A Neutral Heteroleptic Molybdenum Cluster trans-[{Mo6I8}(py)2I4]
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Crystallography
3. Results and Discussion
3.1. Crystal Structure and Literture Analysis
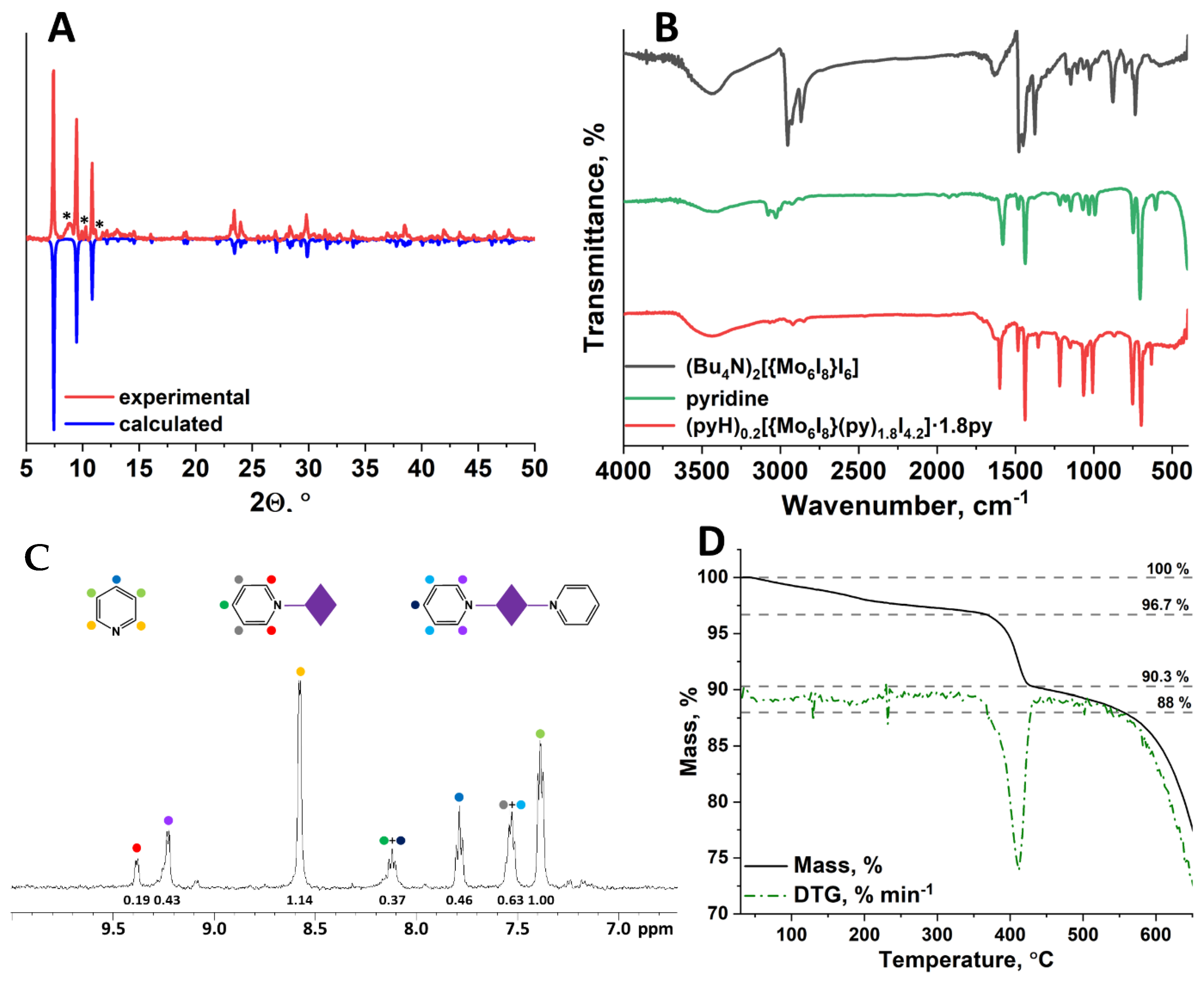
3.2. Synthetic Features and Characterization
3.3. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mikhaylov, M.A.; Sokolov, M.N. Molybdenum Iodides—From Obscurity to Bright Luminescence. Eur. J. Inorg. Chem. 2019, 2019, 4181–4197. [Google Scholar] [CrossRef]
- Marchuk, M.V.; Vorotnikova, N.A.; Vorotnikov, Y.A.; Kuratieva, N.V.; Stass, D.V.; Shestopalov, M.A. Optical Property Trends in a Family of {Mo6I8} Aquahydroxo Complexes. Dalton Trans. 2021, 50, 8794–8802. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubat, P.; Fejfarova, K.; Martincik, J.; Nikl, M.; Lang, K. X-ray Inducible Luminescence and Singlet Oxygen Sensitization by an Octahedral Molybdenum Cluster Compound: A New Class of Nanoscintillators. Inorg. Chem. 2016, 55, 803–809. [Google Scholar] [CrossRef]
- Efremova, O.A.; Vorotnikov, Y.A.; Brylev, K.A.; Vorotnikova, N.A.; Novozhilov, I.N.; Kuratieva, N.V.; Edeleva, M.V.; Benoit, D.M.; Kitamura, N.; Mironov, Y.V.; et al. Octahedral Molybdenum Cluster Complexes with Aromatic Sulfonate Ligands. Dalton Trans. 2016, 45, 15427–15435. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, M.A.; Brylev, K.A.; Abramov, P.A.; Sakuda, E.; Akagi, S.; Ito, A.; Kitamura, N.; Sokolov, M.N. Synthetic Tuning of Redox, Spectroscopic, and Photophysical Properties of {Mo6I8}4+ Core Cluster Complexes by Terminal Carboxylate Ligands. Inorg. Chem. 2016, 55, 8437–8445. [Google Scholar] [CrossRef] [PubMed]
- Akagi, S.; Fujii, S.; Kitamura, N. A Study on the Redox, Spectroscopic, and Photophysical Characteristics of a Series of Octahedral Hexamolybdenum(II) Clusters: [{Mo6X8}Y6]2− (X, Y = Cl, Br, or I). Dalton Trans. 2018, 47, 1131–1139. [Google Scholar] [CrossRef]
- Evtushok, D.V.; Melnikov, A.R.; Vorotnikova, N.A.; Vorotnikov, Y.A.; Ryadun, A.A.; Kuratieva, N.V.; Kozyr, K.V.; Obedinskaya, N.R.; Kretov, E.I.; Novozhilov, I.N.; et al. A Comparative Study of Optical Properties and X-ray Induced Luminescence of Octahedral Molybdenum and Tungsten Cluster Complexes. Dalton Trans. 2017, 46, 11738–11747. [Google Scholar] [CrossRef] [PubMed]
- Vorotnikov, Y.A.; Efremova, O.A.; Vorotnikova, N.A.; Brylev, K.A.; Edeleva, M.V.; Tsygankova, A.R.; Smolentsev, A.I.; Kitamura, N.; Mironov, Y.V.; Shestopalov, M.A. On the Synthesis and Characterisation of Luminescent Hybrid Particles: Mo6 Metal Cluster Complex/SiO2. RSC Adv. 2016, 6, 43367–43375. [Google Scholar] [CrossRef]
- Jackson, J.A.; Turro, C.; Newsham, M.D.; Nocera, D.G. Oxygen Quenching of Electronically Excited Hexanuclear Molybdenum and Tungsten Halide Clusters. J. Phys. Chem. 1990, 94, 4500–4507. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Langmaier, J.; Polívka, T.; Fuciman, M.; Fejfarová, K.; Lang, K. A Comparative Study of the Redox and Excited State Properties of (nBu4N)2[Mo6X14] and (nBu4N)2[Mo6X8(CF3COO)6] (X = Cl, Br, or I). Dalton Trans. 2013, 42, 7224–7232. [Google Scholar] [CrossRef] [PubMed]
- Krasilnikova, A.A.; Shestopalov, M.A.; Brylev, K.A.; Kirilova, I.A.; Khripko, O.P.; Zubareva, K.E.; Khripko, Y.I.; Podorognaya, V.T.; Shestopalova, L.V.; Fedorov, V.E.; et al. Prospects of Molybdenum and Rhenium Octahedral Cluster Complexes as X-ray Contrast Agents. J. Inorg. Biochem. 2015, 144, 13–17. [Google Scholar] [CrossRef]
- Ivanova, M.N.; Vorotnikov, Y.A.; Plotnikova, E.E.; Marchuk, M.V.; Ivanov, A.A.; Asanov, I.P.; Tsygankova, A.R.; Grayfer, E.D.; Fedorov, V.E.; Shestopalov, M.A. Hexamolybdenum Clusters Supported on Exfoliated h-BN Nanosheets for Photocatalytic Water Purification. Inorg. Chem. 2020, 59, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Khatri, O.P.; Cordier, S.; Boukherroub, R.; Jain, S.L. Graphene Oxide Supported Molybdenum Cluster: First Heterogenized Homogeneous Catalyst for the Synthesis of Dimethylcarbonate from CO2 and Methanol. Chem. Eur. J. 2015, 21, 3488–3494. [Google Scholar] [CrossRef]
- Barras, A.; Cordier, S.; Boukherroub, R. Fast Photocatalytic Degradation of Rhodamine B over [Mo6Br8(N3)6]2− Cluster Units under Sun Light Irradiation. Appl. Catal. B Environ. 2012, 123–124, 1–8. [Google Scholar] [CrossRef]
- Feliz, M.; Atienzar, P.; Amela-Cortés, M.; Dumait, N.; Lemoine, P.; Molard, Y.; Cordier, S. Supramolecular Anchoring of Octahedral Molybdenum Clusters onto Graphene and Their Synergies in Photocatalytic Water Reduction. Inorg. Chem. 2019, 58, 15443–15454. [Google Scholar] [CrossRef]
- Nguyen, N.T.K.; Lebastard, C.; Wilmet, M.; Dumait, N.; Renaud, A.; Cordier, S.; Ohashi, N.; Uchikoshi, T.; Grasset, F. A Review on Functional Nanoarchitectonics Nanocomposites Based on Octahedral Metal Atom Clusters (Nb6, Mo6, Ta6, W6, Re6): Inorganic 0D and 2D Powders and Films. Sci. Technol. Adv. Mater. 2022, 23, 547–578. [Google Scholar] [CrossRef] [PubMed]
- Molard, Y. Clustomesogens: Liquid Crystalline Hybrid Nanomaterials Containing Functional Metal Nanoclusters. Acc. Chem. Res. 2016, 49, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Guy, K.; Ehni, P.; Paofai, S.; Forschner, R.; Roiland, C.; Amela-Cortes, M.; Cordier, S.; Laschat, S.; Molard, Y. Lord of The Crowns: A New Precious in the Kingdom of Clustomesogens. Angew. Chem. Int. Ed. 2018, 57, 11692–11696. [Google Scholar] [CrossRef] [PubMed]
- Riehl, L.; Ströbele, M.; Enseling, D.; Jüstel, T.; Meyer, H.-J. Molecular Oxygen Modulated Luminescence of an Octahedro-hexamolybdenum Iodide Cluster having Six Apical Thiocyanate Ligands. Z. Anorg. Allg. Chem. 2016, 642, 403–408. [Google Scholar] [CrossRef]
- Fuhrmann, A.-D.; Seyboldt, A.; Schank, A.; Zitzer, G.; Speiser, B.; Enseling, D.; Jüstel, T.; Meyer, H.-J. Luminescence Quenching of Ligand-Substituted Molybdenum and Tungsten Halide Clusters by Oxygen and Their Oxidation Electrochemistry. Eur. J. Inorg. Chem. 2017, 2017, 4259–4266. [Google Scholar] [CrossRef]
- Amela-Cortes, M.; Paofai, S.; Cordier, S.; Folliot, H.; Molard, Y. Tuned Red NIR Phosphorescence of Polyurethane Hybrid Composites Embedding Metallic Nanoclusters for Oxygen Sensing. Chem. Commun. 2015, 51, 8177–8180. [Google Scholar] [CrossRef] [PubMed]
- Riehl, L.; Seyboldt, A.; Ströbele, M.; Enseling, D.; Jüstel, T.; Westberg, M.; Ogilby, P.R.; Meyer, H.J. A Ligand Substituted Tungsten Iodide Cluster: Luminescence vs. Singlet Oxygen Production. Dalton Trans. 2016, 45, 15500–15506. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, S.; Bigeon, J.; Amela-Cortes, M.; Dumait, N.; Loas, G.h.; Cordier, S.; Molard, Y. Switchable Two-Dimensional Waveguiding Abilities of Luminescent Hybrid Nanocomposites for Active Solar Concentrators. ACS Appl. Mater. Interfaces 2020, 12, 14400–14407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lunt, R.R. Transparent Luminescent Solar Concentrators for Large-Area Solar Windows Enabled by Massive Stokes-Shift Nanocluster Phosphors. Adv. Energy Mater. 2013, 3, 1143–1148. [Google Scholar] [CrossRef]
- Renaud, A.; Jouan, P.-Y.; Dumait, N.; Ababou-Girard, S.; Barreau, N.; Uchikoshi, T.; Grasset, F.; Jobic, S.; Cordier, S. Evidence of the Ambipolar Behavior of Mo6 Cluster Iodides in All-Inorganic Solar Cells: A New Example of Nanoarchitectonic Concept. ACS Appl. Mater. Interfaces 2022, 14, 1347–1354. [Google Scholar] [CrossRef]
- Renaud, A.; Nguyen, T.K.N.; Grasset, F.; Raissi, M.; Guillon, V.; Delabrouille, F.; Dumait, N.; Jouan, P.Y.; Cario, L.; Jobic, S.; et al. Preparation by Electrophoretic Deposition of Molybdenum Iodide Cluster-Based Functional Nanostructured Photoelectrodes for Solar Cells. Electrochim. Acta 2019, 317, 737–745. [Google Scholar] [CrossRef]
- Kirakci, K.; Pozmogova, T.N.; Protasevich, A.Y.; Vavilov, G.D.; Stass, D.V.; Shestopalov, M.A.; Lang, K. A Water-Soluble Octahedral Molybdenum Cluster Complex as a Potential Agent for X-ray Induced Photodynamic Therapy. Biomater. Sci. 2021, 9, 2893–2902. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubáňová, M.; Přibyl, T.; Rumlová, M.; Zelenka, J.; Ruml, T.; Lang, K. A Cell Membrane Targeting Molybdenum-Iodine Nanocluster: Rational Ligand Design toward Enhanced Photodynamic Activity. Inorg. Chem. 2022, 61, 5076–5083. [Google Scholar] [CrossRef]
- Koncošová, M.; Rumlová, M.; Mikyšková, R.; Reiniš, M.; Zelenka, J.; Ruml, T.; Kirakci, K.; Lang, K. Avenue to X-ray-Induced Photodynamic Therapy of Prostatic Carcinoma with Octahedral Molybdenum Cluster Nanoparticles. J. Mater. Chem. B 2022, 10, 3303–3310. [Google Scholar] [CrossRef]
- Vorotnikov, Y.A.; Novikova, E.D.; Solovieva, A.O.; Shanshin, D.V.; Tsygankova, A.R.; Shcherbakov, D.N.; Efremova, O.A.; Shestopalov, M.A. Single-Domain Antibody C7b for Address Delivery of Nanoparticles to HER2-Positive Cancers. Nanoscale 2020, 12, 21885–21894. [Google Scholar] [CrossRef]
- Svezhentseva, E.V.; Vorotnikov, Y.A.; Solovieva, A.O.; Pozmogova, T.N.; Eltsov, I.V.; Ivanov, A.A.; Evtushok, D.V.; Miroshnichenko, S.M.; Yanshole, V.V.; Eling, C.J.; et al. From Photoinduced to Dark Cytotoxicity through an Octahedral Cluster Hydrolysis. Chem. Eur. J. 2018, 24, 17915–17920. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, A.O.; Vorotnikov, Y.A.; Trifonova, K.E.; Efremova, O.A.; Krasilnikova, A.A.; Brylev, K.A.; Vorontsova, E.V.; Avrorov, P.A.; Shestopalova, L.V.; Poveshchenko, A.F.; et al. Cellular Internalisation, Bioimaging and Dark and Photodynamic Cytotoxicity of Silica Nanoparticles Doped by {Mo6I8}4+ Metal Clusters. J. Mater. Chem. B 2016, 4, 4839–4846. [Google Scholar] [CrossRef] [PubMed]
- Vorotnikov, Y.A.; Pozmogova, T.N.; Solovieva, A.O.; Miroshnichenko, S.M.; Vorontsova, E.V.; Shestopalova, L.V.; Mironov, Y.V.; Shestopalov, M.A.; Efremova, O.A. Luminescent Silica Mesoparticles for Protein Transduction. Mat. Sci. Eng. C Mater. 2019, 96, 530–538. [Google Scholar] [CrossRef]
- Beltran, A.; Mikhailov, M.; Sokolov, M.N.; Perez-Laguna, V.; Rezusta, A.; Revillo, M.J.; Galindo, F. A Photobleaching Resistant Polymer Supported Hexanuclear Molybdenum Iodide Cluster for Photocatalytic Oxygenations and Photodynamic Inactivation of Staphylococcus Aureus. J. Mater. Chem. B 2016, 4, 5975–5979. [Google Scholar] [CrossRef]
- Vorotnikova, N.A.; Alekseev, A.Y.; Vorotnikov, Y.A.; Evtushok, D.V.; Molard, Y.; Amela-Cortes, M.; Cordier, S.; Smolentsev, A.I.; Burton, C.G.; Kozhin, P.M.; et al. Octahedral Molybdenum Cluster as a Photoactive Antimicrobial Additive to a Fluoroplastic. Mat. Sci. Eng. C Mater. 2019, 105, 110150. [Google Scholar] [CrossRef]
- Kirakci, K.; Nguyen, T.K.N.; Grasset, F.; Uchikoshi, T.; Zelenka, J.; Kubát, P.; Ruml, T.; Lang, K. Electrophoretically Deposited Layers of Octahedral Molybdenum Cluster Complexes: A Promising Coating for Mitigation of Pathogenic Bacterial Biofilms under Blue Light. ACS Appl. Mater. Interfaces 2020, 12, 52492–52499. [Google Scholar] [CrossRef]
- Vorotnikova, N.A.; Bardin, V.A.; Vorotnikov, Y.A.; Kirakci, K.; Adamenko, L.S.; Alekseev, A.Y.; Meyer, H.J.; Kubat, P.; Mironov, Y.V.; Lang, K.; et al. Heterogeneous Photoactive Antimicrobial Coatings Based on a Fluoroplastic Doped with an Octahedral Molybdenum Cluster Compound. Dalton Trans. 2021, 50, 8467–8475. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Mermer, A.; Keles, T.; Sirin, Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioorg. Chem. 2021, 114, 105076. [Google Scholar] [CrossRef]
- Mironova, A.D.; Mikhailov, M.A.; Brylev, K.A.; Gushchin, A.L.; Sukhikh, T.S.; Sokolov, M.N. Phosphorescent Complexes of {Mo6I8}4+ with Triazolates: [2+3] Cycloaddition of Alkynes to [Mo6I8(N3)6]2−. New J. Chem. 2020, 44, 20620–20625. [Google Scholar] [CrossRef]
- Mironova, A.; Gushchin, A.; Abramov, P.; Eltsov, I.; Ryadun, A.; Sokolov, M. [Mo6I8]4+ Complexes with Tetrazolate Ligands: [3+2] Cycloaddition of Aromatic Nitriles to [Mo6I8(N3)6]2−. Polyhedron 2021, 205, 115282. [Google Scholar] [CrossRef]
- Konovalov, D.I.; Ivanov, A.A.; Vorotnikov, Y.A.; Kuratieva, N.V.; Eltsov, I.V.; Kovalenko, K.A.; Shestopalov, M.A. Self-Assembled Microporous M-HOFs Based on an Octahedral Rhenium Cluster with Benzimidazole. Inorg. Chem. 2021, 60, 14687–14696. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, D.I.; Ivanov, A.A.; Frolova, T.S.; Eltsov, I.V.; Gayfulin, Y.M.; Plunkett, L.; Bazzar, M.; Adawi, A.M.; Bouillard, J.-S.G.; Baiborodin, S.I.; et al. Water-Soluble Rhenium Clusters with Triazoles: The Effect of Chemical Structure on Cellular Internalization and the DNA Binding of the Complexes. Chem. Eur. J. 2020, 26, 13904–13914. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Cordier, S.; Perrin, C. Synthesis and Characterization of Cs2Mo6X14 (X = Br or I) Hexamolybdenum Cluster Halides: Efficient Mo6 Cluster Precursors for Solution Chemistry Syntheses. Z. Anorg. Allg. Chem. 2005, 631, 411–416. [Google Scholar] [CrossRef]
- Bruker. APEX2, Version 1.08, SAINT, Version 07.03, SADABS, Version 02.11, SHELXTL, Version 06.12; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.; Schäfer, H. Die Reaktion von [Mo6Cl8]X4 mit N-Basen. Z. Anorg. Allg. Chem. 1985, 524, 137–143. [Google Scholar] [CrossRef]
- Méry, D.; Plault, L.; Ornelas, C.; Ruiz, J.; Nlate, S.; Astruc, D.; Blais, J.-C.; Rodrigues, J.; Cordier, S.; Kirakci, K.; et al. From Simple Monopyridine Clusters [Mo6Br13(Py-R)][n-Bu4N] and Hexapyridine Clusters [Mo6X8(Py-R)6][OSO2CF3]4 (X = Br or I) to Cluster-Cored Organometallic Stars, Dendrons, and Dendrimers. Inorg. Chem. 2006, 45, 1156–1167. [Google Scholar] [CrossRef]
- Costuas, K.; Garreau, A.; Bulou, A.; Fontaine, B.; Cuny, J.; Gautier, R.; Mortier, M.; Molard, Y.; Duvail, J.L.; Faulques, E.; et al. Combined Theoretical and Time-Resolved Photoluminescence Investigations of [Mo6Bri8Bra6]2− Metal Cluster Units: Evidence of Dual Emission. Phys. Chem. Chem. Phys. 2015, 17, 28574–28585. [Google Scholar] [CrossRef]
- Xin, D.; Matti, T.; Matti, H. Halogen Bonding in Crystal Engineering. In Recent Advances in Crystallography; Benedict, J.B., Ed.; IntechOpen: London, UK, 2012; Volume 262, pp. 143–168. [Google Scholar]
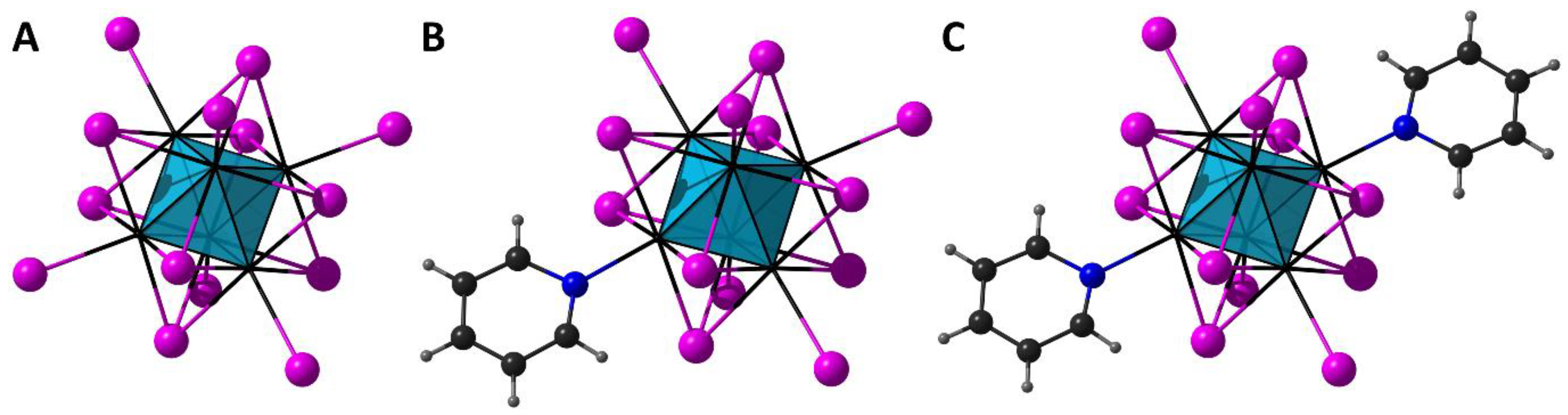


| Cluster Complex | Interatomic Distances, Å | References | |||
|---|---|---|---|---|---|
| Mo–Mo | Mo–Ii | Mo–Ia | Mo–N | ||
| trans-[{Mo6I8}(py)2I4] | 2.675(2)– 2.6900(17) | 2.7656(18)– 2.7891(16) | 2.75(2)– 2.8432(19) | 2.22(3) | this work |
| [{Mo6I8}(N3C2H(COOCH3))6]2− | 2.6721(7)– 2.6794(6) | 2.7590(6)– 2.7815(6) | - | 2.199(4) | [40] |
| [{Mo6I8}(N4C(C6H5))6]2− | 2.6728(10)– 2.6856(9) | 2.7588(8)– 2.7737(9) | 2.844(12)– 2.995(8) # | 2.230(8)– 2.238(9) | [41] |
| [{Mo6I8}(N4C(C6F5))6]2− | 2.6703(12)– 2.6771(12) | 2.7557(9)– 2.7760(11) | - | 2.220(9)– 2.259(9) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchuk, M.V.; Vorotnikov, Y.A.; Ivanov, A.A.; Eltsov, I.V.; Kuratieva, N.V.; Shestopalov, M.A. A Neutral Heteroleptic Molybdenum Cluster trans-[{Mo6I8}(py)2I4]. Symmetry 2022, 14, 2117. https://doi.org/10.3390/sym14102117
Marchuk MV, Vorotnikov YA, Ivanov AA, Eltsov IV, Kuratieva NV, Shestopalov MA. A Neutral Heteroleptic Molybdenum Cluster trans-[{Mo6I8}(py)2I4]. Symmetry. 2022; 14(10):2117. https://doi.org/10.3390/sym14102117
Chicago/Turabian StyleMarchuk, Margarita V., Yuri A. Vorotnikov, Anton A. Ivanov, Ilia V. Eltsov, Natalia V. Kuratieva, and Michael A. Shestopalov. 2022. "A Neutral Heteroleptic Molybdenum Cluster trans-[{Mo6I8}(py)2I4]" Symmetry 14, no. 10: 2117. https://doi.org/10.3390/sym14102117
APA StyleMarchuk, M. V., Vorotnikov, Y. A., Ivanov, A. A., Eltsov, I. V., Kuratieva, N. V., & Shestopalov, M. A. (2022). A Neutral Heteroleptic Molybdenum Cluster trans-[{Mo6I8}(py)2I4]. Symmetry, 14(10), 2117. https://doi.org/10.3390/sym14102117






