Activation-Inhibition Coordination in Neuron, Brain, and Behavior Sequencing/Organization: Implications for Laterality and Lateralization
Abstract
1. Introduction
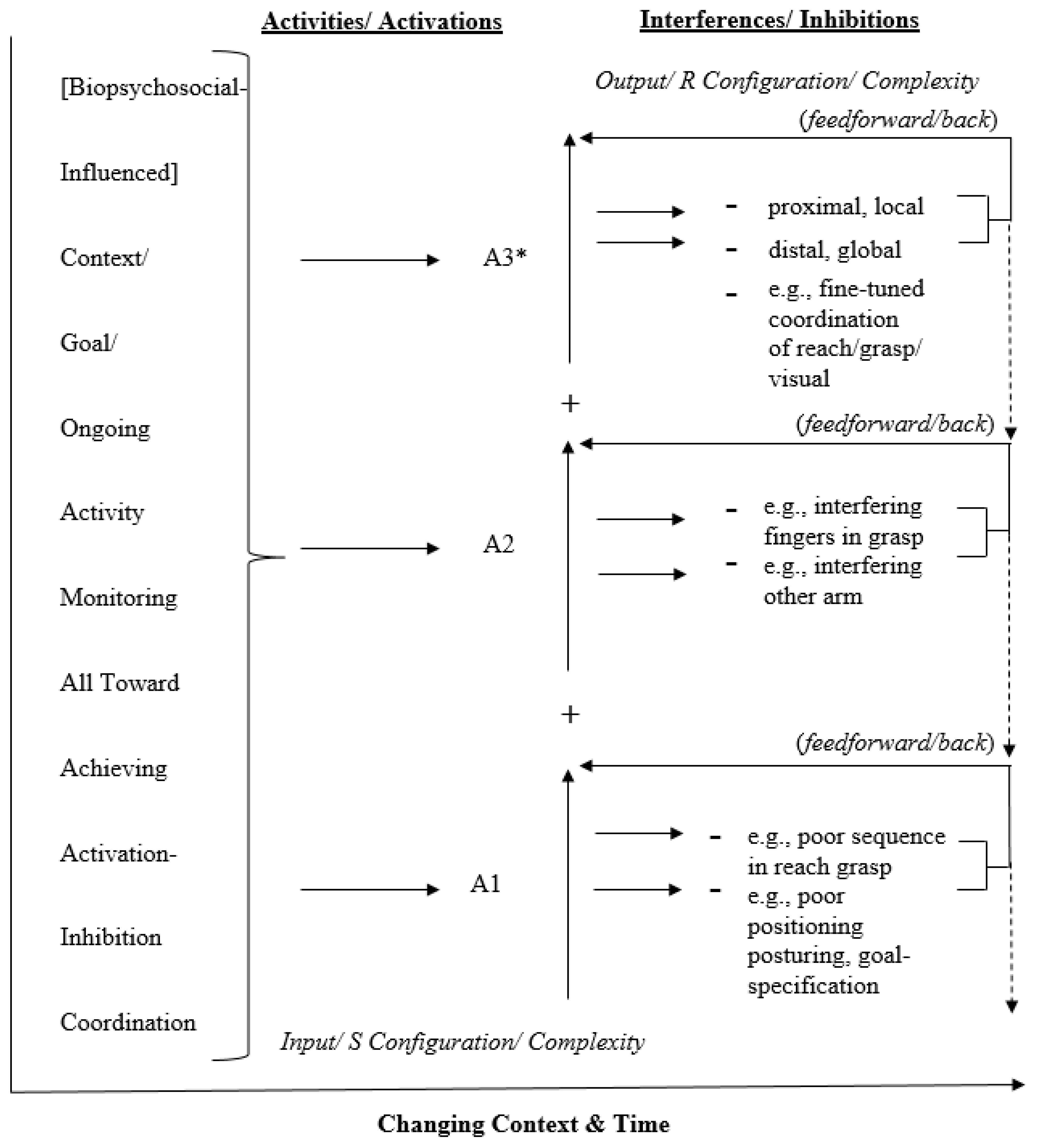
2. Activation-Inhibition Coordination Modeling
2.1. A Left-Hemisphere Activation-Inhibition Coordination Model
2.2. A Generic Activation-Inhibition Coordination Model
3. Clarifications
3.1. Definitions
3.2. Prediction/Testing/Falsifiability
3.3. Interim Conclusion
4. Literature Review
4.1. Past Relevant Literature in Young (2022)
4.2. Recent Relevant Literature
4.2.1. Neuronal
4.2.2. Cortical
4.2.3. Disturbances in Behavior
4.2.4. Lateralization
4.3. Interim Conclusion
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, G.; Bowman, J.G.; Methot, C.; Finlayson, M.; Quintal, J.; Boissonneault, P. Hemispheric specialization development: What (inhibition) and how (parents). In Manual Specialization and the Developing Brain; Young, G., Segalowitz, S.J., Carter, C.M., Trehub, S.E., Eds.; Academic Press: Cambridge, MA, USA, 1983; pp. 119–140. [Google Scholar]
- Young, G.; Gagnon, M. Neonatal laterality, birth stress, familial sinistrality, and left brain inhibition. Dev. Neuropsychol. 1990, 6, 127–150. [Google Scholar] [CrossRef]
- Young, G. Causality and Neo-Stages in Development: Toward Unifying Psychology; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Young, G. Early neuropsychological development: Lateralization of functions—hemispheric specialization. In Developmental Psychology: Cognitive, Perceptuomotor and Neuropsychological Perspectives; Hauert, C.A., Ed.; Elsevier: North Holland, The Netherlands, 1990; pp. 113–181. [Google Scholar]
- Young, G. The development of hemispheric manual specialization. In Cerebral Control of Speech and Limb Movements; Hammond, G.E., Ed.; Elsevier: North Holland, The Netherlands, 1990; pp. 79–139. [Google Scholar]
- Young, G. Development and Causality: Neo-Piagetian Perspectives; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Young, G. Unifying Causality and Psychology: Being, Brain and Behavior; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Swanson, L.W.; Hahn, J.D.; Jeub, L.G.S.; Fortunato, S.; Sporns, O. Subsystem organization of axonal connections within and between the right and left cerebral cortex and cerebral nuclei (endbrain). Proc. Natl. Acad. Sci. USA 2018, 115, E6910–E6919. [Google Scholar] [CrossRef] [PubMed]
- Borsboom, D. Psychometric perspectives on diagnostic systems. J. Clin. Psychol. 2008, 64, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- Werner, K.M.; Inzlicht, M.; Ford, B.Q. Whither inhibition? Curr. Dir. Psychol. Sci. 2022, 31, 333–339. [Google Scholar] [CrossRef]
- Clark, J.M. Contributions of inhibitory mechanisms to unified theory in neuroscience and psychology. Brain Cogn. 1996, 30, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Herdien, L.; Malcolm-Smith, S.; Pileggi, L.-A. Leftward cradling bias in males and its relation to autistic traits and lateralized emotion processing. Brain Cogn. 2021, 147, 105652. [Google Scholar] [CrossRef]
- Hartikainen, K.M. Emotion-attention interaction in the right hemisphere. Brain Sci. 2021, 11, 1006. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, N. Chapter 22—Brain-wide connectivity architecture: Developmental aspects. In Factors Affecting Neurodevelopment: Genetics, Neurology, Behavior, and Diet; Martin, C.R., Preedy, V.R., Rajendram, R., Eds.; Academic Press: Cambridge, Massachusetts, 2021; pp. 247–257. [Google Scholar]
- Young, G. Causality in psychiatry: A hybrid symptom network construct model. Front. Psychiatry 2015, 20, 164. [Google Scholar] [CrossRef]
- Ratnarajah, N.; Rifkin-Graboi, A.; Fortier, M.V.; Chong, Y.S.; Kwek, K.; Saw, S.M.; Godfrey, K.M.; Gluckman, P.D.; Meaney, M.J.; Qiu, A. Structural connectivity asymmetry in the neonatal brain. NeuroImage 2013, 75, 187–194. [Google Scholar] [CrossRef]
- Shine, J.M. Neuromodulatory influences on integration and segregation in the brain. Trends Cogn. Sci. 2019, 23, 572–583. [Google Scholar] [CrossRef]
- Gandolfi, D.; Bigiani, A.; Porro, C.A.; Mapelli, J. Inhibitory plasticity: From molecules to computation and beyond. Int. J. Mol. Sci. 2020, 21, 1805. [Google Scholar] [CrossRef]
- Wehr, M.; Zador, A.M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 2003, 426, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.S.; Nowotny, T.; Abarbanel, H.D.I. Spike-timing-dependent plasticity of inhibitory synapses in the entorhinal cortex. J. Neurophysiol. 2006, 96, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Tatti, R.; Haley, M.S.; Swanson, O.K.; Tselha, T.; Maffei, A. Neurophysiology and regulation of the balance between excitation and inhibition in neocortical circuits. Biol. Psychiatry 2017, 81, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Min, J.O.; Kang, D.-S.; Kim, Y.S.; Jung, G.H.; Park, H.J.; Kim, S.; An, H.; Kwon, J.; Kim, J.; et al. Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/ inhibition balance in cerebellum. Proc. Natl. Acad. Sci. USA 2018, 115, 5004–5009. [Google Scholar] [CrossRef]
- Sharma, H.S.; Muresanu, D.F.; Sahib, S.; Tian, Z.R.; Lafuente, J.V.; Buzoianu, A.D.; Castellani, R.J.; Nozari, A.; Li, C.; Zhang, Z.; et al. Chapter 6—Cerebrolysin restores balance between excitatory and inhibitory amino acids in brain following concussive head injury. Superior neuroprotective effects of TiO2 nanowired drug delivery. In Brain Protection Strategies and Nanomedicine; Sharma, H.S., Sharma, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 266, pp. 211–267. [Google Scholar] [CrossRef]
- Schmidt, J.; Brown, K.E.; Feldman, S.J.; Babul, S.; Zwicker, J.G.; Boyd, L.A. Evidence of altered interhemispheric communication after pediatric concussion. Brain Inj. 2021, 35, 1143–1161. [Google Scholar] [CrossRef]
- Herstel, L.J.; Wierenga, C.J. Network control through coordinated inhibition. Curr. Opin. Neurobiol. 2021, 67, 34–41. [Google Scholar] [CrossRef]
- He, H.-Y.; Cline, H.T. What is excitation/ inhibition and how is it regulated? A case of the elephant and the wiseman. Neurosci. Insights 2019, 13, 1179069519859371. [Google Scholar] [CrossRef]
- Kajiwara, M.; Nomura, R.; Goetze, F.; Kawabata, M.; Isomura, Y.; Akutsu, T.; Shimono, M. Inhibitory neurons exhibit high controlling ability in the cortical microconnectome. PLoS Comput. Biol. 2021, 17, e1008846. [Google Scholar] [CrossRef]
- Rumpel, S.; Triesch, J. The dynamic connectome. e-Neuroforum 2016, 7, 48–53. [Google Scholar] [CrossRef]
- Fortel, I.; Butler, M.; Korthauer, L.E.; Zhan, L.; Ajilore, O.; Sidiropoulos, A.; Wu, Y.; Driscoll, I.; Schonfeld, D.; Leow, A. Inferring excitation-inhibition dynamics using a maximum entropy model unifying brain structure and function. Netw. Neurosci. 2022, 6, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Adesnik, H. Layer-specific excitation/ inhibition balances during neuronal synchronization in the visual cortex. J. Physiol. 2018, 596, 1639–1657. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, S.; Donnelly, I.; Pearson, J. Human brain networks function in connectome-specific harmonic waves. Nat. Commun. 2016, 7, 10340. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.N.; McKendrick, A.M.; Vingrys, A.J. Abnormal inhibition-excitation imbalance in migraine. Cephalalgia 2016, 36, 5–14. [Google Scholar] [CrossRef]
- Li, W. Excitation and inhibition imbalance in Rett syndrome. Front. Neurosci. 2022, 16, 825063. [Google Scholar] [CrossRef]
- Bernardo, P.; Cobb, S.; Coppola, A.; Tomasevic, L.; Di Lazzaro, V.; Bravaccio, C.; Manganelli, F.; Dubbioso, R. Neurophysiological signatures of motor impairment in patients with Rett syndrome. Ann. Neurol. 2020, 87, 763–773. [Google Scholar] [CrossRef]
- Park, B.-Y.; Hong, S.-Y.; Valk, S.L.; Paquola, C.; Benkarim, O.; Bethlehem, R.A.I.; Di Martino, A.; Milham, M.P.; Gozzi, A.; Yeo, B.T.T.; et al. Difference in subcortico-cortical interactions identified from connectome and microcircuit models in autism. Nat. Commun. 2021, 12, 2225. [Google Scholar] [CrossRef]
- Rohr, C.S.; Kamal, S.; Bray, S. Building functional connectivity neuromarkers of behavioral self-regulation across children with and without Autism Spectrum Disorder. Dev. Cogn. Neurosci. 2020, 41, 100747. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, C.; Adamek, J.H.; Crocetti, D.; Mostofsky, S.H.; Ewen, J.B. Altered cortical activation associated with mirror overflow driven by non-dominant hand movement in attention-deficit/ hyperactivity disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 112, 110433. [Google Scholar] [CrossRef]
- Nuzum, N.D.; Teo, W.-P.; Macpherson, H.; Loughman, A.; Szymlek-Gay, E.A.; Hendy, A. Inhibition, excitation and bilateral transfer following a unilateral complex finger-tapping task in young and older adults. Eur. J. Neurosci. 2021, 54, 6608–6617. [Google Scholar] [CrossRef]
- Paitel, E.R.; Nielson, K.A. Temporal dynamics of event-related potentials during inhibitory control characterize age-related neural compensation. Symmetry 2021, 13, 2323. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Ishii, D.; Yamamoto, S.; Okamoto, Y.; Wakatabi, M.; Kohno, Y. Asymmetry of interhemispheric connectivity during rapid movements of right and left hands: A TMS-EEG study. J. Mot. Behav. 2022, 54, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Bondi, D.; Prete, G.; Malatesta, G.; Robazza, C. Laterality in children: Evidence for task-dependent lateralization of motor functions. Int. J. Environ. Res. Public Health 2020, 17, 6705. [Google Scholar] [CrossRef]
- Avena-Koenigsberger, A.; Mišić, B.; Hawkins, R.X.D.; Griffa, A.; Hagmann, P.; Goñi, J.; Sporns, O. Path ensembles and a tradeoff between communication efficiency and resilience in the human connectome. Brain Struct. Funct. 2017, 222, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.S.; The CALM Team; Astle, D.E. Segregation and integration of the functional connectome in neurodevelopmentally ‘at risk’ children. Dev. Sci. 2022, 25, e13209. [Google Scholar] [CrossRef] [PubMed]
- Babik, I. From hemispheric asymmetry through sensorimotor experiences to cognitive outcomes in children with cerebral palsy. Symmetry 2022, 14, 345. [Google Scholar] [CrossRef]
- Huber, K.B.; Marsolek, C.J. Do cerebral motivational asymmetries mediate the relationship between handedness and personality? Laterality Asymmetries Brain Behav. Cogn. 2022, 27, 21–56. [Google Scholar] [CrossRef]
- Fetterman, A.K.; Ode, S.; Robinson, M.D. For which side the bell tolls: The laterality of approach-avoidance associative networks. Motiv. Emot. 2013, 37, 33–38. [Google Scholar] [CrossRef]
- Pérez-Edgar, K.; Kujawa, A.; Nelson, S.K.; Cole, C.; Zapp, D.J. The relation between electroencephalogram asymmetry and attention biases to threat at baseline and under stress. Brain Cogn. 2013, 82, 337–343. [Google Scholar] [CrossRef]
- Garrison, K.E.; Baldwin, C.L.; Schmeichel, B.J.; Harmon-Jones, E. Meta-analysis of the relationship between resting frontal EEG asymmetry and approach/avoidance motivation. University of Albama: Tuscaloosa, Albama, 2018; Unpublished work. [Google Scholar]
- Grimshaw, G.M.; Carmel, D. An asymmetric inhibition model of hemispheric differences in emotional processing. Front. Psychol. 2014, 5, 489. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Schrammen, E.; Grimshaw, G.M.; Berlijin, A.M.; Ocklenburg, S.; Peterburs, J. Response inhibition to emotional faces is modulated by functional hemispheric asymmetries linked to handedness. Brain Cogn. 2020, 145, 105629. [Google Scholar] [CrossRef] [PubMed]
- Neal, L.B.; Gable, P.A. Regulatory control and impulsivity relate to resting frontal activity. Soc. Cogn. Affect. Neurosci. 2017, 12, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.A. Dynamic cerebral processes underlying emotion regulation. Monogr. Soc. Res. Child Dev. 1994, 59, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Maizey, L.; Evans, C.J.; Muhlert, N.; Verbruggen, F.; Chambers, C.; Allen, C.P.G. Cortical and subcortical functional specificity associated with response inhibition. NeuroImage 2020, 220, 117110. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, P.; Malkinson, T.S. Hemispheric lateralization of attention processes in the human brain. Curr. Opin. Psychol. 2019, 29, 90–96. [Google Scholar] [CrossRef]
- Choi, J.M.; Cho, Y.S. Beneficial effect of task-irrelevant threat on response inhibition. Acta Psychol. 2020, 202, 102980. [Google Scholar] [CrossRef]
- Mayer, A.R.; Hanlon, F.M.; Shaff, N.A.; Stephenson, D.D.; Ling, J.M.; Dodd, A.B.; Hogeveen, J.; Quinn, D.K.; Ryman, S.G.; Pirio-Richardson, S. Evidence for asymmetric inhibitory activity during motor planning phases of sensorimotor synchronization. Cortex 2020, 129, 314–328. [Google Scholar] [CrossRef]
- Mancini, C.; Mirabella, G. Handedness does not impact inhibitory control, but movement execution and reactive inhibition are more under a left-hemisphere control. Symmetry 2021, 13, 1602. [Google Scholar] [CrossRef]
- Malatesta, G.; Marzoli, D.; Prete, G.; Tommasi, L. Human lateralization, maternal effects and neurodevelopmental disorders. Front. Behav. Neurosci. 2021, 15, 668520. [Google Scholar] [CrossRef]
- Morange-Majoux, F.; Devouche, E. Neonatal manual specialization in language and music conditions: Consistency with the hemispheric specialization adult model. Early Hum. Dev. 2022, 168, 105575. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jalapa, K.; Han, S.J.; Tawfiq, D.; Cui, M. A dynamic systems perspective towards executive function development: Susceptibility at both ends for inhibitory control. Dev. Psychopathol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Gable, P.A. On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology 2018, 55, e12879. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Fox, N. Asymmetric brain activity discriminates between positive and negative affective stimuli in 10 month old infants. Science 1982, 218, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Krzeczkowski, J.E.; van Lieshout, R.J.; Schmidt, L.A. Transacting brains: Testing an actor–partner model of frontal EEG activity in mother–infant dyads. Dev. Psychopathol. 2020, 34, 969–980. [Google Scholar] [CrossRef]
- Diaz, A.; Swingler, M.M.; Tan, L.; Smith, C.L.; Calkins, S.D.; Bell, M.A. Infant frontal EEG asymmetry moderates the association between maternal behavior and toddler negative affectivity. Infant Behav. Dev. 2019, 55, 88–99. [Google Scholar] [CrossRef]
- Davis, R.; Donati, G.; Finnegan, K.; Boardman, J.P.; Dean, B.; Fletcher-Watson, S.; Forrester, G.S. Social gaze in preterm infants may act as an early indicator of atypical lateralization. Child Dev. 2022, 93, 869–880. [Google Scholar] [CrossRef]
- Hepper, P.G. The developmental origins of laterality: Fetal handedness. Dev. Psychobiol. 2013, 55, 588–595. [Google Scholar] [CrossRef]
- Michel, G.F. Handedness development: A model for investigating the development of hemispheric specialization and interhemispheric coordination. Symmetry 2021, 13, 992. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Hook, M.; Braccini, S.; Schapiro, S.J. Population-level right handedness for a coordinated bimanual task in chimpanzees: Replication and extension in a second colony of apes. Int. J. Primatol. 2003, 24, 677–689. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Phillips, K.A.; Bania, A.; Calcutt, S.E.; Gardner, M.; Russell, J.; Schaeffer, J.; Lonsdorf, E.V.; Ross, S.R.; Schapiro, S.J. Hand preferences for coordinated bimanual actions in 777 great apes: Implications for the evolution of handedness in Hominins. J. Hum. Evol. 2011, 60, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Meguerditchian, A.; Vauclair, J.; Hopkins, W.D. On the origins of human handedness and language: A comparative review of hand preferences for bimanual coordinated actions and gestural communication in nonhuman primates. Dev. Psychobiol. 2013, 55, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Forrester, G.S.; Davis, R.; Malatesta, G.; Todd, B.K. Evolutionary motor biases and cognition in children with and without autism. Sci. Rep. 2020, 10, 17385. [Google Scholar] [CrossRef]
- Forrester, G.S.; Pegler, R.; Thomas, M.S.C.; Mareschal, D. Handedness as a marker of cerebral lateralization in children with and without autism. Behav. Brain Res. 2014, 268, 14–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forrester, G.S.; Quaresmini, C.; Leavens, D.A.; Mareschal, D.; Thomas, M.S.C. Human handedness: An inherited evolutionary trait. Behav. Brain Res. 2013, 237, 200–206. [Google Scholar] [CrossRef]
- Rogers, L.J. Hand and paw preferences in relation to the lateralized brain. Philos. Trans. Biol. Sci. 2009, 364, 943–954. Available online: https://www.jstor.org/stable/40486074 (accessed on 17 July 2022). [CrossRef]
- Malatesta, G.; Marzoli, D.; Rapino, M.; Tommasi, L. The left-cradling bias and its relationship with empathy and depression. Sci. Rep. 2019, 9, 6141. [Google Scholar] [CrossRef]
- Pileggi, L.-A.; Storey, S.; Malcolm-Smith, S. Depressive symptoms disrupt leftward cradling. J. Child Adolesc. Ment. Health 2020, 32, 35–43. [Google Scholar] [CrossRef]
- Pileggi, L.-A.; Malcolm-Smith, S.; Solms, M. Investigating the role of social-affective attachment processes in cradling bias: The absence of cradling bias in children with Autism Spectrum Disorders. Laterality 2015, 20, 154–170. [Google Scholar] [CrossRef]
- Nelson, E.L. Insights into human and nonhuman primate handedness from measuring both hands. Curr. Dir. Psychol. 2022, 31, 154–161. [Google Scholar] [CrossRef]
- Hopkins, W.D. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. J. Comp. Psychol. 1995, 109, 291–297. [Google Scholar] [CrossRef] [PubMed]
| Hemisphere | Type | Description |
|---|---|---|
| Left | Longer term synchrony | Complex, sophisticated, interweaving (see next) |
| Sophisticated synchrony | Sophisticated, subtle interweaving of activation and inhibitory skills, with appropriate activations taking place because of the suppression of interference due to inappropriate alternative behavior, both when selecting adaptive goal-directed activity and during its (movement) transitions. Both subtle competing movements and gross interfering ones are countered and controlled | |
| Altering synchrony | Majorly modifying/disrupting sequential activation-inhibition coordinations | |
| Right | Adjusting synchrony | Minorly adapting/refining sequential activation-inhibition coordinations [could be left hemisphere based, depending on context] |
| Long damping | Full suppression/damping activity over time | |
| Short synchrony | Activation-inhibition synchrony instantaneously or for a short time period. In spatial processes, some information as figure highlighted and some as ground moderated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, G. Activation-Inhibition Coordination in Neuron, Brain, and Behavior Sequencing/Organization: Implications for Laterality and Lateralization. Symmetry 2022, 14, 2051. https://doi.org/10.3390/sym14102051
Young G. Activation-Inhibition Coordination in Neuron, Brain, and Behavior Sequencing/Organization: Implications for Laterality and Lateralization. Symmetry. 2022; 14(10):2051. https://doi.org/10.3390/sym14102051
Chicago/Turabian StyleYoung, Gerald. 2022. "Activation-Inhibition Coordination in Neuron, Brain, and Behavior Sequencing/Organization: Implications for Laterality and Lateralization" Symmetry 14, no. 10: 2051. https://doi.org/10.3390/sym14102051
APA StyleYoung, G. (2022). Activation-Inhibition Coordination in Neuron, Brain, and Behavior Sequencing/Organization: Implications for Laterality and Lateralization. Symmetry, 14(10), 2051. https://doi.org/10.3390/sym14102051







