Encapsulation of Rhodamine 6G Dye Molecules for Affecting Symmetry of Supramolecular Crystals of Melamine-Barbiturate
Abstract
:1. Introduction
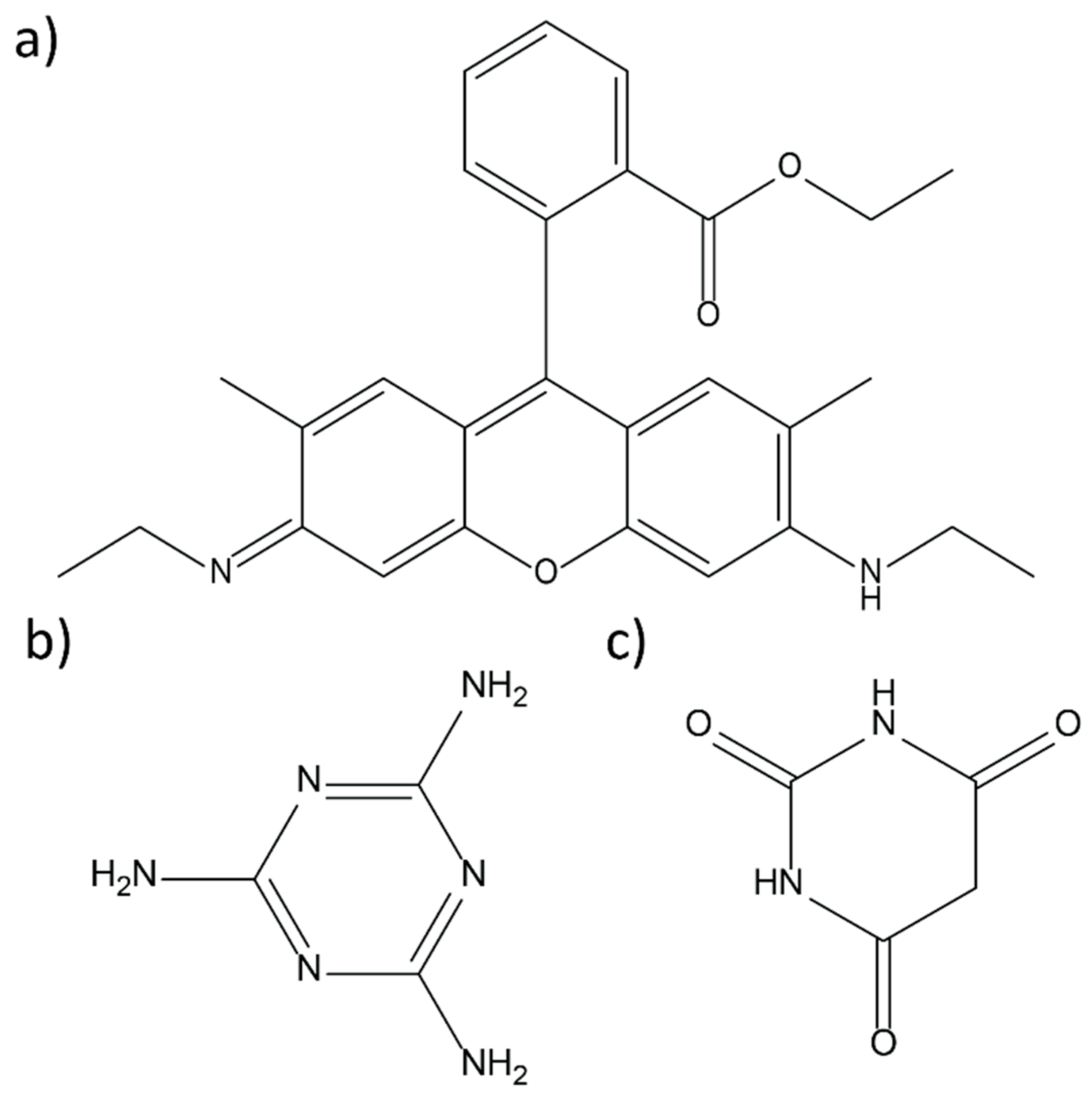
2. Materials and Methods
3. Results and Discussion
3.1. UV-Vis Spectroscopy of Rh6G Containing M-BA
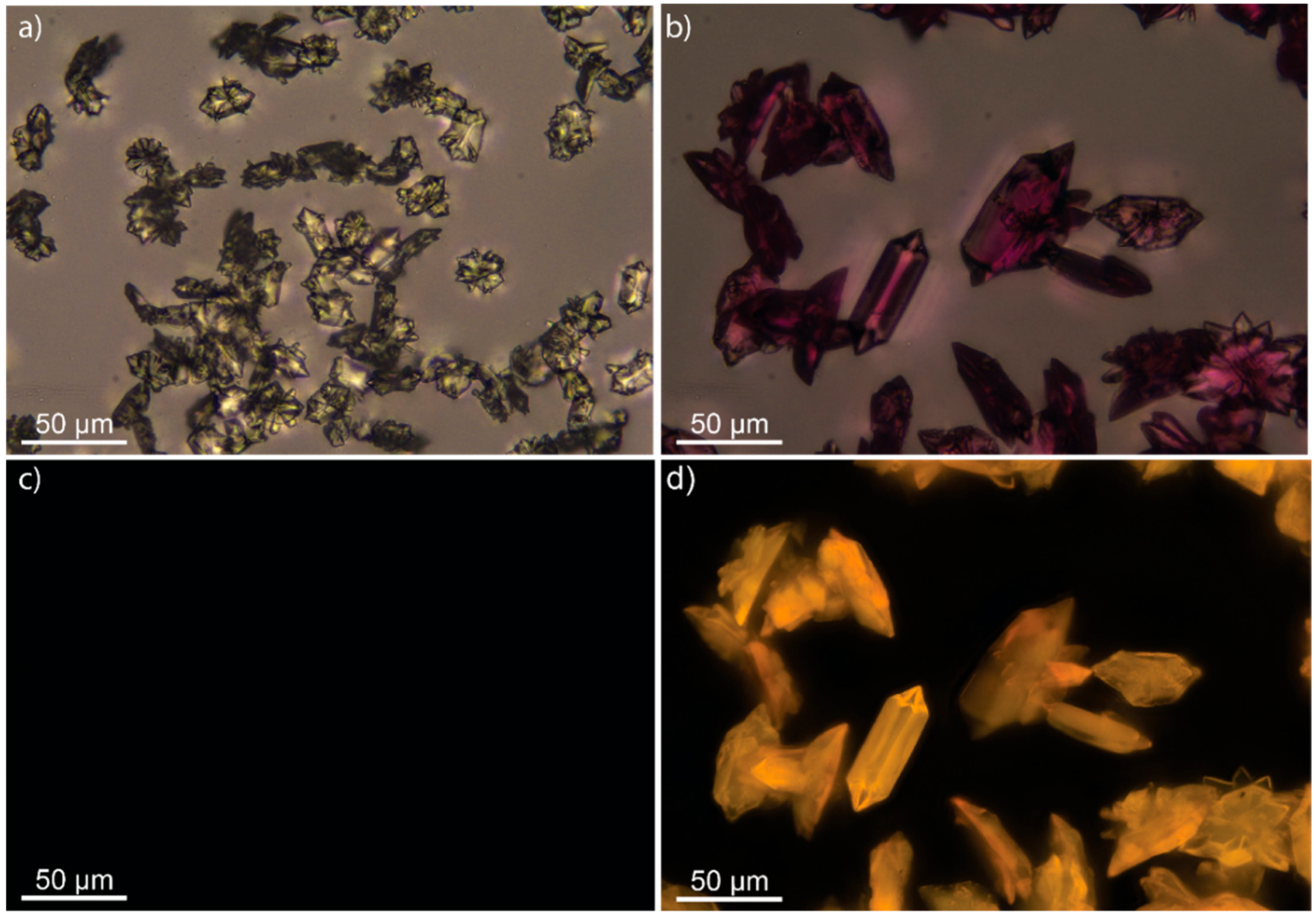
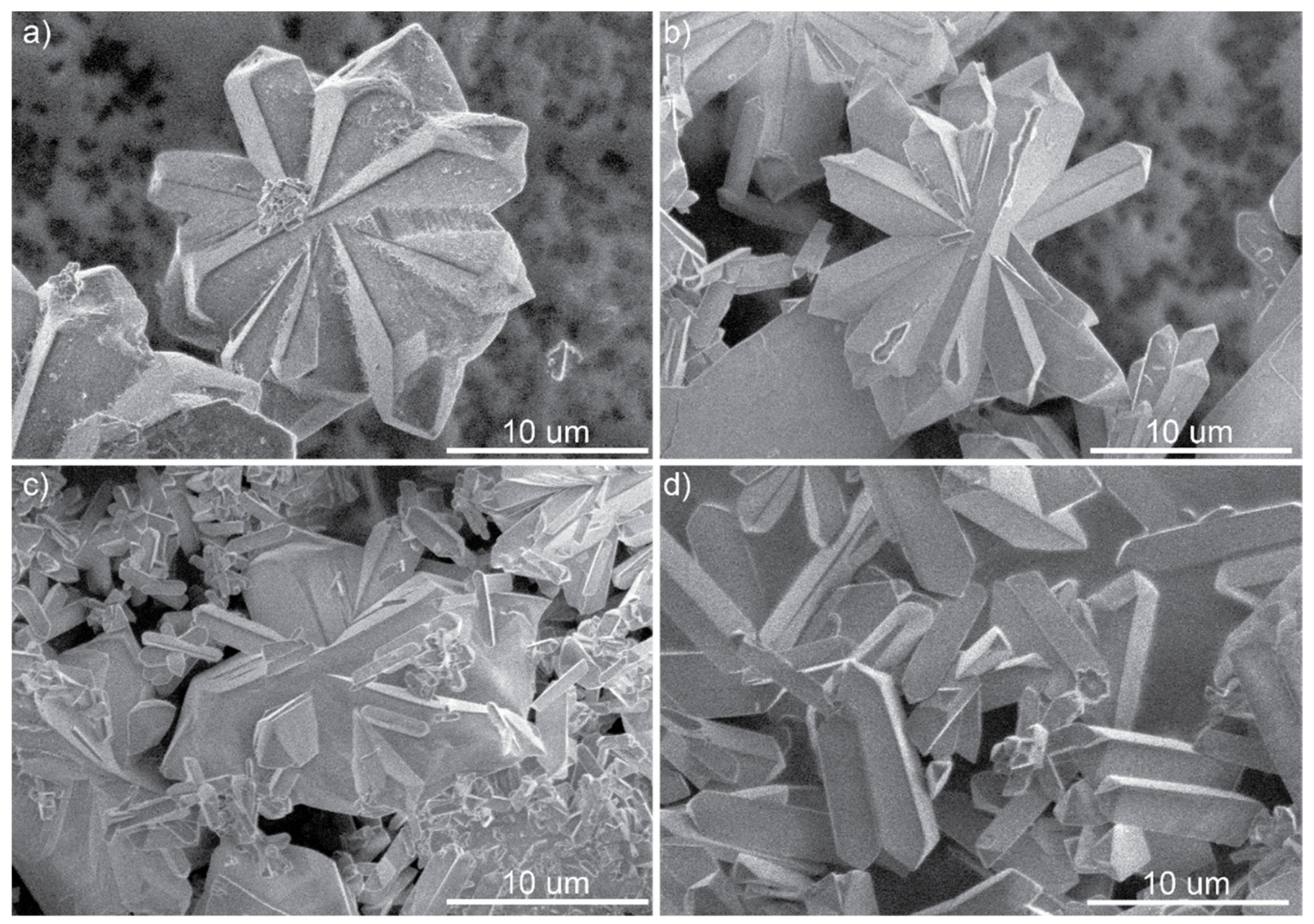
3.2. Scanning Electron Microscopy and Fluorescence Microscope Images of M-BA Crystals with Rh6G
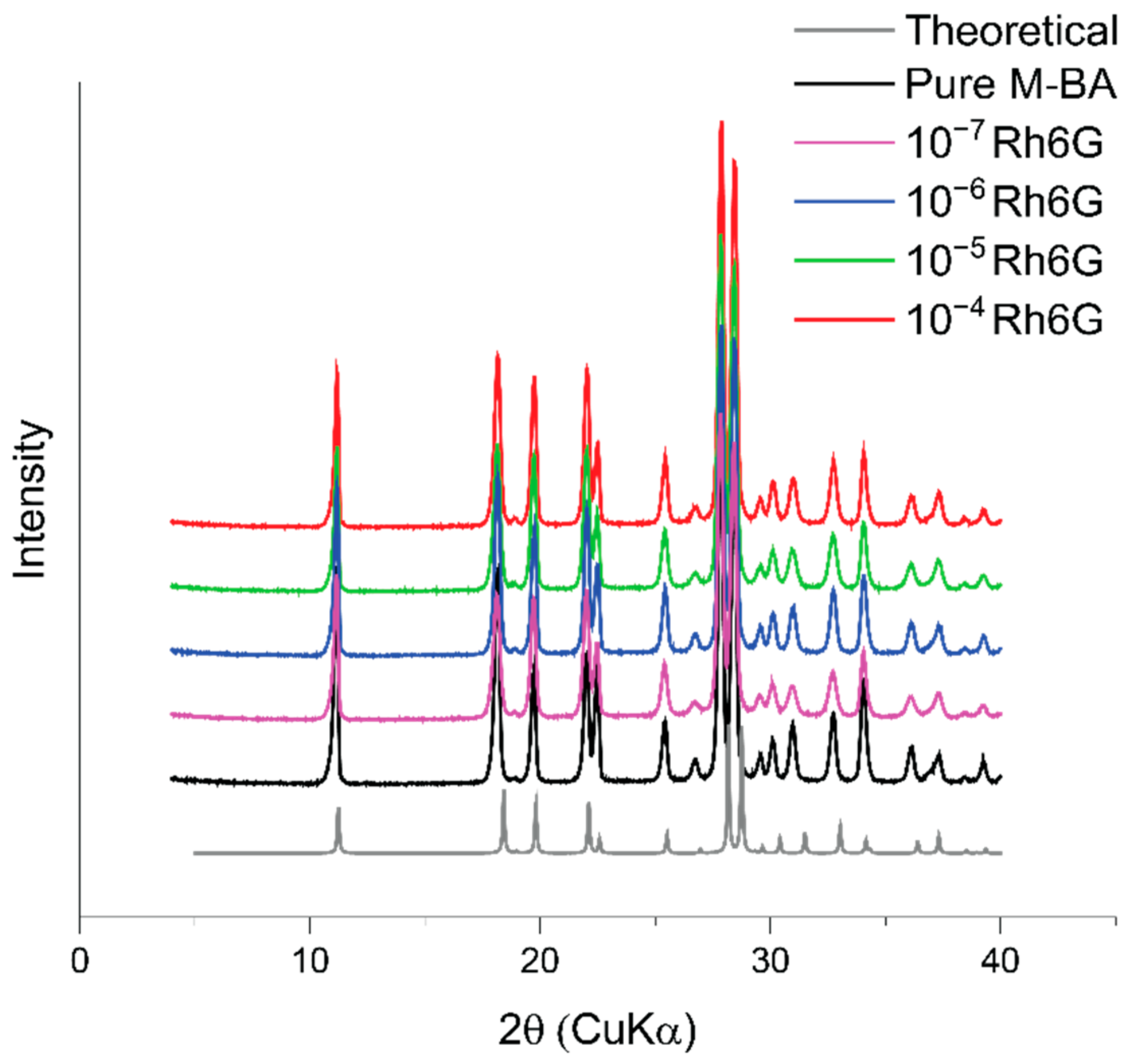
3.3. Powder X-ray Diffraction
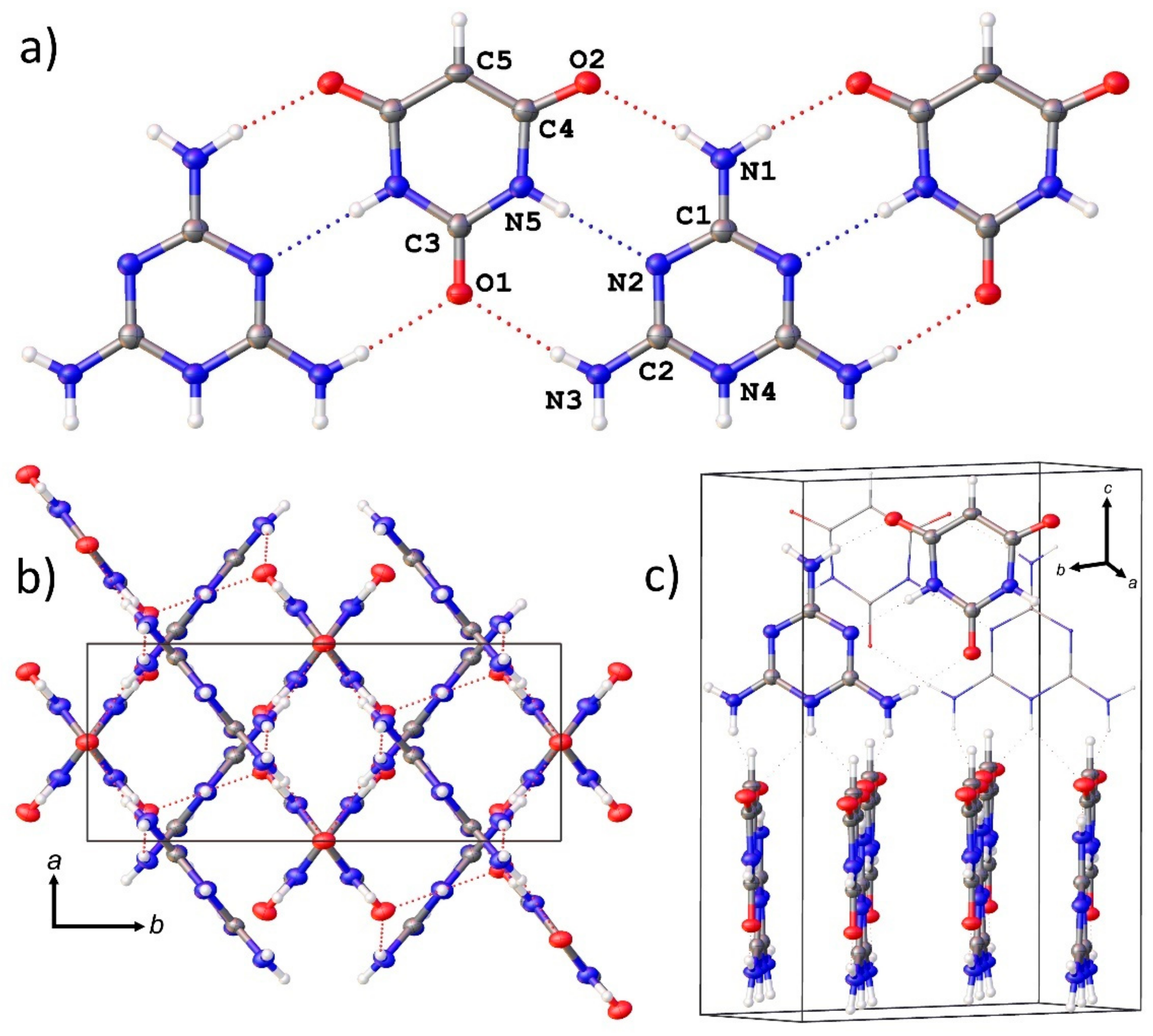
3.4. Single Crystal X-ray Diffraction
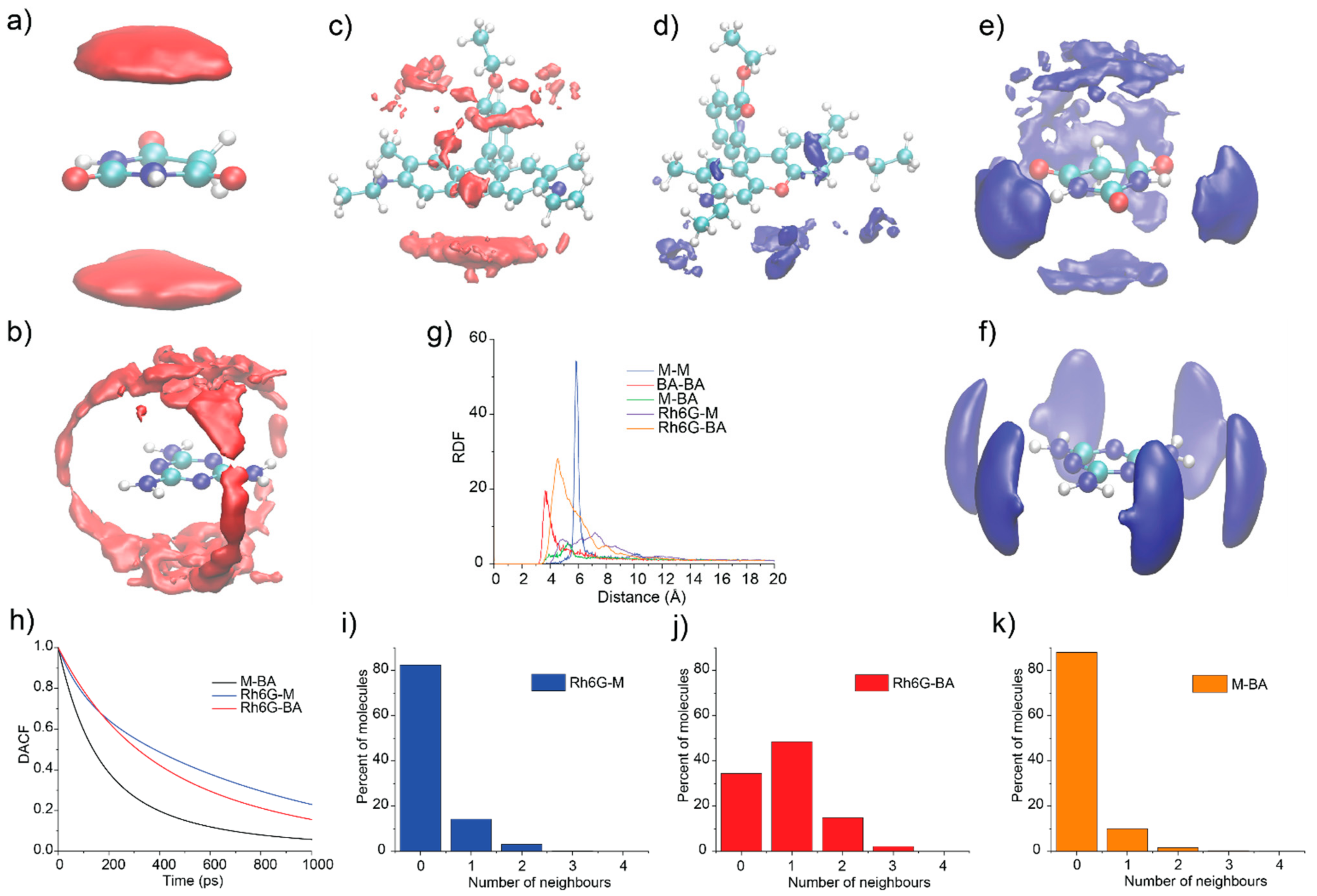
3.5. Quantum Chemistry Calculations
3.6. Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos, J.C.; Pereira, L.C.J.; Waerenborgh, J.C.; Gonçalves, M.C. Encapsulation of Active Molecules in Pharmaceutical Sector: The Role of Ceramic Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128193631. [Google Scholar]
- Ye, C.; Chi, H. A review of recent progress in drug and protein encapsulation: Approaches, applications and challenges. Mater. Sci. Eng. C 2018, 83, 233–246. [Google Scholar] [CrossRef]
- Jiwpanich, S.; Ryu, J.H.; Bickerton, S.; Thayumanavan, S. Noncovalent encapsulation stabilities in supramolecular nanoassemblies. J. Am. Chem. Soc. 2010, 132, 10683–10685. [Google Scholar] [CrossRef] [Green Version]
- Arunkumar, E.; Forbes, C.C.; Smith, B.D. Improving the properties of organic dyes by molecular encapsulation. Eur. J. Org. Chem. 2005, 4051–4059. [Google Scholar] [CrossRef]
- Liu, C.; Gao, C.; Yan, D. Synergistic supramolecular encapsulation of amphiphilic hyperbranched polymer to dyes. Macromolecules 2006, 39, 8102–8111. [Google Scholar] [CrossRef]
- Müller, M.; Zentel, R.; Maka, T.; Romanov, S.G.; Sotomayor Torres, C.M. Dye-containing polymer beads as photonic crystals. Chem. Mater. 2000, 12, 2508–2512. [Google Scholar] [CrossRef]
- Furumi, S. Self-assembled organic and polymer photonic crystals for laser applications. Polym. J. 2013, 45, 579–593. [Google Scholar] [CrossRef]
- Sola-Llano, R.; Gartzia-Rivero, L.; Oliden-Sanchez, A.; Bañuelos, J.; Arbeloa, I.L.; Martínez-Martínez, V. Dye encapsulation into one-dimensional zeolitic materials for optical applications. Chem. Silica Zeolite Based Mater 2019, 229–248. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Etaiw, S.E.D.H. Supramolecular host-guest systems as frameworks for excitation energy transfer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 373–378. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Jockusch, S.; Porel, M. Supramolecular photochemistry in solution and on surfaces: Encapsulation and dynamics of guest molecules and communication between encapsulated and free molecules. Langmuir 2015, 31, 5554–5570. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.K.; Sobolev, Y.I.; Zhang, W.; Zhuang, Q.; Grzybowski, B.A. Enhancing crystal growth using polyelectrolyte solutions and shear flow. Nature 2020, 579, 73–79. [Google Scholar] [CrossRef]
- Björk, J.; Hanke, F.; Palma, C.A.; Samori, P.; Cecchini, M.; Persson, M. Adsorption of aromatic and anti-aromatic systems on graphene through π-π Stacking. J. Phys. Chem. Lett. 2010, 1, 3407–3412. [Google Scholar] [CrossRef]
- Dobberschütz, S.; Nielsen, M.R.; Sand, K.K.; Civioc, R.; Bovet, N.; Stipp, S.L.S.; Andersson, M.P. The mechanisms of crystal growth inhibition by organic and inorganic inhibitors. Nat. Commun. 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shilovskikh, V.V.; Timralieva, A.A.; Nesterov, P.V.; Novikov, A.S.; Sitnikov, P.A.; Konstantinova, E.A.; Kokorin, A.I.; Skorb, E.V. Melamine–barbiturate supramolecular assembly as a pH-dependent organic radical trap material. Chem. A Eur. J. 2020, 26, 16603–16610. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, N.; Kondratyuk, N.; Logunov, M.; Timralieva, A.; Shilovskikh, V.; Skorb, E.V. Insights into the early stages of melamine cyanurate nucleation from aqueous solution. Cryst. Growth Des. 2021, 21, 1984–1992. [Google Scholar] [CrossRef]
- Shilovskikh, V.V.; Timralieva, A.A.; Belogub, E.V.; Konstantinova, E.A.; Kokorin, A.I.; Skorb, E.V. Radical activity of binary melamine-based hydrogen-bonded self-assemblies. Appl. Magn. Reson. 2020, 51, 939–949. [Google Scholar] [CrossRef]
- Mane, S.R.; Sathyan, A.; Shunmugam, R. Barbiturate derived amphiphilic homopolymers: Synthesis, characterization, self-assembly and anticancer drug delivery. Ther. Deliv. 2019, 10, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Bairi, P.; Nandi, A.K. Supramolecular assembly of melamine and its derivatives: Nanostructures to functional materials. RSC Adv. 2014, 4, 1708–1734. [Google Scholar] [CrossRef]
- Rigaku Corporation. CrysAlisPro Software System, Version 1.171.40.50a; Rigaku Oxford Diffraction: Oxford, UK, 2019. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree-Fock and hybrid DFT calculations. A “chain-of-spheres” algorithm for the Hartree-Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Neese, F. An Improvement of the resolution of the identity approximation for the formation of the coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Potential energy functions for atomic-level simulations of water and organic and biomolecular systems. Proc. Natl. Acad. Sci. USA 2005, 102, 6665–6670. [Google Scholar] [CrossRef] [Green Version]
- Dodda, L.S.; Vilseck, J.Z.; Tirado-Rives, J.; Jorgensen, W.L. 1.14∗CM1A-LBCC: Localized bond-charge corrected CM1A charges for condensed-phase simulations. J. Phys. Chem. B 2017, 121, 3864–3870. [Google Scholar] [CrossRef] [Green Version]
- Dodda, L.S.; De Vaca, I.C.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Brehm, M.; Kirchner, B. TRAVIS-A free analyzer and visualizer for monte carlo and molecular dynamics trajectories. J. Chem. Inf. Model. 2011, 51, 2007–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerkowski, J.A.; Seto, C.T.; Wierda, D.A.; Whitesides, G.M. The design of organic structures in the solid state: Hydrogen-bonded molecular “tapes”. J. Am. Chem. Soc. 1990, 112, 9025–9026. [Google Scholar] [CrossRef]
- Zerkowski, J.A.; MacDonald, J.C.; Whitesides, G.M. Investigations into the robustness of secondary and tertiary architecture of hydrogen-bonded crystalline tapes. Chem. Mater. 1994, 6, 1250–1257. [Google Scholar] [CrossRef]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Chukanov, N.V.; Panikorovskii, T.L. Thermal behavior and phase transition of uric acid and its dihydrate form, the common biominerals uricite and tinnunculite. Minerals 2019, 9, 373. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterov, P.V.; Shilovskikh, V.V.; Sokolov, A.D.; Gurzhiy, V.V.; Novikov, A.S.; Timralieva, A.A.; Belogub, E.V.; Kondratyuk, N.D.; Orekhov, N.D.; Skorb, E.V. Encapsulation of Rhodamine 6G Dye Molecules for Affecting Symmetry of Supramolecular Crystals of Melamine-Barbiturate. Symmetry 2021, 13, 1119. https://doi.org/10.3390/sym13071119
Nesterov PV, Shilovskikh VV, Sokolov AD, Gurzhiy VV, Novikov AS, Timralieva AA, Belogub EV, Kondratyuk ND, Orekhov ND, Skorb EV. Encapsulation of Rhodamine 6G Dye Molecules for Affecting Symmetry of Supramolecular Crystals of Melamine-Barbiturate. Symmetry. 2021; 13(7):1119. https://doi.org/10.3390/sym13071119
Chicago/Turabian StyleNesterov, Pavel V., Vladimir V. Shilovskikh, Alexander D. Sokolov, Vladislav V. Gurzhiy, Alexander S. Novikov, Alexandra A. Timralieva, Elena V. Belogub, Nikolay D. Kondratyuk, Nikita D. Orekhov, and Ekaterina V. Skorb. 2021. "Encapsulation of Rhodamine 6G Dye Molecules for Affecting Symmetry of Supramolecular Crystals of Melamine-Barbiturate" Symmetry 13, no. 7: 1119. https://doi.org/10.3390/sym13071119
APA StyleNesterov, P. V., Shilovskikh, V. V., Sokolov, A. D., Gurzhiy, V. V., Novikov, A. S., Timralieva, A. A., Belogub, E. V., Kondratyuk, N. D., Orekhov, N. D., & Skorb, E. V. (2021). Encapsulation of Rhodamine 6G Dye Molecules for Affecting Symmetry of Supramolecular Crystals of Melamine-Barbiturate. Symmetry, 13(7), 1119. https://doi.org/10.3390/sym13071119








