Design, Synthesis and Cytotoxicity Screening of New Thiazole Derivatives as Potential Anticancer Agents through VEGFR-2 Inhibition
Abstract
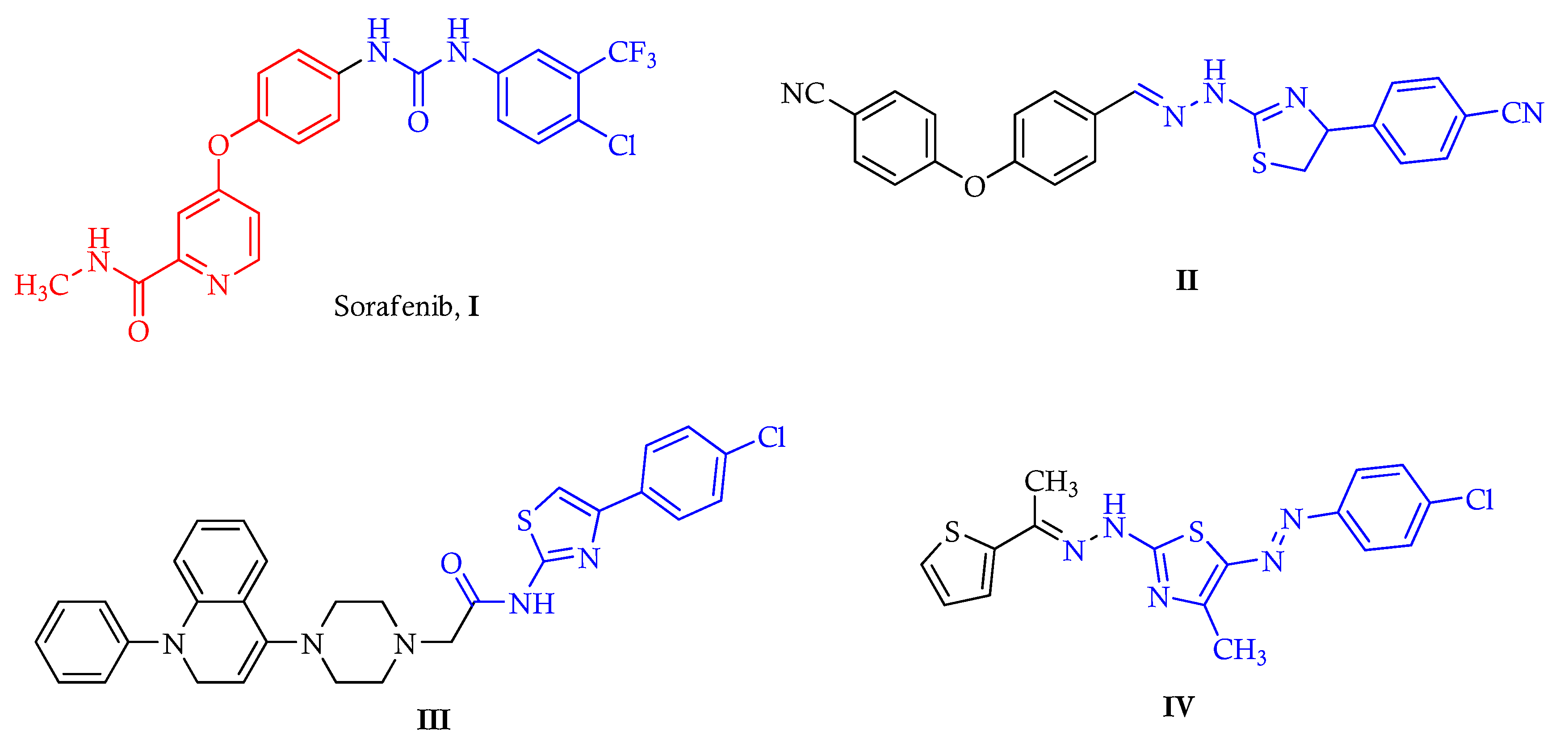
:1. Introduction
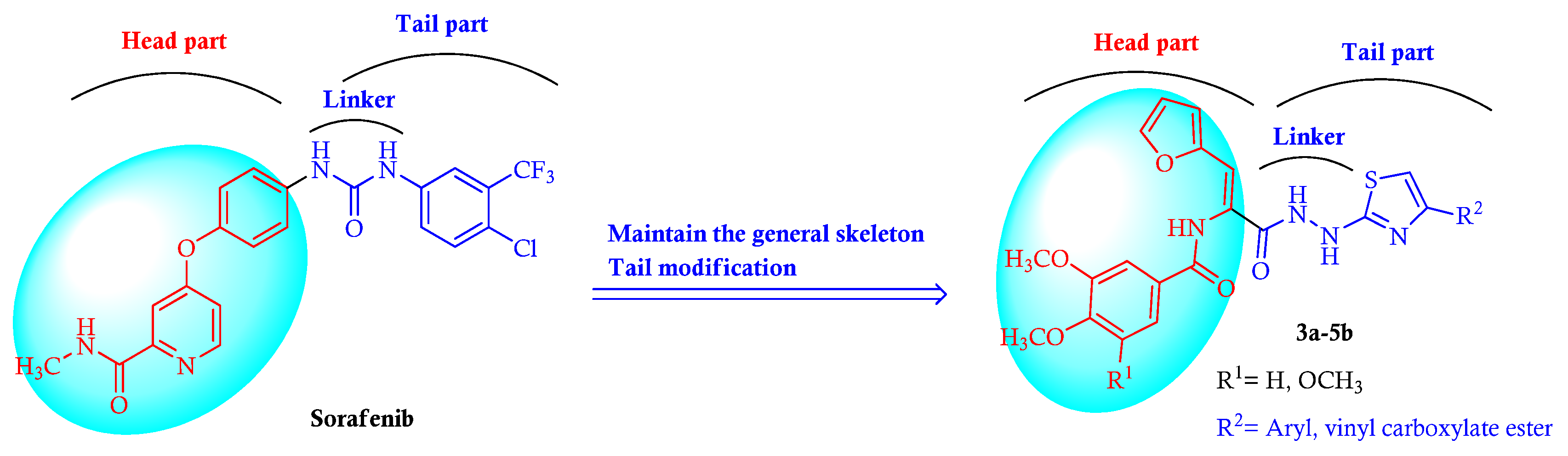
2. Results and Discussion
2.1. Chemistry
2.2. Biology
2.2.1. Cytotoxic Activity against MDA-MB-231 Breast Cancer Cell Line
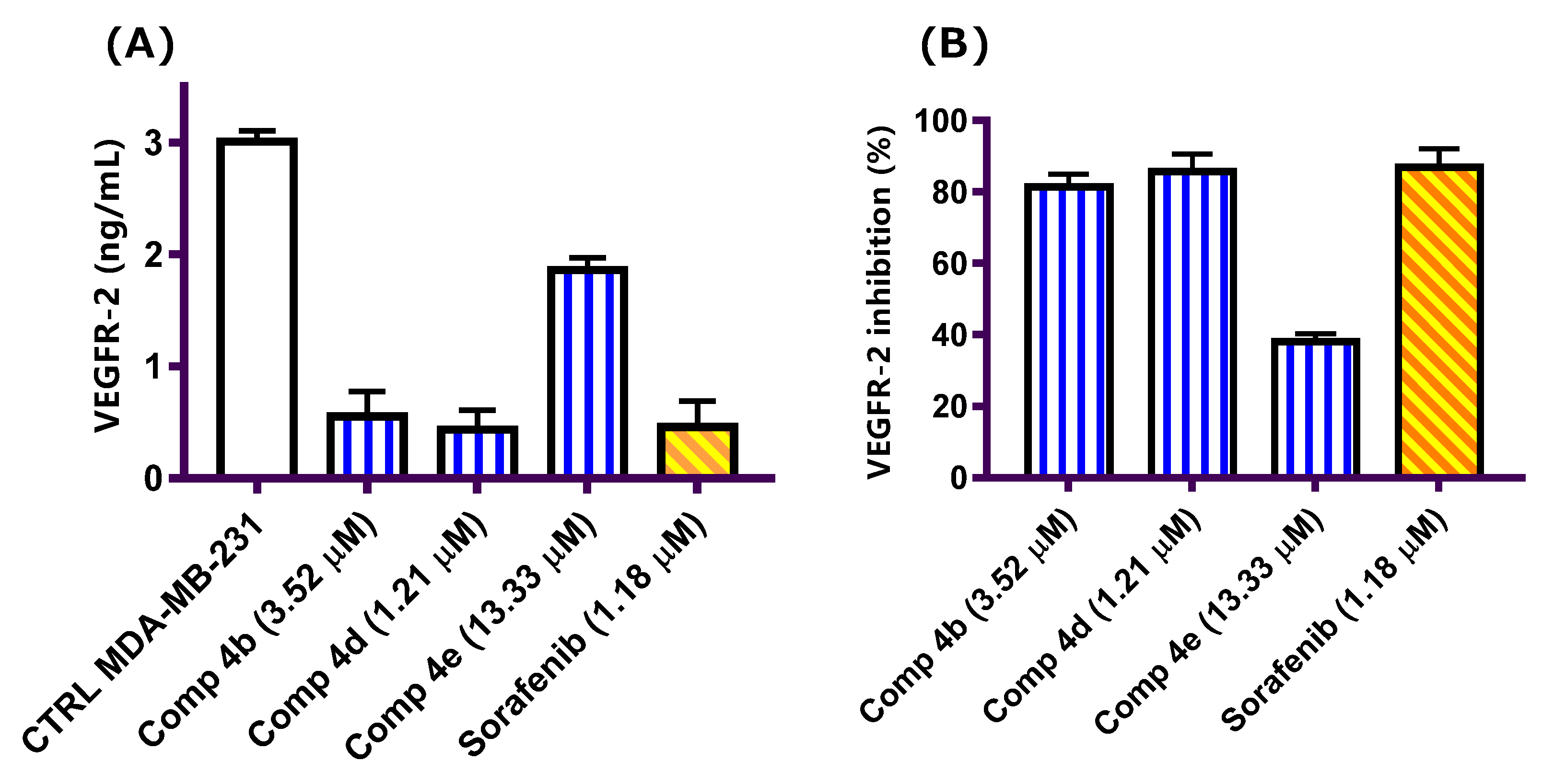
2.2.2. Vascular Endothelium Growth Factor-2 (VEGFR-2) Inhibitory Activity
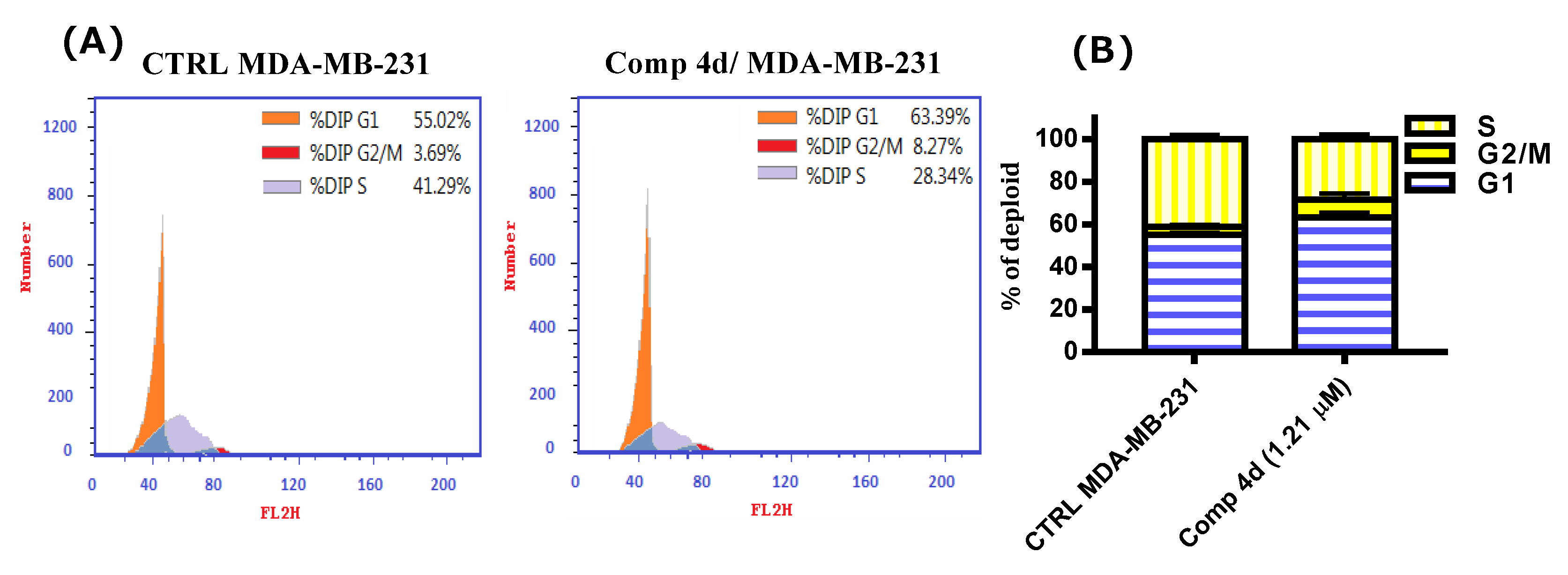
2.2.3. Cell Cycle Analysis of Compound 4d
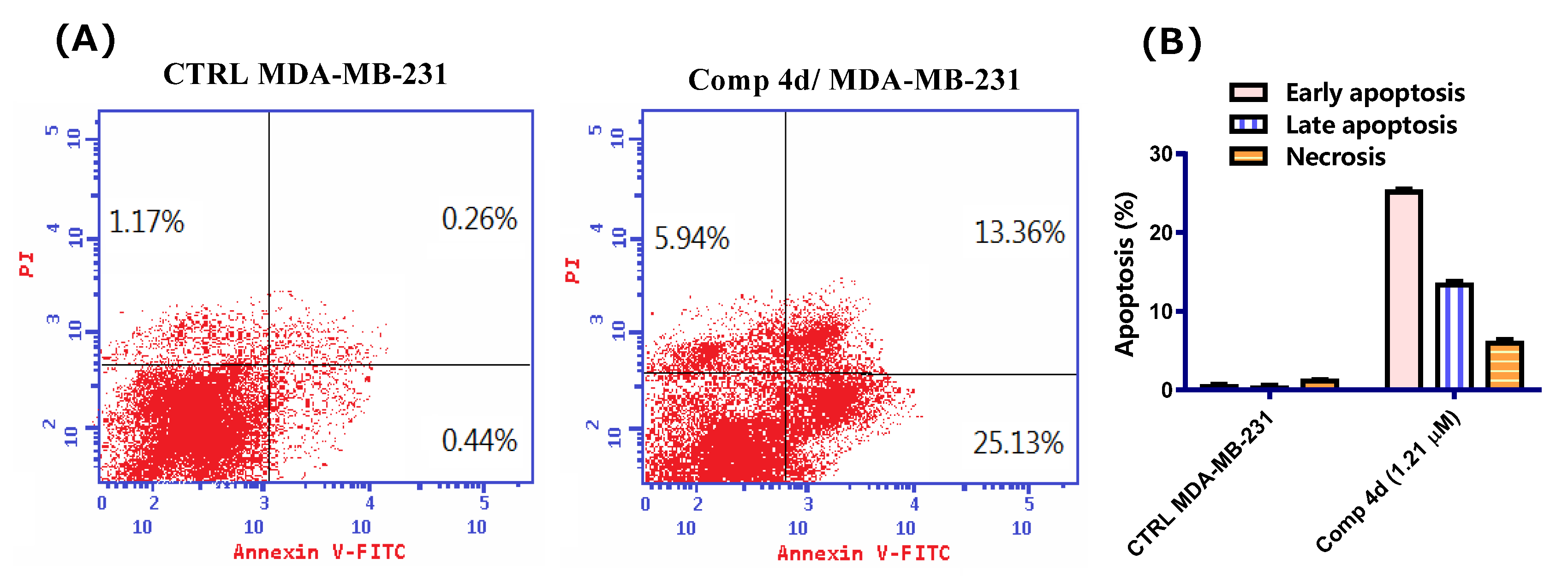
2.2.4. Annexin V/FITC Apoptosis Staining Assay
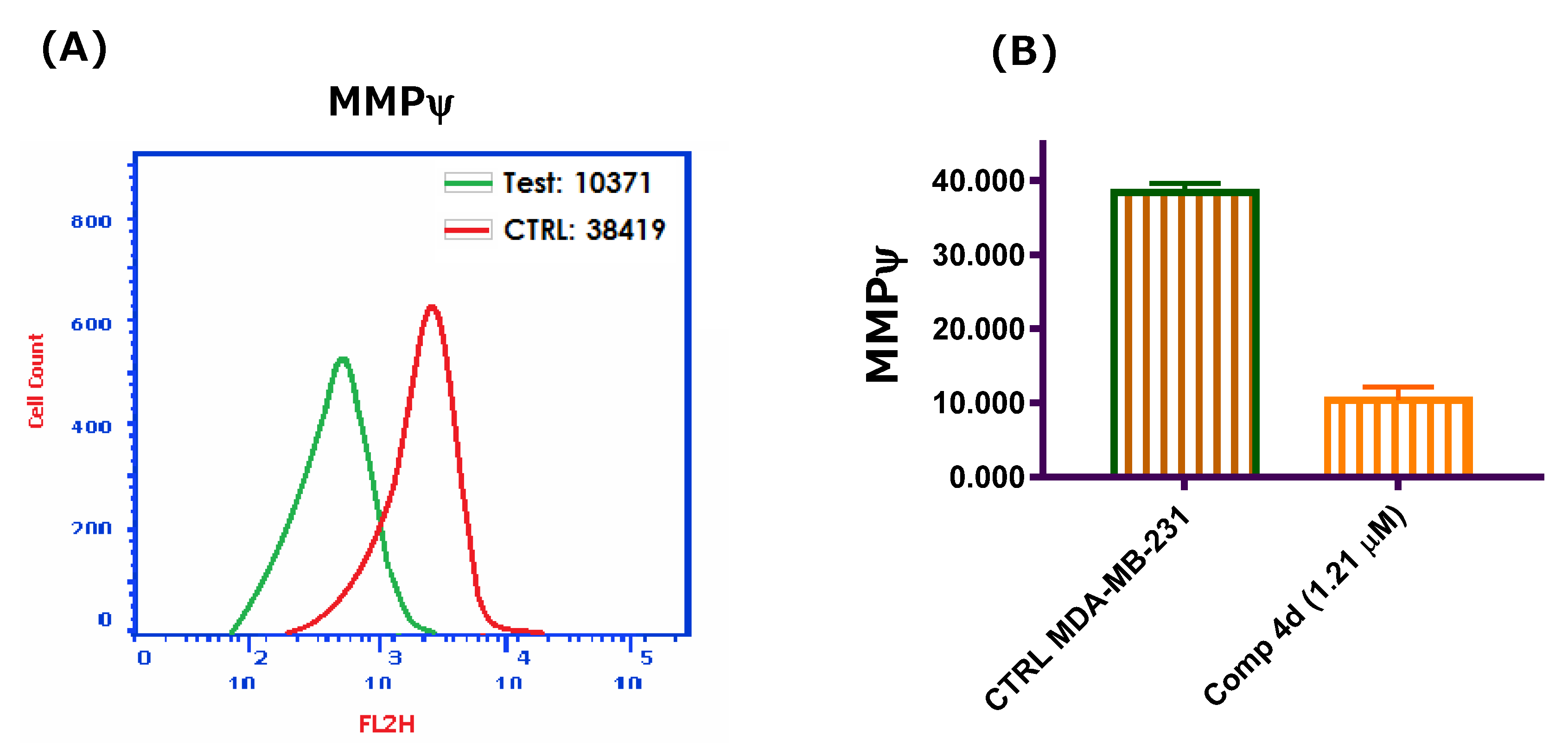
2.2.5. Mitochondrial Membrane Potential (MMP)
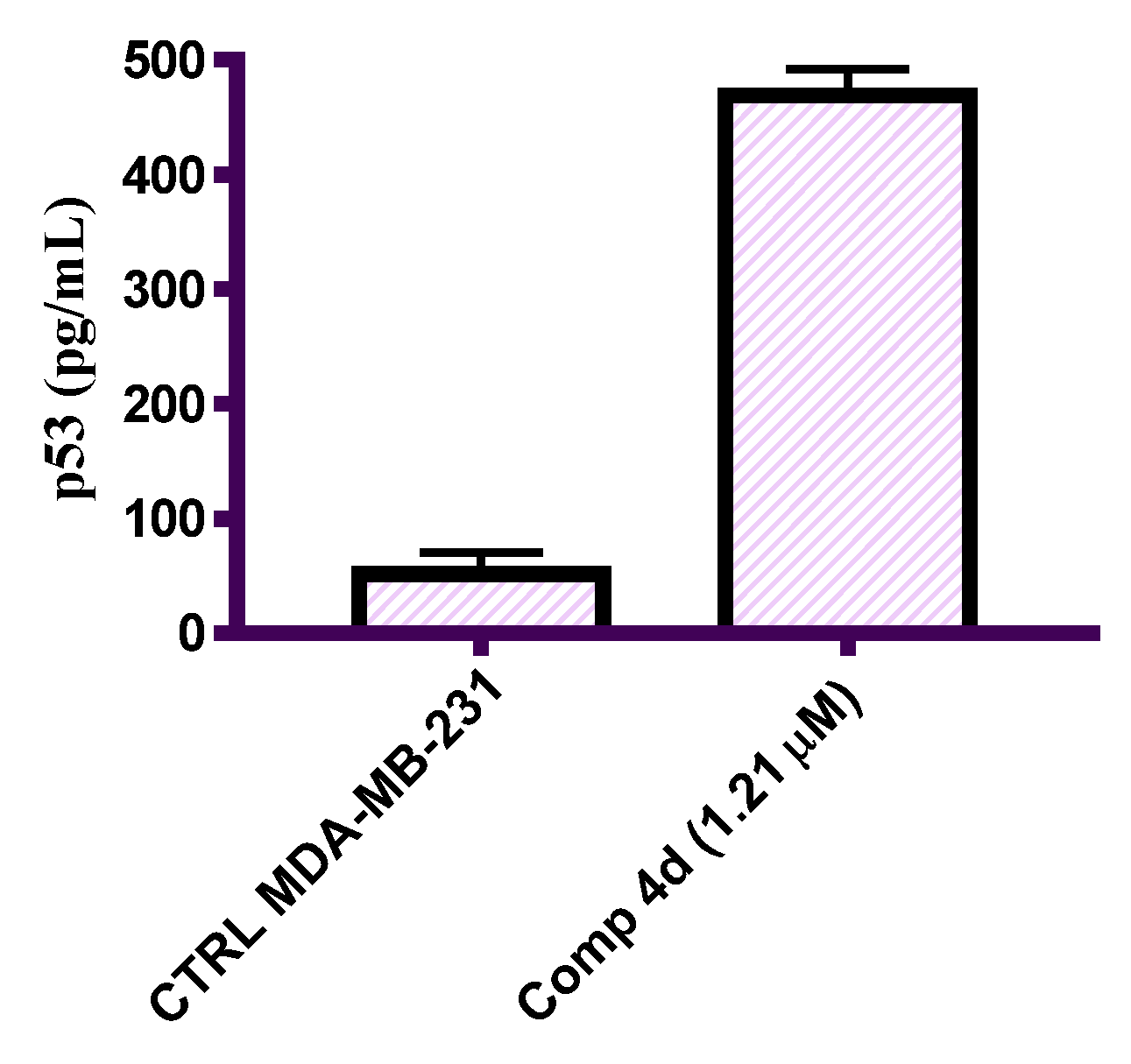
2.2.6. In Vitro ELISA Measurement of the Level of p53
2.2.7. Molecular Docking Study
3. Conclusions
4. Experimental Methods
4.1. Chemistry
4.1.1. General
4.1.2. General Procedure for the Synthesis of (Z)-N-(3-(2-Carbamothioylhydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)arylamides (Z)-2a,b
(Z)-N-(3-(2-Carbamothioylhydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)-3,4-dimethoxybenzamide ((Z)-2a)
(Z)-N-(3-(2-Carbamothioylhydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide ((Z)-2b)
4.1.3. General Procedure for the Synthesis of (Z)-N-(3-(2-(4-Arylthiazol-2-yl)hydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)arylamides 3a-4e
(Z)-N-(3-(2-(4-(4-Chlorophenyl)thiazol-2-yl)hydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)-3,4-dimethoxybenzamide (3a)
(Z)-N-(3-(2-(4-(4-Bromophenyl)thiazol-2-yl)hydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)-3,4-dimethoxybenzamide (3b)
(Z)-N-(1-(Furan-2-yl)-3-(2-(4-(3-nitrophenyl)thiazol-2-yl)hydrazinyl)-3-oxoprop-1-en-2-yl)-3,4-dimethoxybenzamide (3c)
(Z)-N-(1-(Furan-2-yl)-3-oxo-3-(2-(4-phenylthiazol-2-yl)hydrazinyl)prop-1-en-2-yl)-3,4,5-trimethoxybenzamide (4a)
(Z)-N-(3-(2-(4-(4-Chlorophenyl)thiazol-2-yl)hydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (4b)
(Z)-N-(3-(2-(4-(4-Bromophenyl)thiazol-2-yl)hydrazinyl)-1-(furan-2-yl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (4c)
(Z)-N-(1-(Furan-2-yl)-3-(2-(4-(3-nitrophenyl)thiazol-2-yl)hydrazinyl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (4d)
(Z)-N-(1-(Furan-2-yl)-3-(2-(4-(3-methoxyphenyl)thiazol-2-yl)hydrazinyl)-3-oxoprop-1-en-2-yl)-3,4,5-trimethoxybenzamide (4e)
4.1.4. General Procedure for the Synthesis of (E)-Ethyl 2-(2-(2-((Z)-2-(arylamido)-3-(furan-2-yl)acryloyl)hydrazinyl)thiazol-4(5H)-ylidene)acetates 5a,b
(E)-Ethyl 2-(2-(2-((Z)-2-(3,4-dimethoxybenzamido)-3-(furan-2-yl)acryloyl)hydrazinyl)thiazol-4(5H)-ylidene)acetate (5a)
(E)-Ethyl 2-(2-(2-((Z)-3-(furan-2-yl)-2-(3,4,5-trimethoxybenzamido)acryloyl)hydrazinyl)thiazol-4(5H)-ylidene)acetate (5b)
4.2. Biological Study
4.2.1. MTT Cytotoxicity Assay
4.2.2. VEGFR-2 Inhibition Assay
4.2.3. Cell Cycle Analysis
4.2.4. Annexin V/FITC Staining Assay
4.2.5. Mitochondrial Membrane Potential
4.2.6. Effect on p53, Bax and Bcl-2
4.2.7. Molecular Docking Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Joufi, F.; Setia, A.; Salem-Bekhit, M.; Sahu, R.; Alqahtani, F.; Widyowati, R.; Aleanizy, F. Molecular Pathogenesis of Colorectal Cancer with an Emphasis on Recent Advances in Biomarkers, as Well as Nanotechnology-Based Diagnostic and Therapeutic Approaches. Nanomaterials 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- McNevin, C.S.; Cadoo, K.; Baird, A.-M.; Murchan, P.; Sheils, O.; McDermott, R.; Finn, S. Pathogenic BRCA Variants as Biomarkers for Risk in Prostate Cancer. Cancers 2021, 13, 5697. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.K.; Liu, Z.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; et al. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef]
- Klement, G.; Huang, P.; Mayer, B.; Green, S.K.; Man, S.; Bohlen, P.; Hicklin, D.; Kerbel, R.S. Differences in Therapeutic Indexes of Combination Metronomic Chemotherapy and an Anti-VEGFR-2 Antibody in Multidrug-resistant Human Breast Cancer Xenografts1. Clin. Cancer Res. 2002, 8, 221–232. [Google Scholar] [PubMed]
- Shaikh, S.S.; Emens, L.A. Current and emerging biologic therapies for triple negative breast cancer. Expert Opin. Biol. Ther. 2022, 22, 591–602. [Google Scholar] [CrossRef]
- Abdalla, Y.O.A.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, e5794350. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Formica, M.L.; Alfonso, H.G.A.; Palma, S.D. Biological drug therapy for ocular angiogenesis: Anti-VEGF agents and novel strategies based on nanotechnology. Pharmacol. Res. Perspect. 2021, 9, e00723. [Google Scholar] [CrossRef]
- Das, R.; Choithramani, A.; Shard, A. A molecular perspective for the use of type IV tyrosine kinase inhibitors as anticancer therapeutics. Drug Discov. Today 2022, 27, 808–821. [Google Scholar] [CrossRef]
- Cordover, E.; Minden, A.; Lehman, S.; Zhao, O. Signaling pathways downstream to receptor tyrosine kinases: Targets for cancer treatment. J. Cancer Metastasis Treat. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545–112558. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Pathmanathan, S.; Snider, J.; Lyakisheva, A.; Wong, V.; Stagljar, I. Receptor tyrosine kinases and cancer: Oncogenic mechanisms and therapeutic approaches. Oncogene 2021, 40, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Liu, C.-F.; Rao, G.-W. Anti-angiogenic Agents: A Review on Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) Inhibitors. Curr. Med. Chem. 2021, 28, 2540–2564. [Google Scholar] [CrossRef]
- Osude, C.; Lin, L.; Patel, M.; Eckburg, A.; Berei, J.; Kuckovic, A.; Dube, N.; Rastogi, A.; Gautam, S.; Smith, T.J.; et al. Mediating EGFR-TKI Resistance by VEGF/VEGFR Autocrine Pathway in Non-Small Cell Lung Cancer. Cells 2022, 11, 1694. [Google Scholar] [CrossRef]
- Toaldo, M.B.; Salvatore, V.; Marinelli, S.; Palamà, C.; Milazzo, M.; Croci, L.; Venerandi, L.; Cipone, M.; Bolondi, L.; Piscaglia, F. Use of VEGFR-2 Targeted Ultrasound Contrast Agent for the Early Evaluation of Response to Sorafenib in a Mouse Model of Hepatocellular Carcinoma. Mol. Imaging Biol. 2015, 17, 29–37. [Google Scholar] [CrossRef] [PubMed]
- AbdElhameid, M.K.; Labib, M.B.; Negmeldin, A.T.; Al-Shorbagy, M.; Mohammed, M.R. Design, synthesis, and screening of ortho-amino thiophene carboxamide derivatives on hepatocellular carcinomaas VEGFR-2Inhibitors. J. Enzym. Inhib. Med. Chem. 2018, 33, 1472–1493. [Google Scholar] [CrossRef] [PubMed]
- El-Adl, K.; El-Helby, A.-G.A.; Sakr, H.; Ayyad, R.R.; Mahdy, H.A.; Nasser, M.; Abulkhair, H.S.; El-Hddad, S.S.A. Design, synthesis, molecular docking, anticancer evaluations, and in silico pharmacokinetic studies of novel 5-[(4-chloro/2,4-dichloro)benzylidene]thiazolidine-2,4-dione derivatives as VEGFR-2 inhibitors. Arch. Pharm. 2021, 354, e2000279. [Google Scholar] [CrossRef]
- Kassab, A.E.; El-Dash, Y.; Gedawy, E.M. Novel pyrazolopyrimidine urea derivatives: Synthesis, antiproliferative activity, VEGFR-2 inhibition, and effects on the cell cycle profile. Arch. Pharm. 2020, 353, e1900319. [Google Scholar] [CrossRef]
- AbdelHaleem, A.; Mansour, A.O.; AbdelKader, M.; Arafa, R.K. Selective VEGFR-2 inhibitors: Synthesis of pyridine derivatives, cytotoxicity and apoptosis induction profiling. Bioorg. Chem. 2020, 103, 104222–104237. [Google Scholar] [CrossRef]
- Marzouk, A.A.; Abdel-Aziz, S.A.; Abdelrahman, K.S.; Wanas, A.S.; Gouda, A.M.; Youssif, B.G.M.; Abdel-Aziz, M. Design and synthesis of new 1,6-dihydropyrimidin-2-thio derivatives targeting VEGFR-2: Molecular docking and antiproliferative evaluation. Bioorg. Chem. 2020, 102, 104090–104099. [Google Scholar] [CrossRef] [PubMed]
- Dawood, D.H.; Nossier, E.S.; Ali, M.M.; Mahmoud, A.E. Synthesis and molecular docking study of new pyrazole derivatives as potent anti-breast cancer agents targeting VEGFR-2 kinase. Bioorg. Chem. 2020, 101, 103916–103928. [Google Scholar] [CrossRef]
- Tian, Y.; Lei, Y.; Fu, Y.; Sun, H.; Wang, J.; Xia, F. Molecular mechanisms of resistance to tyrosine kinase inhibitors associated with hepatocellular carcinoma. Curr. Cancer Drug Targets 2022, 22, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.E.; Mabrouk, R.R.; Elnagar, M.R.; Farrag, A.M.; Kalaba, M.H.; Sharaf, M.H.; El-Fakharany, E.M.; Bakhotmah, D.A.; Elkaeed, E.B.; Al Ward, M.M.S. New Series of VEGFR-2 Inhibitors and Apoptosis Enhancers: Design, Synthesis and Biological Evaluation. Drug Des. Dev. Ther. 2022, 16, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef] [PubMed]
- Raveesha, R.; Anusuya, A.M.; Raghu, A.V.; Kumar, K.Y.; Kumar, M.G.D.; Prasad, S.B.B.; Prashanth, M.K. Synthesis and characterization of novel thiazole derivatives as potential anticancer agents: Molecular docking and DFT studies. Comput. Toxicol. 2022, 21, 100202–100219. [Google Scholar] [CrossRef]
- Litim, B.; Djahoudi, A.; Meliani, S.; Boukhari, A. Synthesis and potential antimicrobial activity of novel α-aminophosphonates derivatives bearing substituted quinoline or quinolone and thiazole moieties. Med. Chem. Res. 2022, 31, 60–74. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.A.; Taher, E.S.; Lan, P.; El-Koussi, N.A.; Salem, O.I.A.; Gomaa, H.A.M.; Youssif, B.G.M. New pyrimidine/thiazole hybrids endowed with analgesic, anti-inflammatory, and lower cardiotoxic activities: Design, synthesis, and COX-2/sEH dual inhibition. Arch. Pharm. 2022, 355, e2200024. [Google Scholar] [CrossRef]
- Altıntop, M.D.; Sever, B.; Çiftçi, G.A.; Özdemir, A. Design, Synthesis, and Evaluation of a New Series of Thiazole-Based Anticancer Agents as Potent Akt Inhibitors. Molecules 2018, 23, 1318. [Google Scholar] [CrossRef]
- Hassan, A.; Badr, M.; Hassan, H.A.; Abdelhamid, D.; Abuo-Rahma, G.E.D.A. Novel 4-(piperazin-1-yl)quinolin-2(1H)-one bearing thiazoles with antiproliferative activity through VEGFR-2-TK inhibition. Bioorg. Med. Chem. 2021, 40, 116168–116181. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Zidan, A.; Elkaeed, E.B.; Taghour, M.S.; Badawi, W.A. Design, synthesis and docking studies of new hydrazinyl-thiazole derivatives as anticancer and antimicrobial agents. J. Saudi Chem. Soc. 2022, 26, 101488–101502. [Google Scholar] [CrossRef]
- Pandrangi, S.L.; Chittineedi, P.; Chalumuri, S.S.; Meena, A.S.; Mosquera, J.A.N.; Llaguno, S.N.S.; Pamuru, R.R.; Mohiddin, G.J.; Mohammad, A. Role of Intracellular Iron in Switching Apoptosis to Ferroptosis to Target Therapy-Resistant Cancer Stem Cells. Molecules 2022, 27, 3011. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.; Li, W.; Qin, Y.; Yu, T.; Liu, Z.; Zhou, M.; Liu, C.; Qiao, T.; Li, X.; Yousef, R.G.; et al. Inhibition of Vascular Smooth Muscle and Cancer Cell Proliferation by New VEGFR Inhibitors and Their Immunomodulator Effect: Design, Synthesis, and Biological Evaluation. Oxid. Med. Cell. Longev. 2021, 2021, 8321400. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703–718. [Google Scholar] [CrossRef]
- Li, A.M.; Boichard, A.; Kurzrock, R. Mutated TP53 is a marker of increased VEGF expression: Analysis of 7,525 pan-cancer tissues. Cancer Biol. Ther. 2020, 21, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zaki, I.; Masoud, R.E.; Hamoud, M.M.S.; Ali, O.A.A.; Abualnaja, M.; Fayad, E.; Almaaty, A.H.A.; Elnaghia, L.K. Design, synthesis and cytotoxicity screening of new synthesized pyrimidine-5-carbonitrile derivatives showing marked apoptotic effect. J. Mol. Struct. 2022, 1259, 132749–132762. [Google Scholar] [CrossRef]


| Comp No. | IC50 Value (μM) |
|---|---|
| MDA-MB-231 | |
| 3a | 8.12 ± 0.42 |
| 3b | 9.55 ± 0.46 |
| 3c | 7.39 ± 0.41 |
| 4a | 29.77 ± 3.27 |
| 4b | 3.52 ± 0.18 |
| 4c | 4.89 ± 0.24 |
| 4d | 1.21 ± 0.09 |
| 4e | 13.33 ± 0.93 |
| 5a | 24.89 ± 1.19 |
| 5b | 19.25 ± 0.68 |
| Sorafenib | 1.18 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Warhi, T.; Abualnaja, M.; Abu Ali, O.A.; Alyamani, N.M.; Elsaid, F.G.; Shati, A.A.; Albogami, S.; Fayad, E.; Abu Almaaty, A.H.; Mohamed, K.O.; et al. Design, Synthesis and Cytotoxicity Screening of New Thiazole Derivatives as Potential Anticancer Agents through VEGFR-2 Inhibition. Symmetry 2022, 14, 1814. https://doi.org/10.3390/sym14091814
Al-Warhi T, Abualnaja M, Abu Ali OA, Alyamani NM, Elsaid FG, Shati AA, Albogami S, Fayad E, Abu Almaaty AH, Mohamed KO, et al. Design, Synthesis and Cytotoxicity Screening of New Thiazole Derivatives as Potential Anticancer Agents through VEGFR-2 Inhibition. Symmetry. 2022; 14(9):1814. https://doi.org/10.3390/sym14091814
Chicago/Turabian StyleAl-Warhi, Tarfah, Matokah Abualnaja, Ola A. Abu Ali, Najiah M. Alyamani, Fahmy G. Elsaid, Ali A. Shati, Sarah Albogami, Eman Fayad, Ali H. Abu Almaaty, Khaled O. Mohamed, and et al. 2022. "Design, Synthesis and Cytotoxicity Screening of New Thiazole Derivatives as Potential Anticancer Agents through VEGFR-2 Inhibition" Symmetry 14, no. 9: 1814. https://doi.org/10.3390/sym14091814
APA StyleAl-Warhi, T., Abualnaja, M., Abu Ali, O. A., Alyamani, N. M., Elsaid, F. G., Shati, A. A., Albogami, S., Fayad, E., Abu Almaaty, A. H., Mohamed, K. O., Alamoudi, W. M., & Zaki, I. (2022). Design, Synthesis and Cytotoxicity Screening of New Thiazole Derivatives as Potential Anticancer Agents through VEGFR-2 Inhibition. Symmetry, 14(9), 1814. https://doi.org/10.3390/sym14091814







