Contributions of the Right Prefrontal and Parietal Cortices to the Attentional Blink: A tDCS Study
Abstract
:1. Introduction
2. Method
2.1. Participants
2.2. Materials and Apparatus
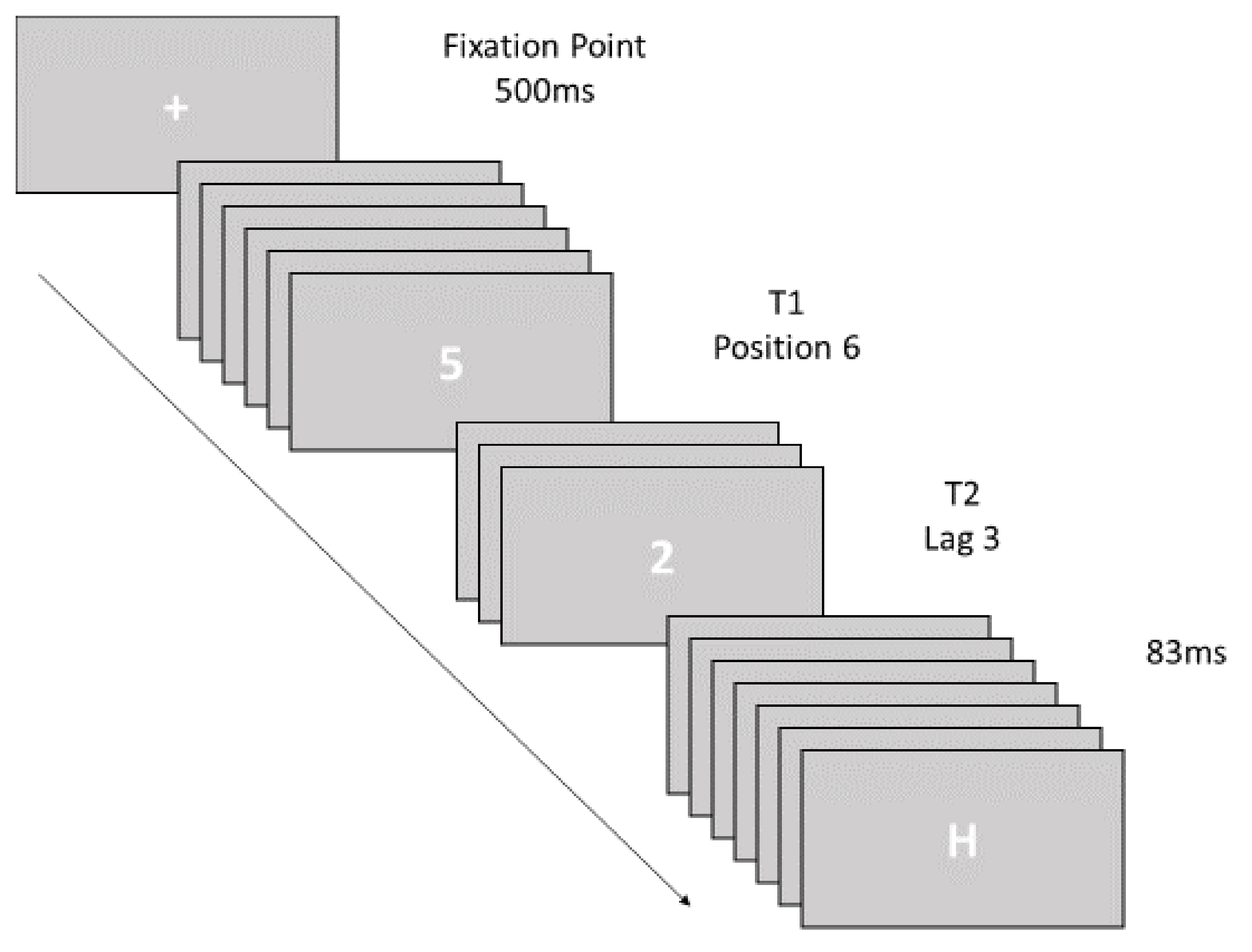
2.2.1. Attentional Blink Task
2.2.2. Online Transcranial Direct Current Stimulation
2.3. Procedure
3. Experimental Design and Data Analyses
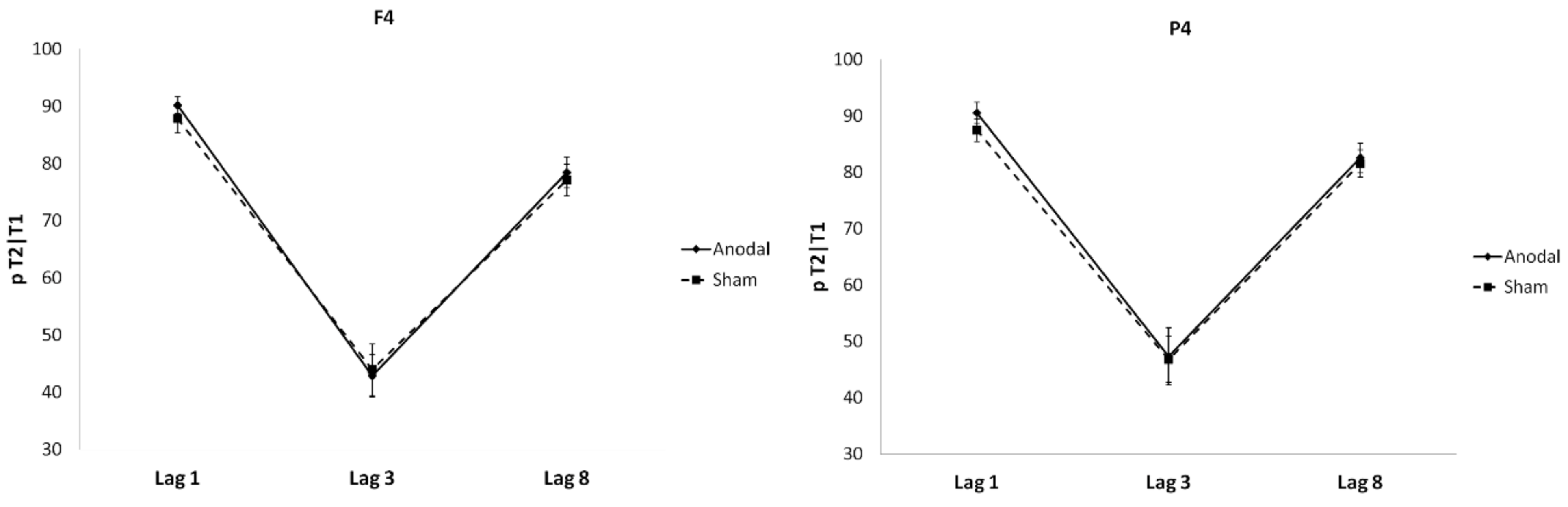
4. Results
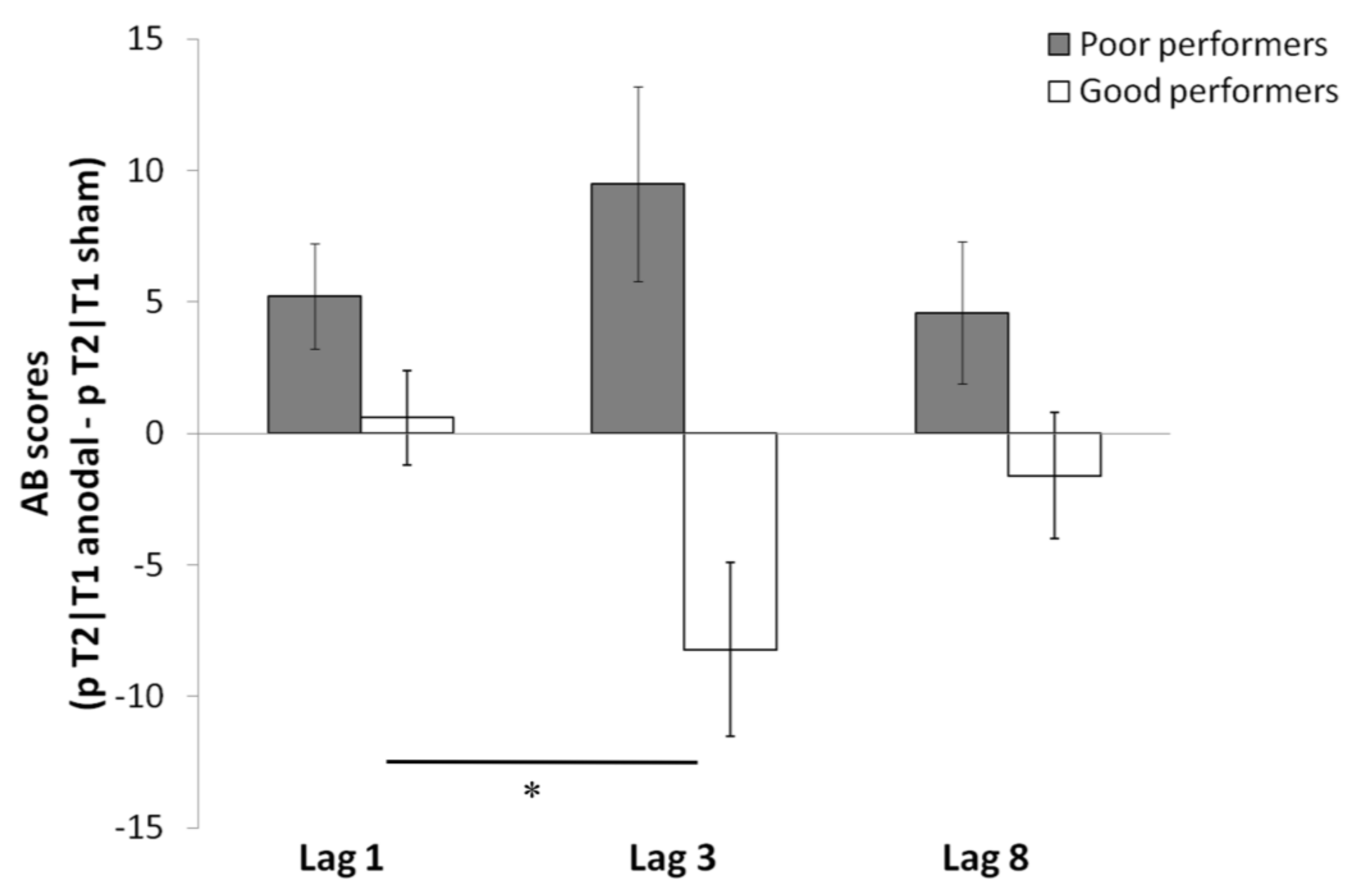
Individual Differences and tDCS Effects on AB and Sparing
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethic Statement
References
- Broadbent, D.E.; Broadbent, M.H.P. From detection to identification: Response to multiple targets in rapid serial visual presentation. Percept. Psychophys. 1987, 42, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond, J.E.; Shapiro, K.L.; Arnell, K.M. Temporary suppression of visual processing in an RSVP task: An attentional blink? J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.M.; Potter, M.C. A two-stage model for multiple target detection in rapid serial visual presentation. J. Exp. Psychol. Hum. Percept. Perform. 1995, 21, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Raymond, J.; Arnell, K. The attentional blink. Trends Cogn. Sci. 1997, 1, 291–296. [Google Scholar] [CrossRef]
- Lasaponara, S.; Dragone, A.; Lecce, F.; Di Russo, F.; Doricchi, F. The “serendipitous brain”: Low expectancy and timing uncertainty of conscious events improve awareness of unconscious ones (evidence from the attentional blink). Cortex 2015, 71, 15–33. [Google Scholar] [CrossRef]
- Jolicœur, P.; Dell’Acqua, R. The Demonstration of Short-Term Consolidation. Cogn. Psychol. 1998, 36, 138–202. [Google Scholar] [CrossRef] [Green Version]
- Di Lollo, V.; Kawahara, J.; Shahab Ghorashi, S.M.; Enns, J.T. The attentional blink: Resource depletion or temporary loss of control? Psychol. Res. 2005, 69, 191–200. [Google Scholar] [CrossRef]
- Olivers, C.N.L.; Meeter, M. A boost and bounce theory of temporal attention. Psychol. Rev. 2008, 115, 836–863. [Google Scholar] [CrossRef]
- Wyble, B.; Bowman, H.; Nieuwenstein, M. The attentional blink provides episodic distinctiveness: Sparing at a cost. J. Exp. Psychol. Hum. Percept. Perform. 2009, 35, 787–807. [Google Scholar] [CrossRef] [Green Version]
- Martens, S.; Wyble, B. The attentional blink: Past, present, and future of a blind spot in perceptual awareness. Neurosci. Biobehav. Rev. 2010, 34, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Fragopanagos, N.; Kockelkoren, S.; Taylor, J.G. A neurodynamic model of the attentional blink. Cogn. Brain Res. 2005, 24, 568–586. [Google Scholar] [CrossRef]
- Taatgen, N.A.; Juvina, I.; Schipper, M.; Borst, J.P.; Martens, S. Too much control can hurt: A threaded cognition model of the attentional blink. Cogn. Psychol. 2009, 59, 1–29. [Google Scholar] [CrossRef]
- Kihara, K.; Ikeda, T.; Matsuyoshi, D.; Hirose, N.; Mima, T.; Fukuyama, H.; Osaka, N. Differential Contributions of the Intraparietal Sulcus and the Inferior Parietal Lobe to Attentional Blink: Evidence from Transcranial Magnetic Stimulation. J. Cogn. Neurosci. 2011, 23, 247–256. [Google Scholar] [CrossRef]
- Mayr, U.; Diedrichsen, J.; Ivry, R.; Keele, S.W. Dissociating Task-set Selection from Task-set Inhibition in the Prefrontal Cortex. J. Cogn. Neurosci. 2006, 18, 14–21. [Google Scholar] [CrossRef]
- Banich, M.T. Executive Function. Curr. Dir. Psychol. Sci. 2009, 18, 89–94. [Google Scholar] [CrossRef]
- Feinstein, J.S.; Stein, M.B.; Castillo, G.N.; Paulus, M.P. From sensory processes to conscious perception. Conscious. Cogn. 2004, 13, 323–335. [Google Scholar] [CrossRef]
- Marois, R.; Yi, D.-J.; Chun, M.M. The Neural Fate of Consciously Perceived and Missed Events in the Attentional Blink. Neuron 2004, 41, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Giesbrecht, B.; Kingstone, A. Right hemisphere involvement in the attentional blink: Evidence from a split-brain patient. Brain Cogn. 2004, 55, 303–306. [Google Scholar] [CrossRef]
- Dell’Acqua, R.; Doro, M.; Dux, P.; Losier, T.; Jolicœur, P. Enhanced frontal activation underlies sparing from the attentional blink: Evidence from human electrophysiology. Psychophysiol. 2016, 53, 623–633. [Google Scholar] [CrossRef]
- Gross, J.; Schmitz, F.; Schnitzler, I.; Kessler, K.; Shapiro, K.; Hommel, B. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 13050–13055. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Jacobson, L.; Koslowsky, M.; Lavidor, M. tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Exp. Brain Res. 2012, 216, 1–10. [Google Scholar] [CrossRef] [PubMed]
- London, R.E.; Slagter, H. Effects of Transcranial Direct Current Stimulation over Left Dorsolateral pFC on the Attentional Blink Depend on Individual Baseline Performance. J. Cogn. Neurosci. 2015, 27, 2382–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- London, R.E.; Slagter, H.A. No Effect of Transcranial Direct Current Stimulation over Left Dorsolateral Prefrontal Cortex on Temporal Attention. J. Cogn. Neurosci. 2021, 33, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Sdoia, S.; Conversi, D.; Pecchinenda, A.; Ferlazzo, F. Access to consciousness of briefly presented visual events is modulated by transcranial direct current stimulation of left dorsolateral prefrontal cortex. Sci. Rep. 2019, 9, 10950. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.; Nitsche, M.; Bermpohl, F.; Antal, A.; Feredoes, E.; Marcolin, M.A.; Rigonatti, S.P.; Silva, M.T.; Paulus, W.; et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005, 166, 23–30. [Google Scholar] [CrossRef]
- Gladwin, T.E.; Uyl, T.D.; Fregni, F.F.; Wiers, R.W. Enhancement of selective attention by tDCS: Interaction with interference in a Sternberg task. Neurosci. Lett. 2012, 512, 33–37. [Google Scholar] [CrossRef]
- MacDonald, A. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef] [Green Version]
- Pecchinenda, A.; Ferlazzo, F.; Lavidor, M. Modulation of selective attention by polarity-specific tDCS effects. Neuropsychologia 2015, 68, 1–7. [Google Scholar] [CrossRef]
- Hommel, B.; Kessler, K.; Schmitz, F.; Gross, J.; Akyürek, E.; Shapiro, K.; Schnitzler, A. How the brain blinks: Towards a neurocognitive model of the attentional blink. Psychol. Res. 2006, 70, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Egner, T.; Monti, J.M.P.; Trittschuh, E.H.; Wieneke, C.A.; Hirsch, J.; Mesulam, M.-M. Neural Integration of Top-Down Spatial and Feature-Based Information in Visual Search. J. Neurosci. 2008, 28, 6141–6151. [Google Scholar] [CrossRef] [Green Version]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Rottschy, C.; Langner, R.; Dogan, I.; Reetz, K.; Laird, A.; Schulz, J.B.; Fox, P.; Eickhoff, S. Modelling neural correlates of working memory: A coordinate-based meta-analysis. NeuroImage 2012, 60, 830–846. [Google Scholar] [CrossRef] [Green Version]
- Rottschy, C.; Caspers, S.; Roski, C.; Reetz, K.; Dogan, I.; Schulz, J.B.; Zilles, K.; Laird, A.R.; Fox, P.; Eickhoff, S.B. Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Struct. Funct. 2012, 218, 1551–1567. [Google Scholar] [CrossRef] [Green Version]
- Dehaene, S.; Changeux, J.-P. Experimental and Theoretical Approaches to Conscious Processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef] [Green Version]
- Driver, J. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition 2001, 79, 39–88. [Google Scholar] [CrossRef]
- Rees, G. Neural correlates of consciousness. Ann. N. Y. Acad. Sci. 2013, 1296, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Patel, G.; Shulman, G.L. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef] [Green Version]
- Bettencourt, K.C.; Xu, Y. Decoding the content of visual short-term memory under distraction in occipital and parietal areas. Nat. Neurosci. 2016, 19, 150–157. [Google Scholar] [CrossRef]
- Li, S.; Cai, Y.; Liu, J.; Li, D.; Feng, Z.; Chen, C.; Xue, G. Dissociated roles of the parietal and frontal cortices in the scope and control of attention during visual working memory. NeuroImage 2017, 149, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.; Hsu, T.-Y.; Chang, C.-F.; Tzeng, O.J.; Hung, D.L.; Muggleton, N.G.; Walsh, V.; Liang, W.-K.; Cheng, S.-K.; Juan, C.-H. Unleashing Potential: Transcranial Direct Current Stimulation over the Right Posterior Parietal Cortex Improves Change Detection in Low-Performing Individuals. J. Neurosci. 2012, 32, 10554–10561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Itthipuripat, S.; Ku, Y. Electrical Stimulation Over Human Posterior Parietal Cortex Selectively Enhances the Capacity of Visual Short-Term Memory. J. Neurosci. 2018, 39, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arciniega, H.; Gözenman, F.; Jones, K.T.; Stephens, J.A.; Berryhill, M.E. Frontoparietal tDCS Benefits Visual Working Memory in Older Adults with Low Working Memory Capacity. Front. Aging Neurosci. 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Lo, O.; Van Donkelaar, P.; Chou, L. Effects of transcranial direct current stimulation over right posterior parietal cortex on attention function in healthy young adults. Eur. J. Neurosci. 2019, 49, 1623–1631. [Google Scholar] [CrossRef]
- Kihara, K.; Kondo, H.M.; Kawahara, J.I. Differential Contributions of GABA Concentration in Frontal and Parietal Regions to Individual Differences in Attentional Blink. J. Neurosci. 2016, 36, 8895–8901. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, E.; Lang, A.-J. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Jospe, K.; Flöel, A.; Lavidor, M. The interaction between embodiment and empathy in facial expression recognition. Soc. Cogn. Affect. Neurosci. 2018, 13, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Parkin, B.L.; Bhandari, M.; Glen, J.C.; Walsh, V. The physiological effects of transcranial electrical stimulation do not apply to parameters commonly used in studies of cognitive neuromodulation. Neuropsychologia 2019, 128, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Polanía, R.; Nitsche, M.A.; Paulus, W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum. Brain Mapp. 2011, 32, 1236–1249. [Google Scholar] [CrossRef]
- Notturno, F.; Marzetti, L.; Pizzella, V.; Uncini, A.; Zappasodi, F. Local and remote effects of transcranial direct current stimulation on the electrical activity of the motor cortical network. Hum. Brain Mapp. 2014, 35, 2220–2232. [Google Scholar] [CrossRef]
- Kunze, T.; Hunold, A.; Haueisen, J.; Jirsa, V.; Spiegler, A. Transcranial direct current stimulation changes resting state functional connectivity: A large-scale brain network modeling study. Neuroimage 2016, 140, 174–187. [Google Scholar] [CrossRef]
- Behrmann, M.; Geng, J.J.; Shomstein, S. Parietal cortex and attention. Curr. Opin. Neurobiol. 2004, 14, 212–217. [Google Scholar] [CrossRef]
- Friedman-Hill, S.R.; Robertson, L.C.; Desimone, R.; Ungerleider, L.G. Posterior parietal cortex and the filtering of distractors. Proc. Natl. Acad. Sci. USA 2003, 100, 4263–4268. [Google Scholar] [CrossRef] [Green Version]
- Menon, V.; Ford, J.M.; Lim, K.; Glover, G.H.; Pfefferbaum, A. Combined event-related fMRI and EEG evidence for temporal—Parietal cortex activation during target detection. NeuroReport 1997, 8, 3029–3037. [Google Scholar] [CrossRef] [Green Version]
- Wojciulik, E.; Kanwisher, N. The Generality of Parietal Involvement in Visual Attention. Neuron 1999, 23, 747–764. [Google Scholar] [CrossRef] [Green Version]
- Petrucci, M.; Pecchinenda, A. Sparing and impairing: Emotion modulation of the attentional blink and the spread of sparing in a 3-target RSVP task. Atten. Percept. Psychophys. 2017, 80, 439–452. [Google Scholar] [CrossRef]
- Pecchinenda, A.; Monachesi, B.; Laeng, B. Fearful expressions of rapidly presented hybrid-faces modulate the lag 1 sparing in the attentional blink. Acta Psychol. 2020, 209, 103124. [Google Scholar] [CrossRef]
- Hsu, T.Y.; Juan, C.H.; Tseng, P. Individual Differences and State-Dependent Responses in Transcranial Direct Current Stimulation. Front. Hum. Neurosci. 2016, 10, 643. [Google Scholar] [CrossRef]
- Rosen, D.S.; Erickson, B.; Kim, Y.E.; Mirman, D.; Hamilton, R.H.; Kounios, J. Anodal tDCS to Right Dorsolateral Prefrontal Cortex Facilitates Performance for Novice Jazz Improvisers but Hinders Experts. Front. Hum. Neurosci. 2016, 10, 579. [Google Scholar] [CrossRef] [Green Version]
- Filmer, H.L.; Ehrhardt, S.E.; Bollmann, S.; Mattingley, J.B.; Dux, P. Accounting for individual differences in the response to tDCS with baseline levels of neurochemical excitability. Cortex 2019, 115, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Benwell, C.S.; Learmonth, G.; Miniussi, C.; Harvey, M.; Thut, G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: Evidence from biparietal tDCS influence on lateralized attention bias. Cortex 2015, 69, 152–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, B.; Kadosh, R.C. Not all brains are created equal: The relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed]
| Group: F4 | Group: P4 | Overall | |
|---|---|---|---|
| Sham tDCS | 84.76 (2.09) | 85.09 (2.09) | 84.93 (1.48) |
| Active tDCS | 85.60 (1.41) | 87.56 (1.41) | 86.58 (1.00) |
| Overall | 85.18 (1.63) | 86.33 (1.63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecchinenda, A.; De Luca, F.; Monachesi, B.; Petrucci, M.; Pazzaglia, M.; Doricchi, F.; Lavidor, M. Contributions of the Right Prefrontal and Parietal Cortices to the Attentional Blink: A tDCS Study. Symmetry 2021, 13, 1208. https://doi.org/10.3390/sym13071208
Pecchinenda A, De Luca F, Monachesi B, Petrucci M, Pazzaglia M, Doricchi F, Lavidor M. Contributions of the Right Prefrontal and Parietal Cortices to the Attentional Blink: A tDCS Study. Symmetry. 2021; 13(7):1208. https://doi.org/10.3390/sym13071208
Chicago/Turabian StylePecchinenda, Anna, Francesca De Luca, Bianca Monachesi, Manuel Petrucci, Mariella Pazzaglia, Fabrizio Doricchi, and Michal Lavidor. 2021. "Contributions of the Right Prefrontal and Parietal Cortices to the Attentional Blink: A tDCS Study" Symmetry 13, no. 7: 1208. https://doi.org/10.3390/sym13071208
APA StylePecchinenda, A., De Luca, F., Monachesi, B., Petrucci, M., Pazzaglia, M., Doricchi, F., & Lavidor, M. (2021). Contributions of the Right Prefrontal and Parietal Cortices to the Attentional Blink: A tDCS Study. Symmetry, 13(7), 1208. https://doi.org/10.3390/sym13071208








