Symmetry of the Human Head—Are Symmetrical Models More Applicable in Numerical Analysis?
Abstract
1. Introduction
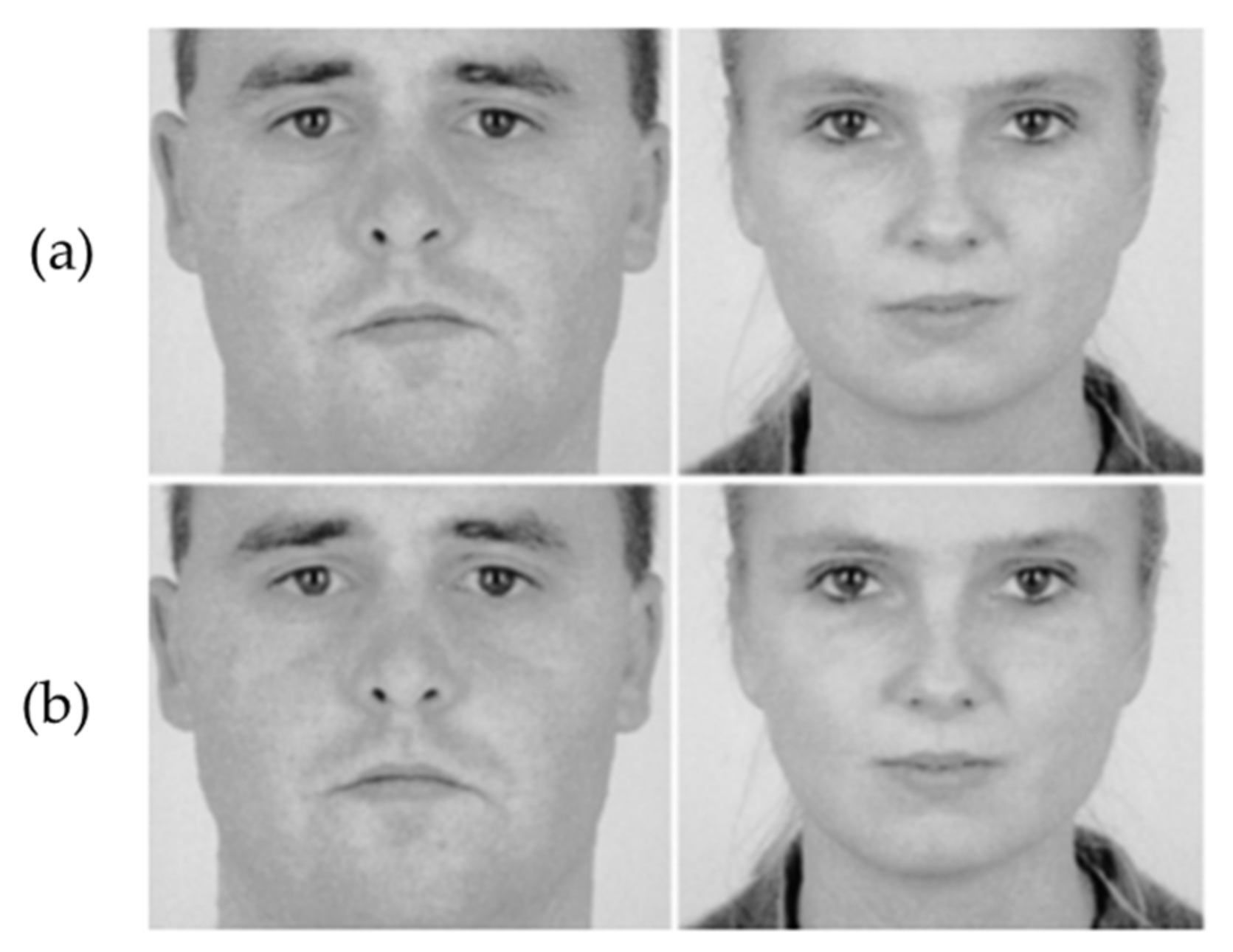
1.1. Facial Symmetry
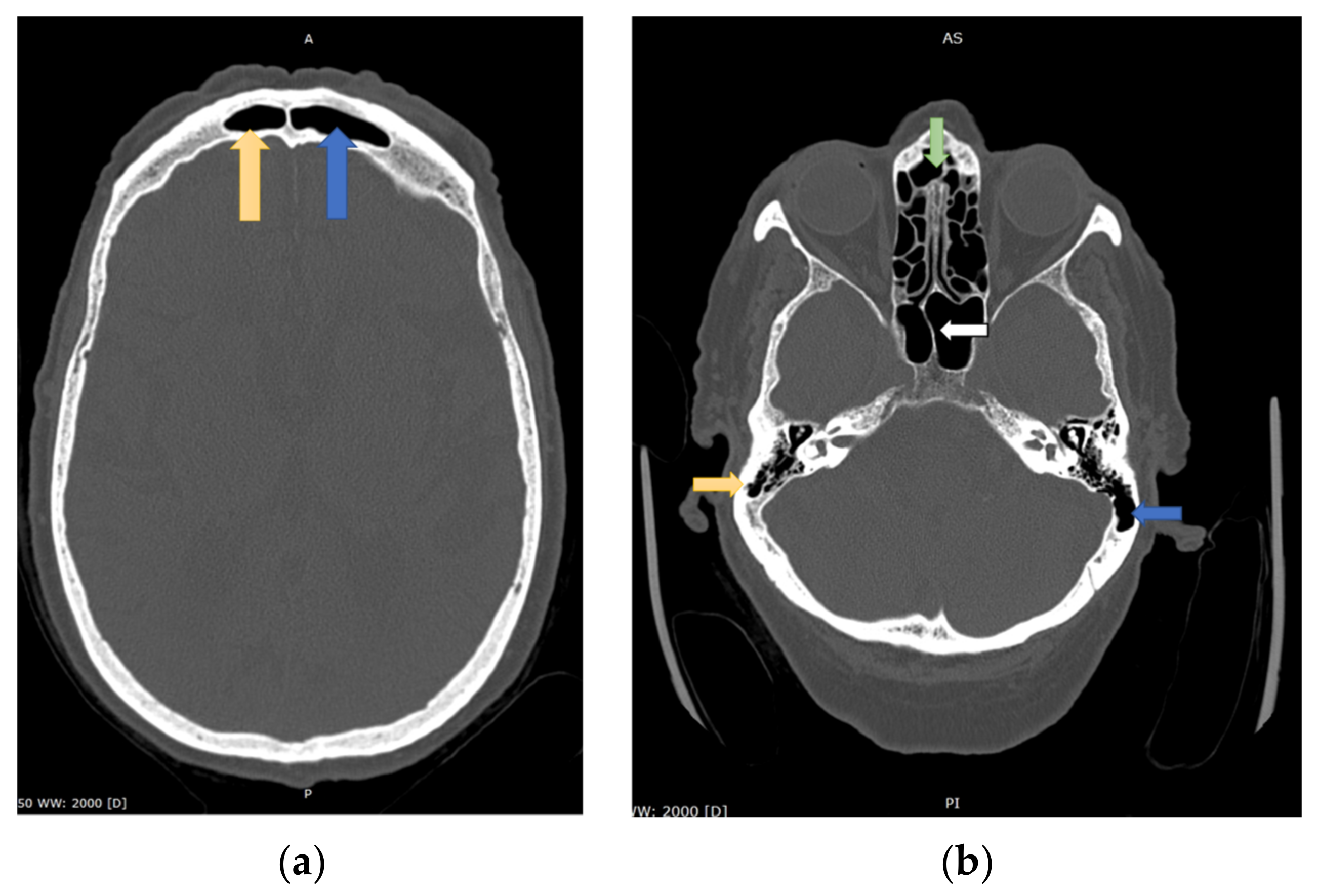
1.2. Skull Symmetry
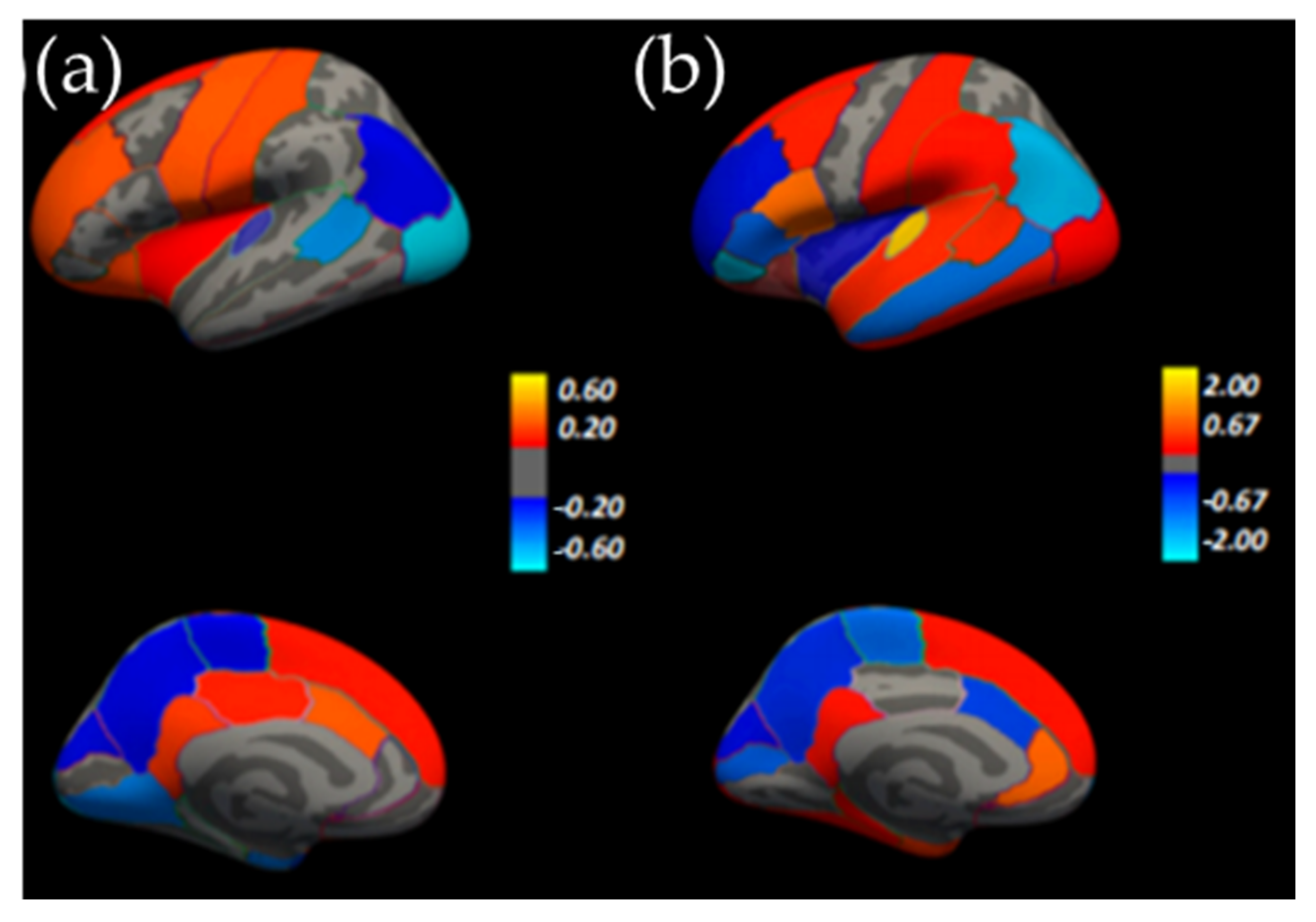
1.3. Brain Symmetry
1.4. Numerical Head Model Symmetry
1.5. Validation of Numerical Models Symmetry
2. Materials and Methods
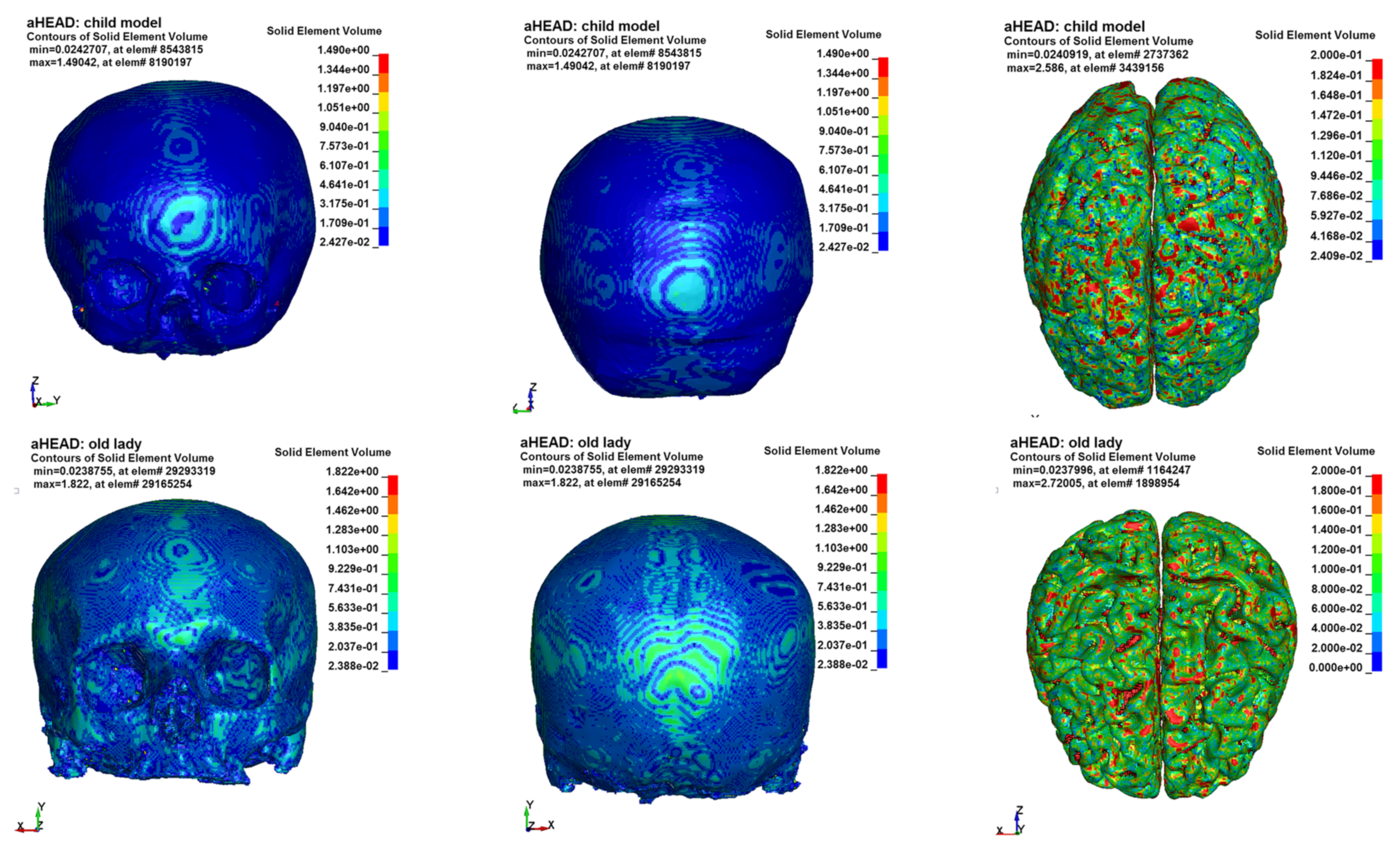
2.1. Development of Numerical Models of the Head of a Young Child and the Elderly
2.2. The Method of Checking the Symmetry in Numerical Models of the Human Head
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Evans, C.S.; Wenderoth, P.; Cheng, K. Detection of bilateral symmetry in complex biological images. Perception 2000, 29, 31–42. [Google Scholar] [CrossRef]
- Kayaert, G.; Wagemans, J. Delayed shape matching benefits from simplicity and symmetry. Vision Res. 2009, 49, 708–717. [Google Scholar] [CrossRef]
- Sasaki, Y.; Vanduffel, W.; Knutsen, T.; Tyler, C.; Tootell, R. Symmetry activates extrastriate visual cortex in human and nonhuman primates. Proc. Natl. Acad. Sci. USA 2005, 102, 3159–3163. [Google Scholar] [CrossRef]
- Makin, A.D.J.; Wilton, M.M.; Pecchinenda, A.; Bertamini, M. Symmetry perception and affective responses: A combined EEG/EMG study. Neuropsychologia 2012, 50, 3250–3261. [Google Scholar] [CrossRef]
- Thornhill, R.; Møller, A.P. Developmental stability, disease and medicine. Biol. Rev. Camb. Philos. Soc. 1997, 72, 497–548. [Google Scholar] [CrossRef]
- Perrett, D.I.; Burt, D.M.; Penton-Voak, I.S.; Lee, K.J.; Rowland, D.A.; Edwards, R. Symmetry and Human Facial Attractiveness. Evol. Hum. Behav. 1999, 20, 295–307. [Google Scholar] [CrossRef]
- Wade, T.J. The Relationships between Symmetry and Attractiveness and Mating Relevant Decisions and Behavior: A review. Symmetry 2010, 2, 1081–1098. [Google Scholar] [CrossRef]
- Gawlikowska, A.; Szczurowski, J.; Czerwiński, F.; Miklaszewska, D.; Adamiec, E.; Dzieciołowska, E. The fluctuating asymmetry of mediaeval and modern human skulls. HOMO- J. Comp. Hum. Biol. 2007, 58, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Chovalopoulou, M.E.; Papageorgopoulou, C.; Bertsatos, A. Cranium asymmetry in a modern Greek population sample of known age and sex. Int. J. Legal Med. 2017, 131, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Myslobodsky, M.S.; Ingraham, L.J.; Weinberger, D.R. Skull asymmetry and handedness in adults: A possibility of their association with lateral head turning in infancy. Percept. Mot. Skills 1987, 65, 415–421. [Google Scholar] [CrossRef]
- Zilles, K.; Dabringhaus, A.; Geyer, S.; Amunts, K.; Qü, M.; Schleicher, A.; Gilissen, E.; Schlaug, G.; Steinmetz, H. Structural Asymmetries in the Human Forebrain and the Forebrain of Non-Human Primates and Rats. Neurosci. Biobehav. Rev. 1996, 20, 593–605. [Google Scholar] [CrossRef]
- Radosevic, D.; Maric, D.; Ivanovic, D. Human skull base asymmetry analysis. Int. J. Morphol. 2020, 38, 1566–1570. [Google Scholar] [CrossRef]
- Distriquin, Y.; Vital, J.M.; Ella, B. Biomechanical analysis of skull trauma and opportunity in neuroradiology interpretation to explain the post-concussion syndrome: Literature review and case studies presentation. Eur. Radiol. Exp. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Kong, X.-Z.; Mathias, S.R.; Guadalupe, T.; Working Group, L.; Glahn, D.C.; Franke, B.; Crivello, F.; Tzourio-Mazoyer, N.; Fisher, S.E.; Thompson, P.M.; et al. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. Proc. Natl. Acad. Sci. USA 2018, 115, E5154–E5163. [Google Scholar] [CrossRef]
- Duboc, V.; Dufourcq, P.; Blader, P.; Roussigné, M. Asymmetry of the Brain: Development and Implications. Annu. Rev. Genet. 2015, 49, 647–672. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N.; Levitsky, W. Human brain: Left-right asymmetries in temporal speech region. Science 1968, 161, 186–187. [Google Scholar] [CrossRef]
- Seidenwurm, D.; Roger Bird, C.; Enzmann, D.R.; Marshall, W.H. Left-Right Temporal Region Asymmetry in Infants and Children. Am. J. Neuroradiol. 1985, 6(5), 777–779. [Google Scholar] [PubMed]
- Chi, J.G.; Dooling, E.C.; Gilles, F.H. Gyral development of the human brain. Ann. Neurol. 1977, 1, 86–93. [Google Scholar] [CrossRef]
- Luders, E.; Narr, K.L.; Thompson, P.M.; Rex, D.E.; Jancke, L.; Toga, A.W. Hemispheric asymmetries in cortical thickness. Cereb. Cortex 2006, 16, 1232–1238. [Google Scholar] [CrossRef]
- Schlaug, G.; Jäncke, L.; Huang, Y.; Steinmetz, H. In vivo evidence of structural brain asymmetry in musicians. Science 1995, 267, 699–701. [Google Scholar] [CrossRef]
- Li, S.; Han, Y.; Wang, D.; Yang, H.; Fan, Y.; Lv, Y.; Tang, H.; Gong, Q.; Zang, Y.; He, Y. Mapping surface variability of the central sulcus in musicians. Cereb. Cortex 2010, 20, 25–33. [Google Scholar] [CrossRef]
- Maguire, E.A.; Woollett, K.; Spiers, H.J. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus 2006, 16, 1091–1101. [Google Scholar] [CrossRef]
- Roe, J.M.; Vidal-Piñeiro, D.; Sørensen, Ø.; Brandmaier, A.M.; Düzel, S.; Gonzalez, H.A.; Kievit, R.A.; Knights, E.; Kuhn, S.; Lindenberger, U.; et al. Asymmetric thinning of the cerebral cortex across the adult lifespan is accelerated in Alzheimer′s Disease. bioRxiv 2020, 1–20. [Google Scholar] [CrossRef]
- Mackiewicz, A.; Banach, M.; Denisiewicz, A.; Bedzinski, R. Comparative studies of cervical spine anterior stabilization systems -Finite element analysis. Clin. Biomech. 2016, 32, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Pałka, Ł.; Kuryło, P.; Klekiel, T.; Pruszyński, P. A mechanical study of novel additive manufactured modular mandible fracture fixation plates-Preliminary Study with finite element analysis. Injury 2020, 51, 1527–1535. [Google Scholar] [CrossRef]
- Klekiel, T.; Będziński, R. Finite element analysis of large deformation of articular cartilage in upper ankle joint of occupant in military vehicles during explosion. Arch. Metall. Mater. 2015, 60, 2115–2121. [Google Scholar] [CrossRef][Green Version]
- Mackiewicz, A.; Sławiński, G.; Niezgoda, T.; Będziński, R. Numerical analysis of the risk of neck injuries caused by IED explosion under the vehicle in military environments. Acta Mech. Autom. 2016, 10, 258–264. [Google Scholar] [CrossRef][Green Version]
- Bukala, J.; Kwiatkowski, P.; Malachowski, J. Numerical analysis of stent expansion process in coronary artery stenosis with the use of non-compliant balloon. Biocybern. Biomed. Eng. 2016, 36, 145–156. [Google Scholar] [CrossRef]
- Sybilski, K.; Małachowski, J. Impact of Disabled Driver’s Mass Center Location on Biomechanical Parameters during Crash. Appl. Sci. 2021, 11, 1427. [Google Scholar] [CrossRef]
- Klekiel, T.; Mackiewicz, A.; Kaczmarek-Pawelska, A.; Skonieczna, J.; Kurowiak, J.; Piasecki, T.; Noszczyk-Nowak, A.; Będziński, R. Novel design of sodium alginate based absorbable stent for the use in urethral stricture disease. J. Mater. Res. Technol. 2020, 9, 9004–9015. [Google Scholar] [CrossRef]
- Arkusz, K.; Klekiel, T.; Sławiński, G.; Będziński, R. Influence of energy absorbers on Malgaigne fracture mechanism in lumbar-pelvic system under vertical impact load. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Arkusz, K.; Klekiel, T.; Niezgoda, T.M.; Będziński, R. The influence of osteoporotic bone structures of the pelvic-hip complex on stress distribution under impact load. Acta Bioeng. Biomech. Orig. Pap. 2018, 20. [Google Scholar] [CrossRef]
- Ghajari, M.; Hellyer, P.J.; Sharp, D.J. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain 2017, 140, 333–343. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, K.H.; Dwarampudi, R.; Omori, K.; Li, T.; Chang, K.; Hardy, W.N.; Khalil, T.B.; King, A.I. Recent advances in brain injury research: A new human head model development and validation. Stapp Car Crash J. 2001, 45, 369–394. [Google Scholar]
- Zhang, L.; Bae, J.; Hardy, W.N.; Monson, K.L.; Manley, G.T.; Goldsmith, W.; Yang, K.H.; King, A.I. Computational study of the contribution of the vasculature on the dynamic response of the brain. Stapp Car Crash J. 2002, 46, 145–164. [Google Scholar] [PubMed]
- Yang, K.H.; Mao, H.; Wagner, C.; Zhu, F.; Chou, C.C.; King, A.I. Modeling of the Brain for Injury Prevention; Springer: Berlin/Heidelberg, Germany, 2011; pp. 69–120. [Google Scholar]
- Kleiven, S.; Hardy, W.N. Correlation of an FE Model of the Human Head with Local Brain Motion—Consequences for Injury Prediction. Stapp Car Crash J. 2002, 46, 123–144. [Google Scholar]
- Kleiven, S. Predictors for traumatic brain injuries evaluated through accident reconstructions. Stapp Car Crash J. 2007, 51, 81–114. [Google Scholar]
- Horgan, T.J.; Gilchrist, M.D. The creation of three-dimensional finite element models for simulating head impact biomechanics. Int. J. Crashworthiness 2003, 8, 353–366. [Google Scholar] [CrossRef]
- Takhounts, E.G.; Ridella, S.A.; Hasija, V.; Tannous, R.E.; Campbell, J.Q.; Malone, D.; Danelson, K.; Stitzel, J.; Rowson, S.; Duma, S. Investigation of traumatic brain injuries using the next generation of simulated injury monitor (SIMon) finite element head model. Stapp Car Crash J. 2008, 52, 1–31. [Google Scholar]
- Zong, Z.; Lee, H.P.; Lu, C. A three-dimensional human head finite element model and power flow in a human head subject to impact loading. J. Biomech. 2006, 39, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Belingardi, G.; Chiandussi, G.; Gaviglio, I. Development and Validation of a New Finite Element Model of Human Head. In Proceedings of the 19th International Technical Conference of the Enhanced Safety of Vehicle (ESV), Washington, DC, USA, 6–9 June 2005. [Google Scholar]
- Li, X.; Zhou, Z.; Kleiven, S. An anatomically detailed and personalizable head injury model: Significance of brain and white matter tract morphological variability on strain. Biomech. Model. Mechanobiol. 2021, 20, 403–431. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhang, L.; Jiang, B.; Genthikatti, V.V.; Jin, X.; Zhu, F.; Makwana, R.; Gill, A.; Jandir, G.; Singh, A.; et al. Development of a Finite Element Human Head Model Partially Validated With Thirty Five Experimental Cases. J. Biomech. Eng. 2013, 135. [Google Scholar] [CrossRef]
- Sahoo, D.; Deck, C.; Willinger, R. Development and validation of an advanced anisotropic visco-hyperelastic human brain FE model. J. Mech. Behav. Biomed. Mater. 2014, 33, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, N.; Nakahira, Y.; Iwamoto, M. Development and validation of a head/brain FE model and investigation of influential factor on the brain response during head impact. Int. J. Veh. Saf. 2016, 9, 1. [Google Scholar] [CrossRef]
- Fernandes, F.A.O.; Tchepel, D.; Alves de Sousa, R.J.; Ptak, M. Development and validation of a new finite element human head model: Yet another head model (YEAHM). Eng. Comput. 2018, 35, 477–496. [Google Scholar] [CrossRef]
- Ratajczak, M.; Ptak, M.; Chybowski, L.; Gawdzińska, K.; Będziński, R. Material and Structural Modeling Aspects of Brain Tissue Deformation under Dynamic Loads. Materials 2019, 12, 271. [Google Scholar] [CrossRef]
- Zhao, W.; Ruan, S.; Li, H.; Cui, S.; He, L.; Li, J. Development and validation of a 5th percentile human head finite element model based on the Chinese population. Int. J. Veh. Saf. 2012, 6, 91. [Google Scholar] [CrossRef]
- Wilhelm, J.; Ptak, M.; Fernandes, F.A.O.; Kubicki, K.; Kwiatkowski, A.; Ratajczak, M.; Sawicki, M.; Szarek, D. Injury Biomechanics of a Child’s Head: Problems, Challenges and Possibilities with a New aHEAD Finite Element Model. Appl. Sci. 2020, 10, 4467. [Google Scholar] [CrossRef]
- Klinich, K.D.; Hulbert, G.M.; Schneider, L.W. Estimating Infant Head Injury Criteria and Impact Response Using Crash Reconstruction and Finite Element Modeling. Stapp Car Crash J. 2002, 46, 165–194. [Google Scholar]
- Roth, S.; Raul, J.-S.; Willinger, R. Biofidelic child head FE model to simulate real world trauma. Comput. Methods Programs Biomed. 2008, 90, 262–274. [Google Scholar] [CrossRef]
- Roth, S.; Vappou, J.; Raul, J.-S.; Willinger, R. Child head injury criteria investigation through numerical simulation of real world trauma. Comput. Methods Programs Biomed. 2009, 93, 32–45. [Google Scholar] [CrossRef]
- Coats, B.; Margulies, S.S.; Ji, S. Parametric study of head impact in the infant. Stapp Car Crash J. 2007, 51, 1–15. [Google Scholar]
- Hardy, W.N.; Foster, C.D.; Mason, M.J.; Yang, K.H.; King, A.I.; Tashman, S. Investigation of Head Injury Mechanisms Using Neutral Density Technology and High-Speed Biplanar X-ray. Stapp Car Crash J. 2001, 45, 337–368. [Google Scholar] [PubMed]
- Fernandes, F.A.O.; Alves de Sousa, R.J.; Ptak, M. Validation of YEAHM. In Head Injury Simulation in Road Traffic Accidents; SpringerBriefs in Applied Sciences and Technology; Springer International Publishing: Cham, Switzerland, 2018; pp. 41–58. ISBN1 978-3-319-89926-8. ISBN2 978-3-319-89925-1. ISSN 2191-530X. [Google Scholar] [CrossRef]
- Nahum, A.M.; Smith, R.; Ward, C.C. Intracranial Pressure Dynamics During Head Impact; SAE Technical Paper 770922; SAE International: Warrendale, PA, USA, 1977. [Google Scholar] [CrossRef]
- Hardy, W.N.; Mason, M.J.; Foster, C.D.; Shah, C.S.; Kopacz, J.M.; Yang, K.H.; King, A.I.; Bishop, J.; Bey, M.; Anderst, W.; et al. A study of the response of the human cadaver head to impact. Stapp Car Crash J. 2007, 51, 17–80. [Google Scholar]
- Fernandes, F.A.O.; Alves de Sousa, R.J.; Ptak, M. Head Injury Simulation in Road Traffic Accidents; SpringerBriefs in Applied Sciences and Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ptak, M.; Kaczyński, P.; Fernandes, F.; de Sousa, R.A. Computer Simulations for Head Injuries Verification After Impact; Springer: Berlin/Heidelberg, Germany, 2017; pp. 431–440. [Google Scholar]
- Cloots, R.J.H.; Gervaise, H.M.T.; Van Dommelen, J.A.W.; Geers, M.G.D. Biomechanics of traumatic brain injury: Influences of the morphologic heterogeneities of the cerebral cortex. Ann. Biomed. Eng. 2008, 36, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Lauret, C.; Hrapko, M.; van Dommelen, J.A.W.; Peters, G.W.M.; Wismans, J.S.H.M. Optical characterization of acceleration-induced strain fields in inhomogeneous brain slices. Med. Eng. Phys. 2009, 31, 392–399. [Google Scholar] [CrossRef]
- Ho, J.; Kleiven, S. Can sulci protect the brain from traumatic injury? J. Biomech. 2009, 42, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Chan-Akeley, R.; Lipari, C.; Kuo, S.-H. Mechanism of Coup and Contrecoup Injuries Induced by a Knock-Out Punch. Math. Comput. Appl. 2020, 25, 22. [Google Scholar] [CrossRef]
- Abaqus Software Hyperelastic Behavior of Rubberlike Materials. Available online: https://abaqus-docs.mit.edu/2017/English/SIMACAEMATRefMap/simamat-c-hyperelastic.htm (accessed on 27 May 2021).


| Author(s) | Model Description | Geometrical Symmetry |
|---|---|---|
| Adult head model | ||
| Zhang et al. [34] | Head geometry of a 50-centile adult man; anatomical drawings. Mass: 4.5 kg; number of elements: 314,500 Linear viscoelastic brain material, elasto-plastic skull material, elastic material for dura matter and skin. | Yes |
| Zhang et al. [35] | Model I and II. Number of elements: 4501 Anatomical drawings. Mass: 4.107 kg. Linear viscoelastic brain material, elastic behaviour for cerebrovascular elements. | Yes |
| King et al. [36] | The newest WSUBIM model, including viscoelastic brain and elastic-plastic skull behaviour, number of elements: 314,500. | Yes |
| Kleiven and Hardy [37] | Finite Element Head Model (KTH FEHM) developed in Kungliga Tekniska Högskolan (Royal Institute of Technology), number of elements: 18,400 Model consisting of skin, skull, cerebrovascular, cerebrospinal fluid (CSF), 11 bridging vein pairs, and simplified neck. Sliding connection between skull and brain. | Yes |
| Kleiven [38] | 11,454 hexahedral elements, 6940 four-node elements, 22 two-node elements truss type Hyperelastic and viscoelastic materials for brain tissue, linear-elastic for skull, skin, and dura matter. | Yes |
| Horgan and Gilchrist [39] | University College Dublin Brain Trauma Model (UCDBTM) model. Consisting of: three-layered skull, dura matter, cerebrospinal fluid, falx, tentorium, separate hemispheres, cerebellum, and brain stem. Linear viscoelastic brain material, elastic material for skull and skin, mixed elements for cerebrospinal fluid. | Yes |
| Takhounts and Eppinger [40] | Number of elements: 45,875; brain model consisting of: skull, dura matter, cerebrospinal fluid based on outer brain layers, and brain. | Yes |
| Zong et al. [41] | Simplified model consisting of three-layered non-uniform skull, incompressible cerebrospinal fluid, and homogenous brain. | Yes |
| Belingardi et al. [42] | Numerical model generated from CT scans of 31 year old patient, composed of scalp, 3-layered-skluu, facial bones, dura matter, CSF, brain tissues, ventricles, falx, and tentorium membrane | Yes |
| Xiaogai Li et al. [43] | Detailed and Personalizable Head Model with Axons for Injury Prediction (ADAPT) is based on ICBM152 template generated from 152 healthy subjects. The head model includes the brain, skull (compact and diploe porous bone), meninges (pia, dura, falx, and tentorium), CSF, and superior sagittal sinus. Hyper-viscoelastic material is prescribed for brain structure. | Yes |
| Mao et al. [44] | Global Human Body Consortium (GHBMC) is based on MRI scans collected from an average adult male. The model consists of facial tissue, scalp, and separate brain structures, such as cerebrum gray, cerebellum, thalamus, brainstem, basal ganglia, CSF, 3rd ventricle, later ventricle, corpus callosum, cerebrum white dura, falx, and pia | Yes |
| Sahoo et al. [45] | Strasbourg University Finite Element Head Model (SUFEHM) is composed of scalp, brain, brainstem, cerebrospinal fluid (CSF), skull, face, and two membranes (the falx and the tentorium). | Yes |
| Atsumi et al. [46] | The Fe head model is an advanced model from the head model of THUMS Ver. 3. The brain consists of separate parts, such as cerebrum, cerebellum, stem, dura, arachnoid, pia, falx, CSF, and superior sagittal sinus. The mesh size and fineness are almost the same as THUMS Ver. 3; contact conditions and material properties are updated to improve computational stability and accuracy to physical model. | Yes |
| Fernandes et al. 2018 [47] | Yet Another Head Model (YEAHM) consists of skull, CSF, and brain. The brain model has all important sections: frontal, parietal, temporal and occipital lobes, cerebrum, cerebellum, corpus callosum, thalamus, midbrain, and brain stem. Nonlinear, viscoelastic model for brain material, hyperelastic model for cerebrospinal fluid, and isotropic linear elastic material for skull material. | No |
| Ratajczak et al. 2019 [48] | αHEAD brain model consisting of skull, dura matter, falx cerebri, tentorium cerebelli, sinus sagittalis superior, bridging veins, hemispheres, and cerebellum. Number of elements: solid—55,117, shell—3784, beam—133 | No |
| Ghajari et al. 2016 [33] | Imperial College London head model based on a 34-year-old male subject, consisting of skin, skull, cerebrospinal fluid, and brain. Falx, tentorium, and pia matter were modelled as shell elements. | No |
| Ji et al. 2015 [49] | Worcester head injury model (WHIM) consists of the scalp, skull, cerebrum, cerebellum, brain stem, corpus callosum, cerebrospinal fluid, ventricles, sinus, falx cerebri, tentorium cerebelli, pia mater, dura mater, facial bone, mandible, facial muscle, masseter, temporalis, submandibular soft tissue, detailed ocular structures, and teeth. The total mass is 3.569 kg. | Yes |
| Child head model | ||
| Wilhelm et al. (aHEAD project 2020) [50] | aHEAD child model—2-year-old child head model. The model consists of: separate hemispheres with white and gray matter, cerebellum, brainstem, pia matter, dura matter, superior sagittal sinus, transversal sinus, bridging veins, cerebrospinal fluid, corpus callosum, and skull divided to lamina interna and externa. The validation process distinguished hyperelastic and viscoelastic material differences. | No |
| (DeSantis) Klinich [51] | Six-month-old child head model, viscoelastic brain material, elastic skull, and skin | No data |
| Roth et al. [52] | Six-month-old child head model, viscoelastic brain material, elastic skull material, cerebrospinal fluid, and skin. Number of elements: solid—69,324, shell—9187 | Yes |
| Roth et al. [53] | Three-year-old child head model, viscoelastic brain material, elastic skull material, cerebrospinal fluid, and skin. Number of elements: solid—23,000, shell—3500 | Yes |
| Coats et al. [54] | One-and-a-half-month-old child head model, Ogden brain material characteristics, elastic skull, and skin | No data |
| Brain Structure No. of FEs and Mass | Child: 2 Year Old—Hemisphere: | Senior: 77 Year Old—Hemisphere: | ||
|---|---|---|---|---|
| Left | Right | Left | Right | |
| White Matter | 233,760 | 245,830 | 231,146 | 237,494 |
| 0.242781 kg | 0.255368 kg | 0.238867 kg | 0.245465 kg | |
 |  |  |  | |
| Grey matter | 208,498 | 201,127 | 313,176 | 311,954 |
| 0.135695 kg | 0.130257 kg | 0.189834 kg | 0.187494 kg | |
 |  |  |  | |
| Total #FEs | 442,258 | 446,957 | 544,322 | 549,448 |
| Total mass | 0.378476 kg | 0.385625 kg | 0.428701 kg | 0.432959 kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, M.; Ptak, M.; Kwiatkowski, A.; Kubicki, K.; Fernandes, F.A.O.; Wilhelm, J.; Dymek, M.; Sawicki, M.; Żółkiewski, S. Symmetry of the Human Head—Are Symmetrical Models More Applicable in Numerical Analysis? Symmetry 2021, 13, 1252. https://doi.org/10.3390/sym13071252
Ratajczak M, Ptak M, Kwiatkowski A, Kubicki K, Fernandes FAO, Wilhelm J, Dymek M, Sawicki M, Żółkiewski S. Symmetry of the Human Head—Are Symmetrical Models More Applicable in Numerical Analysis? Symmetry. 2021; 13(7):1252. https://doi.org/10.3390/sym13071252
Chicago/Turabian StyleRatajczak, Monika, Mariusz Ptak, Artur Kwiatkowski, Konrad Kubicki, Fábio A. O. Fernandes, Johannes Wilhelm, Mateusz Dymek, Marek Sawicki, and Sławomir Żółkiewski. 2021. "Symmetry of the Human Head—Are Symmetrical Models More Applicable in Numerical Analysis?" Symmetry 13, no. 7: 1252. https://doi.org/10.3390/sym13071252
APA StyleRatajczak, M., Ptak, M., Kwiatkowski, A., Kubicki, K., Fernandes, F. A. O., Wilhelm, J., Dymek, M., Sawicki, M., & Żółkiewski, S. (2021). Symmetry of the Human Head—Are Symmetrical Models More Applicable in Numerical Analysis? Symmetry, 13(7), 1252. https://doi.org/10.3390/sym13071252











