Abstract
Prefrontal cortex activity facilitates emotion regulation by cognitive reappraisal. Yet, neuroimaging studies have yielded disparate findings as to whether relatively greater left or right prefrontal activity is more beneficial for reappraisal success. We argue that differences in hemispheric activation during reappraisal efforts may depend on the specific and diverse cognitive strategies utilized to reappraise negative stimuli. In this EEG study, n = 94 participants were randomly assigned to three groups and instructed to either generate problem-oriented reappraisals, positive reinterpretations, or distancing reappraisals for anger-eliciting situations while EEG alpha asymmetry changes in the prefrontal cortex were recorded (F3–F4, F7–F8, and Fp1–Fp2). Engaging in problem orientation yielded a right-lateralized frontal activation pattern and was linked to the highest reappraisal success (percentage of strategy-conforming reappraisals), along with the highest believability ratings. Conversely, engaging in distancing reappraisal yielded a left-lateralized frontal EEG pattern, along with the highest ideational fluency and lowest anger ratings post-reappraisal. No distinct asymmetry pattern emerged for positive reinterpretation; however, this reappraisal condition yielded the lowest reappraisal success and lowest believability ratings. For all groups, higher reappraisal capacity correlated with right-lateralized frontal activity. Frontal EEG alpha asymmetry observed during reappraisal may be a specific function of implemented reappraisal strategy rather than general ideational fluency.
1. Introduction
The ability for cognitive reappraisal of aversive events is often declared a crucial building block for mental health and resilience [1,2]. Cognitive reappraisal involves deliberately viewing an emotionally evocative event (eliciting anger, fear, sadness, etc.) from a different, novel perspective, thereby reducing its emotional impact [3]. A plethora of behavioral studies showed that more habitual reappraisal use in daily life is linked to fewer depressive symptoms, less stress- and anxiety-related psychopathology, and improved cognitive and social functioning [1,3,4,5,6]. Due to its favorable consequences and distinct clinical relevance [7,8], several studies determined that cognitive reappraisal can be trained, highlighting the potential of brief, focused reappraisal interventions for eliciting adaptive behavioral and neuronal changes [9,10,11,12]. Especially from the perspective of intervention and prevention of psychopathology, a large part of research is focused on mapping the specific neuronal mechanisms underlying cognitive reappraisal.
1.1. Brain Activation Underlying Cognitive Reappraisal
FMRI and EEG studies have widely studied the neurofunctional correlates of cognitive reappraisal. Despite some heterogeneity in reappraisal assessment (instructing participants to downregulate negative emotions via reappraisal vs. mapping individual differences in habitual reappraisal use), research has established the prefrontal cortex as a key region for the cognitive restructuring of aversive events [13,14]. Here, it is hypothesized that dorsomedial and dorsolateral as well as ventromedial and ventrolateral prefrontal areas play different intricate parts in exerting cognitive control over emotional arousal in the limbic network, predominantly the amygdala [8,13,15,16,17,18]. These prefrontal regions facilitate cognitive processes such as working memory, response inhibition and selection, switching and updating, and overall cognitive flexibility, which are relevant for successful cognitive reappraisal [18,19,20]. Naturally, cognitive reappraisal also engages other neuronal hubs in the modulation of attention and the attribution of mental states, semantic memory, and representation of emotion goals [13,17,18,21,22,23]. Still, it is the complex architecture of the prefrontal cortex and the diversity of associated executive functions that mostly capture researchers’ attention. For cognitive reappraisal, research focus diverges into two directions: studies targeting the relevance of dorsal vs. ventral prefrontal cortex regions for cognitive reappraisal [24,25,26,27], and studies targeting the relevance of left vs. right prefrontal cortex engagement for cognitive reappraisal [28,29,30]. The present study focuses on the latter approach.
1.2. A Laterality Approach to Cognitive Reappraisal Generation
As a central premise of laterality research, left and right frontal cortex regions are differently involved in modulating aspects of affect, emotion, flexible responding, and psychopathology. In general, it is assumed that relatively greater left over right frontal activation as measured by prefrontal EEG alpha asymmetry indicates a greater ability to adaptively and flexibly modulate emotional responses to challenging environments [31,32,33,34]. In support of this, many EEG alpha asymmetry studies emphasized the importance of left frontal activation for the flexible coping with stressful events [35,36,37,38,39].
Yet, the literature has yielded an interesting disparity of findings for lateralized frontal brain activation during reappraisal efforts. While not directly concerned with laterality, plenty of fMRI studies confirmed the importance of left prefrontal recruitment for effective cognitive reappraisal and downregulation of negative affect [14,40,41,42,43]. Similarly, other studies reported reduced left frontal activation in patients with mood and anxiety disorders, which was believed to reflect inappropriate engagement of the prefrontal regulatory circuitry [16,18]. EEG research seems to agree as well: Choi et al. [44] reported greater relative left frontal activity during cognitive reappraisal compared to viewing negative pictures, with similar findings obtained by Parvaz et al. [45] and Li et al. [30].
However, these findings are challenged by a number of neuroimaging studies that stress the contribution of right prefrontal areas to cognitive reappraisal [23,26,42,46,47,48,49]. In a direct current brain stimulation (tDCS) study, Chen et al. [50] reported that stimulation of the right dorsolateral prefrontal cortex increased cognitive control during cognitive reappraisal, presumably due to increased conflict detection and resolution associated with right frontal areas. Similarly, Falquez et al. [46] identified the right superior frontal gyrus as an indispensable area for successful cognitive reappraisal focused on reducing the personal relevance of negative events. Altogether, it seems to be an open question whether left or right prefrontal engagement is more important for reappraisal success, which requires a closer look at two aspects of cognitive reappraisal: how it is measured (as a tendency/habit or an ability/capacity) and what tactics and strategies make up the rather heterogenous concept of cognitive reappraisal [51,52,53].
1.3. Different Measurement Approaches to Cognitive Reappraisal
Cognitive reappraisal is jointly driven by the tendency or the habit to use cognitive reappraisal for affect regulation in daily life and the actual cognitive capacity to implement cognitive reappraisal successfully [54]. Early reappraisal research focused on what individuals habitually do in their daily lives, i.e., habitual reappraisal use or frequency as indicated by self-report [3]. Later research expanded to what individuals are capable of when it comes to reappraisal implementation, i.e., indirectly assessed or psychometrically scored reappraisal capacity [5,55,56]. Importantly, individuals’ typical use of reappraisal in everyday life cannot be equated with their actual capacity to successfully exercise reappraisal when confronted with adverse scenarios and vice versa, given the, at best, weak correlations between the two [5,51,55,57]. While neuroscience usually tests cognitive reappraisal ability “in vivo” by instructing participants to use reappraisal for the downregulation of negative affect elicited by pictures, which is then compared to e.g., “just watch” conditions, adherence to reappraisal instructions is often only inferred but not verified [20,58,59]. Accordingly, participants “free” interpretation of reappraisal instructions and loose adherence to experimental protocols may account for some variance in the left vs right question of prefrontal cortex engagement during reappraisal.
1.4. Previous EEG Asymmetry Findings for Reappraisal Inventiveness
In recent years, our laboratory investigated prefrontal EEG alpha asymmetry in the context of reappraisal inventiveness—the capacity to spontaneously generate manifold different reappraisals in close to real-life emotional situations [20,23,55,57,60,61,62]. The Reappraisal Inventiveness Test (RIT) developed by Weber et al. [55] constitutes a psychometric performance approach to cognitive reappraisal generation that has participants voice reappraisal ideas out loud while their brain activity is being recorded. The RIT uses a standardized scoring procedure to determine reappraisal fluency (number of non-identical reappraisals), reappraisal flexibility (number of categorically different reappraisals), and reappraisal strategy/quality (e.g., positive reinterpretation or distancing). As a critical advantage, participants’ cognitive reappraisal efforts can be verified by experienced raters, and resulting brain activation can be directly correlated with performance indicators to establish more robust associations between lateralized prefrontal engagement and reappraisal success. In a series of six EEG experiments to date, we repeatedly managed to link participants’ capacity for reappraisal generation in the RIT to frontal EEG alpha asymmetry during their reappraisal efforts.
In our first study, we found higher reappraisal inventiveness (fluency and flexibility) for anger-eliciting stimuli associated with more left-lateralized activity in the lateral prefrontal cortex [20], which supports previous assumptions that successful reappraisal requires response inhibition, effective cognitive switching between ideas, and cognitive control of memory [63,64]. In a second study, we again found that more left frontal activity during anger reappraisal correlated with higher reappraisal fluency [60], and predicted lower chronic stress perception in daily life, underscoring the practical relevance of this brain pattern for stress coping in daily life. Our third EEG study seemed to corroborate the robustness of our reappraisal asymmetry pattern: here, more left-lateralized frontal activity during anger reappraisal predicted lower paranoia proneness, a relationship that was mediated by higher reappraisal fluency [61]. In sum, these first three studies robustly underlined the importance of left frontal activation for versatile reappraisal generation in the RIT [16,18]. Critically however, in subsequent EEG experiments, this association fully reversed. Despite being similarly powered and using similar samples (younger students), RIT items, the same RIT protocol, and an equally experienced set of raters, our studies suddenly found the same measure of higher reappraisal fluency associated with more right-lateralized frontal activity [65,66,67]. See Table 1 for details. Gender, affective states and traits, or personality did not explain the reserved nature of our reappraisal–frontal asymmetry link. However, we considered an alternative explanation for this discrepancy of findings: given the high variability within the emotion regulation strategy of cognitive reappraisal, more left or right prefrontal cortex engagement during reappraisal may more strongly depend on specific cognitive reappraisal strategies/tactics [51,52,53] and less on the process of fluent reappraisal generation per se.

Table 1.
Overview of studies on frontal EEG alpha asymmetry during reappraisal inventiveness.
1.5. Different Cognitive Reappraisal Strategies
Cognitive reappraisal of aversive event can be achieved through multiple different thoughts, strategies, and goals that may have little in common [68]. Noting the variability of strategies within an emotion regulation strategy, research distinguishes positive, situation-focused reappraisal, and distancing/detached reappraisal, which seem related to different outcomes [2,52,69]. Positive reappraisal is focused on reframing situations in a more positive light and “benefit finding” and more directly targets an emotional reinterpretation of an event [62,70]. By comparison, distancing/detached reappraisal strives for a more distanced perspective of an emotionally different observer or a third person, thereby reducing the personal relevance of the stimulus [41,52,68,71]. Studies show that positive reappraisal increases positive emotions while decreasing negative emotions [52,72] and seems to have the most long-term benefits for resilience and well-being [2,6,71,73]. Yet, positive reappraisal is not always effective in decreasing physiological arousal to affective stimuli [72], and it may be cognitively taxing and difficult to implement [74]. By comparison, distancing seems to result in an overall blunted emotion experience, but changes physiological arousal more reliably, and it seems to be more successfully implemented by individuals [52,68,75]. Interestingly, there also seems to be a neuronal divergence of both reappraisal strategies that are relevant to the goal of the present study. Certain fMRI studies find the left ventrolateral prefrontal cortex specifically involved in positive reinterpretation [41,76], arguing that this strategy more strongly relies on the search and selection of alternative meanings, as well as linguistic and semantic processes [48]. By comparison, detached reappraisal seemed to more strongly recruit right frontal areas [41,77]. In their meta-analysis, Powers and LaBar [75] consider the right dorsolateral prefrontal cortex particularly relevant for spatial and attentional control in distancing reappraisal. This research provides another important basis for assuming that lateralized prefrontal cortex engagement during cognitive reappraisal is dependent on the implemented reappraisal strategy and associated cognitive processes.
1.6. The Present Study
To clarify the role of left vs. right prefrontal areas in cognitive reappraisal and help explain our contradictory EEG findings, the present study recorded participants’ frontal EEG alpha asymmetry while they implemented three different cognitive reappraisal strategies: positive reappraisal, distancing/detached reappraisal, and problem-solving. In the RIT framework, problem-solving reappraisal denotes action-based solutions for emotional events focused on taking active steps for analytically solving the problem (see [51]). While it is not often addressed in research, years of experience with the RIT underline participants’ tendency to implement problem-oriented solutions in their reappraisal attempts (“I could do x, y, and z to salvage a situation”) to make themselves aware of their action repertoires and agency, which may help cool down negative affect, particularly anger [55,57,60,62]. In our study, participants were randomly assigned to three groups, who engaged in either positive reinterpretation, distancing reappraisal, or problem-oriented reappraisal for anger-eliciting situations. ANOVA models then compared the three reappraisal groups regarding their EEG alpha asymmetry patterns during reappraisal efforts, cognitive reappraisal performance, anger ratings, reappraisal difficulty and effort, and reappraisal beliefs (merit of their assigned strategy). Importantly, our main goal was to test whether certain cognitive reappraisal strategies may be accompanied by more left-lateralized frontal activity, whereas others are accompanied by more right-lateralized frontal activity, and how these differences may relate to reappraisal-related outcomes.
We posited the following general hypotheses:
Engaging in positive reappraisal would yield more left-lateralized frontal activity, while distancing reappraisal would be accompanied by more right-lateralized frontal activity [41,75,76]. Given that earlier studies with reappraisal inventiveness yielded left-lateralized frontal activity, and content analysis of reappraisal ideas showed a large proportion of problem-oriented reappraisals, we also assumed left-lateralized frontal activity for this strategy [20,60,61].
Positive reappraisal would be more difficult to implement than problem-oriented reappraisal and distancing reappraisal as shown by reappraisal implementation success and subjective difficulty ratings [72,74].
Participants would rate problem-oriented reappraisal and distancing reappraisal as more believable strategies for dealing with anger compared to positive reappraisal [72,74].
2. Materials and Methods
2.1. Participants
The sample size was determined based on effect size estimates between f = 0.24 and f = 0.48 obtained in previous EEG studies relating frontal alpha asymmetry to RIT performance and other relevant outcomes [20,60,61]. A priori power analysis (G*Power) suggested a minimum of n = 78 participants to detect an effect of f = 0.36 (mean from previous studies) in univariate ANOVA models with three groups (α = 0.05, 1 − β = 0.80). In total, n = 114 participants took part in the experiment. However, n = 12 participants had to be removed from analyses due to malfunctions of the audio device recording reappraisal answers, and a further n = 8 participants were removed due to excessive EEG artifacts on one or more frontal channels. The final sample with all required data comprised n = 94 participants (64 women), aged between 18 and 38 years (M = 23.40, SD = 3.80). Our previous studies were mostly based on all-female samples due to ideas that women may be more motivated to downregulate anger for social reasons (possibly translating to greater compliance in laboratory studies), as well as to minimize confounding effects of gender differences in emotion abilities and habits [20,60]. However, several of our behavioral studies also showed that women and men did not differ in their reappraisal inventiveness for anger [61,78] or in their implemented cognitive reappraisal strategies [62], which is why we opted to include both men and women in the present study. Probing frontal asymmetry, reappraisal strategy associations in a mixed-gender sample may also allow for more generalization of our results to the general population. All participants were right-handed as confirmed by the standardized hand dominance test [79], with M = 16.90, SD = 5.70 (Min = 5.76, Max = 31.80). Participants reported no history of neurological or psychiatric illness, abstained from caffeine consumption 2 h prior to their EEG appointment, and came to the session well rested. The majority of participants reported at least high school education (94.6%), and most were students (87.2%) enrolled in various fields at the university (41% psychology). Written, informed consent was provided by all participants, who received either course credit or 20 Euros for their participation. This study was authorized by the local ethics committee of the University of Graz (39/79/63 ex 2020/21).
2.2. Procedure
Participants signed the informed consent form and completed the demographic questionnaire as well as handedness test. Then, they filled in the questionnaires outlined in Section 2.3. Subsequently, EEG electrodes were applied, and EEG was recorded during an initial 2 min resting condition (eyes open). Afterwards, participants were randomly assigned to receive instructions for one out of three cognitive reappraisal strategies: problem-oriented reappraisal (n = 32), positive reinterpretation (n = 31), or distancing reappraisal (n = 31). After instructions and two practice items (~10 min), they performed the reappraisal task while EEG was recorded (5 items, ~25 min in total). Afterwards, participants rated their perceived anger with the RIT situations, difficulty and effortfulness of the task, and reported their reappraisal beliefs. The total experiment duration was ~100 min. Afterwards, participants were offered the opportunity to wash and dry their hair and were compensated for their participation.
2.3. Emotional Assessment Prior to Reappraisal
2.3.1. Depressive Symptoms
The German version of the Center for Epidemiological Studies Depression Scale [80] was used to assess frequency of current depressive symptoms over the past week, such as poor appetite, restless sleep, or feeling lonely. The CES-D consists of 20 items, rated on a scale of 0 (rarely or never) to 3 (most or all the time, α = 0.87). It is highly suitable for measuring sub-clinical depressive experiences in the general population. Mean scores for the entire sample were M = 11.10 (SD = 7.12).
2.3.2. Habitual Reappraisal Use
The German version of the Emotion Regulation Questionnaire (ERQ, [3]) was used to assess participants’ habitual reappraisal use in daily life. Participants answered six items on the reappraisal subscale ranging from 1 (does not apply) to 7 (does apply completely), which were averaged to a mean score of M = 4.80 (SD = 0.81, α = 0.83).
2.4. Reappraisal Inventiveness Test with Three Different Reappraisal Strategies
Participants’ capacity to generate different cognitive reappraisals for anger-eliciting situations was measured with the reappraisal inventiveness test (RIT; [55]). In its original version, the RIT confronts participants with real-life, anger-evoking situations (e.g., a friend promising to water the plants in your apartment while you take a vacation, but not following through, causing most of your plants to die). Participants imagine these situations happening to them and then generate as many different cognitive reinterpretations as possible within a timeframe of 3 min per item. The RIT has been successfully implemented during EEG several times, yielding meaningful associations of brain activity during reappraisal efforts with reappraisal performance, well-being, personality traits, and as a measure of reappraisal training success [12,20,57,60,61].
As a deviation from previous studies, in the present investigation, prior to performing the RIT, participants were explicitly instructed to generate reappraisal ideas according to three different reappraisal strategies: (a) problem-oriented reappraisal, which focuses on thinking about concrete plans and actions to solve the problem at hand (e.g., “I can just go buy new plants” or “Perhaps I can still save some of the plants”), (b) positive reinterpretation, which emphasizes positive aspects of negative scenarios, including advantages or learning experiences (“Now I have room for new decorations”, “That vacation was really great though”, or “At least I learned whom I can trust”) and (c) distancing reappraisal, focused on trivializing the impact of the situation and applying a more detached perspective (e.g., “There are worse situations to come home to”, “Perhaps this could have happened to me as well”, or “In a few weeks, this won’t matter”). See Appendix A for a detailed list of RIT reappraisal categories. Participants were briefly trained in implementing one of the three reappraisal strategies in an experimenter-guided practice trial featuring two anger-eliciting situations, which was performed on a computer screen. They were shown several possible cognitive reappraisal ideas conforming to the respective reappraisal condition (e.g., positive reinterpretation) and then tried to come up with their own strategy-conforming ideas, which were monitored by the experimenter. In total, this brief reappraisal training session lasted ~10 min.
Participants then performed the RIT during EEG, with specific instructions to try and generate only cognitive reappraisal ideas that matched their previous training (e.g., positive reinterpretations). Each trial started with a fixation cross (10 s) followed by the description of an anger-eliciting situation (~20 s), which was supplemented by a matching photograph to allow for better immersion. In the following reappraisal generation phase (3 min per item), participants pressed the space bar whenever they came up with a matching reappraisal idea, briefly verbalized their idea, and then pressed the spacebar again to resume the task. Participants’ answers were recorded by a microphone and later transcribed for analysis. In total, participants worked on five RIT items, which were presented in randomized order.
2.5. Cognitive Reappraisal Capacity
Following the scoring procedure of the RIT [55] and previous relevant research [20,60,61,62], RIT general ideational fluency was used as an index of quantity of generated reappraisal ideas, calculated as the total number of all generated nonidentical reappraisals irrespective of instructed reappraisal strategy (α = 0.93). The fluency index was independently rated by two experienced researchers, with a resulting intraclass correlation (ICC) of 0.99, see [20,57,60,62]. Ideational fluency over all five RIT items and over all groups was M = 27.81 (SD = 9.09). Differences in general ideational fluency among the three different reappraisal groups may indicate that focusing on one particular reappraisal strategy may influence overall reappraisal productivity.
Reappraisal quality/strategy (i.e., problem-orientation, positive reinterpretation, or distancing) was categorized according to the category scheme of the RIT [55], which allows for the differentiation of reappraisal ideas according to their specific content. The inter-rater reliability between the same two raters was ICC = 0.94 for problem-orientation, ICC = 0.93 for positive reinterpretation, and ICC = 0.97 for distancing. Valid reappraisal ideas not matching these three reappraisal strategies (e.g., revenge-related ideation) were not included in the analyses. Reappraisal implementation success was scored by dividing the number of strategy-conforming reappraisal ideas (ideas matching the assigned reappraisal group, e.g., number of distancing reappraisals in the distancing group) by general ideational fluency, ×100. This percentage score represents individuals’ relative reappraisal implementation success and may provide an objective measure of ease and difficulty in generating reappraisals of a particular quality/type. Reappraisal success over all groups was M = 78.24% (SD = 14.83%).
2.6. Post-Reappraisal Assessments
2.6.1. Self-Reported Perceived Anger
Immediately after performing the RIT, participants indicated the amount of anger they would experience when confronted with the depicted situations on a 7-point scale ranging from 0 “not angry at all” to 6 “very angry”. The mean anger rating over all reappraisal groups was M = 4.44, SD = 0.65.
2.6.2. Self-Reported Difficulty and Effortfulness
Participants were asked to indicate how difficult they perceived the task of implementing their assigned cognitive reappraisal strategy on a 17-point Likert scale ranging from 0 “not at all difficult” to 17 “very difficult”. The mean difficulty rating over all reappraisal groups was M = 8.23 (SD = 0.69). Further, participants indicated how much effort they put into the task of generating reappraisals of a specific strategy, again on a 17-point Likert scale ranging from 0 “no effort at all” to 17 “extreme effort”. The mean effort rating was M = 11.84 (SD = 3.64).
2.6.3. Self-Reported Reappraisal Beliefs
Participants’ subjective beliefs about their implemented cognitive reappraisal strategies were assessed with a short, five-item questionnaire called the Reappraisal Beliefs Questionnaire [81]. Participants answer items such as “I believe that these thoughts are suitable for reducing my anger” or “These thoughts are typical for me” on an 8-point Likert Scale ranging from 1 “not at all true” to 7 “completely true”. Higher scores indicate that the credibility of the generated reappraisal ideas is high. The mean score over all reappraisal groups was M = 26.00 (SD = 4.72).
2.7. EEG Recording and Quantification
EEG (international 10–20 system) was recorded from 19 channels using a Brainvision actiCHamp amplifier (Brain Products) with active electrodes in a stretchable cap and a sampling rate of 1000 Hz. The EEG was referenced to the nose and re-referenced offline using an averaged ear reference [82]. Impedance was kept below 30 kΩ for all EEG electrodes, and below 10 kΩ for the ground and reference electrodes. Shifts in EEG alpha asymmetry in the context of emotion regulation and cognitive reappraisal were recorded from three frontal electrode pairs: dorsolateral prefrontal sites (F3–F4), ventrolateral prefrontal sites (F7–F8), and most rostral frontopolar sites (Fp1–Fp2). Only the time frames in which participants mentally generated cognitive reappraisal ideas were used for analysis; reading and speaking intervals were excluded. EOG measures were obtained for the identification of ocular artifacts, with EOG electrodes placed at the supra- and sub-orbit of the right eye (vertical EOG), and at the outer canthi (horizontal EOG). Intervals that indicated ocular and muscle artifacts were eliminated by visual inspection. Power spectra (epoch length 1s, overlapping 50% Hanning window) were averaged across all artifact-free intervals per participant. Following the common approach in the field, analyses focused on the alpha frequency band (8–12 Hz). EEG laterality coefficients indexing relative right versus left sided activation were computed as follows: LC = ((R − L)/(R + L)) × 100, with R denoting alpha power at the right frontal electrode positions (F4, F8, Fp2) and L denoting alpha power at the left frontal electrode positions (F3, F7, Fp1). Thus, positive values indicate higher alpha activity in the right than the left hemisphere, i.e., relatively greater left hemisphere cortical activity [83,84,85]. While being virtually perfectly correlated with lnR—lnL [83,86], the −1 to +1 range of the LC allows for more intuitive interpretations and easier comparisons of data from different studies, frequency bands, and locations [87]. To obtain an index for the EEG alpha asymmetry response (i.e., alpha asymmetry changes in response to generating cognitive reappraisal ideas using different reappraisal strategies), we calculated residualized change scores with linear regressions, using the LC during the resting period to predict the LC during reappraisal generation [60,61]. This approach ensures that the analyzed residual variability was due to the cognitive reappraisal process and implemented reappraisal strategies and not grounded in individual differences at baseline. Further, it controls for measurement error inherent in the use of repeated measures of the same kind.
2.8. Statistical Analyses
For the preliminary analyses, we tested for reappraisal group differences in age, habitual reappraisal use, depressive symptoms (univariate ANOVAS), and gender (chi-squared test). Additionally, Pearson’s correlations for variable relationships over all groups were computed.
For the main analyses, we computed three separate, univariate ANOVAs testing for group differences in frontal EEG alpha asymmetry at dorsolateral frontal (F3/F4), ventrolateral frontal (F7/F8), and frontopolar sites (Fp1/Fp2). Here, the goal was to test whether some cognitive reappraisal strategies produced a left frontal activation pattern, while others produced a right frontal activation pattern. We opted to calculate separate ANOVAs for each electrode position instead of running a general 3 × 3 model (group × site), as analyzing topographically close electrode sites together often leads to variance dilution and may wash out distinct effects on single electrode sites (see [20]).
As our previous EEG asymmetry findings were mostly based on all-female samples, we also ran a supplementary ANOVA model that, along with group, considered gender and the group × gender interaction as additional factors. This main asymmetry analysis was followed up by ANOVA analyses of behavioral group differences in reappraisal performance (ideational fluency and implementation success), and ANOVA analyses of self-reported anger, reappraisal difficulty, reappraisal efforts, and reappraisal beliefs.
Significant ANOVA group effects were followed up by Games–Howell post-hoc tests due to unequal error variances across the groups. Effect sizes are expressed in partial eta squared (ηp2) for ANOVAs and Cohen’s d for t-tests. Results are considered significant if p < 0.05. All analyses were performed with IBM SPSS version 28.
3. Results
3.1. Preliminary Analyses
The three reappraisal groups did not differ in terms of age (F2,91 = 1.74, p = 0.182, η2p = 0.04), depressive symptoms (F2,91 = 0.37, p = 0.690, η2p = 0.01), or habitual reappraisal use (F2,91 = 1.64, p = 0.199, η2p = 0.04). However, there were fewer women (more men) in the problem-oriented reappraisal group than the positive reinterpretation group, and vice versa (X2 = 8.49, p = 0.014).
Over all groups, reappraisal implementation success was correlated with relatively greater right-lateralized activity at all frontal sites; however, only at trend level (dorsolateral: r = −0.20, p = 0.050; ventrolateral: r = −0.20, p = 0.054; and frontopolar: r = −0.20, p = 0.050). Higher perceived anger with the RIT situations was correlated with relatively greater right-lateralized activity at dorsolateral frontal sites (r = −0.20, p = 0.049). No other correlations of frontal EEG alpha asymmetry with behavioral measures were significant (all p’s > 0.163). General ideational fluency was negatively correlated with reappraisal implementation success (r = −0.31, p = 0.002) and with perceived anger ratings (r = −0.25, p = 0.016). Reappraisal implementation success and perceived difficulty ratings were positively correlated at trend level (r = 0.19, p = 0.063). Perceived difficulty and perceived effort ratings were positively correlated (p = 0.29., p = 0.005). For full correlational matrix, see Table 2.

Table 2.
Correlational matrix of all study variables.
3.2. Group Differences in General EEG Alpha Asymmetry Pattern during Reappraisal
Significant group differences in EEG alpha asymmetry were observed for dorsolateral frontal sites (F2,91 = 4.00, p = 0.022, η2p = 0.08) but not for ventrolateral frontal sites (F2,91 = 1.50, p = 0.228, η2p = 0.03) or frontopolar sites (F2,91 = 0.98, p = 0.381, η2p = 0.02). Post-hoc comparisons revealed significant differences between engaging in problem-orientation and engaging in distancing reappraisal: problem-oriented reappraisals was accompanied by right-lateralized frontal activity, while distancing reappraisal was accompanied by left-lateralized frontal activity (p = 0.026). No distinct asymmetry pattern emerged for engaging in positive reinterpretation, which did not differ from problem-orientation (p = 0.526) and distancing reappraisal (p = 0.201). See Table 3 for descriptive statistics and Figure 1 for an illustration of effects.

Table 3.
Descriptive statistics and ANOVA results for reappraisal group comparisons.
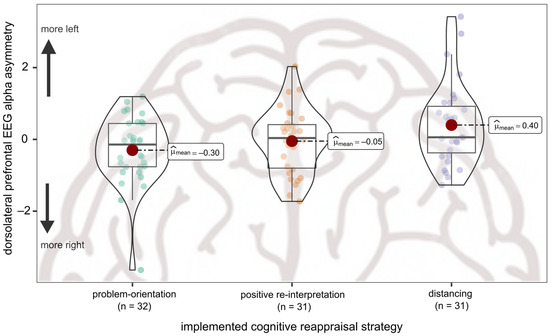
Figure 1.
Differences in dorsolateral frontal EEG alpha asymmetry by cognitive reappraisal group. Implementation of distancing is accompanied by more left-lateralized asymmetry at F3/F4, while implementation of problem-orientation is accompanied by more right-lateralized asymmetry at F3/F4.
Running supplementary ANOVA models with gender and group × gender interactions for each frontal electrode site did not reveal any influence of gender on group differences in frontal asymmetry, neither for dorsolateral frontal (gender: p = 0.663, η2p < 0.01, group × gender: p = 0.803, η2p = 0.01), ventrolateral frontal (gender: p = 0.617, η2p < 0.01, group × gender: p = 0.261, η2p = 0.03), or frontopolar sites (gender: p = 0.347, η2p < 0.01, group × gender = 0.274, η2p = 0.03).
3.3. Group Differences in Reappraisal Performance
There were significant group differences in general ideational fluency (F2,91 = 3.43, p = 0.036, η2p = 0.07). Post hoc comparisons revealed that reappraisal fluency was highest in the distancing group and lowest in the problem-oriented group (p = 0.019). Ideational fluency in the positive-reinterpretation group was higher than in the problem-oriented group (p = 0.205) and lower than the distancing group (p = 0.186); however, these results were not statistically significant.
Significant group differences were also observed for reappraisal implementation success (F2,91 = 4.26, p = 0.017, η2p = 0.09). Post-hoc comparisons showed that implementation success was highest in the problem-orientation group and lowest in the positive reinterpretation group (p = 0.010). Yet, there were no significant differences in reappraisal implementation success between problem-orientation and distancing reappraisal (p = 0.215) or between positive reinterpretation and distancing reappraisal (p = 0.469).
3.4. Group Differences in Anger, Difficulty, Effort, and Reappraisal Beliefs
Significant group differences emerged for perceived anger with the RIT situations (F2,91 = 3.46, p = 0.036, η2p = 0.07), with post-hoc comparisons showing that participants reported experiencing significantly less anger after using distancing reappraisal compared to using problem-oriented reappraisal (p = 0.020) and compared to using positive reinterpretation (p = 0.030). No differences between anger ratings for problem-orientation and positive reinterpretation were observed (p = 0.898).
No group differences emerged for reported difficulty of implementing respective reappraisal strategies (F2,91 = 1.81, p = 0.169, η2p = 0.04) nor for reported implementation efforts (F2,91 = 1.57, p = 0.214, η2p = 0.03).
We observed a trend level effect for group differences in reappraisal beliefs (F2,91 = 2.81, p = 0.065, η2p = 0.06). Post-hoc comparisons showed that using problem-oriented reappraisal for anger-eliciting situations was rated as more believable than using positive reinterpretation (p = 0.032). However, using problem-orientation was rated equally believable to using distancing reappraisal (p = 0.584), and distancing reappraisal was rated equally believable to using positive reinterpretation (p = 0.417). See Table 3 for descriptive statistics and Figure 2 for an illustration of all behavioral effects.
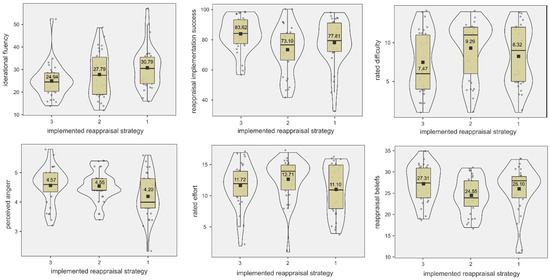
Figure 2.
Differences in RIT performance, anger, rated difficulty, effort, and reappraisal beliefs by cognitive reappraisal group. 1 = distancing, 2 = positive reinterpretation, and 3 = problem-orientation. Mean values are depicted per reappraisal group.
4. Discussion
The present study addressed the inconsistency of left vs. right prefrontal cortex involvement in cognitive reappraisal generation by comparing frontal EEG alpha asymmetry and reappraisal outcomes for three different types of cognitive reappraisal strategies—positive reappraisal, distancing, and problem-oriented reappraisal. Results confirmed our general premise that different cognitive reappraisal strategies engage different cognitive processes and prefrontal cortex activation; however, they only partly aligned with our initial hypotheses. Based on the literature, we expected that positive reappraisal, due to its reliance on stimulus reinterpretation from negative to positive and semantic processing efforts, would be linked to more left-lateralized frontal activity [41,76], while distancing/detached reappraisal, drawing more strongly on attentional control and focus on the self, would be linked to more right-lateralized frontal activity [75]. Based on previous experience with the RIT, we expected left-lateralized frontal activity for problem-oriented reappraisal as well [20,60,61]. However, our asymmetry results diverged from these expectations: In our study, the generation of multiple distancing reappraisals for anger-evoking events was accompanied by greater left-lateralized frontal activity, while the generation of problem-oriented reappraisals was accompanied by more right-lateralized frontal activity. Inventing multiple positive reappraisals for anger regulation did not yield a distinct frontal alpha asymmetry pattern, presumably activating both left and right frontal areas. Several explanations may account for these findings.
4.1. No Distinct Asymmetry Pattern for Positive Reappraisal
One speculation of previous fMRI studies observing left dorsolateral prefrontal cortex activation during positive reinterpretation was that this strategy more strongly relied on internal verbalization of reappraisal ideas than distancing/detached reappraisal. This dominant linguistic and semantic component of positive reappraisal was believed to explain the involvement of left lateral frontal areas [41,48,88]. As a critical difference to most fMRI reappraisal paradigms, the RIT requires participants to verbalize all reappraisal ideas in a coherent and intelligible manner to be subsequently rated for fluency and quality [20,23,55,60]. As such, engaging in distancing reappraisal and problem-orientation should pose similar linguistic demands to positive reinterpretation, which may explain why we did not replicate a left frontal activation pattern specifically for positive reappraisal. Further, reappraisal implementation success (percentage of correct, instruction-conforming reappraisals) was lowest in the positive reappraisal group, as were believability ratings that these reappraisal ideas would be helpful or typical when regulating anger. This confirms previous assumptions that positive reappraisal, although associated with better long-term health outcomes [2,73], may be perceived as difficult to implement [72,74] and may not be a frequently chosen strategy [59,89]. Interestingly, the lack of a distinct asymmetry pattern in the positive reappraisal group may more strongly mimic bilateral alpha activity found for the processing, but not regulation, of negative affective pictures [45], possibly indicating that participants could not implement this strategy effectively for anger regulation.
4.2. More Left Frontal Activity during Distancing Reappraisal
The fact that we observed more left-lateralized frontal activity for distancing reappraisal is interesting, since the involved self-referential processing (to detach oneself from the emotional relevance of a stimulus) and reorienting of visuospatial attention are usually attributed to right frontal cortex areas [41,46,75]. However, other studies parsing lateralized functions of the dorsolateral prefrontal cortex specifically have found its left areas involved in prioritizing personally relevant information in both situations of low and high demands [90], in addition to modulating one’s own responses when goals and rules are not clearly provided by external context [91]. The latter finding is interesting, as in our study, the distancing group scored highest in general reappraisal fluency, irrespective of content (i.e., also non-distancing ideas). It is possible that distancing/detachment instructions in general (“reducing personal relevance”) are less specific than instructions for positive reappraisal (“finding something positive”) and problem-oriented reappraisal (“take actions to fix damage”) and may thus be interpreted more freely by participants who then construe their own reality of distancing approaches. Importantly, this free interpretation to distancing may still produce a rather successful repertoire of reappraisal ideas, as the distancing group also reported the lowest anger ratings post-reappraisal. Prior research also postulated that distancing/detachment may have stronger short-term effects on reducing emotional arousal than positive reappraisal [41,52]. Naturally, our EEG approach can only speculate about modulation of limbic activation by frontal areas during the reappraisal process. Yet, finding the lowest anger ratings in the reappraisal group with more left frontal activity seems to corroborate the importance of left prefrontal recruitment for the successful downregulation of immediate negative affect [14,16,18,30,40,41,42,43].
4.3. More Right Frontal Activity during Problem-Oriented Reappraisal
The right frontal activation previously attributed to the inhibition of negative meaning facilitated by distancing reappraisal [41,42,46], we instead observed for problem-oriented reappraisal. This strategy has not received much attention in other reappraisal taxonomies than the RIT, but it may be most akin to the reappraisal tactic of technical-analytical problem solving proposed by McRae et al. [51]. In our opinion, what qualifies problem-orientation as a crucial, distinct type of reappraisal is that by actively inventing problem solutions for anger-eliciting events, individuals reinforce their own agency and opportunities for control, which may elicit an emotional change even though the solutions are theoretical and not acted out yet [55,62]. As there is little precedence for neuronal correlates of problem-oriented reappraisal, we can only speculate about the observed right-lateralized frontal activity. Previous neuroimaging studies attributed reappraisal-related right frontal activation to more cognitive control necessary for the inhibition of predominant negative affect and thoughts [25,46,50], specifically underlining its inhibitory role for adopting a distanced, third person perspective [46]. However, if we contrast the strategies of problem-oriented and distancing reappraisal, it may be the case that successful problem-oriented reappraisal relies even more strongly on effective inhibitory control and inhibition of unhelpful emotional responses, as participants not only need to emotionally detach themselves from the anger situation, but they also need do produce viable, neutral, and realistic problem solutions via divergent thinking. This may require increased cognitive control, affective response inhibition, and strong engagement of cold regulatory processes, all of which have been linked to the right prefrontal cortex [46,50,92,93,94]. As an additional vital observation, right lateral prefrontal cortex activation has been shown to increase with executive demands [49,95], which may reflect the rather taxing nature of engaging in problem-oriented reappraisal.
Still, participants’ reappraisal implementation success was higher for problem-oriented reappraisal than for positive reappraisal, and comparisons of self-reported reappraisal difficulty and effort yielded no significant group differences. While this challenges the link of right frontal activity to the greater cognitive effort of problem-oriented reappraisal, research has reported significant discrepancies between retrospective and concurrent ratings in emotion tasks, showing that participants may not always be able to accurately report their fleeting emotional experiences [96,97]. As a final perspective, problem-oriented reappraisal received the highest believability ratings, indicating that participants found this strategy to be the most relatable, functional, and typical for dealing with anger-eliciting events. Given that a multitude of previous studies emphasize the prominent effect of individuals’ beliefs on their emotion regulation engagement and regulation effort [98,99], an interlink between greater beliefs and greater (implicit) reappraisal efforts may explain greater involvement of the right frontal cortex in problem-oriented reappraisal.
4.4. Specificity of Findings for the Dorsolateral Prefrontal Electrode Sites
All our alpha asymmetry group effects were only statistically significant for dorsolateral frontal sites. However, reappraisal implementation success for all groups was linked to right-lateralized activity at all frontal sites. One plausible interpretation for this finding is that the (right) ventrolateral and orbitofrontal (frontopolar) cortex facilitate more basic cognitive functions needed for the fluent generation of multiple reappraisals, while the dorsolateral prefrontal cortex facilitates more specific functions that vary by reappraisal content. The primary function of the ventrolateral prefrontal cortex in reappraisal is the inhibition of predominant, but goal-inconsistent, responses in favor of finding alternative interpretations, which also engages semantic memory control and cognitive switching [20,25,27,42]. This modulation of emotional responses is achieved through direct projections to the medial prefrontal cortex–amygdala pathway [16,49]. Orbitofrontal cortex involvement in cognitive reappraisal is attributed to the updating of affective stimulus values and salience of emotional states [41,48,63]. Both predominant response inhibition and updating affective value presumably describe essential, but more general, processes in fluent reappraisal generation. Though we already discussed ideas why distancing and problem-solving reappraisal may diverge in their prefrontal activation, the question remains as to what executive functions the left vs. right dorsolateral prefrontal cortex facilitates that vary by reappraisal content specifically. EEG alpha asymmetry changes in the dorsolateral prefrontal cortex are established as a robust biological correlate of the spontaneous activation of approach and avoidance motivation [86,100,101], with increased left frontal activation reflecting an active problem-oriented approach and increased right frontal activation reflecting avoidance/withdrawal. Yet, this explanation seems too simplistic and counterintuitive when considering the highest reappraisal success and highest believability ratings in the problem-oriented group, which does not converge with avoidance motivation. Instead, it is likely that the specific aspects of working memory, response inhibition, cognitive switching/shifting, attention, and planning that are lateralized in the left vs. right dorsolateral prefrontal cortex are differently involved in specific cognitive reappraisal strategies to a still unknown degree [20,61,63,91,92,102,103]. The nature and extent of this involvement will have to be determined by future investigations using multiple different indicators of executive functioning during the reappraisal process.
4.5. Right Frontal Activity Linked to Reappraisal Performance
The link of reappraisal implementation success to right-lateralized frontal activity converges with some previous evidence from our laboratory [65,66,67] but not with earlier EEG work on reappraisal inventiveness [20,60,61]. Still, stronger involvement of the right frontal cortex in cognitive reappraisal of negative stimuli (i.e., decreasing affect) has been postulated plenty of times [42,46,104]. Ochsner et al. [42] argue that the left prefrontal cortex has a more generic role in reappraisal of both positive and negative stimuli, thus serving crucial functions for both decreasing and increasing emotional responses, while the right prefrontal cortex more specifically facilitates the downregulation of negative emotions. We speculate that reappraisal instructions in our earlier studies [20,60,61] were less elaborate and specific to the downregulation of anger and may have also captured the upregulation of positive emotions or other emotion regulation strategies (e.g., distraction or acceptance), while a later refinement of instructions may have more clearly emphasized the reduction in negative emotions (anger) by reappraisal.
Importantly, while we previously noted that left frontal activity during distancing reappraisal may reflect adaptive prefrontal recruitment for the downregulation of negative affect [16,18,40,41,42], this interpretation may specifically hold participants’ feelings immediately after engaging in cognitive reappraisal (i.e., arousal ratings), and thus, their general capability to modulate their emotional response (amygdala reactivity) by reappraisal short-term. While it may seem contradictory that we find right frontal activity linked to reappraisal implementation success, this may be specific to participants’ theoretical capacity for generating reappraisals fluently and flexibly according to instructions but not necessarily their emotional response. While this general capacity for diverse reappraisal generation is considered an important prerequisite for reappraisal success in daily life—following the idea that a greater pool of available ideas increases the likelihood of finding the reappraisal best suited for a situation—it is not sufficient for mental health effects [57,60,61]. Cognitive reappraisal is a multifactorial process, comprising individuals’ capacity for generating manifold reappraisals (capacity), the effectivity of reappraisal ideas to modulate emotional responses (effectivity), their actual use of reappraisal in daily life (habit), their beliefs in the efficacy of cognitive reappraisal (beliefs), and various other aspects such as cognitive and situational affordances, intensity of emotional stimuli, etc. [54,55,57]. It is a challenge for future research to build a robust framework of which cognitive functions and concomitant brain activity reliably facilitate which aspect of the cognitive reappraisal process.
4.6. Limitations and Future Directions
Some limitations are noted. This study was designed as a between-subject comparison of engagement in different reappraisal strategies. While a within-subject comparisons may appear preferable with regard to statistical power and control of unknown individual differences between groups [105], having all participants engage in all three reappraisal strategies would have likely resulted in carry-over effects from one strategy to the other. Given how successfully cognitive reappraisal can be trained with only brief interventions [9,11,12], repeated engagement with the RIT, even over the course of a few weeks, would have risked diluting strategy-specific effects, as participants are likely to merge strategies and repurpose reappraisal ideas from memory as the experiment progresses. Thus, we believe that in the context of our study, a within-subjects design would be more likely to reveal effects of passive reappraisal rehearsal, and not active reappraisal generation, which may be linked to different brain activation patterns [106]. Further, since our groups did not differ in terms of age, depressive symptoms, or habitual reappraisal use, we are confident that our obtained results are grounded in reappraisal strategy engagement and not in unrelated arbitrary group differences. While the gender composition slightly differed between groups, there are no indications to date that men and women have a different preference or different capacity for producing certain reappraisal strategies [57,73]. Nevertheless, a replication of our results with a different experimental methodology and in larger, more diverse samples is encouraged. On another note, we explicitly stress that it is unclear how much the reappraisal classification scheme of the RIT overlaps with or diverges from other frequent reappraisal taxonomies in the literature [51,52,53,55,68]. By implication, what is classified as distancing or positive reappraisal by some researchers may be attributed to another reappraisal strategy by others. In light of this caveat, we acknowledge that our reported reappraisal group differences may only hold for reappraisal classification in the RIT [55], but not for other, alternative taxonomies (reinterpretation vs. distancing as contrasted by [41,48,52,68]). Still, our findings may provide an interesting perspective to future brain comparisons of reappraisal strategies. On a final note, our findings are limited to using different reappraisal strategies for downregulating anger only. While reappraisal inventiveness in the RIT is believed to generalize over different negative emotions [57], there are assumptions that reappraisal in general or specific reappraisal strategies may work better for downregulating some emotions than they do for others [53,107,108]. In this study, we specifically focused on anger-eliciting stimuli to mimic the RIT material we had most experience with from our previous EEG studies (five studies on anger and one on anxiety), and for which we also found the most robust correlations with behavioral indices of negative affectivity, stress, hostility, and executive functioning [20,23,60,61]. Based on this expertise, we felt more confident in interpreting distinct asymmetry patterns of different reappraisal strategies in the face of anger compared to anxiety. While a mixed emotions approach using both anger and anxiety-eliciting stimuli seems beneficial for a broader investigation of negative emotions in general, this would require a marked increase in RIT items presented during EEG (a minimum of four per emotion), which may prove more taxing to participants at the risk of reducing reappraisal compliance. However, as a next step, the results of this study should be replicated with the anxiety version of the RIT [12,57,62].
5. Conclusions
Successful cognitive reframing of an emotional scenario may take many shapes and forms and engage different cognitive processes and associated brain areas. Our study addressed the issue of diverging laterality effects in prefrontal cortex engagement that was previously reported for reappraisal inventiveness. When measured through the generation of multiple different reappraisal ideas (inventiveness), our findings suggest that distancing reappraisal more strongly recruits left frontal areas and may be more effective in short-term anger reduction, while problem-oriented reappraisal more strongly recruits right frontal areas and is more easily implemented and believable in the face of anger. By contrast, the generation of multiple positive reappraisals seems to rely on bilateral prefrontal activity and appears to be a suboptimal approach for short-term anger regulation. Our findings may help shed light on the role of prefrontal cortex functions for cognitive reappraisal generation and may provide useful neurophysiological indicators for future intervention studies that aim to train individuals’ reappraisal abilities for improving mental health and well-being.
Author Contributions
Conceptualization, I.P. and C.M.P.-S.; methodology, I.P. and C.M.P.-S.; validation, I.P., A.F. and C.M.P.-S.; formal analysis, C.M.P.-S.; investigation, C.M.P.-S.; resources, I.P. and A.F.; data curation, C.M.P.-S.; writing—original draft preparation, C.M.P.-S.; writing—review and editing, A.F. and I.P.; visualization, C.M.P.-S.; supervision, A.F.; funding acquisition, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Austrian Science Fund, grant number P30362.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local ethics committee of the University of Graz (39/79/63 ex 2020/21).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors acknowledge the Open Access Funding by the Austrian Science Fund (FWF).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Reappraisal category scheme as proposed by the RIT.
Overview of RIT anger categories (Weber et al., 2014) [55].
Example situation: “You invite friends over for a meal, but when you step into the kitchen of your flat, the entire kitchen is a mess. Yesterday, your roommate had promised to clean up the kitchen by today. When you go to talk to your roommate, he tells you that he was watching TV and then did not feel like cleaning up anymore.”
| Problem-oriented reappraisal: generate active problem solutions, plan clarification | |
| Prob 1 | Anticipate compensation: it is expected that the wrongdoer compensates the damage |
| Example: “My flatmate has to pay for dinner for me and my friends” | |
| Prob 2 | Plan alternative action: an action is planned, which reduces the impact of the damage |
| Example: “I could just ask my friends if we can meet up in a pub” | |
| Prob 3 | Plan damage control: an action is planned, which limits the damage |
| Example: “I will ask my friends to quickly help me clean up the mess” | |
| Prob 4 | Plan clarification: seeking to talk with the wrongdoer and clarify the situation |
| Example: “I will definitely need to talk to this person about the situation” | |
| Positive Re-Interpretation: generate positive aspects | |
| Pos 5 | Emphasis on general positive aspects: emphasize on positive features of the situation, which are not directly related to the anger-eliciting event |
| Example: “I’m really looking forward to a great evening with my friends” | |
| Pos 6 | Learning experience: to intend to do something different next time, in order to avoid harm |
| Example: “Next time I will make sure the kitchen is clean before I invite people” | |
| Pos 7 | Altruism: focus on the advantage of the situation for the wrongdoer (can also be ironic) |
| Example: “I’m glad my flatmate got to relax, the poor guy really needs it” | |
| Pos 8 | Worst-Case comparison: compare the current event with another, more harmful event and appraise the current event as less negative |
| Example: “At least my flatmate did not forget to turn off the oven and set the kitchen on fire” | |
| Pos 9 | Interpret disadvantage as advantage: find an advantage in the negative situation (e.g., a good opportunity to practice something) |
| Example: “Now we finally have a reason to try out the new restaurant around the corner!” | |
| Pos 10 | Humor: clearly humorous interpretation of the situation, also self-ironic thoughts |
| Example: “If I leave the mess overnight, maybe our apartment ghost will clean it up” | |
| Pos 11 | Appreciation of the wrongdoer: appreciate the wrongdoer for his honesty or things he has done in the past |
| Example: “At least my flatmate is honest about it, usually he is a great guy” | |
| Distancing/Detaching: relativize the meaning of the situation or the guilt of the wrongdoer | |
| Rel 12 | Find an alternative explanation for the wrongdoer’s behavior: interpret the behavior of the wrongdoer as accidental/unintended, find a reason that does not include the intent to harm |
| Example: “He may be busy with a personal problem and does not want to burden me with it” | |
| Rel 13 | Show understanding for the wrongdoer: admit that one could have shown this behavior themselves in a similar situation, explicating state understanding |
| Example: “If my favourite tv show had been on, I would have done the same” | |
| Rel 14 | Compensation: see the wrongdoing as a compensation for a previous action or a fault to be compensated in the future |
| Example: “Last time I forgot to clean the bathroom, now we are even” | |
| Rel 15 | Preserve harmony, protect relationship: desist from anger and confrontation in order not to endanger the relationship with the wrongdoer |
| Example: “This is not worth fighting over, I like living with him too much” | |
| Rel 16 | Trivializing the problem: downplay/deemphasize the harm or harmful intention, also interpret is as external/uncontrollable (fate, bad luck) |
| Example: “It is no big deal, I was not really in the mood to cook anyway” | |
| Rel 17 | Admit partial fault: emphasize on one’s share of blame, focus on what one could have done differently to avoid the situation entirely |
| Example: “It is partly my fault, if I have guests over, it is my responsibility to clean up” | |
| Rel 18 | Devaluation of the wrongdoer: devaluate the wrongdoer as a person by finding them unworthy of anger |
| Example: “He’s not even worth the anger, he is just a general failure” | |
References
- Hu, T.; Zhang, D.; Wang, J.; Mistry, R.; Ran, G.; Wang, X. Relation between emotion regulation and mental health: A meta-analysis review. Psychol. Rep. 2014, 114, 341–362. [Google Scholar] [CrossRef]
- Kalisch, R.; Müller, M.B.; Tüscher, O. A conceptual framework for the neurobiological study of resilience. Behav. Brain Sci. 2015, 38, e92. [Google Scholar] [CrossRef]
- Gross, J.J.; John, O.P. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J. Personal. Soc. Psychol. 2003, 85, 348–362. [Google Scholar] [CrossRef]
- Garnefski, N.; Kraaij, V.; Spinhoven, P. Negative life events, cognitive emotion regulation and emotional problems. Personal. Individ. Differ. 2001, 30, 1311–1327. [Google Scholar] [CrossRef]
- Troy, A.S.; Wilhelm, F.H.; Shallcross, A.J.; Mauss, I.B. Seeing the silver lining: Cognitive reappraisal ability moderates the relationship between stress and depressive symptoms. Emotion 2010, 10, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Riepenhausen, A.; Wackerhagen, C.; Reppmann, Z.C.; Deter, H.-C.; Kalisch, R.; Veer, I.M.; Walter, H. Positive Cognitive Reappraisal in Stress Resilience, Mental Health, and Well-Being: A Comprehensive Systematic Review. Emot. Rev. 2022, 14, 310–331. [Google Scholar] [CrossRef]
- Dryman, M.T.; Heimberg, R.G. Emotion regulation in social anxiety and depression: A systematic review of expressive suppression and cognitive reappraisal. Clin. Psychol. Rev. 2018, 65, 17–42. [Google Scholar] [CrossRef]
- Zilverstand, A.; Parvaz, M.A.; Goldstein, R.Z. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage 2017, 151, 105–116. [Google Scholar] [CrossRef]
- Denny, B.T.; Ochsner, K.N. Behavioral effects of longitudinal training in cognitive reappraisal. Emotion 2014, 14, 425–433. [Google Scholar] [CrossRef]
- Kivity, Y.; Huppert, J.D. Does cognitive reappraisal reduce anxiety? A daily diary study of a micro-intervention with individuals with high social anxiety. J. Consult. Clin. Psychol. 2016, 84, 269–283. [Google Scholar] [CrossRef]
- Ranney, R.M.; Bruehlman-Senecal, E.; Ayduk, O. Comparing the Effects of Three Online Cognitive Reappraisal Trainings on Well-Being. J. Happiness Stud. 2017, 18, 1319–1338. [Google Scholar] [CrossRef]
- Perchtold-Stefan, C.M.; Schertler, M.; Paechter, M.; Fink, A.; Weiss, E.M.; Papousek, I. Learning to be inventive in the face of statistics: A positive reappraisal intervention for statistics anxiety. J. Behav. Ther. Exp. Psychiatry 2023, 82, 101913. [Google Scholar] [CrossRef]
- Buhle, J.T.; Silvers, J.A.; Wager, T.D.; Lopez, R.; Onyemekwu, C.; Kober, H.; Weber, J.; Ochsner, K.N. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex 2014, 24, 2981–2990. [Google Scholar] [CrossRef]
- Opitz, P.C.; Rauch, L.C.; Terry, D.P.; Urry, H.L. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiol. Aging 2012, 33, 645–655. [Google Scholar] [CrossRef]
- Kalisch, R. The functional neuroanatomy of reappraisal: Time matters. Neurosci. Biobehav. Rev. 2009, 33, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.; van Reekum, C.M.; Urry, H.L.; Kalin, N.H.; Davidson, R.J. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007, 27, 8877–8884. [Google Scholar] [CrossRef]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. Neuroimage 2014, 87, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Picó-Pérez, M.; Radua, J.; Steward, T.; Menchón, J.M.; Soriano-Mas, C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Eur. Neuropsychopharmacol. 2017, 27, S693. [Google Scholar] [CrossRef]
- McRae, K.; Gross, J.J.; Weber, J.; Robertson, E.R.; Sokol-Hessner, P.; Ray, R.D.; Gabrieli, J.D.E.; Ochsner, K.N. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012, 7, 11–22. [Google Scholar] [CrossRef]
- Papousek, I.; Weiss, E.M.; Perchtold, C.M.; Weber, H.; de Assunção, V.L.; Schulter, G.; Lackner, H.K.; Fink, A. The capacity for generating cognitive reappraisals is reflected in asymmetric activation of frontal brain regions. Brain Imaging Behav. 2017, 11, 577–590. [Google Scholar] [CrossRef]
- Messina, I.; Bianco, S.; Sambin, M.; Viviani, R. Executive and semantic processes in reappraisal of negative stimuli: Insights from a meta-analysis of neuroimaging studies. Front. Psychol. 2015, 6, 145523. [Google Scholar] [CrossRef]
- Morawetz, C.; Bode, S.; Baudewig, J.; Jacob, A.M.; Heekeren, H.R. Neural representation of emotion regulation goals. Hum. Brain Mapp. 2016, 37, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Perchtold, C.M.; Papousek, I.; Koschutnig, K.; Rominger, C.; Weber, H.; Weiss, E.M.; Fink, A. Affective creativity meets classic creativity in the scanner. Hum. Brain Mapp. 2018, 39, 393–406. [Google Scholar] [CrossRef]
- He, Z.; Li, S.; Mo, L.; Zheng, Z.; Li, Y.; Li, H.; Zhang, D. The VLPFC-Engaged Voluntary Emotion Regulation: Combined TMS-fMRI Evidence for the Neural Circuit of Cognitive Reappraisal. J. Neurosci. 2023, 43, 6046–6060. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.M.; Morello, L.Y.N.; Boggio, P.S. Ventrolateral but not Dorsolateral Prefrontal Cortex tDCS effectively impact emotion reappraisal—Effects on Emotional Experience and Interbeat Interval. Sci. Rep. 2018, 8, 15295. [Google Scholar] [CrossRef]
- Steward, T.; Martínez-Zalacaín, I.; Mestre-Bach, G.; Sánchez, I.; Riesco, N.; Jiménez-Murcia, S.; Fernández-Formoso, J.A.; Veciana de las Heras, M.; Custal, N.; Menchón, J.M.; et al. Dorsolateral prefrontal cortex and amygdala function during cognitive reappraisal predicts weight restoration and emotion regulation impairment in anorexia nervosa. Psychol. Med. 2020, 52, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mo, L.; Bi, R.; He, Z.; Chen, Y.; Xu, F.; Xie, H.; Zhang, D. The VLPFC versus the DLPFC in Downregulating Social Pain Using Reappraisal and Distraction Strategies. J. Neurosci. 2021, 41, 1331–1339. [Google Scholar] [CrossRef]
- Cao, D.; Qian, Z.; Tang, Y.; Wang, J.; Jiang, T.; Li, Y. Neural indicator of positive reappraisal: A TMS-EEG study over the left VLPFC. J. Affect. Disord. 2022, 300, 418–429. [Google Scholar] [CrossRef]
- Cheng, S.; Qiu, X.; Li, S.; Mo, L.; Xu, F.; Zhang, D. Different Roles of the Left and Right Ventrolateral Prefrontal Cortex in Cognitive Reappraisal: An Online Transcranial Magnetic Stimulation Study. Front. Hum. Neurosci. 2022, 16, 928077. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Cao, D.; Qian, Z.; Tang, Y.; Wang, J. TMS-EEG signatures of facilitated cognitive reappraisal in emotion regulation by left ventrolateral prefrontal cortex stimulation. Neuropsychologia 2023, 184, 108560. [Google Scholar] [CrossRef]
- Allen, J.J.B.; Reznik, S.J. Frontal EEG asymmetry as a promising marker of depression vulnerability: Summary and methodological considerations. Curr. Opin. Psychol. 2015, 4, 93–97. [Google Scholar] [CrossRef]
- Peterson, C.K.; Gravens, L.C.; Harmon-Jones, E. Asymmetric frontal cortical activity and negative affective responses to ostracism. Soc. Cogn. Affect. Neurosci. 2010, 6, 277–285. [Google Scholar] [CrossRef]
- Papousek, I.; Reiser, E.M.; Weber, B.; Freudenthaler, H.H.; Schulter, G. Frontal brain asymmetry and affective flexibility in an emotional contagion paradigm. Psychophysiology 2012, 49, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Reznik, S.J.; Allen, J.J.B. Frontal asymmetry as a mediator and moderator of emotion: An updated review. Psychophysiology 2018, 55, e12965. [Google Scholar] [CrossRef] [PubMed]
- Blackhart, G.C.; Kline, J.P. Individual differences in anterior EEG asymmetry between high and low defensive individuals during a rumination/distraction task. Personal. Individ. Differ. 2005, 39, 427–437. [Google Scholar] [CrossRef]
- Goodman, R.N.; Rietschel, J.C.; Lo, L.-C.; Costanzo, M.E.; Hatfield, B.D. Stress, emotion regulation and cognitive performance: The predictive contributions of trait and state relative frontal EEG alpha asymmetry. Int. J. Psychophysiol. 2013, 87, 115–123. [Google Scholar] [CrossRef]
- Jackson, D.C.; Mueller, C.J.; Dolski, I.; Dalton, K.M.; Nitschke, J.B.; Urry, H.L.; Rosenkranz, M.A.; Ryff, C.D.; Singer, B.H.; Davidson, R.J. Now You Feel It, Now You Don’t: Frontal Brain Electrical Asymmetry and Individual Differences in Emotion Regulation. Psychol. Sci. 2003, 14, 612–617. [Google Scholar] [CrossRef]
- Lopez-Duran, N.L.; Nusslock, R.; George, C.; Kovacs, M. Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology 2012, 49, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Papousek, I.; Weiss, E.M.; Schulter, G.; Fink, A.; Reiser, E.M.; Lackner, H.K. Prefrontal EEG alpha asymmetry changes while observing disaster happening to other people: Cardiac correlates and prediction of emotional impact. Biol. Psychol. 2014, 103, 184–194. [Google Scholar] [CrossRef]
- Dillon, D.G.; Pizzagalli, D.A. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Res. Neuroimaging 2013, 212, 99–107. [Google Scholar] [CrossRef]
- Dörfel, D.; Lamke, J.-P.; Hummel, F.; Wagner, U.; Erk, S.; Walter, H. Common and differential neural networks of emotion regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: A comparative fMRI investigation. Neuroimage 2014, 101, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1–E24. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Paul, B.; Schneider, W.; Siegle, G.J. Neural Correlates of Three Neurocognitive Intervention Strategies: A Preliminary Step Towards Personalized Treatment for Psychological Disorders. Cogn. Ther. Res. 2012, 37, 657–672. [Google Scholar] [CrossRef]
- Choi, D.; Sekiya, T.; Minote, N.; Watanuki, S. Relative left frontal activity in reappraisal and suppression of negative emotion: Evidence from frontal alpha asymmetry (FAA). Int. J. Psychophysiol. 2016, 109, 37–44. [Google Scholar] [CrossRef]
- Parvaz, M.A.; MacNamara, A.; Goldstein, R.Z.; Hajcak, G. Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cogn. Affect. Behav. Neurosci. 2012, 12, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Falquez, R.; Couto, B.; Ibanez, A.; Freitag, M.T.; Berger, M.; Arens, E.A.; Lang, S.; Barnow, S. Detaching from the negative by reappraisal: The role of right superior frontal gyrus (BA9/32). Front. Behav. Neurosci. 2014, 8, 165. [Google Scholar] [CrossRef]
- He, Z.; Lin, Y.; Xia, L.; Liu, Z.; Zhang, D.; Elliott, R. Critical role of the right VLPFC in emotional regulation of social exclusion: A tDCS study. Soc. Cogn. Affect. Neurosci. 2018, 13, 357–366. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Ray, R.D.; Cooper, J.C.; Robertson, E.R.; Chopra, S.; Gabrieli, J.D.E.; Gross, J.J. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 2004, 23, 483–499. [Google Scholar] [CrossRef]
- Silvers, J.A.; Weber, J.; Wager, T.D.; Ochsner, K.N. Bad and worse: Neural systems underlying reappraisal of high- and low-intensity negative emotions. Soc. Cogn. Affect. Neurosci. 2015, 10, 172–179. [Google Scholar] [CrossRef]
- Chen, L.; Oei, T.P.; Zhou, R. The cognitive control mechanism of improving emotion regulation: A high-definition tDCS and ERP study. J. Affect. Disord. 2023, 332, 19–28. [Google Scholar] [CrossRef]
- McRae, K.; Ciesielski, B.; Gross, J.J. Unpacking cognitive reappraisal: Goals, tactics, and outcomes. Emotion 2012, 12, 250–255. [Google Scholar] [CrossRef]
- Shiota, M.N.; Levenson, R.W. Turn down the volume or change the channel? Emotional effects of detached versus positive reappraisal. J. Personal. Soc. Psychol. 2012, 103, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Vishkin, A.; Hasson, Y.; Millgram, Y.; Tamir, M. One Size Does Not Fit All: Tailoring Cognitive Reappraisal to Different Emotions. Pers. Soc. Psychol. Bull. 2020, 46, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Silvers, J.A.; Guassi Moreira, J.F. Capacity and tendency: A neuroscientific framework for the study of emotion regulation. Neurosci. Lett. 2019, 693, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Loureiro de Assunção, V.; Martin, C.; Westmeyer, H.; Geisler, F.C. Reappraisal inventiveness: The ability to create different reappraisals of critical situations. Cogn. Emot. 2014, 28, 345–360. [Google Scholar] [CrossRef]
- Rowlands, L.; Coetzer, R.; Turnbull, O.H. Good things better? Reappraisal and discrete emotions in acquired brain injury. Neuropsychol. Rehabil. 2020, 30, 1947–1975. [Google Scholar] [CrossRef]
- Perchtold, C.M.; Papousek, I.; Fink, A.; Weber, H.; Rominger, C.; Weiss, E.M. Gender Differences in Generating Cognitive Reappraisals for Threatening Situations: Reappraisal Capacity Shields Against Depressive Symptoms in Men, but Not Women. Front. Psychol. 2019, 10, 430348. [Google Scholar] [CrossRef]
- Demaree, H.A.; Robinson, J.L.; Pu, J.; Allen, J.J.B. Strategies actually employed during response-focused emotion regulation research: Affective and physiological consequences. Cogn. Emot. 2006, 20, 1248–1260. [Google Scholar] [CrossRef]
- Opitz, P.C.; Cavanagh, S.R.; Urry, H.L. Uninstructed emotion regulation choice in four studies of cognitive reappraisal. Personal. Individ. Differ. 2015, 86, 455–464. [Google Scholar] [CrossRef]
- Perchtold, C.M.; Fink, A.; Rominger, C.; Weber, H.; de Assunção, V.L.; Schulter, G.; Weiss, E.M.; Papousek, I. Reappraisal inventiveness: Impact of appropriate brain activation during efforts to generate alternative appraisals on the perception of chronic stress in women. Anxiety Stress Coping 2018, 31, 206–221. [Google Scholar] [CrossRef]
- Perchtold, C.M.; Weiss, E.M.; Rominger, C.; Fink, A.; Weber, H.; Papousek, I. Cognitive reappraisal capacity mediates the relationship between prefrontal recruitment during reappraisal of anger-eliciting events and paranoia-proneness. Brain Cogn. 2019, 132, 108–117. [Google Scholar] [CrossRef]
- Perchtold-Stefan, C.M.; Fink, A.; Rominger, C.; Weiss, E.M.; Papousek, I. More habitual physical activity is linked to the use of specific, more adaptive cognitive reappraisal strategies in dealing with stressful events. Stress Health 2020, 36, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Badre, D.; Wagner, A.D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 2007, 45, 2883–2901. [Google Scholar] [CrossRef] [PubMed]
- Malooly, A.M.; Genet, J.J.; Siemer, M. Individual differences in reappraisal effectiveness: The role of affective flexibility. Emotion 2013, 13, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Zirngast, J. A Spark of Hope: Can Positive Thinking Help to Calm Down?—A Short RIT Intervention. Master’s Thesis, University of Graz, Graz, Austria, 2019. [Google Scholar]
- Schertler, M. Introducing a Reappraisal Inventiveness Intervention for Statistics Anxiety: Effects on Emotion Regulation, Anxiety, and EEG Alpha Asymmetry. Master’s Thesis, University of Graz, Graz, Austria, 2020. [Google Scholar]
- Bauer, J. How Thinking Shapes Feeling: Reappraisal Strategies and Their Associations with EEG Alpha Asymmetry, Motivational Traits, and Attentional Control. Master’s Thesis, University of Graz, Graz, Austria, 2020. [Google Scholar]
- Shiota, M.N.; Levenson, R.W. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychol. Aging 2009, 24, 890–900. [Google Scholar] [CrossRef]
- Willroth, E.C.; Hilimire, M.R. Differential effects of self- and situation-focused reappraisal. Emotion 2016, 16, 468–474. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Bunge, S.A.; Gross, J.J.; Gabrieli, J.D.E. Rethinking Feelings: An fMRI Study of the Cognitive Regulation of Emotion. J. Cogn. Neurosci. 2002, 14, 1215–1229. [Google Scholar] [CrossRef]
- Moskowitz, J.T.; Hult, J.R.; Bussolari, C.; Acree, M. What works in coping with HIV? A meta-analysis with implications for coping with serious illness. Psychol. Bull. 2009, 135, 121–141. [Google Scholar] [CrossRef]
- Rompilla, D.B.; Hittner, E.F.; Stephens, J.E.; Mauss, I.; Haase, C.M. Emotion regulation in the face of loss: How detachment, positive reappraisal, and acceptance shape experiences, physiology, and perceptions in late life. Emotion 2022, 22, 1417–1434. [Google Scholar] [CrossRef]
- Nowlan, J.S.; Wuthrich, V.M.; Rapee, R.M. Positive reappraisal in older adults: A systematic literature review. Aging Ment. Health 2015, 19, 475–484. [Google Scholar] [CrossRef]
- Troy, A.S.; Shallcross, A.J.; Brunner, A.; Friedman, R.; Jones, M.C. Cognitive reappraisal and acceptance: Effects on emotion, physiology, and perceived cognitive costs. Emotion 2018, 18, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.P.; LaBar, K.S. Regulating emotion through distancing: A taxonomy, neurocognitive model, and supporting meta-analysis. Neurosci. Biobehav. Rev. 2019, 96, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Li, Y.; Tang, Y. Functional specificity of the left ventrolateral prefrontal cortex in positive reappraisal: A single-pulse transcranial magnetic stimulation study. Cogn. Affect. Behav. Neurosci. 2021, 21, 793–804. [Google Scholar] [CrossRef]
- Koenigsberg, H.W.; Fan, J.; Ochsner, K.N.; Liu, X.; Guise, K.; Pizzarello, S.; Dorantes, C.; Tecuta, L.; Guerreri, S.; Goodman, M.; et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia 2010, 48, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Perchtold, C.M.; Weiss, E.M.; Rominger, C.; Feyaerts, K.; Ruch, W.; Fink, A.; Papousek, I. Humorous cognitive reappraisal: More benign humour and less “dark” humour is affiliated with more adaptive cognitive reappraisal strategies. PLoS ONE 2019, 14, e0211618. [Google Scholar] [CrossRef]
- Steingrüber, H.J.; Lienert, G.A. Hand-Dominanz-Test; Hogrefe: Göttingen, Germany, 1971. [Google Scholar]
- Hautzinger, M.; Bailer, M. Allgemeine Depressionsskala: ADS Manual; Beltz-Test-GmbH: Göttingen, Germany, 1993. [Google Scholar]
- De Assunção, V.L. Reappraisal als Fähigkeit-Entwicklung des Reappraisal Inventiveness Test. Ph.D. Thesis, University of Greifswald, Greifswald, Germany, 2017. [Google Scholar]
- Hagemann, D. Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biol. Psychol. 2004, 67, 157–182. [Google Scholar] [CrossRef]
- Allen, J.J.; Coan, J.A.; Nazarian, M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol. 2004, 67, 183–218. [Google Scholar] [CrossRef]
- Harmon-Jones, E. Unilateral right-hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology 2006, 43, 598–603. [Google Scholar] [CrossRef]
- Scheeringa, R.; Fries, P.; Petersson, K.-M.; Oostenveld, R.; Grothe, I.; Norris, D.G.; Hagoort, P.; Bastiaansen, M.C. Neuronal Dynamics Underlying High- and Low-Frequency EEG Oscillations Contribute Independently to the Human BOLD Signal. Neuron 2011, 69, 572–583. [Google Scholar] [CrossRef]
- Papousek, I.; Wimmer, S.; Lackner, H.K.; Schulter, G.; Perchtold, C.M.; Paechter, M. Trait positive affect and students’ prefrontal EEG alpha asymmetry responses during a simulated exam situation. Biol. Psychol. 2019, 148, 107762. [Google Scholar] [CrossRef]
- Pivik, R.T.; Broughton, R.J.; Coppola, R.; Davidson, R.J.; Fox, N.; Nuwer, M.R. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology 1993, 30, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Klaus, J.; Schutter, D.J.L.G. The Role of Left Dorsolateral Prefrontal Cortex in Language Processing; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2017. [Google Scholar]
- Sheppes, G.; Scheibe, S.; Suri, G.; Radu, P.; Blechert, J.; Gross, J.J. Emotion regulation choice: A conceptual framework and supporting evidence. J. Exp. Psychol. Gen. 2014, 143, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.; Wang, H.T.; Murphy, C.; Ho, N.S.P.; Wang, X.; Sormaz, M.; Karapanagiotidis, T.; Leech, R.M.; Bernhardt, B.; Margulies, D.S.; et al. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nat. Commun. 2019, 10, 3816. [Google Scholar] [CrossRef]
- Kaller, C.P.; Rahm, B.; Spreer, J.; Weiller, C.; Unterrainer, J.M. Dissociable Contributions of Left and Right Dorsolateral Prefrontal Cortex in Planning. Cereb. Cortex 2011, 21, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef]
- Feeser, M.; Prehn, K.; Kazzer, P.; Mungee, A.; Bajbouj, M. Transcranial Direct Current Stimulation Enhances Cognitive Control During Emotion Regulation. Brain Stimul. 2014, 7, 105–112. [Google Scholar] [CrossRef]
- Vanderhasselt, M.-A.; Baeken, C.; van Schuerbeek, P.; Luypaert, R.; de Raedt, R. Inter-individual differences in the habitual use of cognitive reappraisal and expressive suppression are associated with variations in prefrontal cognitive control for emotional information: An event related fMRI study. Biol. Psychol. 2013, 92, 433–439. [Google Scholar] [CrossRef]
- Rottschy, C.; Langner, R.; Dogan, I.; Reetz, K.; Laird, A.R.; Schulz, J.B.; Fox, P.T.; Eickhoff, S.B. Modelling neural correlates of working memory: A coordinate-based meta-analysis. NeuroImage 2012, 60, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.B.; Kuppens, P.; Sheeber, L.B. Heart rate responses to parental behavior in depressed adolescents. Biol. Psychol. 2012, 90, 80–87. [Google Scholar] [CrossRef]
- Papousek, I.; Schulter, G.; Rominger, C.; Fink, A.; Weiss, E.M. The fear of other persons’ laughter: Poor neuronal protection against social signals of anger and aggression. Psychiatry Res. 2016, 235, 61–68. [Google Scholar] [CrossRef]
- Ford, B.Q.; Gross, J.J. Emotion regulation: Why beliefs matter. Can. Psychol. Psychol. Can. 2018, 59, 1–14. [Google Scholar] [CrossRef]
- Veilleux, J.C.; Hyde, K.C.; Chamberlain, K.D.; Higuera, D.E.; Schreiber, R.E.; Warner, E.A.; Clift, J.B. The “thinking threshold”: A therapeutic concept guided by emotion regulation flexibility. Pract. Innov. 2022, 7, 28–39. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Gable, P.A.; Peterson, C.K. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biol. Psychol. 2010, 84, 451–462. [Google Scholar] [CrossRef]
- Kelley, N.J.; Hortensius, R.; Schutter, D.J.; Harmon-Jones, E. The relationship of approach/avoidance motivation and asymmetric frontal cortical activity: A review of studies manipulating frontal asymmetry. Int. J. Psychophysiol. 2017, 119, 19–30. [Google Scholar] [CrossRef]
- Shackman, A.J.; McMenamin, B.W.; Maxwell, J.S.; Greischar, L.L.; Davidson, R.J. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychol. Sci. 2009, 20, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Vanderhasselt, M.-A.; de Raedt, R.; Baeken, C. Dorsolateral prefrontal cortex and Stroop performance: Tackling the lateralization. Psychon. Bull. Rev. 2009, 16, 609–612. [Google Scholar] [CrossRef]
- Moore, M.; Iordan, A.D.; Hu, Y.; Kragel, J.E.; Dolcos, S.; Dolcos, F. Localized or diffuse: The link between prefrontal cortex volume and cognitive reappraisal. Soc. Cogn. Affect. Neurosci. 2016, 11, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Charness, G.; Gneezy, U.; Kuhn, M.A. Experimental methods: Between-subject and within-subject design. J. Econ. Behav. Organ. 2012, 81, 1–8. [Google Scholar] [CrossRef]
- Wu, X.; Guo, T.; Tan, T.; Zhang, W.; Qin, S.; Fan, J.; Luo, J. Superior emotional regulating effects of creative cognitive reappraisal. NeuroImage 2019, 200, 540–551. [Google Scholar] [CrossRef]
- Katzir, M.; Eyal, T. When stepping outside the self is not enough: A self-distanced perspective reduces the experience of basic but not of self-conscious emotions. J. Exp. Soc. Psychol. 2013, 49, 1089–1092. [Google Scholar] [CrossRef]
- Theurel, A.; Gentaz, E. The regulation of emotions in adolescents: Age differences and emotion-specific patterns. PLoS ONE 2018, 13, e0195501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).