Ab Initio Computations of O and AO as well as ReO2, WO2 and BO2-Terminated ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 (001) Surfaces
Abstract
:1. Introduction
2. Computation Methods and Materials
3. Ab Initio Computation Results for ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 Bulk Properties
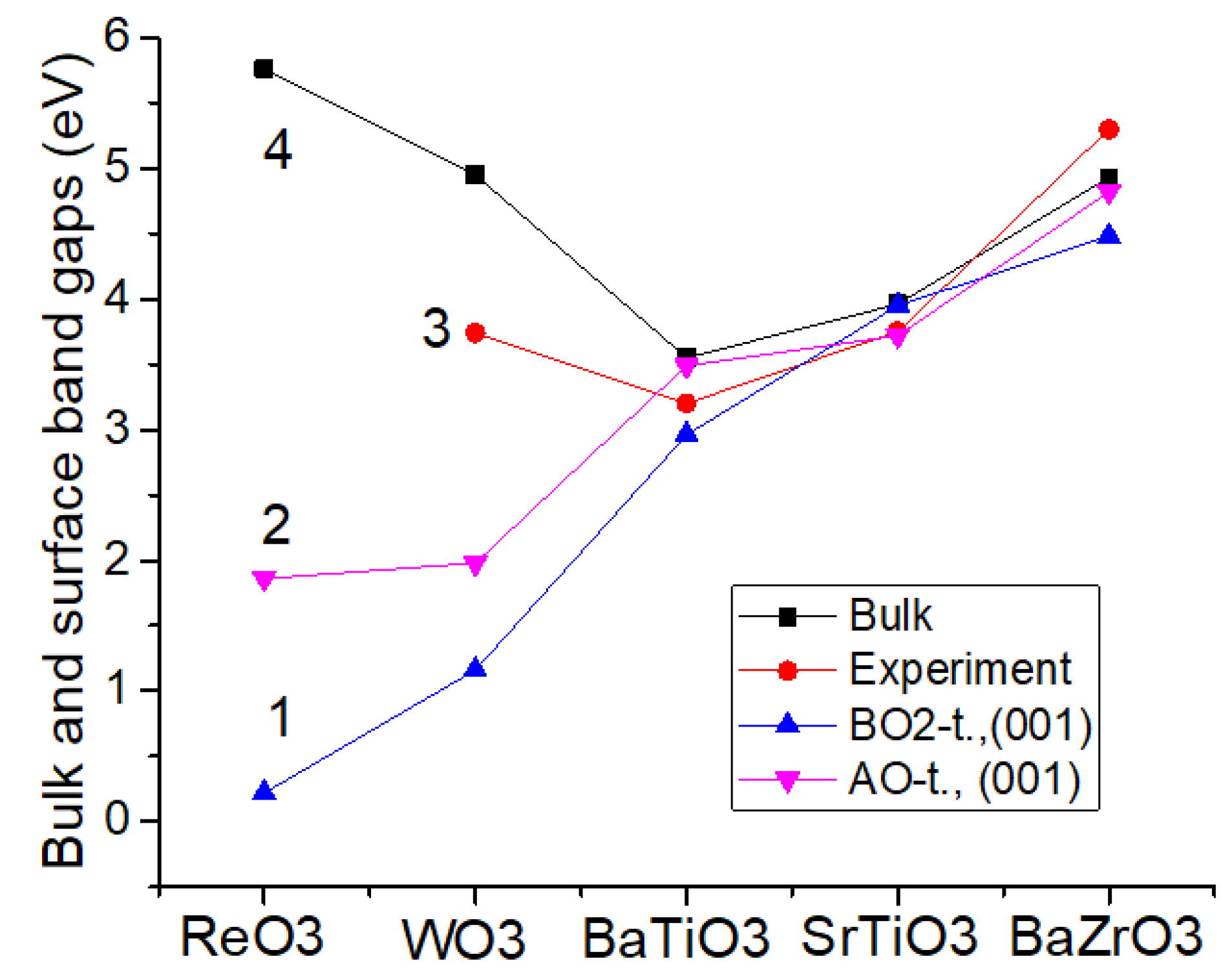
4. Ab Initio Computation Results for the BO2 and O-Terminated ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 (001) Surfaces
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eglitis, R.I.; Vanderbilt, D. Ab initio calculations of the atomic and electronic structure of CaTiO3 (001) and (011) surfaces. Phys. Rev. B 2008, 78, 155420. [Google Scholar] [CrossRef] [Green Version]
- Erdman, N.; Warschkow, O.; Asta, M.; Poeppelmeier, K.R.; Ellis, D.E.; Marks, L.D. Surface Structures of SrTiO3 (001): A TiO2-rich Reconstruction with a c (4 × 2) Unit Cell. J. Am. Chem. Soc. 2003, 125, 10050–10056. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Lee, Y.; Kim, S.; Yoon, Y.; Kim, Y.; Park, S.C. Surface termination of BaTiO3 (111) single crystal: A combined DFT and XPS study. Appl. Surf. Sci. 2022, 578, 152018. [Google Scholar] [CrossRef]
- Brik, M.G.; Ma, C.G.; Krasnenko, V. First-principles calculations of the structural and electronic properties of the cubic CaZrO3 (001) surfaces. Surf. Sci. 2013, 608, 146–153. [Google Scholar] [CrossRef]
- Sambrano, J.R.; Longo, V.M.; Longo, E.; Taft, C.A. Electronic and structural properties of the (001) SrZrO3 surface. J. Mol. Struct. THEOCHEM 2007, 813, 49–56. [Google Scholar] [CrossRef]
- Piskunov, S.; Eglitis, R.I. First principles hybrid DFT calculations of BaTiO3/SrTiO3 (001) interface. Solid State Ion. 2015, 274, 29–33. [Google Scholar] [CrossRef]
- Zhao, X.; Selloni, A. Structure and stability of NaTaO3 (001) and KTaO3 (001) surfaces. Phys. Rev. Mater. 2019, 3, 015801. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Kleperis, J.; Purans, J.; Popov, A.I.; Jia, R. Ab initio calculations of CaZrO3 (011) surfaces: Systematic trends in polar (011) surface calculations of ABO3 perovskites. J. Mater. Sci. 2020, 55, 203–217. [Google Scholar] [CrossRef]
- Lazaro, S.D.; Longo, E.; Sambrano, J.R.; Beltrán, A. Structural and electronic properties of PbTiO3 slabs: A DFT periodic study. Surf. Sci. 2004, 552, 149–159. [Google Scholar] [CrossRef]
- Jia, W.; Vikhnin, V.S.; Liu, H.; Kapphan, S.; Eglitis, R.; Usvyat, D. Critical effects in optical response due to charge transfer vibronic excitions and their structure in perovskite-like systems. J. Lumin. 1999, 83, 109–113. [Google Scholar] [CrossRef]
- Gellé, F.; Chirita, R.; Mertz, D.; Rastei, M.V.; Dinia, A.; Colis, S. Guideline to atomically flat TiO2-terminated SrTiO3 (001) surfaces. Surf. Sci. 2018, 677, 39–45. [Google Scholar] [CrossRef]
- Mesquita, W.D.; Oliveira, M.C.D.; Assis, M.; Ribeiro, R.A.P.; Eduardo, A.C.; Teodoro, M.D.; Marques, G.E.; Júnior, M.G.; Longo, E.; Gurgel, M.F.D.C. Unraveling the relationship between bulk structure and exposed surfaces and its effect on the electronic structure and photoluminescent properties of Ba0.5Sr0.5TiO3: A joint experimental and theoretical approach. Mater. Res. Bull. 2021, 143, 111442. [Google Scholar] [CrossRef]
- Zhang, R.; Hwang, G.S. First-principles mechanistic study of the initial growth of SrO by atomic layer deposition on TiO2-terminated SrTiO3 (001). J. Phys. Chem. C 2020, 124, 28116. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Popov, A.I.; Jia, R. Tendencies in ABO3 perovskite and SrF2, BaF2 and CaF2 bulk and surface F-center ab initio computations at high symmetry cubic structure. Symmetry 2021, 13, 1920. [Google Scholar] [CrossRef]
- Meng, J.; Lan, Z.Y.; Lin, Q.Y.; Chen, T.; Chen, X.; Wei, X.; Lu, Y.H.; Li, J.X.; Zhang, Z. Cubic-like BaZrO3 nanocrystals with exposed {001}/{011} facets and tuned electronic band structure for enhanced photocatalytic hydrogen production. J. Mater. Sci. 2019, 54, 1967–1976. [Google Scholar] [CrossRef]
- Carlotto, S.; Natile, M.M.; Glisenti, A.; Vittadini, A. Adsorption of small molecules at the cobalt-doped SrTiO3 (001) surface: A first-principles investigation. Surf. Sci. 2015, 633, 68–76. [Google Scholar] [CrossRef]
- Kotomin, E.A.; Piskunov, S.; Zhukovskii, Y.F.; Eglitis, R.I.; Gopejenko, A.; Ellis, D.E. The electronic properties of an oxygen vacancy at ZrO2-terminated (001) surfaces of a cubic PbZrO3: Computer simulations from the first principles. Phys. Chem. Chem. Phys. 2008, 10, 4258–4263. [Google Scholar] [CrossRef]
- Saghayezhian, M.; Sani, S.M.R.; Zhang, J.; Plummer, E.W. Rumpling and enhanced covalency at the SrTiO3 (001) surface. J. Phys. Chem. C 2019, 123, 8086–8091. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Piskunov, S. First principles calculations of SrZrO3 bulk and ZrO2-terminated (001) surface F centers. Comput. Condens. Matter 2016, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Krainyukova, N.V.; Butskii, V.V. RHEED study of stepped (001) surface of strontium titanate. Appl. Surf. Sci. 2004, 235, 32–37. [Google Scholar] [CrossRef]
- Sokolov, M.; Eglitis, R.I.; Piskunov, S.; Zhukovskii, Y.F. Ab initio hybrid DFT calculations of BaTiO3 bulk and BaO-terminated (001) surface F-centers. Int. J. Mod. Phys. B 2017, 31, 1750251. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.N.; Machala, M.L.; Bluhm, H.; Chuech, W.C. Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions. Nat. Commun. 2015, 6, 6097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eglitis, R.I.; Popov, A.I. Ab initio calculations for the polar (001) surfaces of YAlO3. Nucl. Instr. Methods B 2018, 434, 1–5. [Google Scholar] [CrossRef]
- Kim, J.S.; Yang, J.H.; Kim, B.K.; Kim, Y.C. Proton conduction at BaO-terminated (001) BaZrO3 surface using density functional theory. Solid State Ion. 2015, 275, 19–22. [Google Scholar] [CrossRef]
- Piskunov, S.; Eglitis, R.I. Comparative ab initio calculations of SrTiO3/BaTiO3 and SrZrO3/PbZrO3 (001) heterostructures. Nucl. Instr. Methods B 2016, 374, 20–23. [Google Scholar] [CrossRef]
- Erdman, N.; Poeppelmeier, K.R.; Asta, M.; Warschkov, O.; Ellis, D.E.; Marks, L.D. The structure and chemistry of the TiO2-rich surface of SrTiO3 (001). Nature 2002, 419, 55–58. [Google Scholar] [CrossRef]
- Eglitis, R.I. Ab initio hybrid DFT calculations of BaTiO3, PbTiO3, SrZrO3 and PbZrO3 (111) surfaces. Appl. Surf. Sci. 2015, 358, 556–562. [Google Scholar] [CrossRef]
- Maalaoui, A.; Said, O.B.; Akriche, S.T.; Al-Deyab, S.S.; Rzaigui, M. Synthesis, Characterization, Fluorescence and Antibacterial Activity of the Re(VII) Complex [ReO3(phen)(H2PO4)]·H2O. Z. Für Nat. B 2012, 67, 1178–1184. [Google Scholar] [CrossRef]
- Duan, G.; Chen, L.; Jing, Z.; Luna, P.D.; Wen, L.; Zhang, L.; Xu, J.; Li, Z.; Yang, Z.; Zhou, R. Robust Antibacterial Activity of Tungsten Oxide (WO3-x) Nanodots. Chem. Res. Toxicol. 2019, 32, 1357–1366. [Google Scholar] [CrossRef]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids 2018, 116, 137–147. [Google Scholar] [CrossRef]
- Alam, N.N.; Malik, N.A.; Samat, M.H.; Hussin, N.H.; Jaafar, N.K.; Radzwan, A.; Mohyedin, M.Z.; Haq, B.U.; Ali, A.M.M.; Hassan, O.; et al. Underlaying mechanism of surface (001) cubic ATiO3 (A = Pb, Sn) in enhancing thermoelectric performance of thin-film applications using density functional theory. Surf. Interfaces 2021, 27, 101524. [Google Scholar] [CrossRef]
- Enterkin, J.A.; Subramanian, A.K.; Russell, B.C.; Castell, M.R.; Poeppelmeier, K.R.; Marks, L.D. A homologous series of structures on the surface of SrTiO3 (110). Nat. Mater. 2010, 9, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Isakoviča, I.; Gopejenko, A.; Ivanova, A.; Začinskis, A.; Eglitis, R.I.; D’yachkov, P.N.; Piskunov, S. Time-Dependent Density Functional Theory Calculations of N- and S-Doped TiO2 Nanotube for Water-Splitting Applications. Nanomaterials 2021, 11, 2900. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Young, J.D.; Jong, C.H.; Kim, D.; Hong, J.; Kim, B.K.; Lee, J.; Son, J.W.S.; Shim, J.H. Demonstrating the Potential of Yttrium-Doped Barium Zirconate Electrolyte for High-Performance Fuell Cells. Nat. Commun. 2017, 8, 14553. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily Processed Protonic Ceramic Fuell Cells with High Performance at Low Temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Shu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly Efficient Reversible Protonic Ceramic Electrochemical Cells for Power Generation and Fuel Production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.I.; Haile, S.M. Exceptional Power Density and Stability at Intermediate Temperature in Protonic Ceramic Fuell Cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly Durable, Coking and Sulfur Tolerant, Fuel-Flexible Protonic Ceramic Fuell Cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef]
- Morendo, S.H.; Zanón, R.; Escolástico, S.; Yuste-Tirados, I.; Malerød-Fjeld, K.; Vestre, P.K.; Coors, W.G.; Martinez, A.; Norby, T.; Serra, J.M.; et al. Direct Conversion of Methane to Aromatics in a Catalytic co-ionic Membrane Reactor. Science 2016, 353, 563–566. [Google Scholar]
- Malerød-Fjeld, K.; Clark, D.; Yuste-Tirados, I.; Zanon, R.; Catalán-Martinez, D.; Beeaff, D.; Morejudo, S.H.; Vestre, P.K.; Norby, T.; Haugsrud, R.; et al. Thermo-Electrochemical Production of Compressed Hydrogen from Methane with Near-Zero Energy Loss. Nat. Energy 2017, 2, 923–931. [Google Scholar] [CrossRef]
- Dawber, M.; Rabe, K.M.; Scott, J.F. Physics of thin-film ferroelectric oxides. Rev. Mod. Phys. 2005, 77, 1083–1130. [Google Scholar] [CrossRef] [Green Version]
- Cohen, R.E. Origin of ferroelectricity in perovskite oxides. Nature 1992, 358, 136–138. [Google Scholar] [CrossRef]
- Zhong, W.; Vanderbilt, D.; Rabe, K.M. Phase Transitions in BaTiO3 from First Principles. Phys. Rev. Lett. 1994, 73, 1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, W.; Vanderbilt, D.; Rabe, K.M. First-principles theory of ferroelectric phase transitions for perovskites: The case of BaTiO3. Phys. Rev. B 1995, 52, 6301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, M.C.; Ribeiro, R.A.P.; Longo, E.; Bomio, M.R.D.; Motta, F.V.; Lazaro, S.R.D. Temperature dependence on phase evolution in the BaTiO3 polytypes studied using ab initio calculations. Int. J. Quantum Chem. 2020, 120, e26054. [Google Scholar] [CrossRef]
- Meyer, B.; Padilla, J.; Vanderbilt, D. Theory of PbTiO3, BaTiO3 and SrTiO3 surfaces. Faraday Discuss. 1999, 114, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Heifets, E.; Ho, J.; Merinov, B. Density functional simulation of the BaZrO3 (011) surface structure. Phys. Rev. B 2007, 75, 155431. [Google Scholar] [CrossRef] [Green Version]
- Heifets, E.; Kotomin, E.A.; Maier, J. Semi-empirical simulations of surface relaxation for perovskite titanates. Surf. Sci. 2000, 462, 19–35. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Kotomin, E.A.; Borstel, G. Quantum chemical modelling of perovskite solid solutions. J. Phys. Condens. Matter 2000, 12, L431–L434. [Google Scholar] [CrossRef] [Green Version]
- Meyer, B.; Vanderbilt, D. Ab initio study of BaTiO3 and PbTiO3 surfaces in external electric fields. Phys. Rev. B 2001, 63, 205426. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Pang, Q.; Xu, K.W.; Ji, V. First-principles study of the (110) polar surface of cubic PbTiO3. Comput. Mater. Sci. 2009, 44, 1360–1365. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Gabrusenoks, J.; Popov, A.I.; Jia, R. Comparative ab initio calculation of ReO3, SrZrO3, BaZrO3, PbZrO3 and CaZrO3 (001) surfaces. Crystals 2020, 10, 745. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Jia, R. Comparative Hybrid Hartree-Fock-DFT Calculations of ReO3, SrTiO3, BaTiO3, PbTiO3 and CaTiO3 (001) Surfaces. Integr. Ferroelectr. 2021, 220, 9–17. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Jia, R. Comparative Hybrid Hartree-Fock-DFT Calculations of WO2-Terminated Cubic WO3 as well as SrTiO3, BaTiO3, PbTiO3 and CaTiO3 (001) surfaces. Crystals 2021, 11, 455. [Google Scholar] [CrossRef]
- Cora, F.; Stachiotti, M.G.; Catlow, C.R.A. Transition Metal Oxide Chemistry: Electronic Structure of WO3, ReO3 and NaWO3. J. Phys. Chem. B 1997, 101, 3945–3952. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Jehng, J.M.; Wachs, I.E. In Situ Raman Spectroscopy of Supported Transition Metal Oxide Catalysts: 18O2-16O2 Isotopic Labeling Studies. J. Phys. Chem. B 2000, 104, 7382–7387. [Google Scholar] [CrossRef] [Green Version]
- Koffyberg, F.P.; Dwight, K.; Wold, A. Interband transitions of semiconducting oxides determined from photoelectrolysis spectra. Solid State Commun. 1979, 30, 433–437. [Google Scholar] [CrossRef]
- Wemple, S.H. Polarized Fluctuations and the Optical-Absorption Edge in BaTiO3. Phys. Rev. B 1970, 2, 2679–2689. [Google Scholar] [CrossRef]
- Benthem, K.; Elsässer, C.; French, R.H. Bulk electronic structure of SrTiO3: Experiment and Theory. J. Appl. Phys. 2001, 90, 6156–6164. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J. Band offsets of wide-band-gap oxides and implications for future electronic devices. J. Vacuum. Sci. Technol. B 2000, 18, 1785–1791. [Google Scholar] [CrossRef]
- Schirber, J.E.; Morosin, B. “Compressibility Collapse” Transition in ReO3. Phys. Rev. Lett. 1979, 42, 1485–1487. [Google Scholar] [CrossRef]
- Balászi, C.; Farkas-Jahnke, M.; Kotsis, I.; Petrás, L.; Pfeifer, J. The observation of cubic tungsten trioxide at high-temperature dehydration of tungstic acid hydrate. Solid State Ion. 2001, 141–142, 411–416. [Google Scholar] [CrossRef]
- Edwards, J.W.; Speiser, R.; Johnston, H.L. Structure of Barium Titanate at Elevated Temperatures. J. Am. Chem. Soc. 1951, 73, 2934–2935. [Google Scholar] [CrossRef]
- Okazaki, A.; Scheel, H.J.; Müller, K.A. The lattice constant vs. temperature relation around the 105 K transition of a flux-grown SrTiO3 crystal. Phase Trans. 1985, 5, 207–218. [Google Scholar]
- Mathews, M.D.; Mirza, E.B.; Momin, A.C. High-temperature X-ray diffractometric studies of CaZrO3, SrZrO3 and BaZrO3. J. Mater. Sci. Lett. 1991, 10, 305–306. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B 1989, 33, 8800–8802, Erratum in Phys. Rev. B 1989, 40, 3399. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Saunders, V.R.; Dovesi, R.; Roetti, C.; Causa, N.; Harrison, N.M.; Orlando, R.; Zicovich-Wilson, C.M. CRYSTAL-2009 User Manual; University of Torino: Torino, Italy, 2009. [Google Scholar]
- Monkhorst, H.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Piskunov, S.; Heifets, E.; Eglitis, R.I.; Borstel, G. Bulk properties and electronic structure of SrTiO3, BaTiO3, PbTiO3 perovskites: An ab initio HF/DFT study. Comput. Mater. Sci. 2004, 29, 165–178. [Google Scholar] [CrossRef]
- Piskunov, S.; Kotomin, E.A.; Heifets, E.; Maier, J.; Eglitis, R.I.; Borstel, G. Hybrid DFT calculations of the atomic and electronic structure for ABO3 perovskite (001) surfaces. Surf. Sci. 2005, 575, 75–88. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Popov, A.I. Systematic trends in (001) surface ab initio calculations of ABO3 perovskites. J. Saudi Chem. Soc. 2018, 22, 459–468. [Google Scholar] [CrossRef]
- Vassilyeva, A.F.; Eglitis, R.I.; Kotomin, E.A.; Dauletbekova, A.K. Ab initio calculations of MgF2 (001) and (011) surface structure. Phys. B Condens. Matter 2010, 405, 2125–2127. [Google Scholar] [CrossRef]
- Rubloff, G.W. Far-Ultraviolet Reflectence Spectra and the Electronic Structure of Ionic Crystals. Phys. Rev. B 1972, 5, 662–684. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Lisitsyna, L.A.; Popov, A.I.; Kotomin, E.A.; Abuova, F.U.; Akilbekov, A.; Maier, J. Stabilization of primary mobile radiation defects in MgF2 crystals. Nucl. Instrum. Methods B 2016, 374, 24–28. [Google Scholar] [CrossRef]
- Slater, J.C. A Simplification of the Hartree-Fock Method. Phys. Rev. 1951, 81, 385–390. [Google Scholar] [CrossRef]
- Dovesi, R.; Orlando, R.; Roetti, C.; Pisani, C.; Saunders, V.R. The Periodic Hartree-Fock Method and Its Implementation in the Crystal Code. Phys. Stat. Sol. B 2000, 217, 63–88. [Google Scholar] [CrossRef]
- Dirac, P.A.M. Note on Exchange Phenomena in the Thomas Atom. Proc. Camb. Phil. Soc. 1930, 26, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in Fortran 77, 2nd ed.; Cambridge University Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Noguera, C. Polar oxide surfaces. J. Phys. Condens. Matter 2000, 12, R367. [Google Scholar] [CrossRef]
- Pojani, A.; Finocchi, F.; Noguera, C. Polarity on the SrTiO3 (111) and (110) surfaces. Surf. Sci. 1999, 442, 179–198. [Google Scholar] [CrossRef]
- Pojani, A.; Finocchi, F.; Noguera, C. A theoretical study of the unreconstructed polar (111) face of SrTiO3. Appl. Surf. Sci. 1999, 142, 177–181. [Google Scholar] [CrossRef]
- Cora, F.; Patel, A.; Harrison, N.M.; Dovesi, R.; Catlow, C.R.A. An ab initio Hartree-Fock study of the cubic and tetragonal phases of bulk tungsten trioxide. J. Am. Chem. Soc. 1996, 118, 12174–12182. [Google Scholar] [CrossRef]
- Mayer, I. Bond Order and Valence: Relations to Mulliken’s Population Analysis. Int. J. Quantum Chem. 1984, 26, 151–154. [Google Scholar] [CrossRef]
- Bochicchio, R.C.; Reale, H.F. On the nature of crystalline bonding: Extension of statistical population analysis to two- and three- dimensional crystalline systems. J. Phys. B 1993, 26, 4871–4883. [Google Scholar] [CrossRef]
- Shi, H.; Chang, L.; Jia, R.; Eglitis, R.I. Ab initio calculations of the transfer and aggregation of F centers in CaF2. J. Phys. Chem. C 2012, 116, 4832–4839. [Google Scholar] [CrossRef]
- Grigorjeva, L.; Millers, D.K.; Pankratov, V.; Williams, R.T.; Eglitis, R.I.; Kotomin, E.A. Experimental and theoretical studies of polaron optical properties in KNbO3 perovskites. Solid State Commun. 2004, 129, 691–696. [Google Scholar] [CrossRef]
- Eglitis, R.I. Comparative First-Principles Calculations of SrTiO3, BaTiO3, PbTiO3 and CaTiO3 (001), (011) and (111) Surfaces. Ferroelectrics 2015, 483, 53–67. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Vanderbilt, D. Ab initio calculations of BaTiO3 and PbTiO3 (001) and (011) surface structures. Phys. Rev. B 2007, 76, 155439. [Google Scholar] [CrossRef] [Green Version]
- Eglitis, R.I.; Vanderbilt, D. First-principles calculations of atomic and electronic structure of SrTiO3 (001) and (011) surfaces. Phys. Rev. B 2008, 77, 195408. [Google Scholar] [CrossRef]
- Eglitis, R.I. First-principles calculations of BaZrO3 (001) and (011) surfaces. J. Phys. Condens. Matter 2007, 19, 356004. [Google Scholar] [CrossRef] [Green Version]
- Padilla, J.; Vanderbilt, D. Ab initio study of SrTiO3 surfaces. Surf. Sci. 1998, 418, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Kunc, K.; Lee, M.H. Structural relaxation and longitudinal dipole moment of SrTiO3 (001) (1 × 1) surfaces. Phys. Rev. B 2000, 62, 10409. [Google Scholar] [CrossRef]
- Bickel, N.; Schmidt, G.; Heinz, K.; Müller, K. Ferroelectric relaxation of the SrTiO3 (100) surface. Phys. Rev. Lett. 1993, 62, 2009–2012. [Google Scholar] [CrossRef]
- Hikita, T.; Hanada, T.; Kudo, M.; Kawai, M. Structure and electronic state of the TiO2 and SrO terminated SrTiO3 (100) surfaces. Surf. Sci. 1993, 287–288, 377–381. [Google Scholar] [CrossRef]
- Charlton, G.; Brennan, S.; Muryn, C.A.; McGrath, R.; Norman, D.; Turner, T.S.; Thorthon, G. Surface relaxation of SrTiO3 (001). Surf. Sci. 2000, 457, L376–L380. [Google Scholar] [CrossRef]



| Crystal | Symmetry in RT | BandGap (Γ-Γ) (eV) in RT | Trans. T (K) to Cubic Phase | Exp. Latt. Con. (Å), Cubic Ph. |
|---|---|---|---|---|
| ReO3 | Cubic [55] | Unknown | Cubic from liquid helium T till 673 K | 3.747 Å [61] |
| WO3 | Monoclinic [56] | 3.74 eV [57] | Unknown | 3.71–3.75 Å [62] |
| BaTiO3 | Tetragonal ↔ orthorhombic (278 K) | 3.38 eV (∥ c); 3.27 eV (⟂ c) [58] | 403 K [45] | 4.004 Å—474 K [63] |
| SrTiO3 | Cubic | 3.75 eV [59] | 110 K [45] | 3.898 Å—110 K [64] |
| BaZrO3 | Cubic | 5.3 eV [60] | Cubic, all T | 4.199 Å RT [65] |
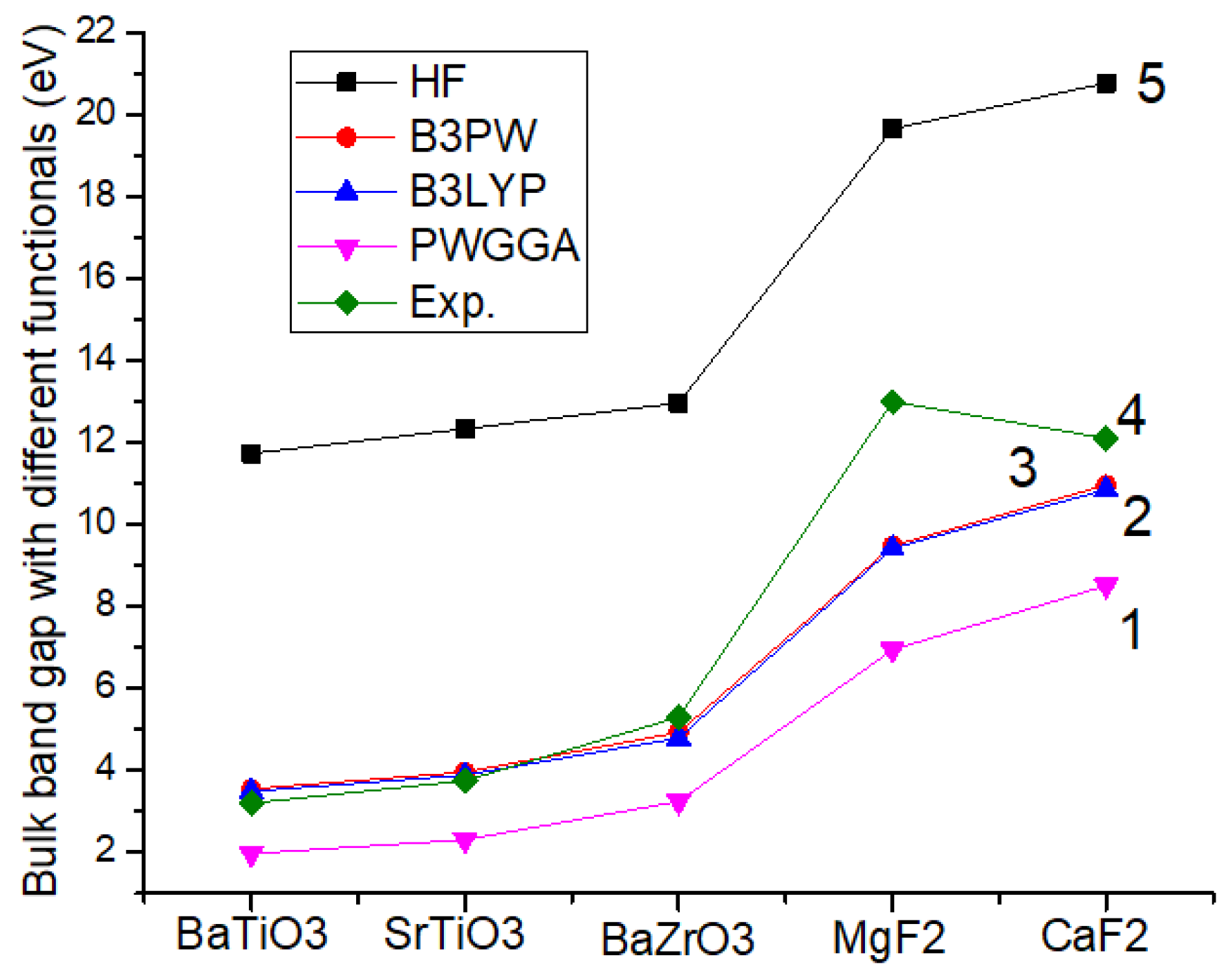
| Approach | BaTiO3 | SrTiO3 | BaZrO3 | MgF2 | CaF2 |
|---|---|---|---|---|---|
| HF | 11.73 | 12.33 | 12.96 | 19.65 | 20.77 |
| B3PW | 3.55 | 3.96 | 4.93 | 9.48 | 10.96 |
| B3LYP | 3.49 | 3.89 | 4.79 | 9.42 | 10.85 |
| PWGGA | 1.97 | 2.31 | 3.24 | 6.94 | 8.51 |
| Experiment | 3.2 [72] | 3.75 [59] | 5.3 [60] | 13.0 [76] | 12.1 [75] |
| Crystal | ReO3 | WO3 | BaTiO3 | SrTiO3 | BaZrO3 | |
|---|---|---|---|---|---|---|
| Atom | Property | B3LYP | B3LYP | B3PW | B3PW | B3PW |
| A | Q | - | - | +1.797 | +1.871 | +1.815 |
| P | - | - | −0.034 | −0.010 | −0.012 | |
| O | Q | −0.794 | −1.032 | −1.388 | −1.407 | −1.316 |
| P | +0.212 | +0.142 | +0.098 | +0.088 | +0.108 | |
| B | Q | +2.382 | +3.095 | +2.367 | +2.351 | +2.134 |
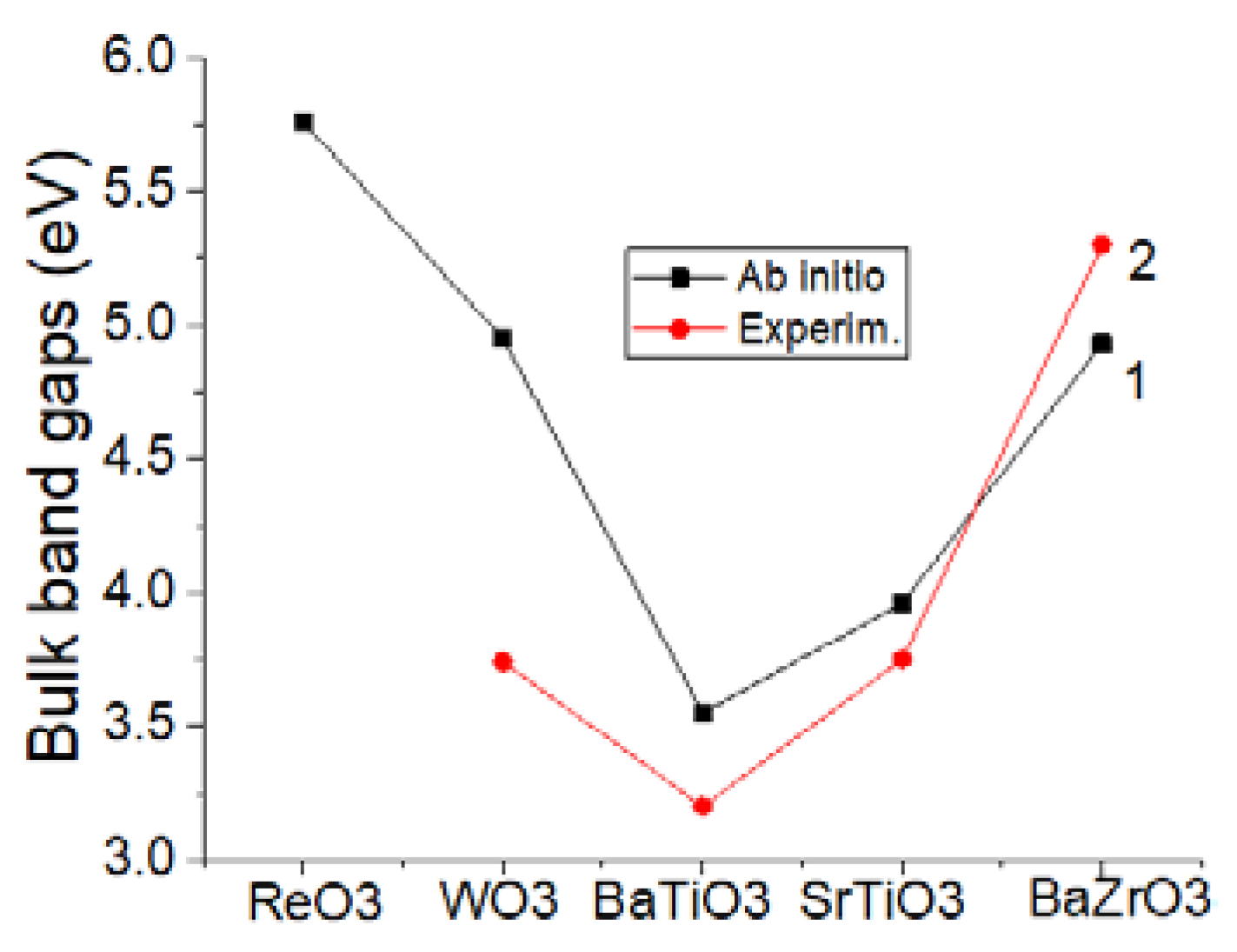
| Material | Theoretical Γ-Γ Bulk Gap (eV) | Experimental Γ-Γ Bulk Gap (eV) |
|---|---|---|
| ReO3 | 5.76 eV (B3LYP) | Unknown |
| WO3 | 4.95 eV (B3LYP) | 3.74 eV [57] |
| BaTiO3 | 3.55 eV (B3PW) | 3.2 eV [72] |
| SrTiO3 | 3.96 eV (B3PW) | 3.75 eV [59] |
| BaZrO3 | 4.93 eV (B3PW) | 5.3 eV [60] |
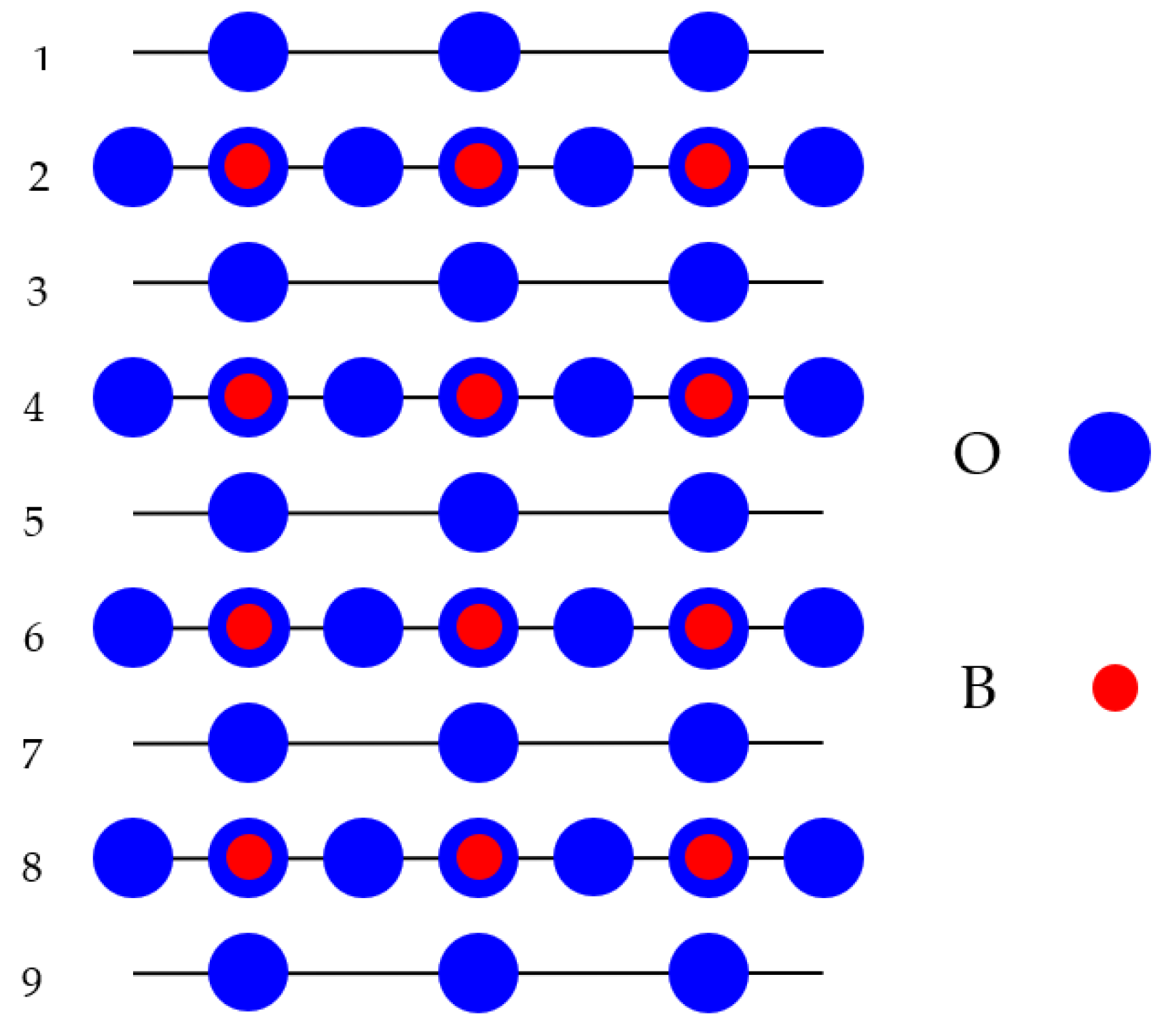
| Computed (001) Surf. | ReO3 | WO3 | BaTiO3 | SrTiO3 | BaZrO3 | |
|---|---|---|---|---|---|---|
| Layer | Atom | ReO2-Ter. | WO2-Ter. | TiO2-Ter. | TiO2-Ter. | ZrO2-Ter. |
| 1 | B | −3.19 | −2.07 | −3.08 | −2.25 | −1.79 |
| O | −1.17 | +0.42 | −0.35 | −0.13 | −1.70 | |
| 2 | A | No atom | No atom | +2.51 | +3.55 | +1.94 |
| O | −0.32 | +0.11 | +0.38 | +0.57 | +0.85 | |
| 3 | B | −0.17 | −0.01 | - | - | −0.03 |
| O | −0.11 | 0.00 | - | - | 0.00 | |
| Computed (001) Surf. | ReO3 | WO3 | BaTiO3 | SrTiO3 | BaZrO3 | |
|---|---|---|---|---|---|---|
| Layer | Atom | O-Termin. | O-Termin. | BaO-Ter. | SrO-Ter. | BaO-Ter. |
| 1 | A | No atom | No atom | −1.99 | −4.84 | −4.30 |
| O | −3.73 | −4.24 | −0.63 | +0.84 | −1.23 | |
| 2 | B | +2.71 | +2.65 | +1.74 | +1.75 | +0.47 |
| O | −0.53 | −0.11 | +1.40 | +0.77 | +0.18 | |
| 3 | A | No atom | No atom | - | - | −0.01 |
| O | −0.44 | −0.48 | - | - | −0.14 | |
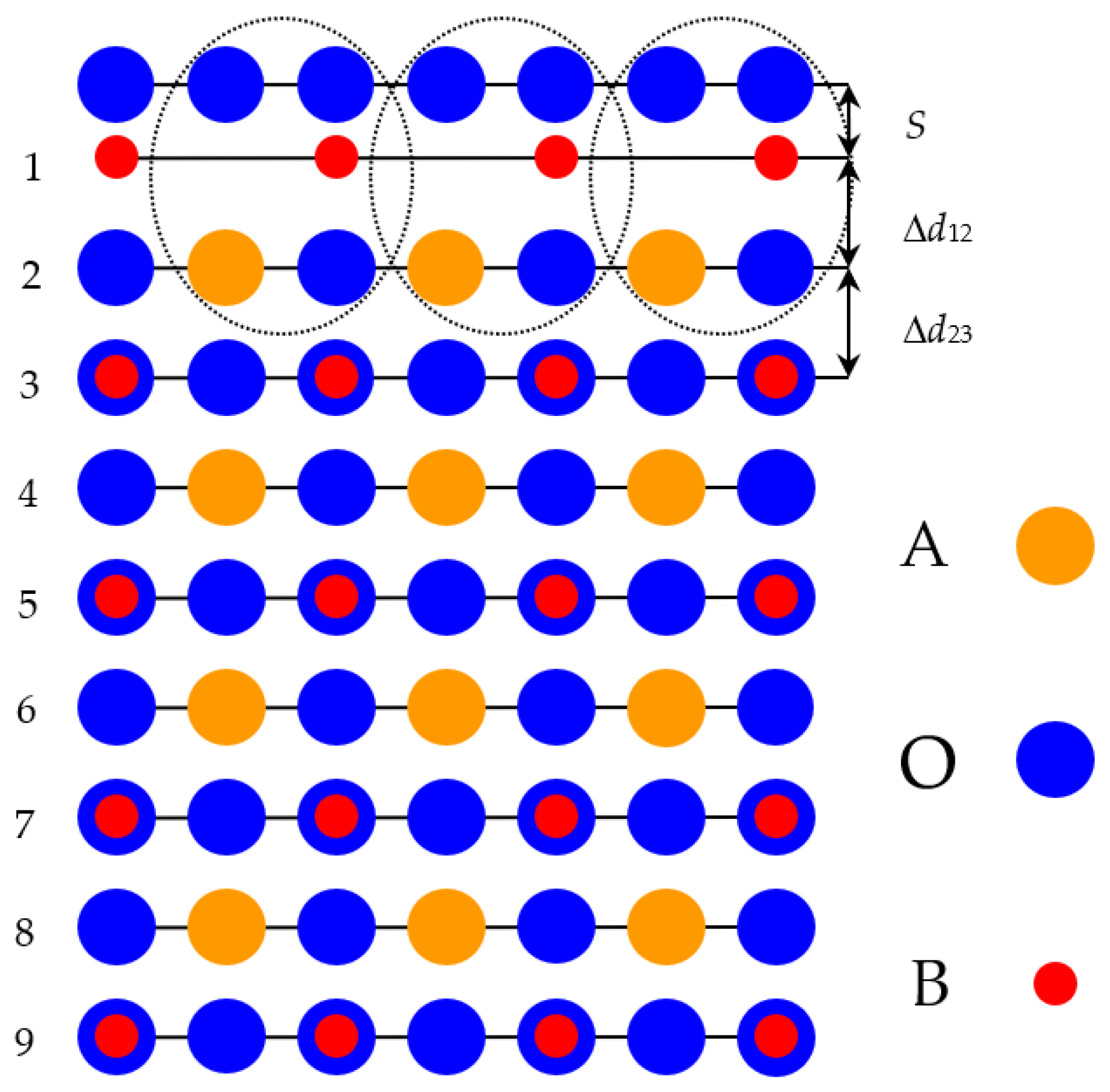
| SrO-Terminated SrTiO3 (001) Surface | |||
|---|---|---|---|
| s | Δd12 | Δd23 | |
| Our B3PW results | +5.66 | −6.58 | +1.75 |
| Ab initio [94] | +5.8 | −6.9 | +2.4 |
| Ab initio [95] | +7.7 | −8.6 | +3.3 |
| Shell model [48] | +8.2 | −8.6 | +3.0 |
| LEED exp. [96] | 4.1 ± 2 | −5 ± 1 | 2 ± 1 |
| RHEED exp. [97] | 4.1 | 2.6 | 1.3 |
| SXRD exp. [98] | 1.3 ± 12.1 | −0.3 ± 3.6 | −6.7 ± 2.8 |
| Material | Functional | Re-O, W-O and B-O Chemical Bond Populations (in e) | |
|---|---|---|---|
| Bulk | ReO2, WO2, BO2-Term. (001) Surfaces | ||
| ReO3 | B3LYP | 0.212 | 0.170 |
| WO3 | B3LYP | 0.142 | 0.108 |
| BaTiO3 | B3PW | 0.098 | 0.126 |
| SrTiO3 | B3PW | 0.088 | 0.118 |
| BaZrO3 | B3PW | 0.108 | 0.132 |
| Material | Functional | Bulk (Γ-Γ) | Exp. (Γ-Γ) | BO2-T. (001) | AO-T. (001) |
|---|---|---|---|---|---|
| ReO3 | B3LYP | 5.76 | No data | 0.22 | 1.86 |
| WO3 | B3LYP | 4.95 | 3.74 | 1.16 | 1.98 |
| BaTiO3 | B3PW | 3.55 | 3.2 | 2.96 | 3.49 |
| SrTiO3 | B3PW | 3.96 | 3.75 | 3.95 | 3.72 |
| BaZrO3 | B3PW | 4.93 | 5.3 | 4.48 | 4.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eglitis, R.I.; Purans, J.; Popov, A.I.; Bocharov, D.; Chekhovska, A.; Jia, R. Ab Initio Computations of O and AO as well as ReO2, WO2 and BO2-Terminated ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 (001) Surfaces. Symmetry 2022, 14, 1050. https://doi.org/10.3390/sym14051050
Eglitis RI, Purans J, Popov AI, Bocharov D, Chekhovska A, Jia R. Ab Initio Computations of O and AO as well as ReO2, WO2 and BO2-Terminated ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 (001) Surfaces. Symmetry. 2022; 14(5):1050. https://doi.org/10.3390/sym14051050
Chicago/Turabian StyleEglitis, Roberts I., Juris Purans, Anatoli I. Popov, Dmitry Bocharov, Anastasiia Chekhovska, and Ran Jia. 2022. "Ab Initio Computations of O and AO as well as ReO2, WO2 and BO2-Terminated ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 (001) Surfaces" Symmetry 14, no. 5: 1050. https://doi.org/10.3390/sym14051050
APA StyleEglitis, R. I., Purans, J., Popov, A. I., Bocharov, D., Chekhovska, A., & Jia, R. (2022). Ab Initio Computations of O and AO as well as ReO2, WO2 and BO2-Terminated ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 (001) Surfaces. Symmetry, 14(5), 1050. https://doi.org/10.3390/sym14051050








