Mathematical Assessment of the Impact of the Imperfect Vaccination on Diphtheria Transmission Dynamics
Abstract
1. Introduction
2. Model Formulation
3. Analysis of the Diphtheria-Vaccine Model
3.1. Basic Properties
3.2. Disease Free Equilibrium and Reproductive Number
3.3. Endemic Equilibrium and Local Stability
3.4. Global Stability Analysis
3.5. Vaccine-Induced Herd Immunity Threshold
4. Numerical Simulations
4.1. Appropriate Model Parameters
4.2. Sensitivity Analysis of the Reproductive Number
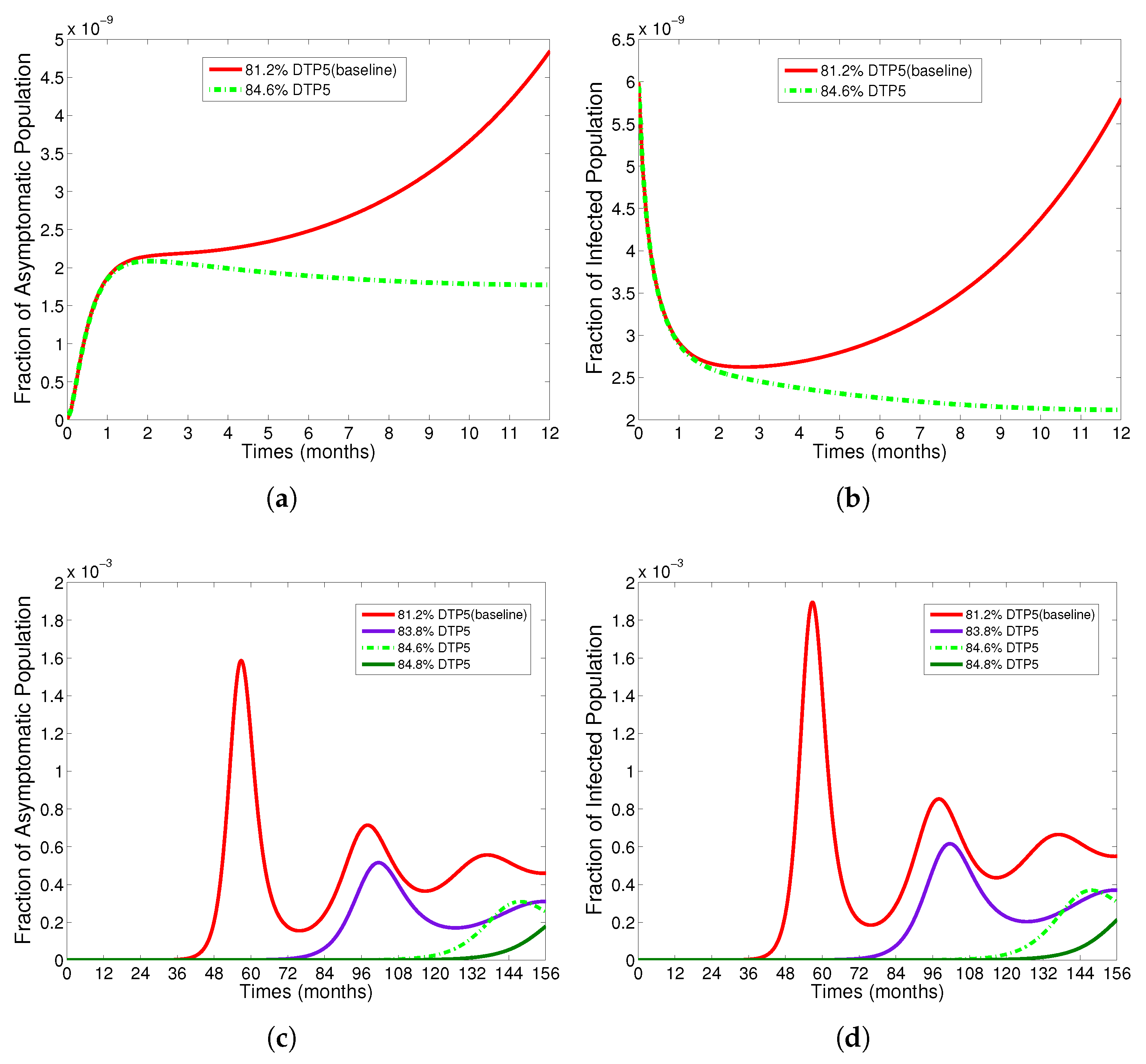
4.3. Effect of Vaccination Rate
4.4. Effect of Asymptomatic Individuals on Spreading Diphtheria
5. Discussion and Conclusions
- (i)
- The threshold value called the basic reproductive number under vaccination of the diphtheria-vaccine model, denoted by , is derived by using the next generation method. It is found that the disease-free equilibrium of the diphtheria-vaccine model is globally asymptomatically stable whenever in the sense that routine vaccination against diphtheria can lead to the effective control or elimination of diphtheria if it can bring (and maintain) . Furthermore, the critical rate of vaccination () and the threshold vaccine-induced community herd immunity of the proposed model () are derived. It is found that is identical to the formula of herd immunity (also called community immunity) which is a new result found in this study.
- (ii)
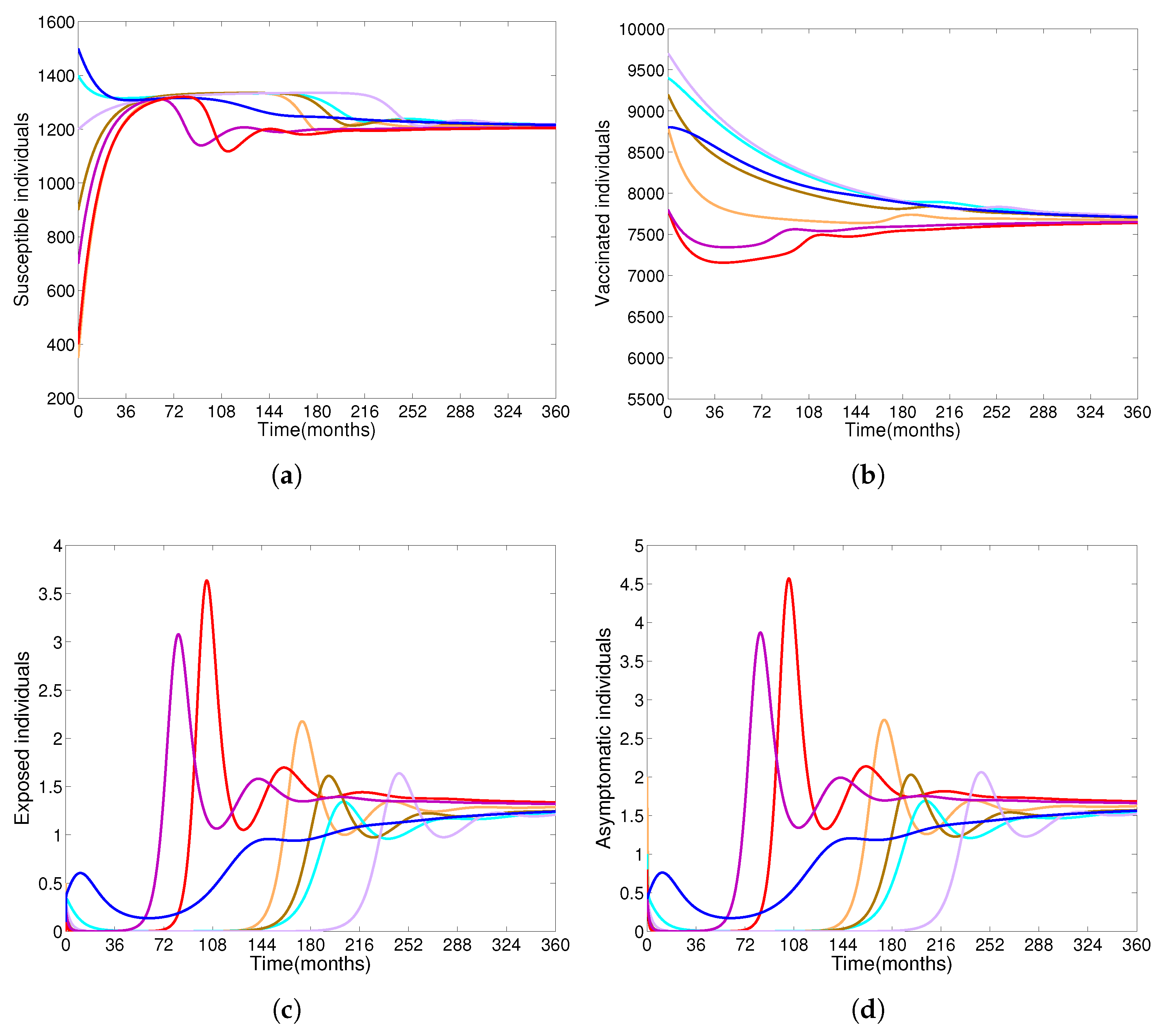
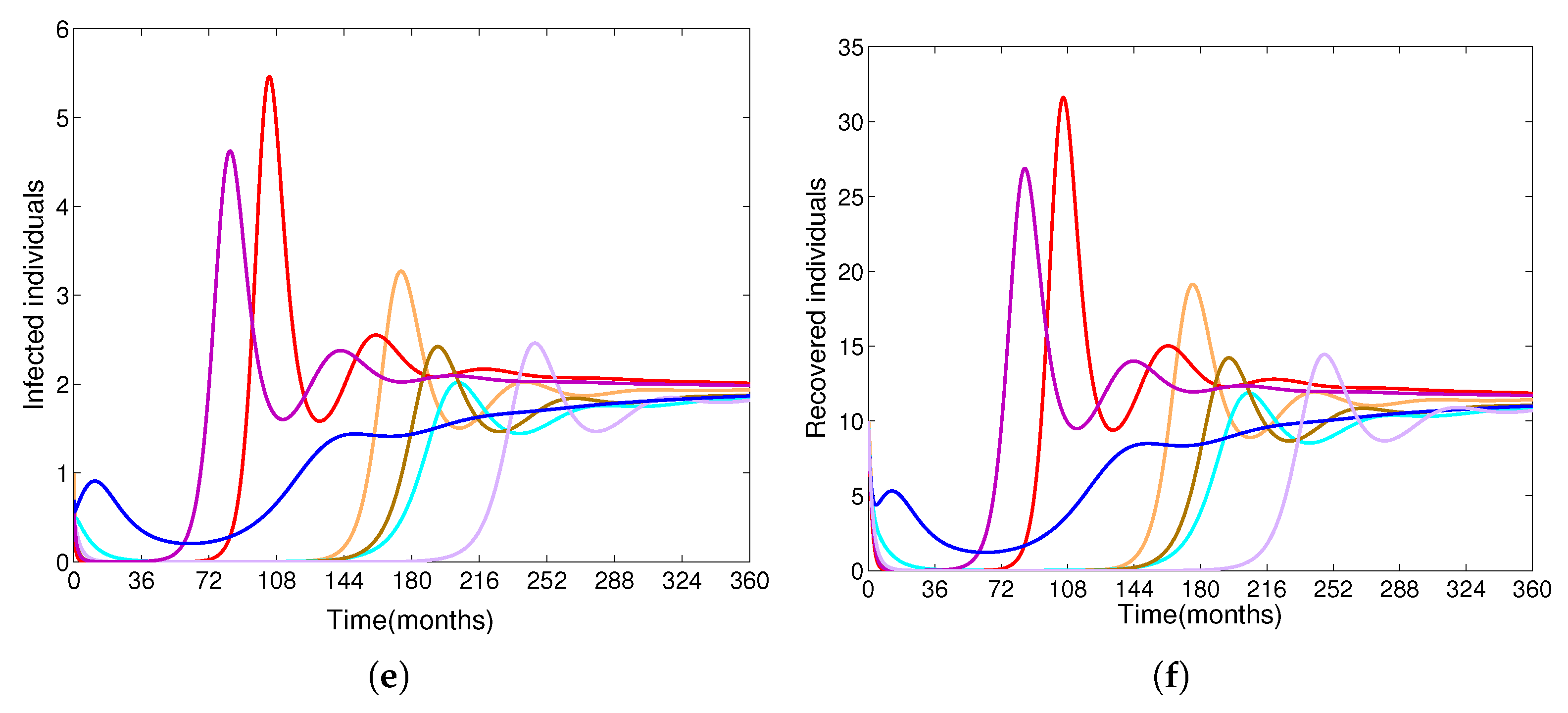
- Based on constructing the suitable Lyapunov functions, it is found that the endemic equilibrium of the proposed model is globally asymptotically stable whenever . The epidemiological implication of these results is that the community transmission of diphtheria can be significantly curtailed if and the disease still persists in the community if , that is, the vaccination program adopted is not effective. The implication of global stability of equilibrium is verified that the solution of the diphtheria-vaccine model converges to the correct equilibria irrespective of the initial sizes of the six state variables. Our simulations show that if the initial sizes of sub-populations have fluctuated, they will affect fast (slow) convergence to a correct equilibrium. This finding is interesting because the initial sizes of sub-populations, especially, susceptible and vaccinated individuals are major factors in either eliminating diphtheria or controlling diphtheria spreads before the next vaccine type is taken.
- (iii)
- The appropriate model parameters given in Table 1 are obtained by comparing the cumulative number of diphtheria cases produced by the diphtheria-vaccine model with the real cases in Thailand in 2018. The sensitivity analysis of has demonstrated that the rate of vaccination is the most sensitive to . Contour plots of suggest the combined control measures should be addressed on the rate of vaccination and the incubation period of asymptomatic individuals. Further, we consider the asymptomatic class as a separate population because this population can spread the infection without being sick themselves. The study results obtained indicate that the incubation period of asymptomatic individuals has an impact on the optimal vaccination coverage level needed for diphtheria eradication, see Table 2. Our simulations also show that when the vaccination coverage level is greater than the threshold value , asymptomatic individuals still persist in the community for some period of time, even though the infected individuals decrease and are eventually eliminated. In epidemiology, this result supports the possibility of asymptomatic infection being related to antibody decay due to waning and not boosting immunity [54]. Although it is well known that a low rate of vaccination has had an impact on the vaccination proportion resulting in the duration of diphtheria protection, this is the first time to investigate the impact of asymptomatic infection on the vaccination coverage for the past and recent vaccination coverage levels have an effect on the duration of diphtheria’s protection, and it is also the cause of discovering the patients in the different age groups [6]. Therefore, our study suggests that the officers involving disease control should be concerned not only with maintaining the coverage level needed for the primary vaccination but they should be concerned to maintain the boosting vaccination coverage level for all adults every 10 years at least the threshold coverage level () in order to significantly halt the spread of diphtheria in the community.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Proof of Theorem 4
References
- Diphtheria. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/dip.pdf (accessed on 10 June 2021).
- Immunization Vaccines and Biologicals Diphtheria. Available online: https://www.who.int/data/gho/data/themes/immunization (accessed on 11 March 2021).
- Choe, Y.J.; Vidor, E.; Manson, C. Post-marketing surveillance of Tetravalent Diphtheria-Tetanus-Acellular pertussis and inactivated poliovirus (DTaP-IPV) vaccine in South Korea, 2009 to 2015. Infect Dis. Ther. 2022, 11, 1479–1492. [Google Scholar] [CrossRef]
- Hamborsky, J.; Kroger, A.; Wolfe, C. Diphtheria. In Epidemiology and Prevention of Vaccine-Preventable Diseases, 5th ed.; CDC: Atlanta, GA, USA, 2015; pp. 107–118. [Google Scholar]
- World Health Organization. Diphtheria Reported Cases. 2019. Available online: https://apps.who.int/gho/data/view.main.1520_41?lang=en (accessed on 14 August 2021).
- National Disease Surveillance. (Report 506), Bureau of Epidemiology, Ministry of Public Health, Thailand. Diphtheria. Available online: http://doe.moph.go.th/surdata/disease.php?dcontent=old&ds=23 (accessed on 15 December 2021).
- Wanlapakorn, N.; Ngaovithunvong, V.; Thongmee, T.; Vichaiwattana, P.; Vongpunsawad, S.; Poovorawan, Y. Seroprevalence of antibodies to pertussis toxin among different age groups in Thailand after 37 years of universal whole-cell pertussis vaccination. PLoS ONE 2016, 12, e0148338. [Google Scholar] [CrossRef]
- World Health Organization. Global Vaccine Action Plan 2011–2020; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Clarke, K.E.N.; MacNeil, A.; Hadler, S.; Scott, C.; Tiwari, T.S.P.; Cherian, T. Global epidemiology of diphtheria, 2000–2017. Emerg. Infect. Dis. 2019, 25, 1834–1842. [Google Scholar] [CrossRef]
- Annual Report. 2017. Available online: https://apps-doe.moph.go.th/boeeng/download/AESR-6112-24.pdf (accessed on 10 January 2021).
- Annaul Epidemiological Surveillance Report. 2018. Available online: https://apps-doe.moph.go.th/boeeng/download/AW_Annual_Mix%206212_14_r1.pdf (accessed on 1 March 2021).
- Annual Epidemiological Surveillance Report. 2019. Available online: https://apps-doe.moph.go.th/boeeng/download/MIX_AESR_2562.pdf (accessed on 8 May 2021).
- Nanthavong, N.; Black, A.; Nouanthong, P.; Souvannaso, C.; Vilivong, K.; Muller, C.; Goossens, S.; Quet, F.; Buisson, Y. Diphtheria in Lao PDR: Insufficient coverage or ineffective vaccine? PLoS ONE 2015, 10, e0121749. [Google Scholar] [CrossRef] [PubMed]
- Kaji, A.; Parker, D.; Chu, C.; Thayatkawin, W.; Suelaor, J.; Charatrueangrongkun, R.; Salathibuppha, K.; Nosten, F.; McGready, R. Immunization Coverage in Migrant School Children Along the Thailand-Myanmar Border. J. Immigr. Minor. Health 2016, 18, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Hanvatananukul, P.; Prasarakee, C.; Sarachai, S.; Aurpibul, L.; Sintupat, K.; Khampan, R.; Saheng, J.; Sudjaritruk, T. Seroprevalence of antibodies against diphtheria, tetanus, and pertussis among healthy Thai adolescents. Int. J. Infect. Dis. 2020, 96, 422–430. [Google Scholar] [CrossRef]
- Sein, C.; Tiwari, T.; Macneil, A.; Wannemuehler, K.; Soulaphy, C.; Souliphone, P.; Reyburn, R.; Gonzalez, A.R.; Watkins, M.; Goodson, J.L. Diphtheria outbreak in Lao People’s Democratic Republic, 2012–2013. Vaccine 2016, 34, 4321–4326. [Google Scholar] [CrossRef]
- Ohyver, M.; Pudjihastuti, H. Modeling the number of diphtheria cases in East Java province using zero-inflated poisson regression. Procedia Comput. Sci. 2018, 135, 643–647. [Google Scholar] [CrossRef]
- Jhancy, M.; Satyavani, A.; Manikyamba, D.; Deepthi, K.T. Re-emergence of diphtheria—An outbreak from east Godavari District, Andhra Pradesh. Pediatr. Infect. Dis. 2015, 7, 33–35. [Google Scholar] [CrossRef]
- Operational Guidelines of Prevention, Control and Treatment of Diphtheria. 2020. Available online: https://ddc.moph.go.th/uploads/files/1161920200220072136.pdf (accessed on 9 October 2021).
- Sen, M.; Ibeas, A.; Quesada, S. On vaccination controls for the SEIR epidemic model. Commun. Nonlinear Sci. Numer. Simul. 2012, 17, 2637–2658. [Google Scholar] [CrossRef]
- Bai, Z.; Zhou, Y. Global dynamics of an SEIRS epidemic model with periodic vaccination and seasonal contact rate. Nonlinear Anal. Real World Appl. 2012, 13, 1060–1068. [Google Scholar] [CrossRef]
- Bentaleb, D.; Amine, S. Lyapunov function and global stability for a two-strain SEIR model with bilinear and nonmonotone incidence. Int. J. Biomath. 2019, 12, 1950021. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Z.; Zhang, F. Global stability and hopf bifurcations of an SEIR epidemiological model with logistic growth and time delay. Appl. Math. Comput. 2015, 269, 332–342. [Google Scholar] [CrossRef]
- Earn, D.; Rohani, P.; Bolker, B.; Grenfell, B. A simple model for complex dynamical transitions in epidemics. Science 2000, 287, 667–670. [Google Scholar] [CrossRef]
- Teitelbaulm, M.; Edmunds, M. Immunization and vaccine-preventable illness, Unites States, 1992–1997. Stat. Bull. Metrop. Insur. Co. 1999, 80, 13–20. [Google Scholar]
- Wright, S. Pertussis infection in adults. South. Med. J. 1998, 91, 702–708. [Google Scholar] [CrossRef]
- Mossong, J.; Nokes, D.; Edmunds, W.; Cox, M.; Ratman, S.; Muller, C. Modelling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am. J. Epidemiol. 1999, 150, 1238–1249. [Google Scholar] [CrossRef]
- Knox, E.G. Strategy for rubella vaccination. Int. J. Epidemiol. 1980, 9, 13–23. [Google Scholar] [CrossRef]
- Samsuzzoha, M.; Singh, M.; Lucy, D. Uncertainty and sensitivity Analysis of the basic reproduction number of a vaccinated epidemic model of Influenza. Appl. Math. Model. 2013, 37, 903–915. [Google Scholar] [CrossRef]
- Tan, X.; Yuan, L.; Zhou, J.; Zheng, Y.; Yang, F. Modeling the initial transmission dynamics of influenza A H1N1 in Guangdong Province, China. Int. J. Infect. Dis. 2013, 17, e479–e484. [Google Scholar] [CrossRef][Green Version]
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A 1927, 115, 700–721. [Google Scholar]
- Alexander, M.E.; Moghadas, S.M.; Rohani, P.; Summers, A.R. Modelling the effect of a booster vaccination on disease epidemiology. J. Math. Biol. 2006, 52, 290–306. [Google Scholar] [CrossRef]
- Moghadas, S.M.; Gumel, A.B. A mathematical study of a model for childhood diseases with non-permanent immunity. J. Comput. Appl. Math. 2003, 157, 347–363. [Google Scholar] [CrossRef]
- Wang, Z.; Röst, G.; Moghadas, S.M. Delay in booster schedule as a control parameter in vaccination dynamics. J. Math. Biol. 2019, 79, 2157–2182. [Google Scholar] [CrossRef]
- Nudee, K.; Chinviriyasit, W.; Chinviriyasit, S. The effect of backward bifurcation in controlling measles transmission by vaccination. Chaos Solit. Fractals 2019, 123, 400–412. [Google Scholar] [CrossRef]
- Béraud, G. Mathematical models and vaccination strategies. Vaccine 2018, 36, 5366–5372. [Google Scholar] [CrossRef]
- Ho, S.H.; He, D.; Eftimie, R. Mathematical models of transmission dynamics and vaccine strategies in Hong Kong during the 2017–2018 winter influenza season. J. Theor. Biol. 2019, 476, 74–94. [Google Scholar] [CrossRef]
- Zaman, G.; Kang, Y.H.; Cho, G.; Jung, I.H. Optimal strategy of vaccination & treatment in an SIR epidemic model. Math. Comput. Simul. 2017, 136, 63–77. [Google Scholar]
- Huang, S.; Chen, F.; Chen, L. Global dynamics of a network-based SIQRS epidemic model with demographics and vaccination. Commun. Nonlinear Sci. Numer. Simul. 2017, 43, 296–310. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, D.; Hayat, T.; Alsaedi, A. Stationary distribution of a stochastic delayed SVEIR epidemic model with vaccination and saturation incidence. Phys. A Stat. Mech. Appl. 2018, 512, 849–863. [Google Scholar] [CrossRef]
- De la Sen, M.; Quesada, S.A.; Ibeas, A.; Nistal, R. On an SEIADR epidemic model with vaccination, treatment and dead-infectious corpses removal controls. Math. Comput. Simul. 2019, 163, 47–79. [Google Scholar]
- Zhou, L.; Wang, Y.; Xiao, Y.; Li, M.Y. Global dynamics of a discrete age-structured SIR epidemic model with applications to measles vaccination strategies. Math. Biosci. 2019, 308, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, X.; Pan, Q.; Wang, L. Optimal strategy for a dose-escalation vaccination against COVID-19 in refugee camps. AIMS Math. 2022, 7, 9288–9310. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Tian, T.; Zhang, X. Mathematical modeling and analysis of tumor chemotherapy. Symmetry 2022, 14, 704. [Google Scholar] [CrossRef]
- Korobeinikov, A. Lyapunov functions and global properties for SEIR and SEIS epidemic models. Math. Med. Biol. 2004, 21, 75–83. [Google Scholar] [CrossRef]
- Logistic Growth Model, Mathematical Association of America. Available online: https://www.maa.org/press/periodicals/loci/joma/logistic-growth-model (accessed on 10 January 2021).
- Tsoularis, A.; Wallace, J. Analysis of logistic growth models. Math Biosci. 2002, 179, 21–55. [Google Scholar] [CrossRef]
- Zhonghua, Z.; Yaohong, S. Qualitative analysis of a SIR epidemic model with saturated treatment rate. J. Appl. Math. Comput. 2010, 34, 177–194. [Google Scholar] [CrossRef]
- Li, J.; Teng, Z.; Wang, G.; Zhang, L.; Hu, C. Stability and bifurcation analysis of an SIR epidemic model with logistic growth and saturated treatment. Chaos Solit. Fractals 2017, 99, 63–71. [Google Scholar] [CrossRef]
- Arenasa, A.R.; Thackara, N.B.; Haskellb, E.C. The logistic growth model as an approximating model for viral load measurements of influenza A virus. Math. Comput. Simul. 2017, 133, 206–222. [Google Scholar] [CrossRef]
- Guo, Z.K.; Huo, H.F.; Xiang, H. Hopf bifurcation of an age-structured HIV infection model with logistic target-cell growth. J. Biol. Dyn. 2019, 13, 362–384. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Pan, S.; Zhao, P.; Han, L.; Wang, X.; Hemerik, L.; Knops, J.; van der Werf, W. Outbreak analysis with a logistic growth model shows COVID-19 suppression dynamics in China. PLoS ONE 2020, 15, e0235247. [Google Scholar] [CrossRef]
- Wu, K.; Darcet, D.; Wang, Q.; Sornette, D. Generalized logistic growth modeling of the COVID-19 outbreak: Comparing the dynamics in the 29 provinces in China and in the rest of the world. Nonlinear Dyn. 2020, 101, 1561. [Google Scholar] [CrossRef]
- Glass, K.; Grenfell, B. Waning immunity and subclinical measles infections in England. Vaccine 2004, 22, 4110–4116. [Google Scholar] [CrossRef]
- Leung, K.; Trapman, P.; Britton, T. Who is the infector? Epidemic models with symptomatic and asymptomatic cases. Math. Biosci. 2018, 301, 190–198. [Google Scholar] [CrossRef]
- Zhou, T. Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Lyapunov, A.M. The General Problem of the Stability of Motion; Taylor & Francis: London, UK, 1992. [Google Scholar]
- Korobeinikov, A.; Wake, G.C. Lyapunov functions and global stability for SIR, SIRS, and SIS epidemiological models. Appl. Math. Lett. 2002, 15, 955–960. [Google Scholar] [CrossRef]
- Magal, P.; McCluskey, C.C.; Webb, G.F. Lyapunov functional and global asymptotic stability for an infection-age model. Appl. Anal. 2010, 89, 1109–1140. [Google Scholar] [CrossRef]
- Enatsu, Y.; Nakata, Y.; Muroya, Y. Lyapunov functional techniques for the global stability analysis of a delayed SIRS epidemic model. Nonlinear Anal. Real World Appl. 2012, 13, 2120–2133. [Google Scholar] [CrossRef]
- Shuai, Z.; van den Driessche, P. Global stability of infectious disease models using lyapunov functions. SIAM J. Appl. Math. 2013, 73, 1513–1532. [Google Scholar] [CrossRef]
- Kuniya, T.; Wang, J. Lyapunov functions and global stability for a spatially diffusive SIR epidemic model. Appl. Anal. 2016, 96, 1935–1960. [Google Scholar] [CrossRef]
- Islam, Z.; Ahmed, S.; Rahman, M.M.; Karim, M.F.; Amin, M. Global stability analysis and parameter estimation for a diphtheria model: A case study of an epidemic in Rohingya refugee camp in Bangladesh. Comput. Math. Methods Med. 2022, 2022, 6545179. [Google Scholar] [CrossRef]
- Truelove, S.A.; Keegan, L.T.; Moss, W.J.; Macher, L.H.C.E.; Azman, A.S.; Lessler, J. Clinical and epidemiological aspects of diphtheria: A systematic review and pooled analysis. Clin. Infect. Dis. 2020, 71, 89–97. [Google Scholar] [CrossRef]
- Number of Births from Registration by Sex Region Province: 2010–2019. Available online: http://statbbi.nso.go.th/staticreport/page/sector/en/01.aspx (accessed on 18 October 2021).
- Public Health Statistics. 2017. Available online: http://www.pcko.moph.go.th/Health-Statistics/stratistics60.pdf (accessed on 17 August 2021).
- Lakshmikantham, V.; Leela, S.; Martynyuk, A.A. Stability Analysis of Nonlinear Systems; Marcel Dekker Inc.: Abington, UK, 1989. [Google Scholar]
- Hethcote, H.W. The mathematics of infectious disease. SIAM Rev. 2000, 42, 599–653. [Google Scholar] [CrossRef]
- van den Driessche, P.; Watmough, J. Reproduction numbers and sub-treshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Diekmann, O.; Heesterbeek, J.A.P.; Metz, J.A.J. On the Definition and the Computation of the Basic Reproduction Ratio R0 in Models for Infectious Diseases in Heterogeneous Populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Scherer, A.; McLean, A. Mathematical models of vaccination. Br. Med. Bull. 2002, 62, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans Dynamics and Control, 2nd ed.; Oxford University Press Inc.: Oxford, UK, 1991. [Google Scholar]
- LaSalle, J.P. The Stability of Dynamical Systems; Hamilton Press: Venice, CA, USA, 1976. [Google Scholar]
- Anderson, R.M.; May, R.M. Vaccination and herd immunity to infectious diseases. Nature 1985, 318, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M. The concept of herd immunity and the design of community-based immunization programmes. Vaccine 1992, 10, 928–935. [Google Scholar] [CrossRef]
- Murray, J.D. Mathematical Biology; Springer: New York, NU, USA, 1989; pp. 702–704. [Google Scholar]
- Draper, N.R.; Smith, H. Applied Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Vaccine Information Statement DTP. Available online: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/dtap.html (accessed on 7 November 2021).
- Vaccine Information Statement Td. Available online: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/td.pdf (accessed on 1 May 2021).
- US Centers for Disease Control and Prevention. Review of the Epidemiology of Diphtheria-2000-2016. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/2017/sage-meeting-of-april-2017/background-docs/session-diphtheria/1.-review-of-the-epidemiology-of-diphtheria—2000-2016-pdf-829kb.pdf?sfvrsn=9ba4f061_3k (accessed on 17 August 2021).
- Carr, J. Applications Centre Manifold Theory; Spinger: New York, NY, USA, 1981. [Google Scholar]
- Castillo-Chavez, C.; Song, B. Dynamical Models of Tuberculosis and Their Applications. Math. Biosci. Eng. 2004, 1, 361–404. [Google Scholar] [CrossRef] [PubMed]
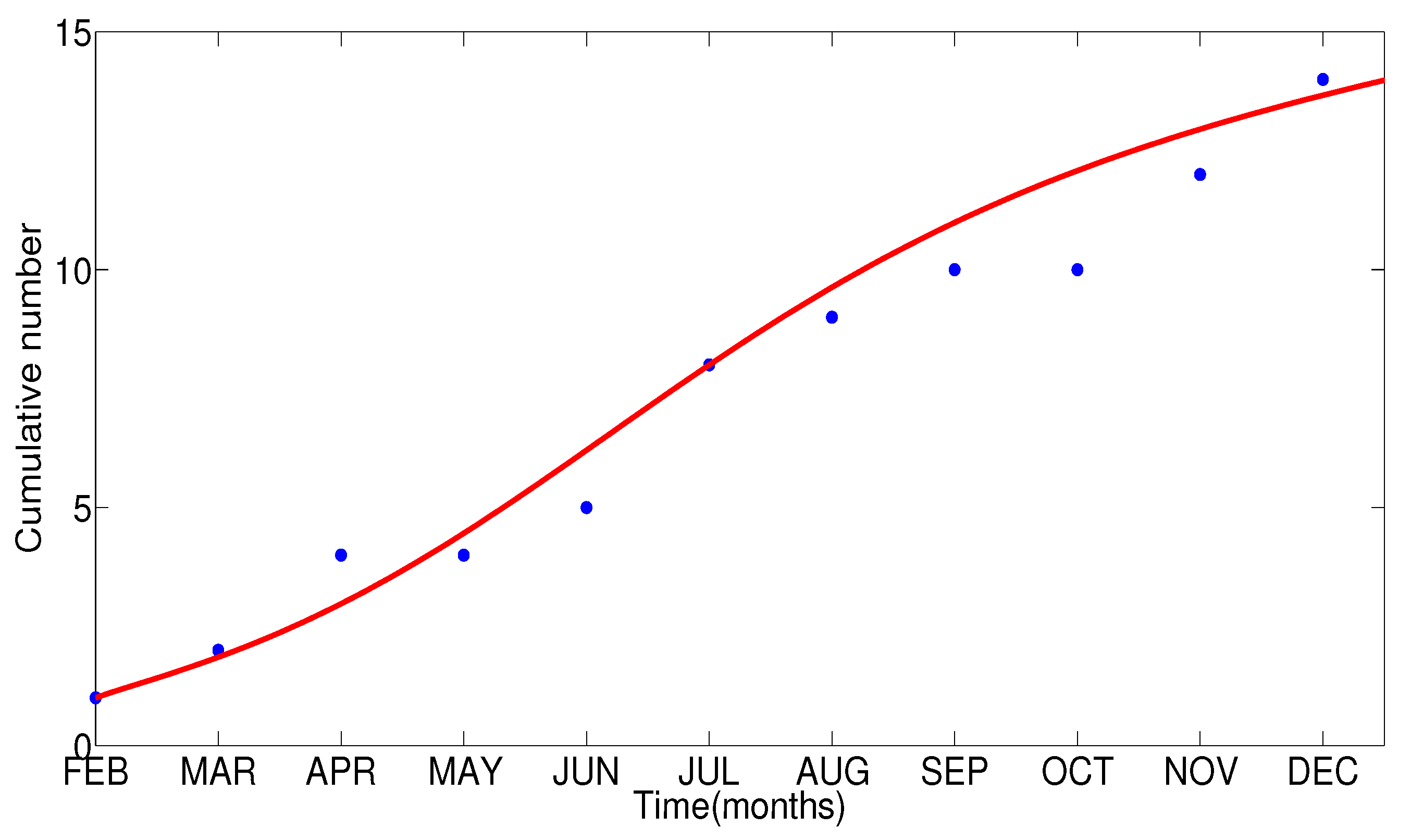
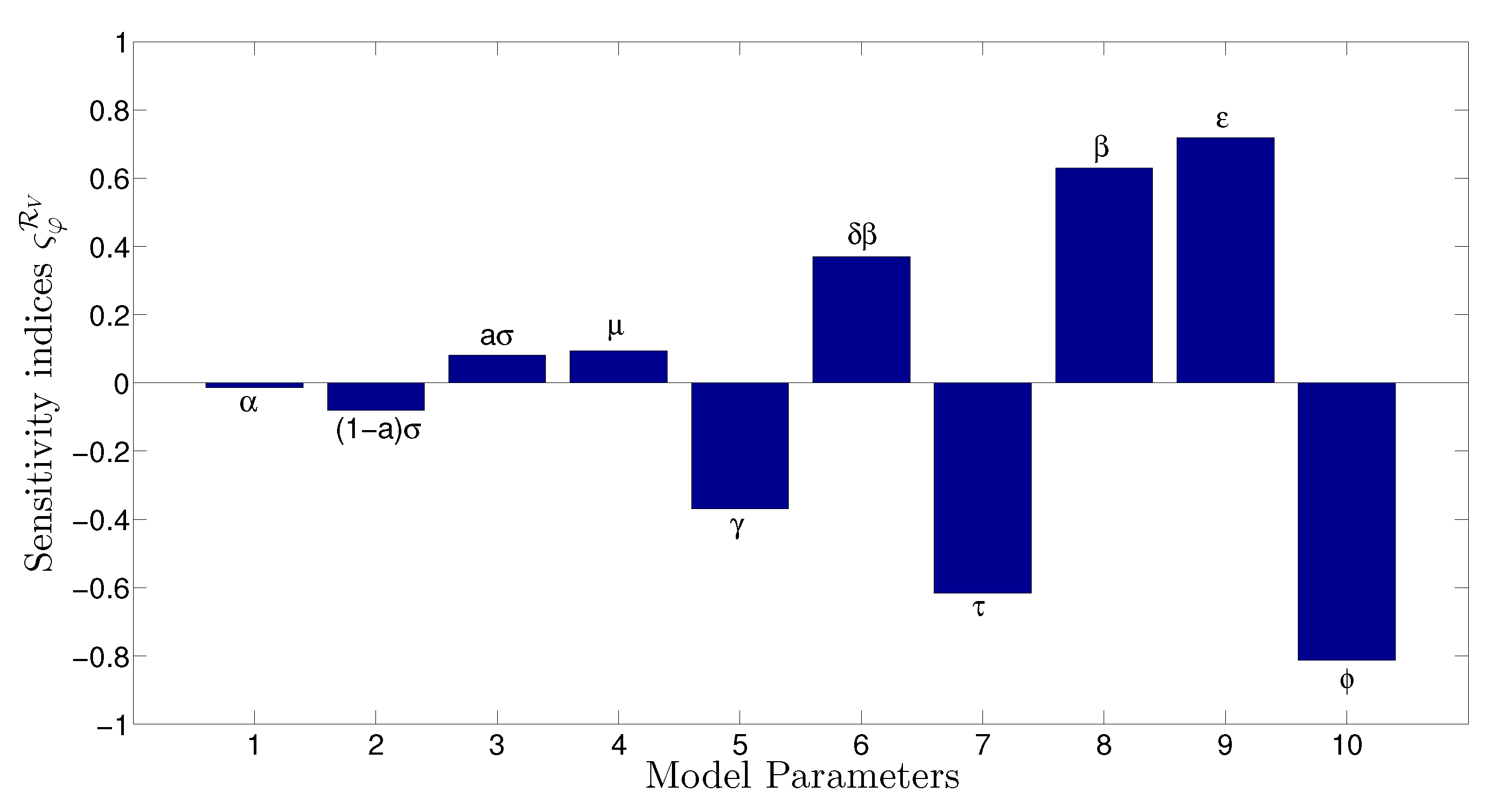
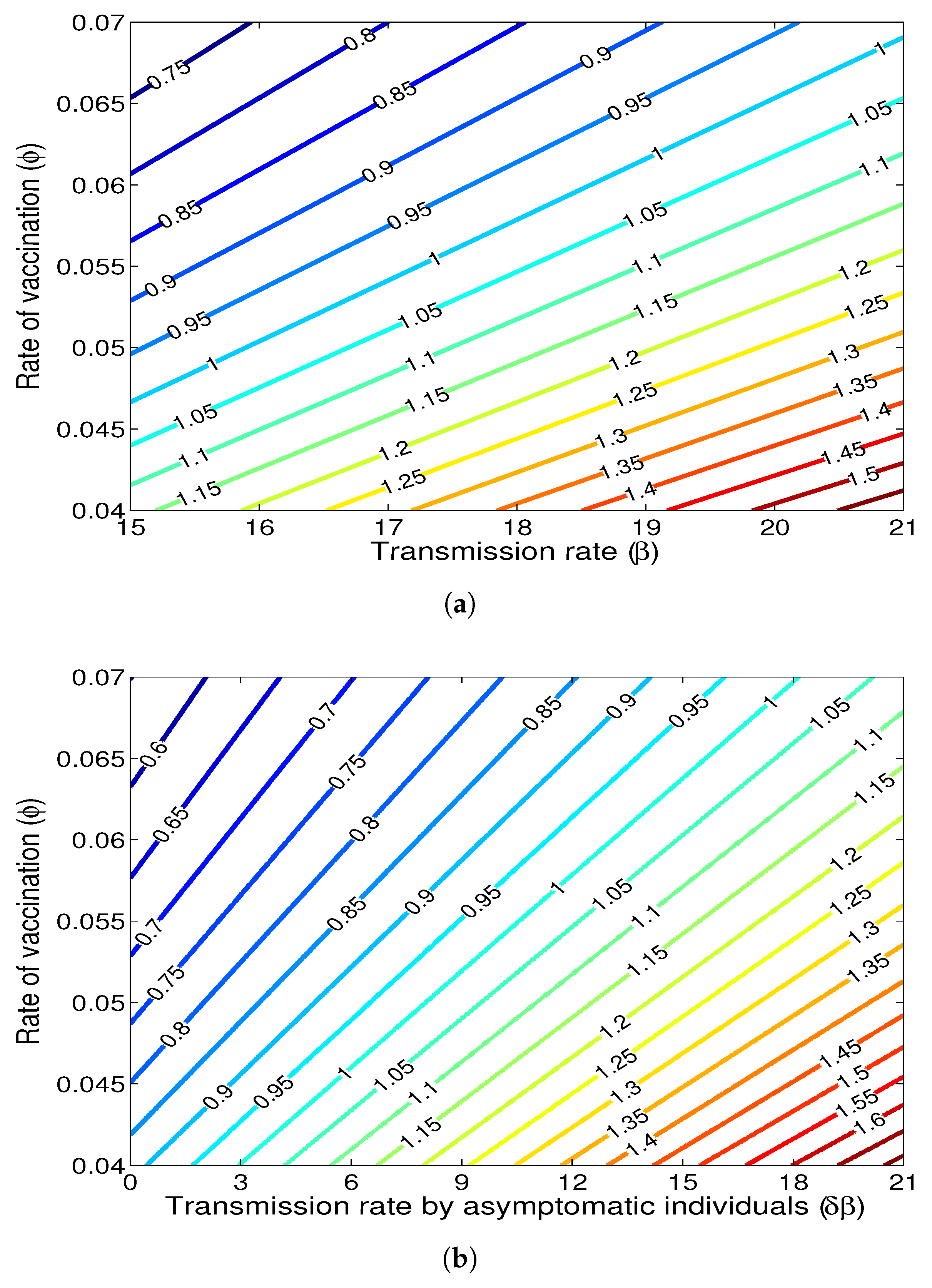


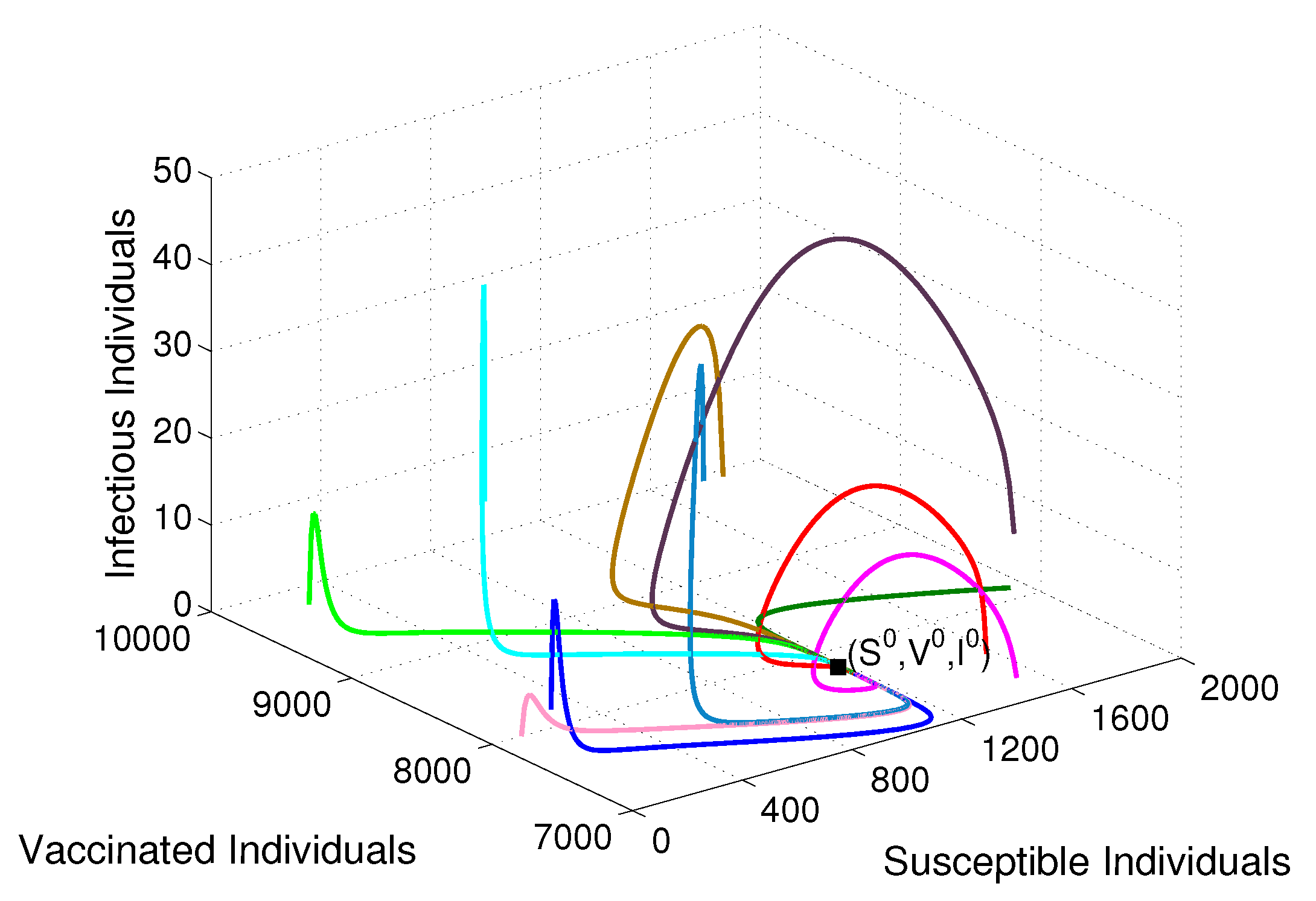

| Parameter | Definition | Value | Reference |
|---|---|---|---|
| Transmission rate | 18.5 | data fit | |
| a | Proportion of infectious population, | 0.55 | [64] |
| Modification parameter, | 0.7 | [64] | |
| Rate of vaccination | 0.0406 | [10] | |
| r | Birth rate | 0.0101 | [65] |
| Rate of progression from the exposed class to either the asymptomatic class or the infected class | 6 | [1] | |
| Natural death rate | 0.0011 | [66] | |
| Diphtheria mortality rate | [1] | ||
| Rate of waning vaccine | [1] | ||
| Rate of progression from the recovered class to the vaccinated class | [1] | ||
| Recovered rate of asymptomatic individuals | [1] | ||
| Recovered rate of infected individuals | [1] | ||
| K | Carrying capacity | - |
| 5.94 | 1.1398 | 0.0476 | 0.8351 | 0.8347 | 1 | 4 | 0 |
| 4.26 | 1.2657 | 0.0539 | 0.8515 | 0.8509 | 3 | 6 | 2 |
| 2.70 | 1.3826 | 0.0597 | 0.8640 | 0.8633 | 4 | 5 | 6 |
| 2.10 | 1.4276 | 0.0619 | 0.8683 | 0.8675 | 4 | 4 | 7 |
| 1.26 | 1.4905 | 0.0651 | 0.8739 | 0.8730 | 5 | 3 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanchanarat, S.; Chinviriyasit, S.; Chinviriyasit, W. Mathematical Assessment of the Impact of the Imperfect Vaccination on Diphtheria Transmission Dynamics. Symmetry 2022, 14, 2000. https://doi.org/10.3390/sym14102000
Kanchanarat S, Chinviriyasit S, Chinviriyasit W. Mathematical Assessment of the Impact of the Imperfect Vaccination on Diphtheria Transmission Dynamics. Symmetry. 2022; 14(10):2000. https://doi.org/10.3390/sym14102000
Chicago/Turabian StyleKanchanarat, Siwaphorn, Settapat Chinviriyasit, and Wirawan Chinviriyasit. 2022. "Mathematical Assessment of the Impact of the Imperfect Vaccination on Diphtheria Transmission Dynamics" Symmetry 14, no. 10: 2000. https://doi.org/10.3390/sym14102000
APA StyleKanchanarat, S., Chinviriyasit, S., & Chinviriyasit, W. (2022). Mathematical Assessment of the Impact of the Imperfect Vaccination on Diphtheria Transmission Dynamics. Symmetry, 14(10), 2000. https://doi.org/10.3390/sym14102000





