Maladaptive One-Leg Balance Control in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assessments
2.3. Primary and Secondary Outcomes
2.4. Statistical Analysis
3. Results
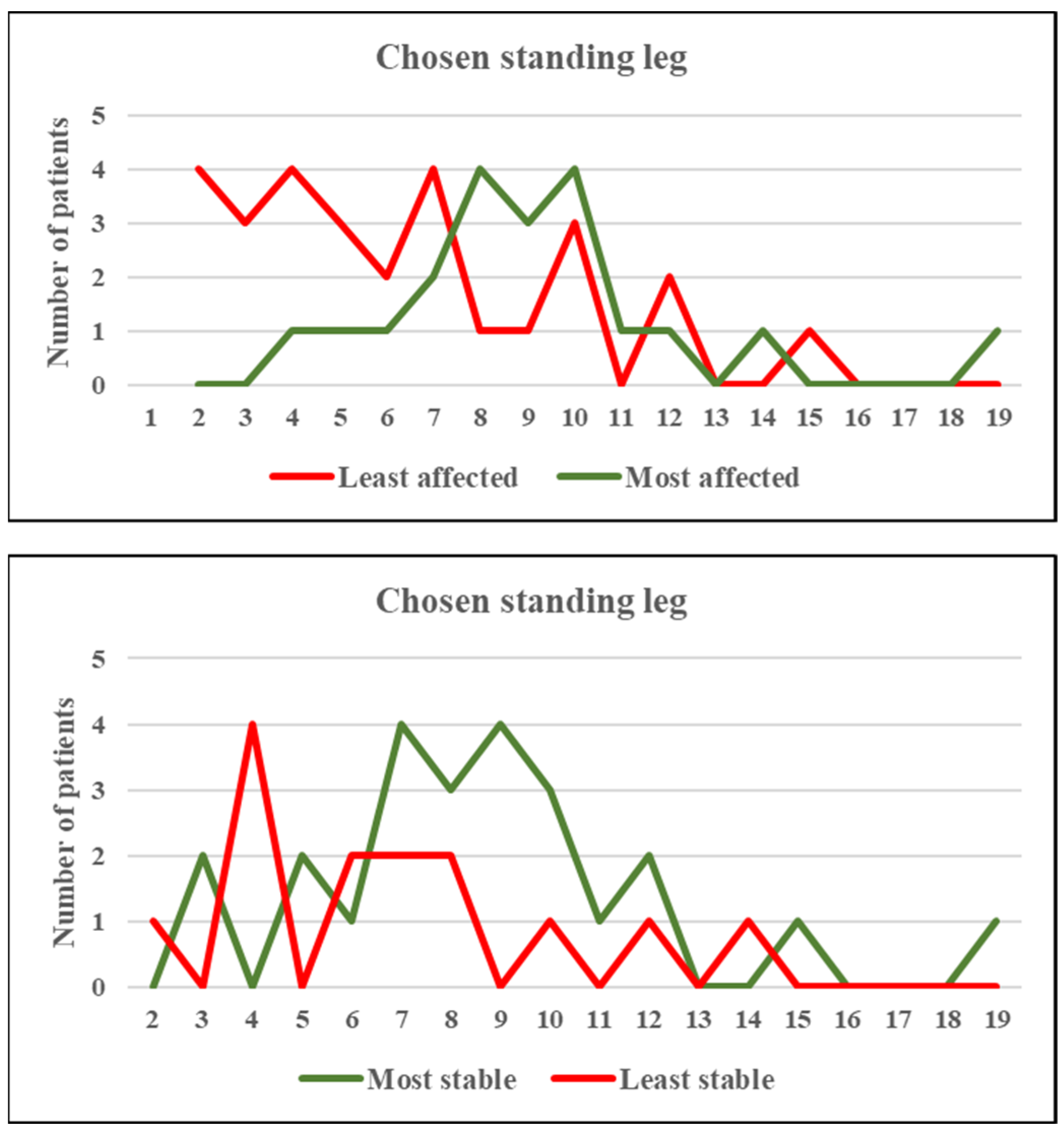
3.1. Whole Group Analyses
3.2. Secondary Outcomes
4. Discussion
4.1. One-Leg Stance Strategy in Patients with PD
4.2. Maladaptive One-Leg Stance Strategy in People with PD
4.3. Influence of Patients’ Characteristics on One-Leg Stance
4.4. Limits of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Debû, B.; Godeiro, C.D.O.; Lino, J.C.; Moro, E. Managing Gait, Balance, and Posture in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2018, 18, 23. [Google Scholar] [CrossRef]
- Marcori, A.J.; Monteiro, P.H.M.; A Oliveira, J.; Doumas, M.; A Teixeira, L. Single Leg Balance Training: A Systematic Review. Percept. Mot. Ski. 2022, 129, 232–252. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Williams, T.F.; Mayewski, R. Fall risk index for elderly patients based on number of chronic disabilities. Am. J. Med. 1986, 80, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.J.; Ms, S.J.W.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.; Garry, P.J. One-Leg Balance Is an Important Predictor of Injurious Falls in Older Persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef]
- Riemann, B.L.; Schmitz, R. The relationship between various modes of single leg postural control assessment. Int. J. Sports Phys. Ther. 2012, 7, 257–266. [Google Scholar] [PubMed]
- Gabbard, S.H.C. Examining the Stabilising Characteristics of Footedness. Laterality 1997, 2, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gabbard, C.; Hart, S. A Question of Foot Dominance. J. Gen. Psychol. 1996, 123, 289–296. [Google Scholar] [CrossRef]
- Wang, Z.; Newell, K.M. Footedness exploited as a function of postural task asymmetry. Laterality 2013, 18, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Previc, F.H. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol. Rev. 1991, 98, 299–334. [Google Scholar] [CrossRef]
- Peters, M. Footedness: Asymmetries in foot preference and skill and neuropsychological assessment of foot movement. Psychol. Bull. 1988, 103, 179–192. [Google Scholar] [CrossRef]
- Elias, L.J.; Bryden, M.; Bulman-Fleming, M. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia 1998, 36, 37–43. [Google Scholar] [CrossRef] [PubMed]
- van Melick, N.; Meddeler, B.M.; Hoogeboom, T.J.; Nijhuis-van der Sanden, M.W.G.; van Cingel, R.E.H. How to determine leg dominance: The agreement between self-reported and observed performance in healthy adults. PLoS ONE 2017, 12, e0189876. [Google Scholar] [CrossRef]
- Bacelar, A.M.; Teixeira, L.A. Footedness across ages: Distinction between mobilization and stabilization tasks. Laterality 2014, 20, 141–153. [Google Scholar] [CrossRef]
- Gentry, V.; Gabbard, C. Foot-Preference Behavior: A Developmental Perspective. J. Gen. Psychol. 1995, 122, 37–45. [Google Scholar] [CrossRef]
- Gabbard, C. Foot Laterality in Children, Adolescents, and Adults. Laterality 1996, 1, 199–206. [Google Scholar] [CrossRef]
- Carey, D.P.; Smith, D.T.; Martin, D.; Smith, G.; Skriver, J.; Rutland, A.; Shepherd, J.W. The bi-pedal ape: Plasticity and asymmetry in footedness. Cortex 2009, 45, 650–661. [Google Scholar] [CrossRef]
- Castrioto, A.; Piscicelli, C.; Pérennou, D.; Krack, P.; Debû, B. The pathogenesis of Pisa syndrome in Parkinson’s disease. Mov. Disord. 2014, 29, 1100–1107. [Google Scholar] [CrossRef]
- Boonstra, T.A.; Schouten, A.C.; Van Vugt, J.P.P.; Bloem, B.R.; Van Der Kooij, H. Parkinson’s disease patients compensate for balance control asymmetry. J. Neurophysiol. 2014, 112, 3227–3239. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Freidl, W.; Fazekas, F.; Reinhart, B.; Grieshofer, P.; Koch, M.; Eber, B.; Schumacher, M.; Polmin, K.; Lechner, H. The Mattis Dementia Rating Scale: Normative data from 1,001 healthy volunteers. Neurology 1994, 44, 964. [Google Scholar] [CrossRef]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fung, V.S.C. Postural Instability in Patients with Parkinson’s Disease. CNS Drugs 2013, 27, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Beuter, A.; Hernández, R.; Rigal, R.; Modolo, J.; Blanchet, P.J. Postural Sway and Effect of Levodopa in Early Parkinson’s Disease. Can. J. Neurol. Sci. J. Can. des Sci. Neurol. 2008, 35, 65–68. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Mancini, M. Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Mov. Disord. 2013, 28, 1544–1551. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Yarnall, A.; Coleman, S.; Burn, D.; Rochester, L. Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naïve cohort. Mov. Disord. 2016, 31, 1829–1836. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Nutt, J.G.; Carlson-Kuhta, P.; Stephens, M.; Horak, F.B. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp. Neurol. 2009, 215, 334–341. [Google Scholar] [CrossRef]
- Nieuwboer, A.; Giladi, N. Characterizing freezing of gait in Parkinson’s disease: Models of an episodic phenomenon. Mov. Disord. 2013, 28, 1509–1519. [Google Scholar] [CrossRef]
- Zawadka-Kunikowska, M.; Zalewski, P.; Klawe, J.J.; Pawlak, J.; Tafil-Klawe, M.; Kędziora-Kornatowska, K.; Newton, J.L. Age-related changes in cognitive function and postural control in Parkinson’s disease. Aging Clin. Exp. Res. 2014, 26, 505–510. [Google Scholar] [CrossRef]
- Dirnberger, G.; Jahanshahi, M. Executive dysfunction in Parkinson’s disease: A review. J. Neuropsychol. 2013, 7, 193–224. [Google Scholar] [CrossRef]
- Marchese, R.; Bove, M.; Abbruzzese, G. Effect of cognitive and motor tasks on postural stability in Parkinson’s disease: A posturographic study. Mov. Disord. 2003, 18, 652–658. [Google Scholar] [CrossRef]
- Lee, J.M.; Koh, S.-B.; Chae, S.W.; Seo, W.-K.; Kwon, D.Y.; Kim, J.H.; Oh, K.; Baik, J.S.; Park, K.W. Postural Instability and Cognitive Dysfunction in Early Parkinson’s Disease. Can. J. Neurol. Sci. J. Can. des Sci. Neurol. 2012, 39, 473–482. [Google Scholar] [CrossRef]
- Kelly, V.; Johnson, C.; McGough, E.; Shumway-Cook, A.; Horak, F.; Chung, K.; Espay, A.; Revilla, F.; Devoto, J.; Wood-Siverio, C.; et al. Association of cognitive domains with postural instability/gait disturbance in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 692–697. [Google Scholar] [CrossRef]
- Leandri, M.; Campbell, J.; Molfetta, L.; Barbera, C.; Tabaton, M. Relationship Between Balance and Cognitive Performance in Older People. J. Alzheimer’s Dis. 2015, 45, 705–707. [Google Scholar] [CrossRef]
- Lord, S.R.; Delbaere, K.; Sturnieks, D.L. Aging. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 157–171. [Google Scholar] [CrossRef]
| Age (years) | 57.5 ± 9.6 |
| Gender (M/W) | 36/14 |
| PD duration (years) | 7.5 ± 3.6 |
| MDS-UPDRS III ON Med (/132) | 21.8 ± 7.8 |
| MDS-UPDRS IV (/24) | 7.0 ± 3.9 |
| MDRS (/144) | 138.8 ± 3.8 |
| Medication (LEDD) | 1004.1 ± 424.8 |
| FOG/Falls (Number of patients) | 9/15 |
| Total Sample (N = 50) | Least Affected Body Side (N = 28) | Most Affected Body Side (N = 22) | |
|---|---|---|---|
| Disease duration (years) | 7.5 ± 3.6 | 4.7 ± 1.8 | 10.4 ± 2.6 * |
| Stance duration preferred leg (sec) | 26.2 ± 13.9 | 29.9 ± 13.1 | 21.4 ± 13.5 * |
| Stance duration opposite leg (sec) | 25.8 ± 15.3 | 32.1 ± 12.1 | 19.0 ± 16.1 * |
| Stance duration most stable leg (sec) | 29.8 ± 13.6 | 33.2 ± 10.6 | 26.3 ± 15.5 |
| Stance duration least stable leg (sec) | 22.2 ± 14.7 ß | 26.5 ± 13.1 ß | 18.0 ± 15.2 ß |
| Total Sample (N = 50) | G1 (N = 25) | G2 (N = 25) | |
|---|---|---|---|
| Disease duration (years) | 7.5 ± 3.6 | 5.8 ± 3.0 | 9.5 ± 3.2 β |
| UPDRS III score (/132) | 21.8 ± 7.8 | 21.2 ± 7.4 | 22.1 ± 8.4 |
| MDRS score (/144) | 138.8 ± 3.8 | 139.1 ± 4.0 | 138.1 ± 3.5 |
| Stance duration preferred leg (sec) | 26.2 ± 13.9 | 31.4 ± 12.0 | 20.1 ± 13.8 * |
| Stance duration opposite leg (sec) | 25.8 ± 15.3 | 32.6 ± 10.1 | 17.8 ± 16.7 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevrier, E.; Moro, E.; Pelissier, P.; Castrioto, A.; Krack, P.; Fraix, V.; Debû, B. Maladaptive One-Leg Balance Control in Parkinson’s Disease. Symmetry 2022, 14, 2511. https://doi.org/10.3390/sym14122511
Chevrier E, Moro E, Pelissier P, Castrioto A, Krack P, Fraix V, Debû B. Maladaptive One-Leg Balance Control in Parkinson’s Disease. Symmetry. 2022; 14(12):2511. https://doi.org/10.3390/sym14122511
Chicago/Turabian StyleChevrier, Eric, Elena Moro, Pierre Pelissier, Anna Castrioto, Paul Krack, Valérie Fraix, and Bettina Debû. 2022. "Maladaptive One-Leg Balance Control in Parkinson’s Disease" Symmetry 14, no. 12: 2511. https://doi.org/10.3390/sym14122511
APA StyleChevrier, E., Moro, E., Pelissier, P., Castrioto, A., Krack, P., Fraix, V., & Debû, B. (2022). Maladaptive One-Leg Balance Control in Parkinson’s Disease. Symmetry, 14(12), 2511. https://doi.org/10.3390/sym14122511






