Binaural Background Noise Enhances Neuromagnetic Responses from Auditory Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
2.3. Procedure
2.4. MEG Data Acquisition and Analysis
2.5. Experimental Design and Statistical Analysis
3. Results
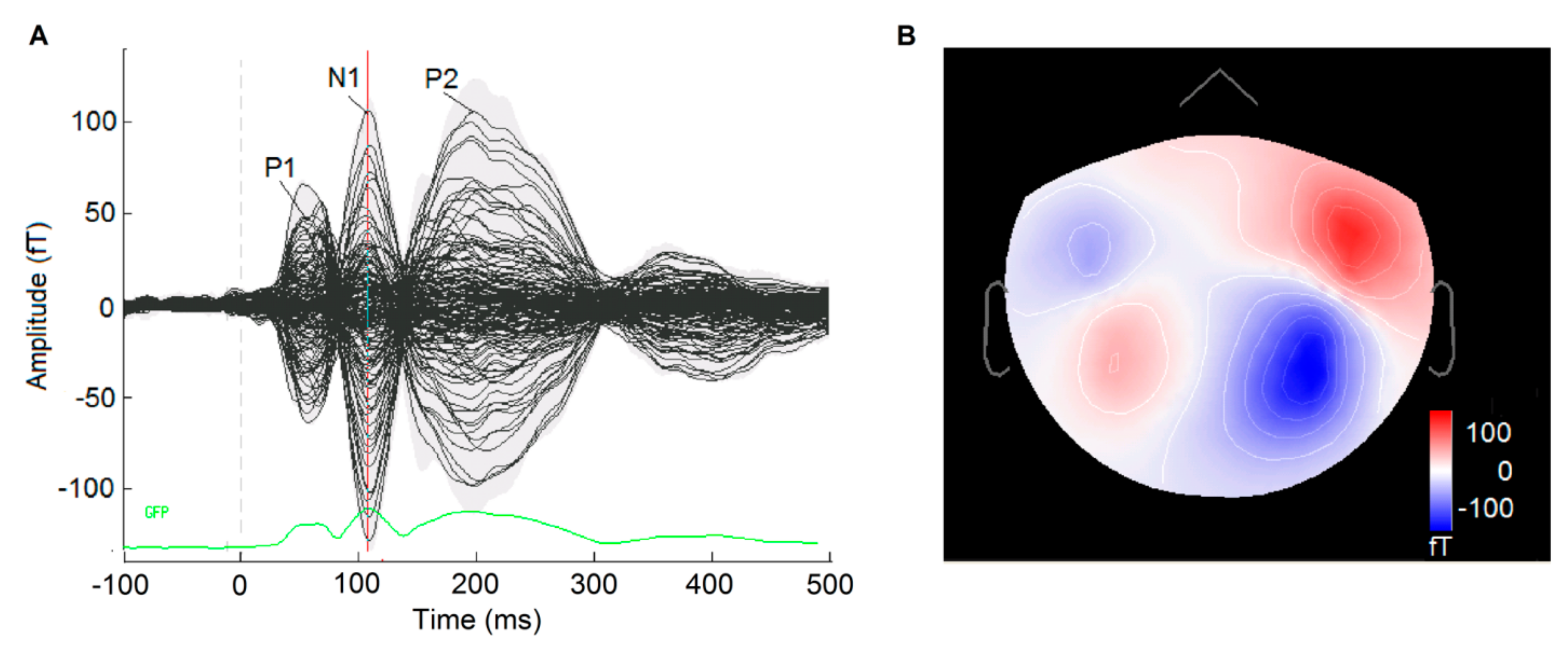
3.1. Auditory-Evoked Responses
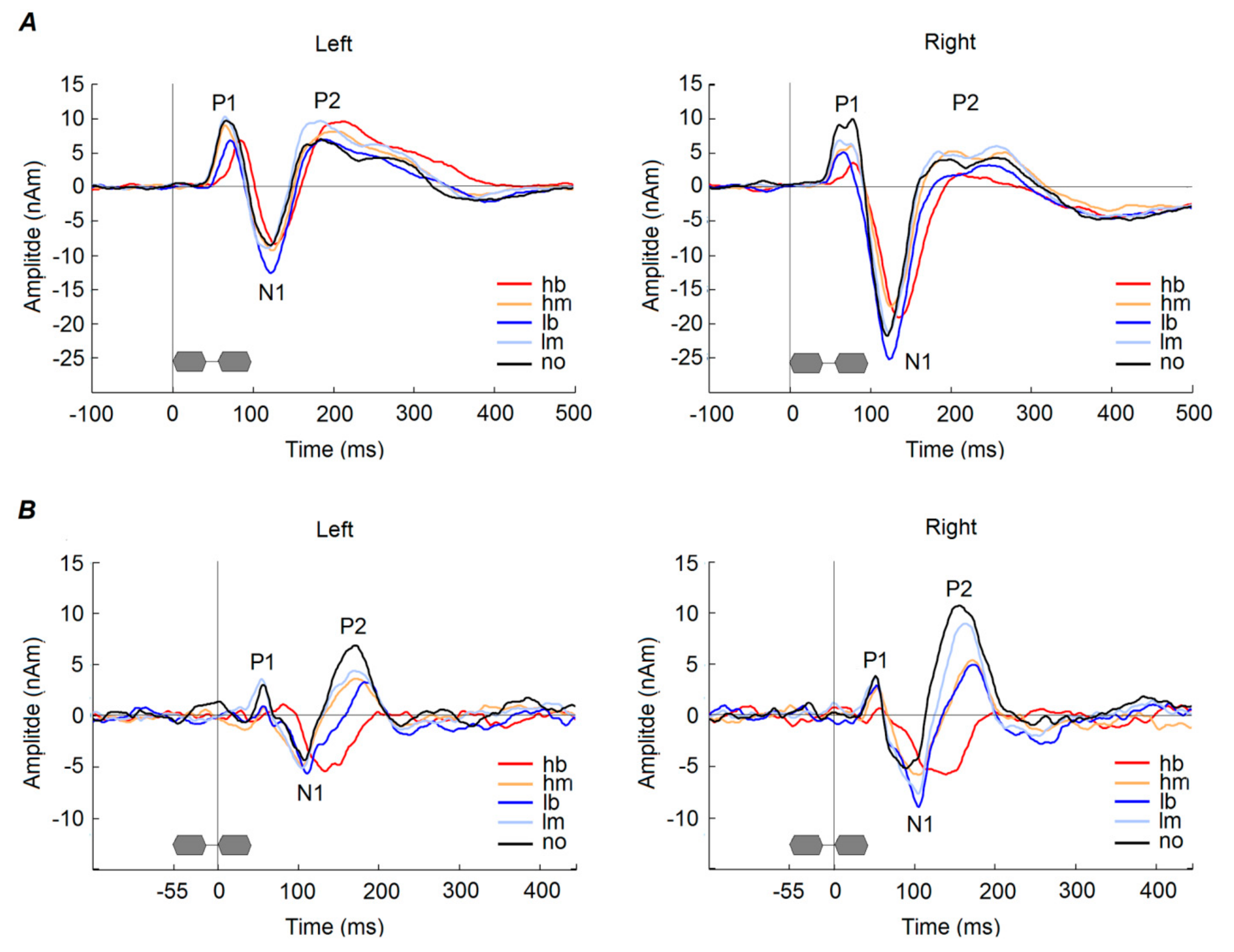
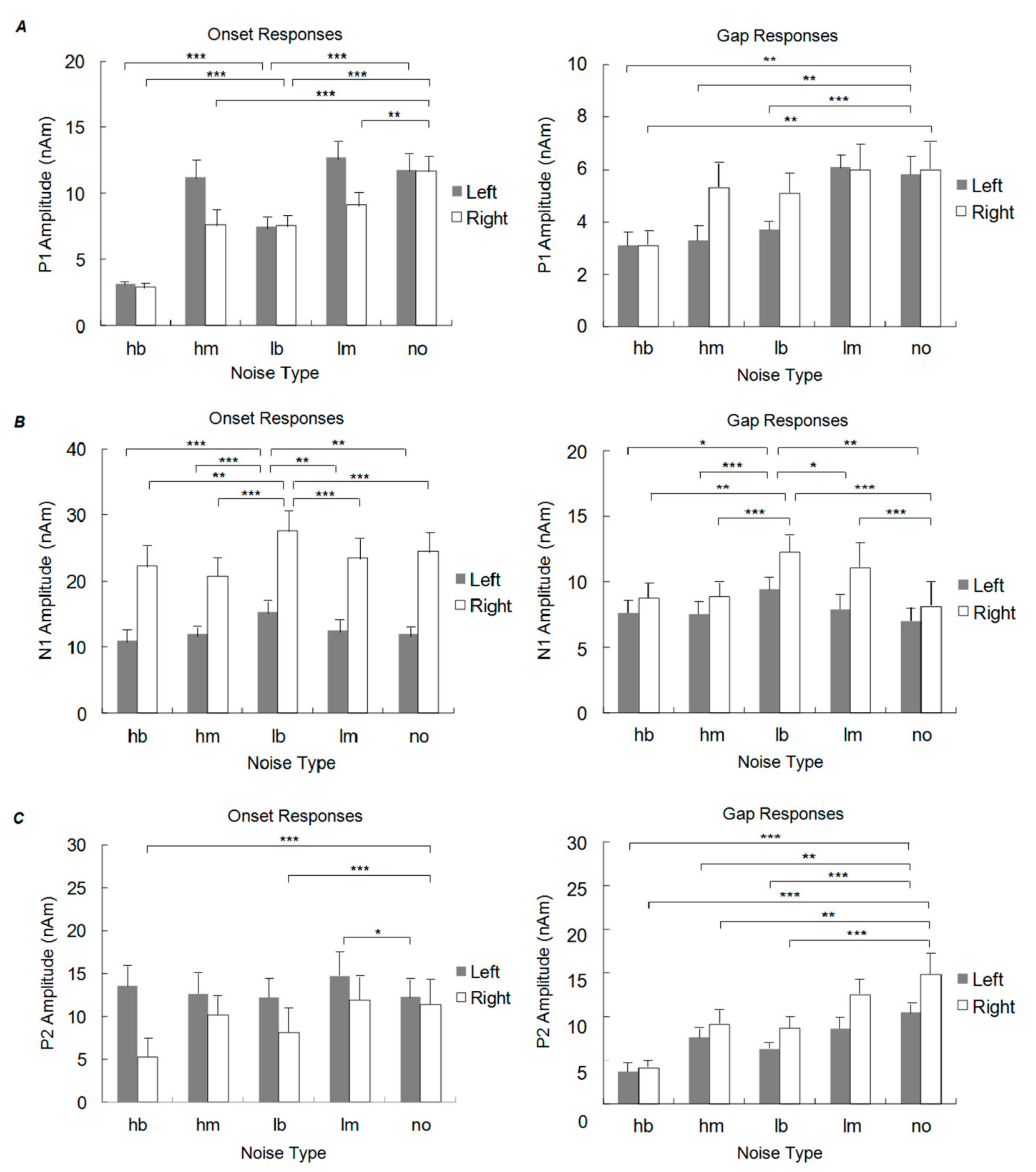
3.1.1. P1 Onset Response
3.1.2. N1 Onset Response
3.1.3. P2 Onset Response
3.1.4. P1 Gap Response
3.1.5. N1 Gap Response
3.1.6. P2 Gap Response
3.2. Gamma (35–45 Hz) Synchronization
4. Discussion
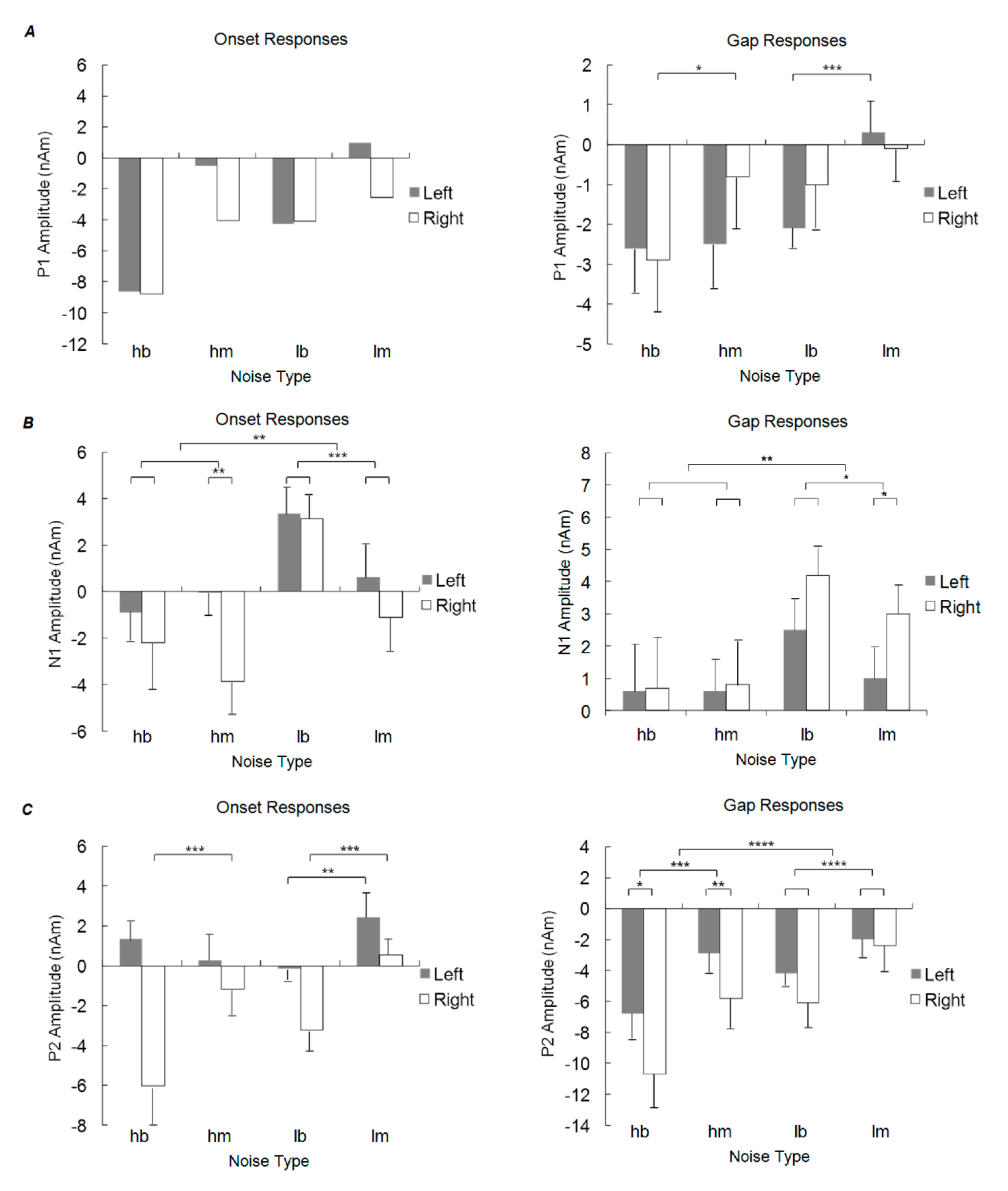
4.1. Concurrent Noise Conditions
4.2. P1 Responses
4.3. Evoked Gamma-Band Responses
4.4. N1 Responses
4.5. P2 Responses
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, H.; Morgan, C.T.; Hawkins, J.E.; Galambos, R.; Smith, F.W. Temporary deafness following exposure to loud tones and noise. Laryngoscope 1946, 56, 19–21. [Google Scholar] [CrossRef]
- Phillips, D.P. Temporal response features of cat auditory cortex neurons contributing to sensitivity to tones delivered in the presence of continuous noise. Hear. Res. 1985, 19, 253–268. [Google Scholar] [CrossRef]
- Phillips, D.P. Neural representation of sound amplitude in the auditory cortex: Effects of noise masking. Behav. Brain Res. 1990, 37, 197–214. [Google Scholar] [CrossRef]
- Billings, C.J.; Tremblay, K.L.; Stecker, G.C.; Tolin, W.M. Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hear. Res. 2009, 254, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Kaplan-Neeman, R.; Kishon-Rabin, L.; Henkin, Y.; Muchnik, C. Identification of syllables in noise: Electrophysiological and behavioral correlates. J. Acoust. Soc. Am. 2006, 120, 926–933. [Google Scholar] [CrossRef]
- Whiting, K.A.; Martin, B.A.; Stapells, D.R. The effects of broadband noise masking on cortical event-related potentials to speech sounds /ba/ and /da. Ear Hear. 1998, 19, 218–231. [Google Scholar] [CrossRef]
- Zeng, F.G.; Fu, Q.J.; Morse, R. Human hearing enhanced by noise. Brain Res. 2000, 869, 251–255. [Google Scholar] [CrossRef]
- Näätänen, R.; Picton, T. The n1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology 1987, 24, 375–425. [Google Scholar] [CrossRef] [PubMed]
- Koerner, T.K.; Zhang, Y. Effects of background noise on inter-trial phase coherence and auditory n1-p2 responses to speech stimuli. Hear. Res. 2015, 328, 113–119. [Google Scholar] [CrossRef]
- Alain, C.; McDonald, K.; Van Roon, P. Effects of age and background noise on processing a mistuned harmonic in an otherwise periodic complex sound. Hear. Res. 2012, 283, 126–135. [Google Scholar] [CrossRef]
- Alain, C.; Quan, J.; McDonald, K.; Van Roon, P. Noise-induced increase in human auditory evoked neuromagnetic fields. Eur. J. Neurosci. 2009, 30, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Alain, C.; Roye, A.; Salloum, C. Effects of age-related hearing loss and background noise on neuromagnetic activity from auditory cortex. Front. Syst. Neurosci. 2014, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Billings, C.J.; Gordon, S.Y.; McMillan, G.P.; Gallun, F.J.; Molis, M.R.; Konrad-Martin, D. Noise-induced enhancement of envelope following responses in normal-hearing adults. J. Acoust. Soc. Am. 2020, 147, EL201. [Google Scholar] [CrossRef]
- Papesh, M.A.; Billings, C.J.; Baltzell, L.S. Background noise can enhance cortical auditory evoked potentials under certain conditions. Clin. Neurophysiol. 2015, 126, 1319–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parbery-Clark, A.; Marmel, F.; Bair, J.; Kraus, N. What subcortical-cortical relationships tell us about processing speech in noise. Eur. J. Neurosci. 2011, 33, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.L.; Garnier, S.; Micheyl, C.; Lina, G.; Chays, A.; Chéry-Croze, S. Auditory efferents involved in speech-in-noise intelligibility. NeuroReport 1997, 8, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Poveda, E.A. Olivocochlear efferents in animals and humans: From anatomy to clinical relevance. Front. Neurol. 2018, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H.; Stracke, H.; Ross, B.; Kakigi, R.; Pantev, C. Left hemispheric dominance during auditory processing in a noisy environment. BMC Biol. 2007, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Ross, B.; Miyazaki, T.; Fujioka, T. Interference in dichotic listening: The effect of contralateral noise on oscillatory brain networks. Eur. J. Neurosci. 2012, 35, 106–118. [Google Scholar] [CrossRef]
- Adler, L.E.; Olincy, A.; Waldo, M.; Harris, J.G.; Griffith, J.; Stevens, K.; Flach, K.; Nagamoto, H.; Bickford, P.; Leonard, S.; et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr. Bull. 1998, 24, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Freedman, R.; Adler, L.E.; Gerhardt, G.A.; Waldo, M.; Baker, N.; Rose, G.M.; Drebing, C.; Nagamoto, H.; Bickford-Wimer, P.; Franks, R. Neurobiological studies of sensory gating in schizophrenia. Schizophr. Bull. 1987, 13, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Freedman, R.; Adler, L.E.; Myles-Worsley, M.; Nagamoto, H.T.; Miller, C.; Kisley, M.; McRae, K.; Cawthra, E.; Waldo, M. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch. Gen. Psychiatry 1996, 53, 1114–1121. [Google Scholar] [CrossRef]
- Boutros, N.N.; Korzyukov, O.; Jansen, B.; Feingold, A.; Bell, M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res. 2004, 126, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Jerger, K.; Biggins, C.; Fein, G. P50 suppression is not affected by attentional manipulations. Biol. Psychiatry 1992, 31, 365–377. [Google Scholar] [CrossRef]
- Wan, L.; Friedman, B.H.; Boutros, N.N.; Crawford, H.J. P50 sensory gating and attentional performance. Int J. Psychophysiol. 2008, 67, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, R.; Waldo, M.; Bickford-Wimer, P.; Nagamoto, H. Elementary neuronal dysfunctions in schizophrenia. Schizophr. Res. 1991, 4, 233–243. [Google Scholar] [CrossRef]
- Hall, J.W., 3rd; Grose, J.H. The relation between gap detection, loudness, and loudness growth in noise-masked normal-hearing listeners. J. Acoust. Soc. Am. 1997, 101, 1044–1049. [Google Scholar] [CrossRef]
- Shailer, M.J.; Moore, B.C. Detection of temporal gaps in bandlimited noise: Effects of variations in bandwidth and signal-to-masker ratio. J. Acoust. Soc. Am. 1985, 77, 635–639. [Google Scholar] [CrossRef]
- Ross, B.; Schneider, B.; Snyder, J.S.; Alain, C. Biological markers of auditory gap detection in young, middle-aged, and older adults. PLoS ONE 2010, 5, e10101. [Google Scholar] [CrossRef] [Green Version]
- Clementz, B.A.; Blumenfeld, L.D.; Cobb, S. The gamma band response may account for poor p50 suppression in schizophrenia. NeuroReport 1997, 8, 3889–3893. [Google Scholar] [CrossRef]
- Kisley, M.A.; Cornwell, Z.M. Gamma and beta neural activity evoked during a sensory gating paradigm: Effects of auditory, somatosensory and cross-modal stimulation. Clin. Neurophysiol. 2006, 117, 2549–2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.M.; Keil, A.; Kissler, J.; Gruber, T. Suppression of the auditory middle-latency response and evoked gamma-band response in a paired-click paradigm. Exp. Brain Res. 2001, 136, 474–479. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for meg/eeg analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic statistical parametric mapping: Combining fmri and meg for high-resolution imaging of cortical activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Tadel, F.; Bock, E.; Niso, G.; Mosher, J.C.; Cousineau, M.; Pantazis, D.; Leahy, R.M.; Baillet, S. Meg/eeg group analysis with brainstorm. Front. Neurosci. 2019, 13, 76. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.X. A better way to define and describe morlet wavelets for time-frequency analysis. NeuroImage 2019, 199, 81–86. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Aranibar, A. Evaluation of event-related desynchronization (erd) preceding and following voluntary self-paced movement. Electroencephalogr. Clin. Neurophysiol. 1979, 46, 138–146. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related eeg/meg synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Pantev, C.; Makeig, S.; Hoke, M.; Galambos, R.; Hampson, S.; Gallen, C. Human auditory evoked gamma-band magnetic fields. Proc. Natl. Acad. Sci. USA 1991, 88, 8996–9000. [Google Scholar] [CrossRef] [Green Version]
- White, P.M.; Yee, C.M. P50 sensitivity to physical and psychological state influences. Psychophysiology 2006, 43, 320–328. [Google Scholar] [CrossRef]
- Bruyns-Haylett, M.; Luo, J.; Kennerley, A.J.; Harris, S.; Boorman, L.; Milne, E.; Vautrelle, N.; Hayashi, Y.; Whalley, B.J.; Jones, M.; et al. The neurogenesis of p1 and n1: A concurrent eeg/lfp study. NeuroImage 2017, 146, 575–588. [Google Scholar] [CrossRef] [Green Version]
- Korzyukov, O.; Pflieger, M.E.; Wagner, M.; Bowyer, S.M.; Rosburg, T.; Sundaresan, K.; Elger, C.E.; Boutros, N.N. Generators of the intracranial p50 response in auditory sensory gating. NeuroImage 2007, 35, 814–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.X.; Edgar, J.C.; Thoma, R.J.; Hanlon, F.M.; Moses, S.N.; Lee, R.R.; Paulson, K.M.; Weisend, M.P.; Irwin, J.G.; Bustillo, J.R.; et al. Predicting eeg responses using meg sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clin. Neurophysiol. 2003, 114, 835–850. [Google Scholar] [CrossRef]
- Reite, M.; Teale, P.; Zimmerman, J.; Davis, K.; Whalen, J. Source location of a 50 msec latency auditory evoked field component. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 490–498. [Google Scholar] [CrossRef]
- Woods, D.L.; Alain, C.; Covarrubias, D.; Zaidel, O. Frequency-related differences in the speed of human auditory processing. Hear. Res. 1993, 66, 46–52. [Google Scholar] [CrossRef]
- Ninomiya, H.; Sato, E.; Onitsuka, T.; Hayashida, T.; Tashiro, N. Auditory p50 obtained with a repetitive stimulus paradigm shows suppression to high-intensity tones. Psychiatry Clin. Neurosci. 2000, 54, 493–497. [Google Scholar] [CrossRef]
- Weate, S.J.; Moore, J.L.; Drake, M.E., Jr. Effect of frontal and temporal seizure foci on p50 auditory evoked potentials. Clin. Electroencephalogr. 1995, 26, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.E.; Hoffer, L.; Nagamoto, H.T.; Waldo, M.C.; Kisley, M.A.; Giffith, J.M. Yohimbine impairs p50 auditory sensory gating in normal subjects. Neuropsychopharmacology 1994, 10, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.S.; Munk, M.H.; Engel, A.K. Cognitive functions of gamma-band activity: Memory match and utilization. Trends Cogn. Sci 2004, 8, 347–355. [Google Scholar] [CrossRef]
- Bertrand, O.; Tallon-Baudry, C. Oscillatory gamma activity in humans: A possible role for object representation. Int. J. Psychophysiol. 2000, 38, 211–223. [Google Scholar] [CrossRef]
- Elliott, M.A.; Muller, H.J. Evidence for 40-hz oscillatory short-term visual memory revealed by human reaction-time measurements. J. Exp. Psychol. Learn. Mem. Cogn. 2000, 26, 703–718. [Google Scholar] [CrossRef]
- Ross, B.; Fujioka, T. 40-hz oscillations underlying perceptual binding in young and older adults. Psychophysiology 2016, 53, 974–990. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.; Herdman, A.T.; Pantev, C. Stimulus induced desynchronization of human auditory 40-hz steady-state responses. J. Neurophysiol. 2005, 94, 4082–4093. [Google Scholar] [CrossRef] [Green Version]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef]
- Ross, B.; Dobri, S.; Schumann, A. Speech-in-noise understanding in older age: The role of inhibitory cortical responses. Eur. J. Neurosci. 2019, 51, 891–908. [Google Scholar] [CrossRef]
- Narici, L.; Carozzo, S.; Lopez, L.; Ogliastro, C.; Sannita, W.G. Phase-locked oscillatory approximately 15- to 30-hz response to transient visual contrast stimulation: Neuromagnetic evidence for cortical origin in humans. Neuroimage 2003, 19, 950–958. [Google Scholar] [CrossRef]
- Sannita, W.G.; Lopez, L.; Piras, C.; Di Bon, G. Scalp-recorded oscillatory potentials evoked by transient pattern-reversal visual stimulation in man. Electroencephalogr. Clin. Neurophysiol. 1995, 96, 206–218. [Google Scholar] [CrossRef]
- Howard, M.F.; Poeppel, D. Hemispheric asymmetry in mid and long latency neuromagnetic responses to single clicks. Hear. Res. 2009, 257, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, B.; Herdman, A.T.; Pantev, C. Right hemispheric laterality of human 40 hz auditory steady-state responses. Cereb. Cortex 2005, 15, 2029–2039. [Google Scholar] [CrossRef]
- Kanno, A.; Nakasato, N.; Fujiwara, S.; Yoshimoto, T. Right hemispheric dominancy in the auditory evoked magnetic fields for pure-tone stimuli. Brain Nerve 1996, 48, x1–x243. [Google Scholar]
- Shaw, M.E.; Hämäläinen, M.S.; Gutschalk, A. How anatomical asymmetry of human auditory cortex can lead to a rightward bias in auditory evoked fields. NeuroImage 2013, 74, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kisley, M.A.; Noecker, T.L.; Guinther, P.M. Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology 2004, 41, 604–612. [Google Scholar] [CrossRef]
- Boutros, N.N.; Belger, A. Midlatency evoked potentials attenuation and augmentation reflect different aspects of sensory gating. Biol. Psychiatry 1999, 45, 917–922. [Google Scholar] [CrossRef]
- Billings, C.J.; Bennett, K.O.; Molis, M.R.; Leek, M.R. Cortical encoding of signals in noise: Effects of stimulus type and recording paradigm. Ear Hear. 2011, 32, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ross, B. Auditory cortex responses to interaural time differences in the envelope of low-frequency sound, recorded with meg in young and older listeners. Hear. Res. 2018, 370, 22–39. [Google Scholar] [CrossRef]
- Luck, S.J.; Hillyard, S.A. Electrophysiological correlates of feature analysis during visual search. Psychophysiology 1994, 31, 291–308. [Google Scholar] [CrossRef]
- Alain, C.; Roye, A.; Arnott, S.A. Middle and late auditory evoked responses: What are they telling us on central auditory disorders. In Disorders of Peripheral and Central Auditory Processing: Handbook of Clinical Neurophysiology; Celesia, G.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 10, pp. 177–199. [Google Scholar]
- Alain, C.; Ross, B. The role of neuroelectric and neuromagnetic recordings in assessing learning and rehabilitation effects. In Cognitive Neurorehabilitation, Evidence and Applications, 2nd ed.; Stuss, D.T., Winocur, G., Eds.; Cambridge University Press: New York, NY, USA, 2008; pp. 183–199. [Google Scholar]
- Wegel, R.L.; Lane, C.E. Auditory masking of one pure tone by another. Phys. Rev. 1924, 23, 266–285. [Google Scholar] [CrossRef]


| Source | df | Error df | Amplitude | Latency | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | |||||||
| Onset Responses | P1 | H | 1 | 14 | 1.624 | 0.223 | 0.104 | 6.037 | 0.028 | 0.301 |
| N | 4 | 56 | 62.575 | <0.001 | 0.817 | 1.280 | 0.289 | 0.084 | ||
| H × N | 4 | 56 | 3.269 | 0.018 | 0.189 | 2.117 | 0.091 | 0.131 | ||
| N1 | H | 1 | 14 | 27.353 | <0.001 | 0.661 | 6.811 | 0.021 | 0.327 | |
| N | 4 | 56 | 5.481 | 0.001 | 0.281 | 13.272 | <0.001 | 0.487 | ||
| H × N | 4 | 56 | 1.663 | 0.171 | 0.106 | 1.341 | 0.266 | 0.087 | ||
| P2 | H | 1 | 14 | 4.050 | 0.064 | 0.224 | 6.171 | 0.026 | 0.306 | |
| N | 4 | 56 | 5.622 | 0.001 | 0.287 | 2.570 | 0.048 | 0.155 | ||
| H × N | 4 | 56 | 6.237 | <0.001 | 0.308 | 0.884 | 0.479 | 0.059 | ||
| Gap Responses | P1 | H | 1 | 14 | 4.920 | 0.044 | 0.260 | 10.010 | 0.007 | 0.417 |
| N | 4 | 56 | 5.151 | 0.001 | 0.269 | 8.523 | <0.001 | 0.378 | ||
| H × N | 4 | 56 | 1.246 | 0.302 | 0.082 | 1.261 | 0.296 | 0.083 | ||
| N1 | H | 1 | 14 | 1.739 | 0.208 | 0.111 | 3.620 | 0.078 | 0.205 | |
| N | 4 | 56 | 4.116 | 0.005 | 0.227 | 20.250 | <0.001 | 0.592 | ||
| H × N | 4 | 56 | 0.970 | 0.431 | 0.065 | 6.110 | <0.001 | 0.304 | ||
| P2 | H | 1 | 14 | 7.287 | 0.017 | 0.342 | 1.699 | 0.213 | 0.108 | |
| N | 4 | 56 | 17.427 | <0.001 | 0.555 | 25.131 | <0.001 | 0.642 | ||
| H × N | 4 | 56 | 2.438 | 0.058 | 0.148 | 0.696 | 0.598 | 0.047 | ||
| Source | df | Error df | F | p | |
|---|---|---|---|---|---|
| H | 1 | 14 | 0.550 | 0.471 | 0.038 |
| N | 4 | 56 | 3.765 | 0.009 | 0.212 |
| H × N | 4 | 56 | 3.200 | 0.020 | 0.186 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Alain, C.; Ross, B. Binaural Background Noise Enhances Neuromagnetic Responses from Auditory Cortex. Symmetry 2021, 13, 1748. https://doi.org/10.3390/sym13091748
Shen D, Alain C, Ross B. Binaural Background Noise Enhances Neuromagnetic Responses from Auditory Cortex. Symmetry. 2021; 13(9):1748. https://doi.org/10.3390/sym13091748
Chicago/Turabian StyleShen, Dawei, Claude Alain, and Bernhard Ross. 2021. "Binaural Background Noise Enhances Neuromagnetic Responses from Auditory Cortex" Symmetry 13, no. 9: 1748. https://doi.org/10.3390/sym13091748
APA StyleShen, D., Alain, C., & Ross, B. (2021). Binaural Background Noise Enhances Neuromagnetic Responses from Auditory Cortex. Symmetry, 13(9), 1748. https://doi.org/10.3390/sym13091748







