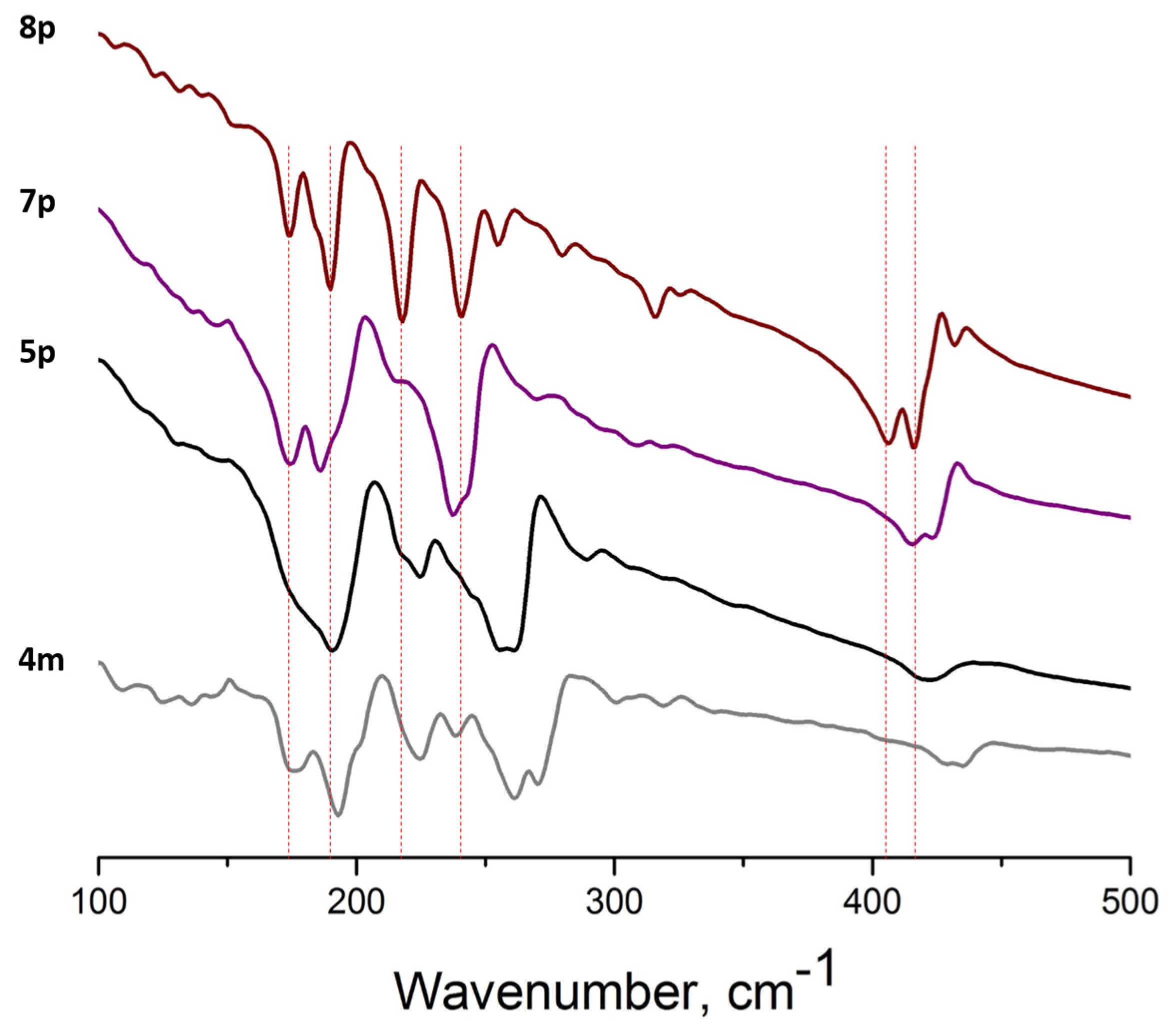
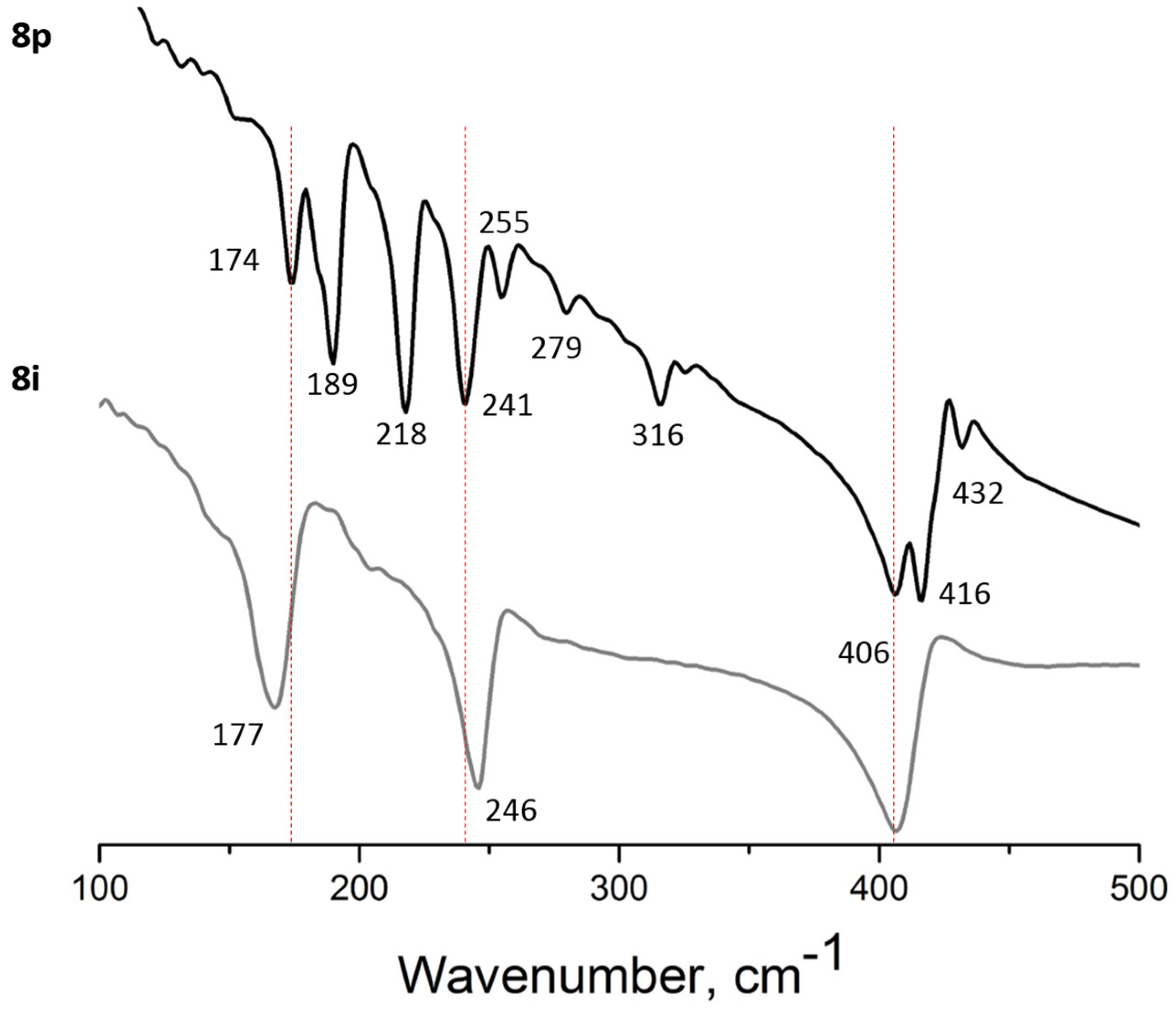
3.2. Far IR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, T.G. Hexanuclear and higher nuclearity clusters of the Groups 4–7 metals with stabilizing π-donor ligands. Coord. Chem. Rev. 2003, 243, 213–235. [Google Scholar] [CrossRef]
- Prokopuk, N.; Shriver, D.F. The Octahedral M6Y8 And M6Y12 Clusters of Group 4 and 5 Transition Metals. Adv. Inorg. Chem. 1998, 46, 1–49. [Google Scholar] [CrossRef]
- Saito, T. Rhenium sulfide cluster chemistry. J. Chem. Soc. Dalton. Trans. 1999, 97–106. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Boubekeur, K.; Uriel, S.; Batail, P. Chemistry of Hexanuclear Rhenium Chalcohalide Clusters. Chem. Rev. 2001, 101, 2037–2066. [Google Scholar] [CrossRef]
- Perrin, A.; Perrin, C. Low-Dimensional Frameworks in Solid State Chemistry of Mo6 and Re6 Cluster Chalcohalides. Eur. J. Inorg. Chem. 2011, 2011, 3848–3856. [Google Scholar] [CrossRef]
- Gray, T.G.; Rudzinski, C.M.; Meyer, E.E.; Holm, R.H.; Nocera, D.G. Spectroscopic and Photophysical Properties of Hexanuclear Rhenium(III) Chalcogenide Clusters. J. Am. Chem. Soc. 2003, 125, 4755–4770. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Ueda, Y.; Ishizaka, S.; Yamada, K.; Aniya, M.; Sasaki, Y. Temperature Dependent Emission of Hexarhenium(III) Clusters [Re6(μ3-S)8X6]4− (X = Cl−, Br−, and I−): Analysis by Four Excited Triplet-State Sublevels. Inorg. Chem. 2005, 44, 6308–6313. [Google Scholar] [CrossRef]
- Wilson, W.B.; Stark, K.; Johnson, D.B.; Ren, Y.; Ishida, H.; Cedeño, D.L.; Szczepura, L.F. Photophysical Properties of a Series of Rhenium Selenide Cluster Complexes Containing Nitrogen-Donor Ligands. Eur. J. Inorg. Chem. 2014, 2014, 2254–2261. [Google Scholar] [CrossRef]
- Efremova, O.A.; Brylev, K.A.; Kozlova, O.; White, M.S.; Shestopalov, M.A.; Kitamura, N.; Mironov, Y.V.; Bauer, S.; Sutherland, A.J. Polymerisable octahedral rhenium cluster complexes as precursors for photo/electroluminescent polymers. J. Mater. Chem. C 2014, 2, 8630–8638. [Google Scholar] [CrossRef]
- Ryzhikov, M.R.; Gayfulin, Y.M.; Ulantikov, A.A.; Arentov, D.O.; Kozlova, S.G.; Mironov, Y.V. Evolution of the Electronic Structure of the trans-[Re6S8bipy4Cl2] Octahedral Rhenium Cluster during Reduction. Molecules 2023, 28, 3658. [Google Scholar] [CrossRef]
- Tarasenko, M.S.; Naumov, N.G.; Virovets, A.V.; Naumov, D.Y.; Kuratieva, N.V.; Mironov, Y.V.; Ikorskii, V.N.; Fedorov, V.E. New coordination polymers based on paramagnetic cluster anions [Re6Se8(CN)6]3− and rare earth cations: The synthesis and structure of [{Ln(H2O)3)}{Re6Se8(CN)6}]·3.5H2O. J. Struct. Chem. 2005, 46, S137–S144. [Google Scholar] [CrossRef]
- Echeverría, C.; Becerra, A.; Nuñez-Villena, F.; Muñoz-Castro, A.; Stehberg, J.; Zheng, Z.; Arratia-Perez, R.; Simon, F.; Ramírez-Tagle, R. The paramagnetic and luminescent [Re6Se8I6]3− cluster. Its potential use as an antitumoral and biomarker agent. New J. Chem. 2012, 36, 927–932. [Google Scholar] [CrossRef]
- Ly, G.T.; Choi, J.; Kim, Y.; Kim, Y.; Kim, S.; Yang, S.-H.; Kim, S.-J. One-dimensional lead iodide hybrid stabilized by inorganic hexarhenium cluster cations as a new broad-band emitter. RSC Adv. 2021, 11, 24580–24587. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Lee, K.; Choi, B.; Meggiolaro, D.; Liu, F.; Nuckolls, C.; Pasupathy, A.; De Angelis, F.; Batail, P.; Roy, X.; et al. Superatomic Two-Dimensional Semiconductor. Nano Lett. 2018, 18, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.E.; Hughbanks, T. Electronic Transitions in [Re6S8X6]4− (X = Cl, Br, I): Results from Time-Dependent Density Functional Theory and Solid-State Calculations. Inorg. Chem. 2006, 45, 8273–8282. [Google Scholar] [CrossRef] [PubMed]
- Telford, E.J.; Russell, J.C.; Swann, J.R.; Fowler, B.; Wang, X.; Lee, K.; Zangiabadi, A.; Watanabe, K.; Taniguchi, T.; Nuckolls, C.; et al. Doping-Induced Superconductivity in the van der Waals Superatomic Crystal Re6Se8Cl2. Nano Lett. 2020, 20, 1718–1724. [Google Scholar] [CrossRef]
- Kirakci, K.; Shestopalov, M.A.; Lang, K. Recent developments on luminescent octahedral transition metal cluster complexes towards biological applications. Coord. Chem. Rev. 2023, 481, 215048. [Google Scholar] [CrossRef]
- Stass, D.V.; Vorotnikova, N.A.; Shestopalov, M.A. Direct observation of x-ray excited optical luminescence from a Re6 metal cluster in true aqueous solution: The missing link between material characterization and in vivo applications. J. Appl. Phys. 2021, 129, 183102. [Google Scholar] [CrossRef]
- Krasilnikova, A.A.; Solovieva, A.O.; Ivanov, A.A.; Trifonova, K.E.; Pozmogova, T.N.; Tsygankova, A.R.; Smolentsev, A.I.; Kretov, E.I.; Sergeevichev, D.S.; Shestopalov, M.A.; et al. Comprehensive study of hexarhenium cluster complex Na4[{Re6Te8}(CN)6]—In terms of a new promising luminescent and X-ray contrast agent. Nanomed. NBM 2017, 13, 755–763. [Google Scholar] [CrossRef]
- Molard, Y.; Dorson, F.; Brylev, K.A.; Shestopalov, M.A.; Le Gal, Y.; Cordier, S.; Mironov, Y.V.; Kitamura, N.; Perrin, C. Red-NIR Luminescent Hybrid Poly(methyl methacrylate) Containing Covalently Linked Octahedral Rhenium Metallic Clusters. Chem. Eur. J. 2010, 16, 5613–5619. [Google Scholar] [CrossRef]
- Amela-Cortes, M.; Cordier, S.; Naumov, N.G.; Mériadec, C.; Artzner, F.; Molard, Y. Hexacyano octahedral metallic clusters as versatile building blocks in the design of extended polymeric framework and clustomesogens. J. Mater. Chem. C 2014, 2, 9813–9823. [Google Scholar] [CrossRef]
- Molard, Y. Clustomesogens: Liquid Crystalline Hybrid Nanomaterials Containing Functional Metal Nanoclusters. Acc. Chem. Res. 2016, 49, 1514–1523. [Google Scholar] [CrossRef]
- Suh, M.-J.; Vien, V.; Huh, S.; Kim, Y.; Kim, S.-J. Mesolamellar Phases Containing [Re6Q8(CN)6]4– (Q = Te, Se, S) Cluster Anions. Eur. J. Inorg. Chem. 2008, 2008, 686–692. [Google Scholar] [CrossRef]
- Kubeil, M.; Stephan, H.; Pietzsch, H.-J.; Geipel, G.; Appelhans, D.; Voit, B.; Hoffmann, J.; Brutschy, B.; Mironov, Y.V.; Brylev, K.A.; et al. Sugar-Decorated Dendritic Nanocarriers: Encapsulation and Release of the Octahedral Rhenium Cluster Complex [Re6S8(OH)6]4−. Chem. Asian J. 2010, 5, 2507–2514. [Google Scholar] [CrossRef]
- Nguyen, N.T.K.; Lebastard, C.; Wilmet, M.; Dumait, N.; Renaud, A.; Cordier, S.; Ohashi, N.; Uchikoshi, T.; Grasset, F. A review on functional nanoarchitectonics nanocomposites based on octahedral metal atom clusters (Nb6, Mo6, Ta6, W6, Re6): Inorganic 0D and 2D powders and films. Sci. Technol. Adv. Mater. 2022, 23, 547–578. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Evans, A.M.; Meirzadeh, E.; Han, S.Y.; Russell, J.C.; Wiscons, R.A.; Bartholomew, A.K.; Reed, D.A.; Zangiabadi, A.; Steigerwald, M.L.; et al. Site-Selective Surface Modification of 2D Superatomic Re6Se8. J. Am. Chem. Soc. 2022, 144, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Cordier, S.; Fabre, B.; Molard, Y.; Fadjie-Djomkam, A.B.; Tournerie, N.; Ledneva, A.; Naumov, N.G.; Moreac, A.; Turban, P.; Tricot, S.; et al. Covalent Anchoring of Re6Se8i Cluster Cores Mono layers on Modified n- and p-Type Si(111) Surfaces: Effect of Coverage on Electronic Properties. J. Phys. Chem. C 2010, 114, 18622–18633. [Google Scholar] [CrossRef]
- Shores, M.P.; Beauvais, L.G.; Long, J.R. Cluster-Expanded Prussian Blue Analogues. J. Am. Chem. Soc 1999, 121, 775–779. [Google Scholar] [CrossRef]
- Beauvais, L.G.; Shores, M.P.; Long, J.R. Cyano-Bridged Re6Q8 (Q = S, Se) Cluster-Metal Framework Solids: A New Class of Porous Materials. Chem. Mater. 1998, 10, 3783–3786. [Google Scholar] [CrossRef]
- Fedorov, V.E.; Naumov, N.G.; Mironov, Y.V.; Virovets, A.V.; Artemkina, S.B.; Efremova, O.A.; Peak, U.-H. Inorganic coordination polymers based on chalcocyanyde cluster complexes. J. Struct. Chem. 2002, 43, 669–684. [Google Scholar] [CrossRef]
- Cordier, S.; Molard, Y.; Brylev, K.A.; Mironov, Y.V.; Grasset, F.; Fabre, B.; Naumov, N.G. Advances in the Engineering of Near Infrared Emitting Liquid Crystals and Copolymers, Extended Porous Frameworks, Theranostic Tools and Molecular Junctions Using Tailored Re6 Cluster Building Blocks. J. Clust. Sci. 2015, 26, 53–81. [Google Scholar] [CrossRef]
- Cheplakova, A.M.; Kovalenko, K.A.; Shestopalov, M.A.; Brylev, K.A.; Fedin, V.P. Rhenium octahedral clusters in mesoporous MIL-101: Luminescence and sorption properties. Russ. Chem. Bull. 2014, 63, 1487–1492. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Falaise, C.; Shmakova, A.A.; Leclerc, N.; Cordier, S.; Molard, Y.; Mironov, Y.V.; Shestopalov, M.A.; Abramov, P.A.; Sokolov, M.N.; et al. Cyclodextrin-Assisted Hierarchical Aggregation of Dawson-type Polyoxometalate in the Presence of {Re6Se8} Based Clusters. Inorg. Chem. 2020, 59, 11396–11406. [Google Scholar] [CrossRef] [PubMed]
- Selby, H.D.; Roland, B.K.; Zheng, Z. Ligand-Bridged Oligomeric and Supramolecular Arrays of the Hexanuclear Rhenium Selenide Clusters−Exploratory Synthesis, Structural Characterization, and Property Investigation. Acc. Chem. Res. 2003, 36, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Selby, H.D.; Zheng, Z. New directions of cluster chemistry—The story of the [Re6(μ3-Se)8]2+ clusters. Comments Inorg. Chem. 2005, 26, 75–102. [Google Scholar] [CrossRef]
- Xie, J.; Wang, L.; Anderson, J.S. Heavy chalcogenide-transition metal clusters as coordination polymer nodes. Chem. Sci. 2020, 11, 8350–8372. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, Y.M.; Gayfulin, Y.M.; Kovalenko, K.A.; Samsonenko, D.G.; van Leusen, J.; Korolkov, I.V.; Fedin, V.P.; Mironov, Y.V. Multifunctional Metal–Organic Frameworks Based on Redox-Active Rhenium Octahedral Clusters. Inorg. Chem. 2018, 57, 2072–2084. [Google Scholar] [CrossRef]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- van Lenthe, E.; Ehlers, A.; Baerends, E.-J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Slougui, A.; Perrin, A.; Sergent, M. Structure of potassium hexarhenium nonabromide pentasulfide: KRe6S5Br9. Acta Cryst. Sect. C 1992, 48, 1917–1920. [Google Scholar] [CrossRef]
- Slougui, A.; Ferron, S.; Perrin, A.; Sergent, M. Anionic charge effects on the crystal structures of Re6 octahedral cluster based compounds: The structure of the cubic bromide K2Re6S6Br8. J. Clust. Sci. 1997, 8, 349–359. [Google Scholar] [CrossRef]
- Solodovnikov, S.F.; Yarovoi, S.S.; Mironov, Y.V.; Vironets, A.V.; Fedorov, V.E. Unusual Disordering of Potassium Ions in the Structures of Cluster Rhenium Thiohalides K3[Re6S7Br7] and K4[Re6S8Cl6]. J. Struct. Chem. 2004, 45, 865–873. [Google Scholar] [CrossRef]
- Pilet, G.; Cordier, S.; Perrin, C.; Perrin, A. Single-crystal structure of Cs2Re6S6Br8 rhenium thiobromide with acentric Re6 octahedral cluster units. J. Struct. Chem. 2007, 48, 680–689. [Google Scholar] [CrossRef]
- Leduc, L.; Perrin, A.; Sergent, M.; Le Traon, F.; Pilet, J.C.; Le Traon, A. Rhenium octahedral clusters: Characterization of Re6Se4Cl10 and the parent compound Re6Se4Br10. Mater. Lett. 1985, 3, 209–215. [Google Scholar] [CrossRef]
- Perricone, A.; Slougui, A.; Perrin, A. Rhenium octahedral clusters: The systems Re-S-Br and M-Re-S-Br (M = Na, K, RB, Cs). Solid State Sci. 1999, 1, 657–666. [Google Scholar] [CrossRef]
- Perrin, A.; Leduc, L.; Sergent, M. Halogen bridged Re8L8 units in octahedral cluster rhenium chalcohalides. Eur. J. Solid State Inorg. Chem 1991, 28, 919–931. [Google Scholar]
- Fischer, C.; Alonso-Vante, N.; Fiechter, S.; Tributsch, H.; Reck, G.; Schulz, W. Structure and photoelectrochemical properties of semiconducting rhenium cluster chalcogenides: Re6X8Br2 (X = S, Se). J. Alloys Compd. 1992, 178, 305–314. [Google Scholar] [CrossRef]
- Schäfer, H.; Schnering, H.G.v. Metall-Metall-Bindungen bei niederen Halogeniden, Oxyden und Oxydhalogeniden schwerer Übergangsmetalle Thermochemische und strukturelle Prinzipien. Angew. Chem. 1964, 76, 833–849. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuda, A.; Ito, Y.; Ishizaka, S.; Shinoda, S.; Tsukube, H.; Kitamura, N.; Shinohara, A. Photoluminescent Properties of Chalcobromide−capped Octahedral Hexarhenium(III) Complexes [{Re6Q8−nBrn}Br6]n−4 (Q = Se, n = 1−3; Q = S, n = 1, 2). Inorg. Chem. 2010, 49, 3473–3481. [Google Scholar] [CrossRef]
- Yarovoi, S.S.; Solodovnikov, S.F.; Tkachev, S.V.; Mironov, Y.V.; Fedorov, V.E. Synthesis, structure, and 77Se NMR study of the (PPh4)2[Re6Se6Br8] complex. Russ. Chem. Bull. 2003, 52, 68–72. [Google Scholar] [CrossRef]
- Griffith, W.P.; Kiernan, P.M.; O’Hare, B.P.; Brégeault, J.M. Vibrational spectra of the [Re(CN)7]4−, [V(CN)7]4−, [Nb(CN)6]4−, [Re4S4(CN)12]4− and [Re4Se4(CN)12]4− ions. J. Mol. Struct. 1978, 46, 307–317. [Google Scholar] [CrossRef]
| Polymer Structure | Ionic Structure | Isomers (Inner S/Br Positions) |
|---|---|---|
| x = 8 | ||
| Re6S8Br2 (8p) [{Re6Si6Si–a2/2}S2/2a–iBra–a4/2] * 3D polymer | Cs4Re6S8Br6 (8i) Cs4{Re6Si8}Bra6 |  |
| x = 7 | ||
| Re6S7Br4 (7p) {Re6Si7Bri}Bra6/2 3D polymer | Cs3Re6S7Br7 (7i) Cs3{Re6S7iBri}Bra6 |  |
| x = 6 | ||
| – | M2Re6S6Br8 (M = Cs or K) (6i) M2{Re6Si6Bri2}Bra6 |  |
| x = 5 | ||
| Re6S5Br8 (5p) {Re6Si5Bri3}Bra4Bra2/2 1D chains | KRe6S5Br9 (5i) K{Re6Si5Bri3}Bra6 | 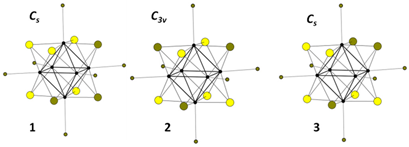 |
| x = 4 | ||
| Re6S4Br10 (4m) {Re6Si4Bri4}Bra6 Neutral molecules |  | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledneva, A.Y.; Ivanova, M.N.; Poltarak, P.A.; Yarovoy, S.S.; Kolesov, B.A.; Fedorov, V.E.; Naumov, N.G. Systematic Study of Vibrational Spectra of Octahedral Rhenium Clusters {Re6S8-xBrx}Bry (x = 0, 1, 2, 3, 4) with Mixed Sulfur/Bromine Inner Ligands. Symmetry 2023, 15, 1791. https://doi.org/10.3390/sym15091791
Ledneva AY, Ivanova MN, Poltarak PA, Yarovoy SS, Kolesov BA, Fedorov VE, Naumov NG. Systematic Study of Vibrational Spectra of Octahedral Rhenium Clusters {Re6S8-xBrx}Bry (x = 0, 1, 2, 3, 4) with Mixed Sulfur/Bromine Inner Ligands. Symmetry. 2023; 15(9):1791. https://doi.org/10.3390/sym15091791
Chicago/Turabian StyleLedneva, Alexandra Yu., Mariia N. Ivanova, Pavel A. Poltarak, Spartak S. Yarovoy, Boris A. Kolesov, Vladimir E. Fedorov, and Nikolay G. Naumov. 2023. "Systematic Study of Vibrational Spectra of Octahedral Rhenium Clusters {Re6S8-xBrx}Bry (x = 0, 1, 2, 3, 4) with Mixed Sulfur/Bromine Inner Ligands" Symmetry 15, no. 9: 1791. https://doi.org/10.3390/sym15091791
APA StyleLedneva, A. Y., Ivanova, M. N., Poltarak, P. A., Yarovoy, S. S., Kolesov, B. A., Fedorov, V. E., & Naumov, N. G. (2023). Systematic Study of Vibrational Spectra of Octahedral Rhenium Clusters {Re6S8-xBrx}Bry (x = 0, 1, 2, 3, 4) with Mixed Sulfur/Bromine Inner Ligands. Symmetry, 15(9), 1791. https://doi.org/10.3390/sym15091791







