Symmetry between Structure–Antibacterial Effect of Polymers Functionalized with Phosphonium Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation and Characterization
2.2. Preparation of Bacterial Cultures Used during Antibacterial Tests
3. Results and Discussions
3.1. Characterization of Prepared Material by FT-IR
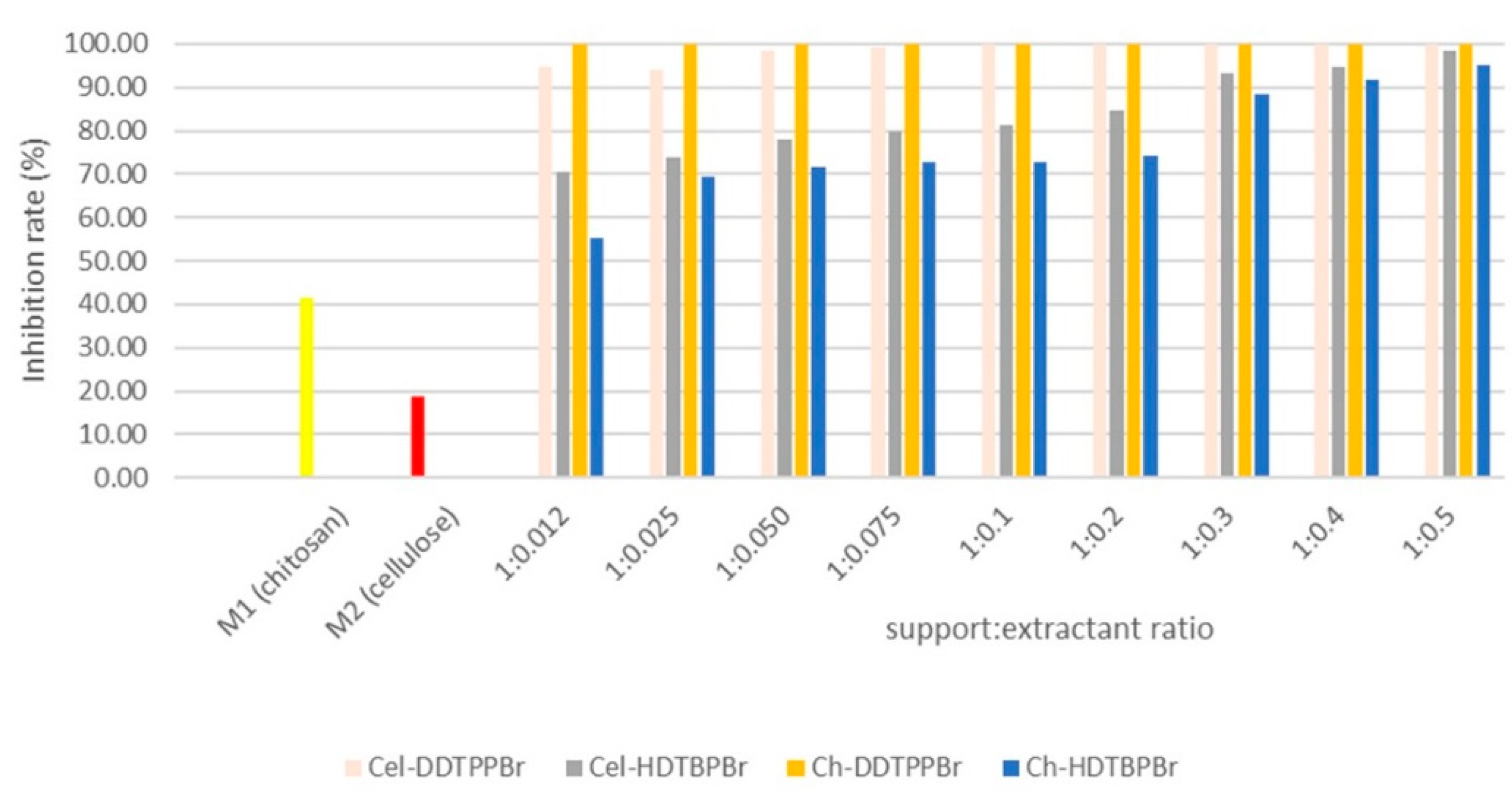
3.2. Antimicrobial Effect of Prepared Materials
3.2.1. Case of a Heterotrophic Inoculum
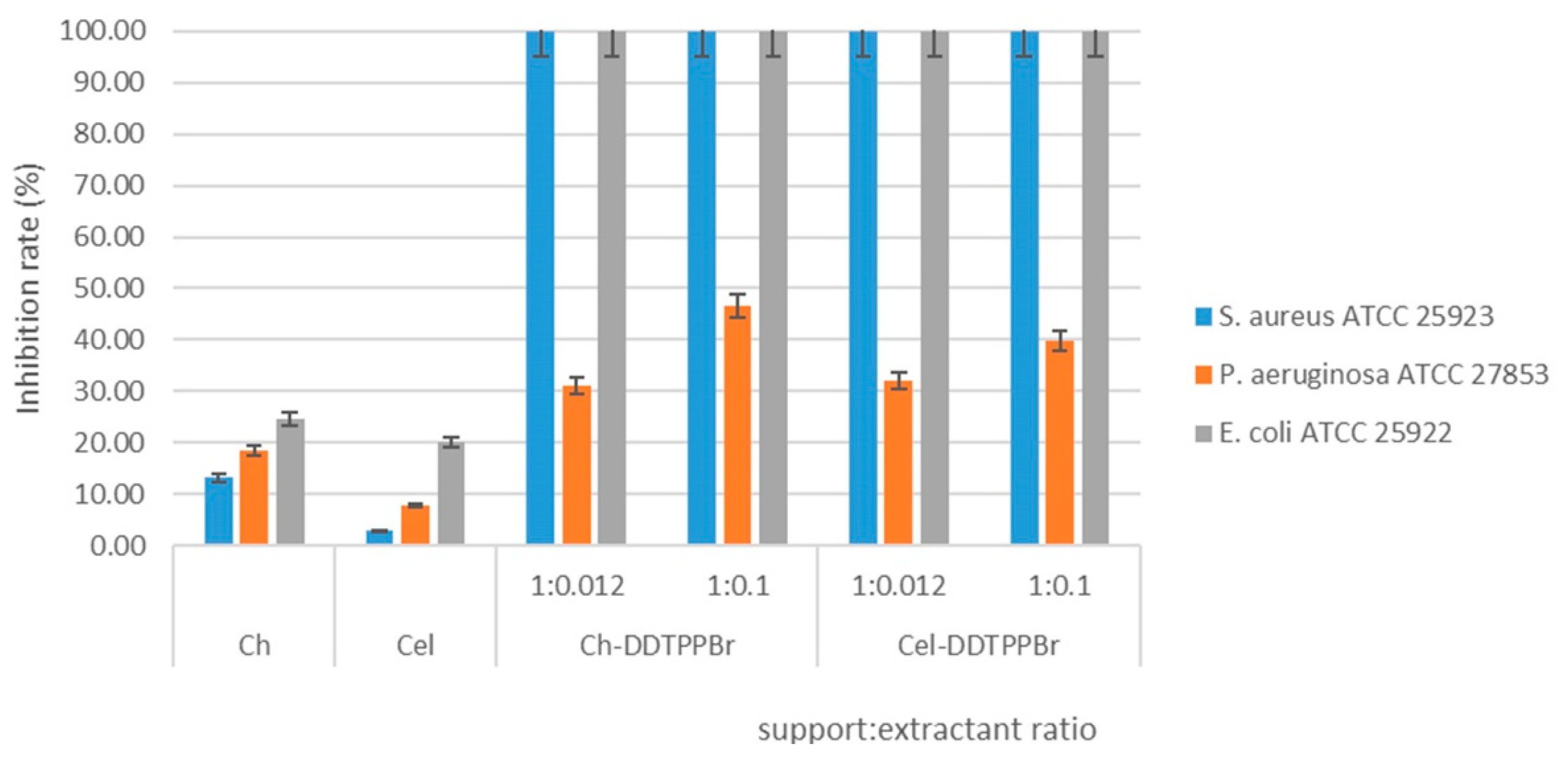
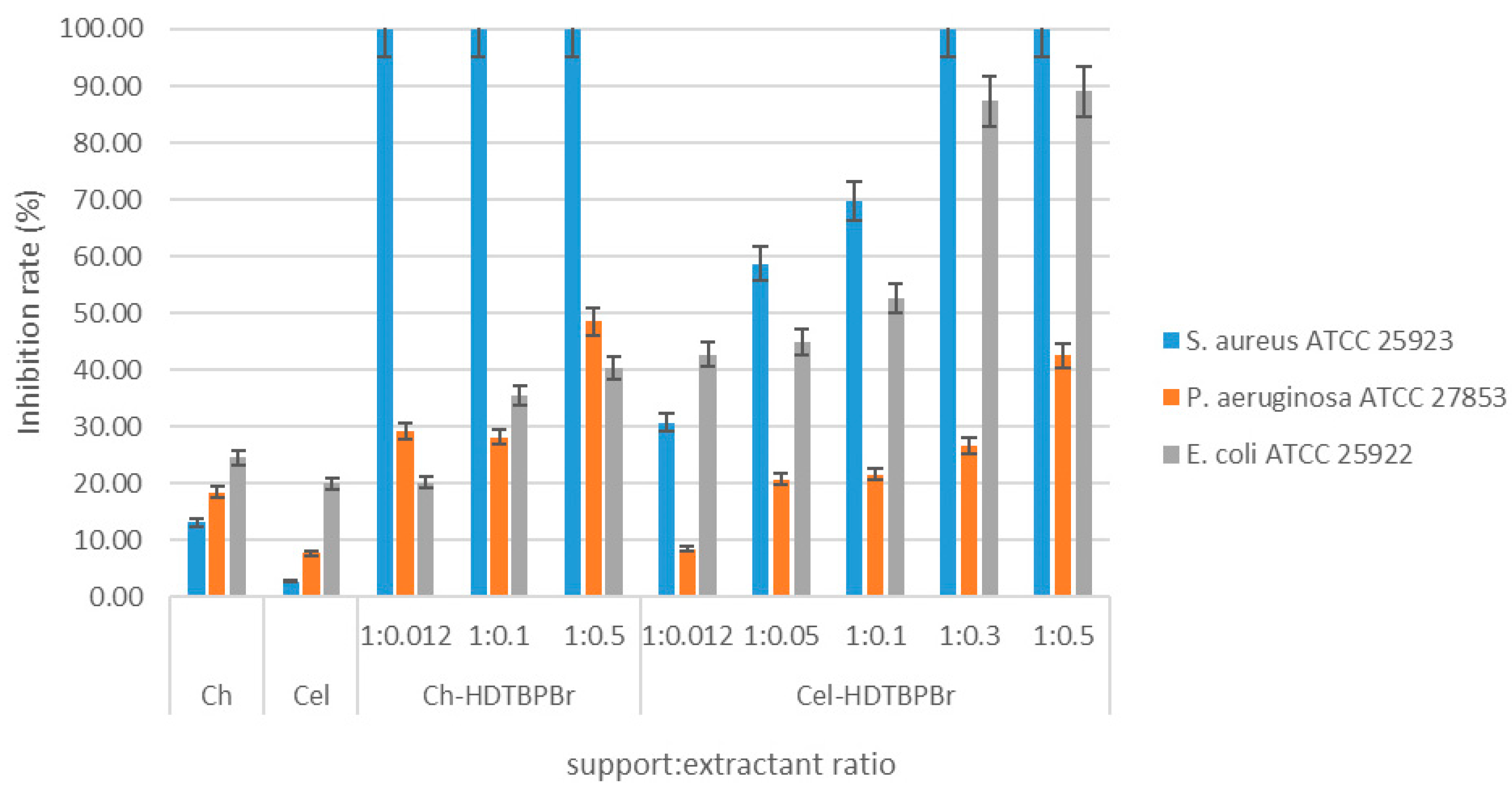
3.2.2. Case of Reference Strains
Antimicrobial Effect of Ch-DDTPPBr and Cel-DDTPPBr
Antimicrobial Effect of Ch-HDTBPBr and Cel-HDTBPBr
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cheba, B.A. Chitin and Chitosan: Marine Biopolymers with Unique Properties and Versatile Applications. Glob. J. Biotechnol. Biochem. 2011, 6, 149–153. [Google Scholar]
- Fernandes, S.C.; Freire, C.S.; Silvestre, A.J.; Neto, C.P.; Gandini, A. Novel materials based on chitosan and cellulose. Polym. Int. 2011, 60, 875–882. [Google Scholar] [CrossRef]
- Khattak, S.; Wahid, F.; Liu, L.-P.; Jia, S.-R.; Chu, L.-Q.; Xie, Y.-Y.; Li, Z.-X.; Zhong, C. Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Akca, G.; Özdemir, A.; Öner, Z.G.; Şenel, S. Comparison of different types and sources of chitosan for the treatment of infections in the oral cavity. Res. Chem. Intermed. 2018, 44, 4811–4825. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Biotechnol. 2013, 35, 155–175. [Google Scholar] [CrossRef]
- Kostag, M.; El Seoud, O.A. Sustainable biomaterials based on cellulose, chitin and chitosan composites—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent Advances of Chitosan and Its Derivatives for Novel Applications in Food Science. J. Food Processing Beverages 2013, 1, 13. [Google Scholar]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and applications of chitosan and cellulose composite materials. J. Environ. Manag. 2022, 301, 113850. [Google Scholar] [CrossRef] [PubMed]
- Phisalaphong, M.; Jatupaiboon, N. Biosynthesis and characterization of bacteria cellulose–chitosan film. Carbohydr. Polym. 2008, 74, 482–488. [Google Scholar] [CrossRef]
- Coma, V.; Freire, V.; Silvestre, A. Recent advances on the development of antibacterial polysaccharide-based materials. In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1751–1803. [Google Scholar]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. VI. Antibacterial activity of fibers surface-treated with phosphonium salts containing trimethoxysilane groups. J. Appl. Polym. Sci. 1994, 52, 641–647. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Dong, F.; Li, Q.; Guo, Z. Design, synthesis of novel chitosan derivatives bearing quaternary phosphonium salts and evaluation of antifungal activity. Int. J. Biol. Macromol. 2017, 102, 704–711. [Google Scholar] [CrossRef]
- Xue, Y.; Pan, Y.; Xiao, H.; Zhao, Y. Novel quaternary phosphonium-type cationic polyacrylamide and elucidation of dual-functional antibacterial/antiviral activity. RSC Adv. 2014, 4, 46887–46895. [Google Scholar] [CrossRef]
- Csóka, L.; Božanić, D.K.; Božanić, D.K.; Nagy, V.; Dimitrijević-Branković, S.; Luyt, A.S.; Grozdits, G.; Djoković, V. Viscoelastic properties and antimicrobial activity of cellulose fiber sheets impregnated with Ag nanoparticles. Carbohydr. Polym. 2012, 90, 1139–1146. [Google Scholar] [CrossRef]
- Sperandeo, P.; Bosco, F.; Clerici, F.; Polissi, A.; Gelmi, M.L.; Romanelli, A. Covalent Grafting of Antimicrobial Peptides onto Microcrystalline Cellulose. ACS Appl. Bio Mater. 2020, 3, 4895–4901. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bousserrhine, N.; Alphonse, V.; Le Floch, F.; Mechiche, Y.C.; Menidjel, I.; Carbonnier, B. Chitosan based self-assembled nanocapsules as antibacterial agent. Colloids Surf. B-Biointerfaces 2019, 181, 158–165. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. III. Immobilization of phosphonium salts by surface photografting and antibacterial activity of the surface-treated polymer films. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1467–1472. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, H.M.; El-Zairy, E.M.R. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polim.-Cienc. E Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Kabanov, V.L.; Novinyuk, L.V. Chitosan application in food technology: A review of recent advances. Food Syst. 2020, 3, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. Sustain. Agric. Rev. 2019, 35, 49–123. [Google Scholar]
- Tian, B.; Liu, Y. Chitosan-based biomaterials: From discovery to food application. Polym. Adv. Technol. 2020, 31, 2408–2421. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups. Polymers 2018, 10, 395. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, M. Three-dimensional macroporous calcium phosphate bioceramics with nested chitosan sponges for load-bearing bone implants. J. Biomed. Mater. Res. 2002, 61, 1–8. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Shukur, K.; Haj, N. Preparation and Bioactivity Assessment of Chitosan-1-Acetic Acid-5-Flurouracil Conjugates as Cancer Prodrugs. Molecules 2017, 22, 1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huan, L.; Xiao, L.; Yang, G. Chitosan Application in Textile Processing. Curr. Trends Fash. Technol. Text. Eng. 2018, 4, 555635. [Google Scholar]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef]
- Wilts, E.M.; Herzberger, J.; Long, T.E. Addressing water scarcity: Cationic polyelectrolytes in water treatment and purification. Polym. Int. 2018, 67, 799–814. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Gan, W.; Zhou, J.; Zhang, L. Flocculation Properties and Antimicrobial Activities of Quaternized Celluloses Synthesized in NaOH/Urea Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 1242–1246. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, N.S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Koshani, R.; Tavakolian, M.; van de Ven, T.G.M. Cellulose-based dispersants and flocculants. J. Mater. Chem. B 2020, 8, 10502–10526. [Google Scholar] [CrossRef] [PubMed]
- Fauzani, D.; Notodarmojo, S.; Handajani, M.; Helmy, Q.; Kardiansyah, T. Cellulose in natural flocculant applications: A review. J. Phys. Conf. Ser. 2021, 2047, 012030. [Google Scholar] [CrossRef]
- Terhi, S. Functionalized Nanocelluloses in Wastewater Treatment Applications. Ph.D. Thesis, Faculty of Technology, University of Oulu: Tampere, Finland, 2015. [Google Scholar]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Muntean, D. Antimicrobial Activities of Chitosan Derivatives. Pharmaceutics 2021, 13, 1639. [Google Scholar] [CrossRef] [PubMed]
- Nemes, N.S.; Ardean, C.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Paul, C.; Duda-Seiman, D.; Muntean, D. Antimicrobial activity of cellulose based materials. Polymers 2022, 14, 735. [Google Scholar] [CrossRef] [PubMed]
- Cortina, J.L.; Miralles, N.; Sastre, A.; Aguilar, M.; Profumo, A.; Pesavento, M. Solvent-impregnated resins containing di-(2,4,4-trimethylpentyl) phosphonic acid I. Comparative study of di-(2,4,4-trimethylpentyl)phosphinic acid adsorbed into Amberlite XAD-2 and dissolved in toluene. React. Polym. 1993, 21, 89–101. [Google Scholar] [CrossRef]
- Cortina, J.L.; Miralles, N.; Sastre, A.M.; Aguilar, M.; Profumo, A.; Pesavento, M. Solvent-impregnated resins containing di-(2,4,4-trimethylpentyl) phosphinic acid II. Study of the distribution equilibria of Zn(II), Cu(II) and Cd(II). React. Polym. 1993, 21, 103–116. [Google Scholar] [CrossRef]
- Cortina, J.L.; Miralles, N.; Aguilar, M.; Sastre, M. Solvent impregnated resins containing di(2-ethylhexyl)phosphoric acid. I. Preparation and study of the retention and distribution of the extractant on the resin. Solvent Extr. Ion Exch. 1994, 12, 349–369. [Google Scholar] [CrossRef]
- Kabay, N.; Cortina, J.L.; Trochimczuk, A.; Streat, M. Solvent-impregnated resins (SIRs)—Methods of preparation and their applications. React. Funct. Polym. 2010, 70, 484–496. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 09, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Negrea, A.; Gabor, A.; Davidescu, C.M.; Ciopec, M.; Negrea, P.; Duteanu, N.; Barbulescu, A. Rare Earth Elements Removal from Water Using Natural Polymers. Sci. Rep. 2018, 8, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, P.A. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. R. Soc. Med. 2002, 95 (Suppl. 41), 22–26. [Google Scholar] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumariya, R.; Sood, S.K.; Rajput, Y.S.; Saini, N.; Garsa, A.K. Increased membrane surface positive charge and altered membrane fluidity leads to cationic antimicrobial peptide resistance in Enterococcus faecalis. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 1367–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. Journal Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Mengatto, L.; Ferreyra, M.G.; Rubiolo, A.; Rintoul, I.; Luna, J. Hydrophilic and hydrophobic interactions in cross-linked chitosan membranes. Mater. Chem. Phys. 2013, 139, 181–186. [Google Scholar] [CrossRef]
- Hanpanich, O.; Wongkongkatep, P.; Pongtharangkul, T.; Wongkongkatep, J. Turning hydrophilic bacteria into biorenewable hydrophobic material with potential antimicrobial activity via interaction with chitosan. Bioresour. Technol. 2017, 230, 97–102. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Thewes, N.; Loskill, P.; Jung, P.; Peisker, H.; Bischoff, M.; Herrmann, M.; Jacobs, K. Hydrophobic interaction governs unspecific adhesion of staphylococci: A single cell force spectroscopy study. Beilstein J. Nanotechnol. 2014, 5, 1501–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Petterson, T.; Illergård, J.; Ek, M.; Wågberg, L. Influence of Cellulose Charge on Bacteria Adhesion and Viability to PVAm/CNF/PVAm-Modified Cellulose Model Surfaces. Biomacromolecules 2019, 20, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Morsi, R.E.; Al-Sabagh, A.M. Surface active properties of chitosan and its derivatives. Colloids Surf. B Biointerfaces 2009, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sibusiso, A.; Blessing, A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
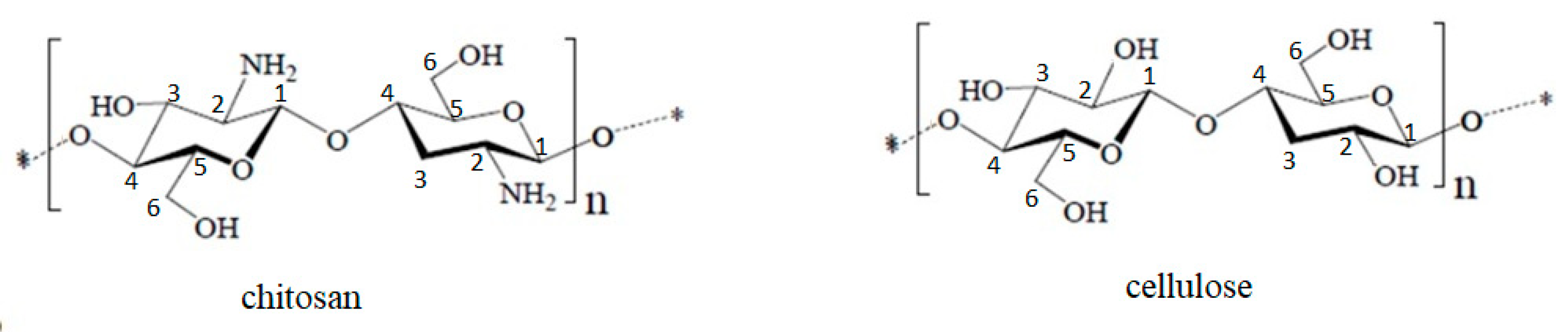
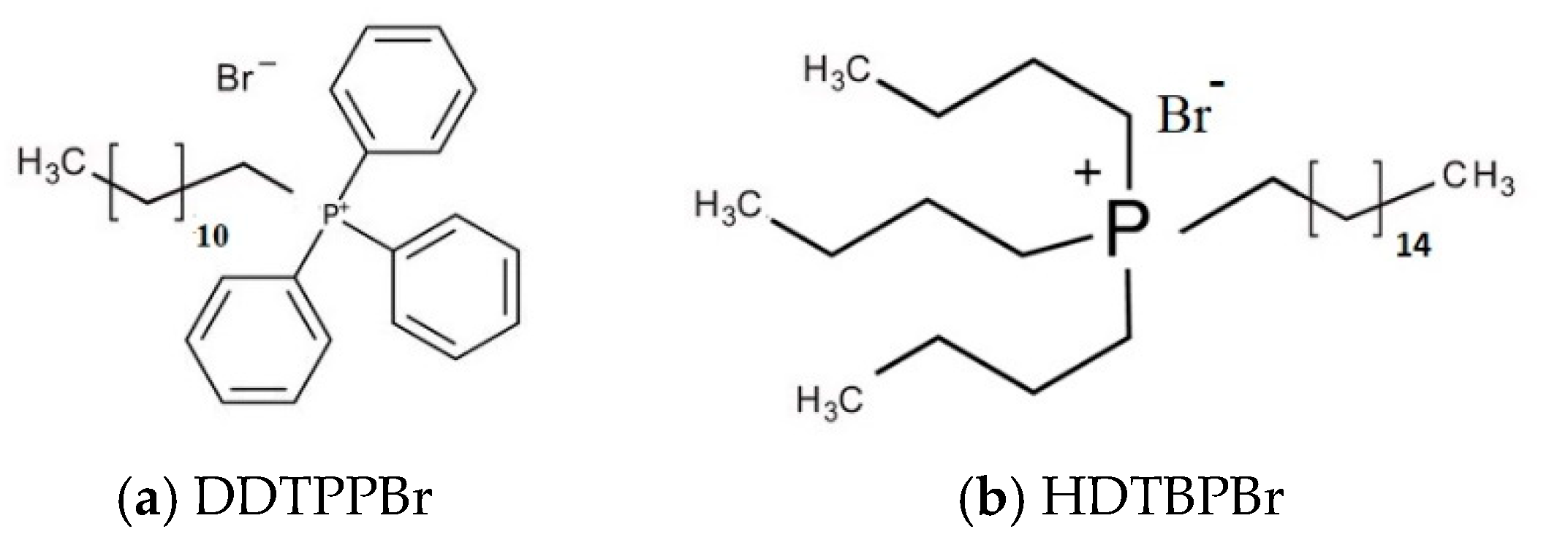
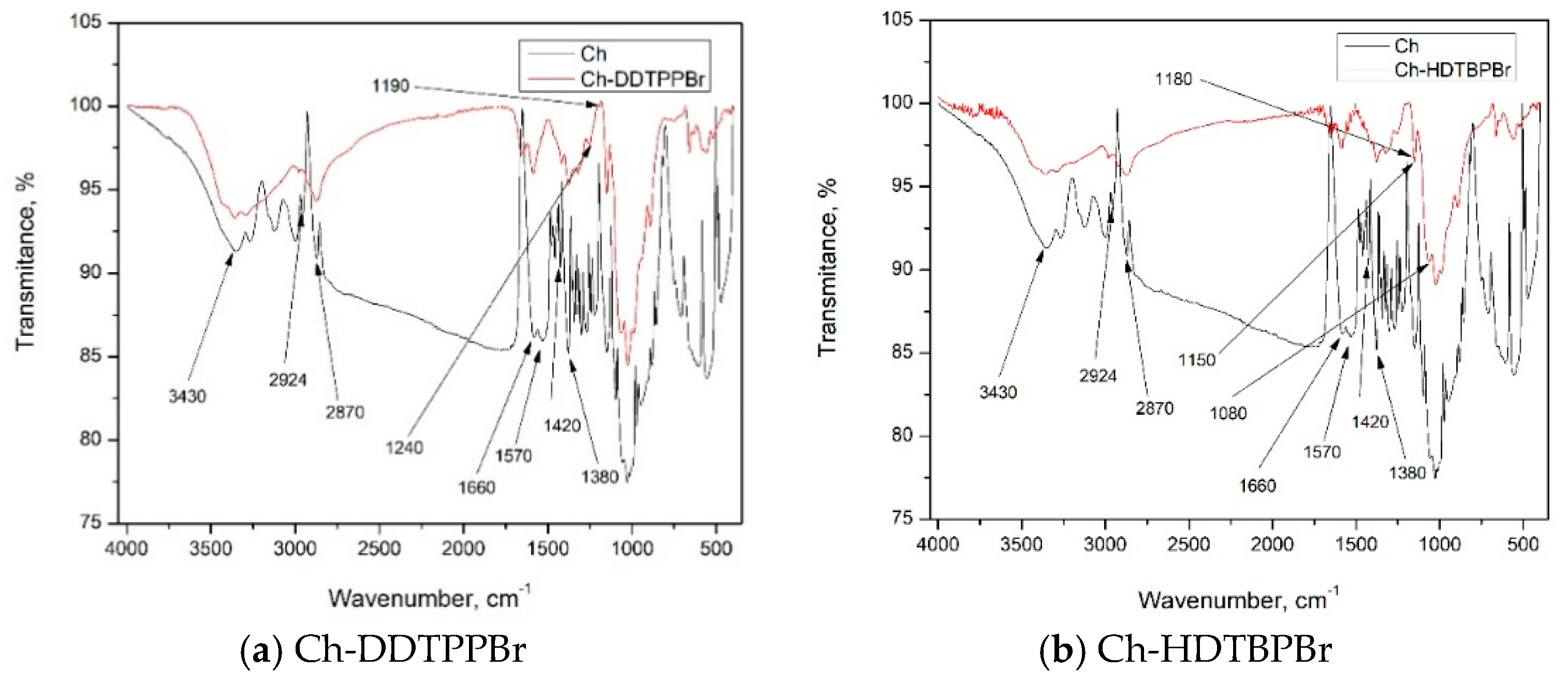
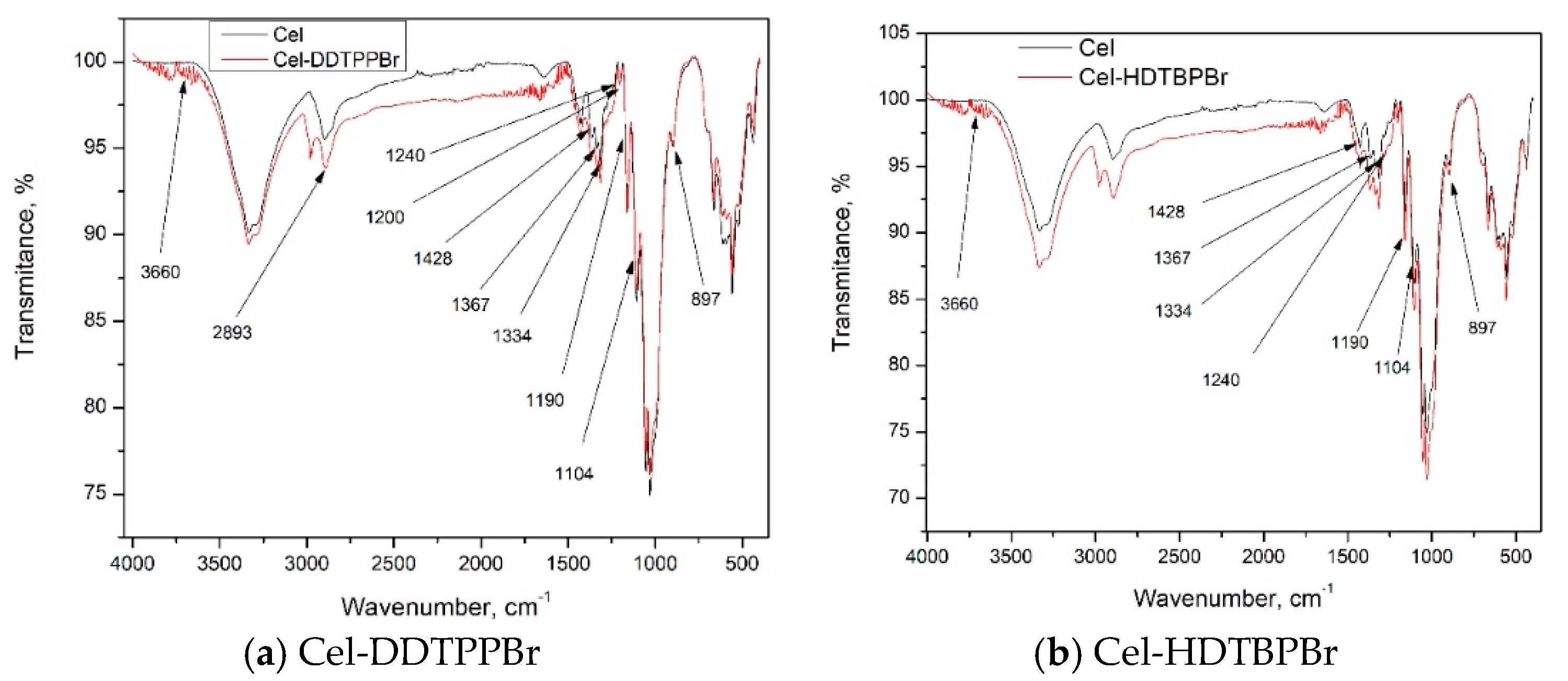
| Group | FT-IR Bands (cm−1) | Observations | |
|---|---|---|---|
| Chitosan | |||
| CH2–OH | 1380–1420 | ||
| N–H | 1570 | ||
| C=O | 1660 | ||
| C–H | 2870; 2924 | ||
| O–H | 3430 | ||
| Cellulose (Cel) | |||
| O–H | 3660 | Large band | |
| C–H | 2893 | Small plateau; stretching vibrations in polysaccharides | |
| CH2 | 1428; 1367 |  | Vibrations specific to the crystalline structure of cellulose |
| C–O | 1334; 1158 | ||
| O–C–O | 1104; 1027 | ||
| 897 | Amorphous region in cellulose | ||
| OH2 | 1600–900 |  | Water molecules vibrations |
| 1633 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemeş, N.S.; Ardean, C.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Musta, V.F.; Duţeanu, N.; Negrea, P.; Muntean, D. Symmetry between Structure–Antibacterial Effect of Polymers Functionalized with Phosphonium Salts. Symmetry 2022, 14, 572. https://doi.org/10.3390/sym14030572
Nemeş NS, Ardean C, Davidescu CM, Negrea A, Ciopec M, Musta VF, Duţeanu N, Negrea P, Muntean D. Symmetry between Structure–Antibacterial Effect of Polymers Functionalized with Phosphonium Salts. Symmetry. 2022; 14(3):572. https://doi.org/10.3390/sym14030572
Chicago/Turabian StyleNemeş, Nicoleta Sorina, Cristina Ardean, Corneliu Mircea Davidescu, Adina Negrea, Mihaela Ciopec, Virgil Filaret Musta, Narcis Duţeanu, Petru Negrea, and Delia Muntean. 2022. "Symmetry between Structure–Antibacterial Effect of Polymers Functionalized with Phosphonium Salts" Symmetry 14, no. 3: 572. https://doi.org/10.3390/sym14030572
APA StyleNemeş, N. S., Ardean, C., Davidescu, C. M., Negrea, A., Ciopec, M., Musta, V. F., Duţeanu, N., Negrea, P., & Muntean, D. (2022). Symmetry between Structure–Antibacterial Effect of Polymers Functionalized with Phosphonium Salts. Symmetry, 14(3), 572. https://doi.org/10.3390/sym14030572










