Does Double Biofeedback Affect Functional Hemispheric Asymmetry and Activity? A Pilot Study
Abstract
1. Introduction
1.1. Hemispheric Asymmetry
1.2. Biofeedback
1.3. Double Biofeedback
1.4. Biofeedback and Hemispheric Asymmetry
2. Materials and Methods
2.1. Participants
2.2. Computer Laterometry
- Δt min L (µs)—lead-lag delay when the virtual auditory space stimulus started shifting from the vertex with left ear advance.
- Δt min R (µs)—lead-lag delay when the virtual auditory space stimulus started shifting from the vertex with right ear advance.
- Δt max L (µs)—lead-lag delay at extreme lateralization with left-ear advance.
- Δt max R (µs)—lead-lag delay at extreme lateralization with right-ear advance.
- Δt rash L (µs)—lead-lag delay at the ‘echo’-effect with left-ear advance.
- Δt rash L (µs)—lead-lag delay at the ‘echo’-effect with right-ear advance.
- Functional hemispheric asymmetry, as reflected in K min, K max, K echo.
- Functional hemispheric activity, as reflected in Δt min, Δt max, Δt echo and interpreted as lability, excitability, and stability.
2.3. Double Biofeedback
2.4. Study Design
- The participant was asked to wear headphones and LED glasses. The headphones were used for laterometry as well as to play double biofeedback sound stimuli.
- The evaluation of functional hemispheric asymmetry and activity with the help of laterometry.
- Double biofeedback
- (1)
- The recording of narrow-frequency components within the pre-defined EEG range (4–20 Hz with 0.1 Hz frequency increase every three seconds) that are dominant for a particular participant. Total recording duration was equal to 480 s.
- (2)
- General double biofeedback mode where the current amplitude of the respective EEG oscillator from the pre-defined range (4–20 Hz) is transformed into feedback sound signals and the current amplitude of the respective alpha EEG oscillator is used to modulate the intensity of sinusoidal light signals that are generated at the frequency rate of this oscillator. Total recoding duration was equal to 300 s.
- The evaluation of the functional hemispheric asymmetry and activity with the help of laterometry.
2.5. Data Analysis
3. Results and Discussion
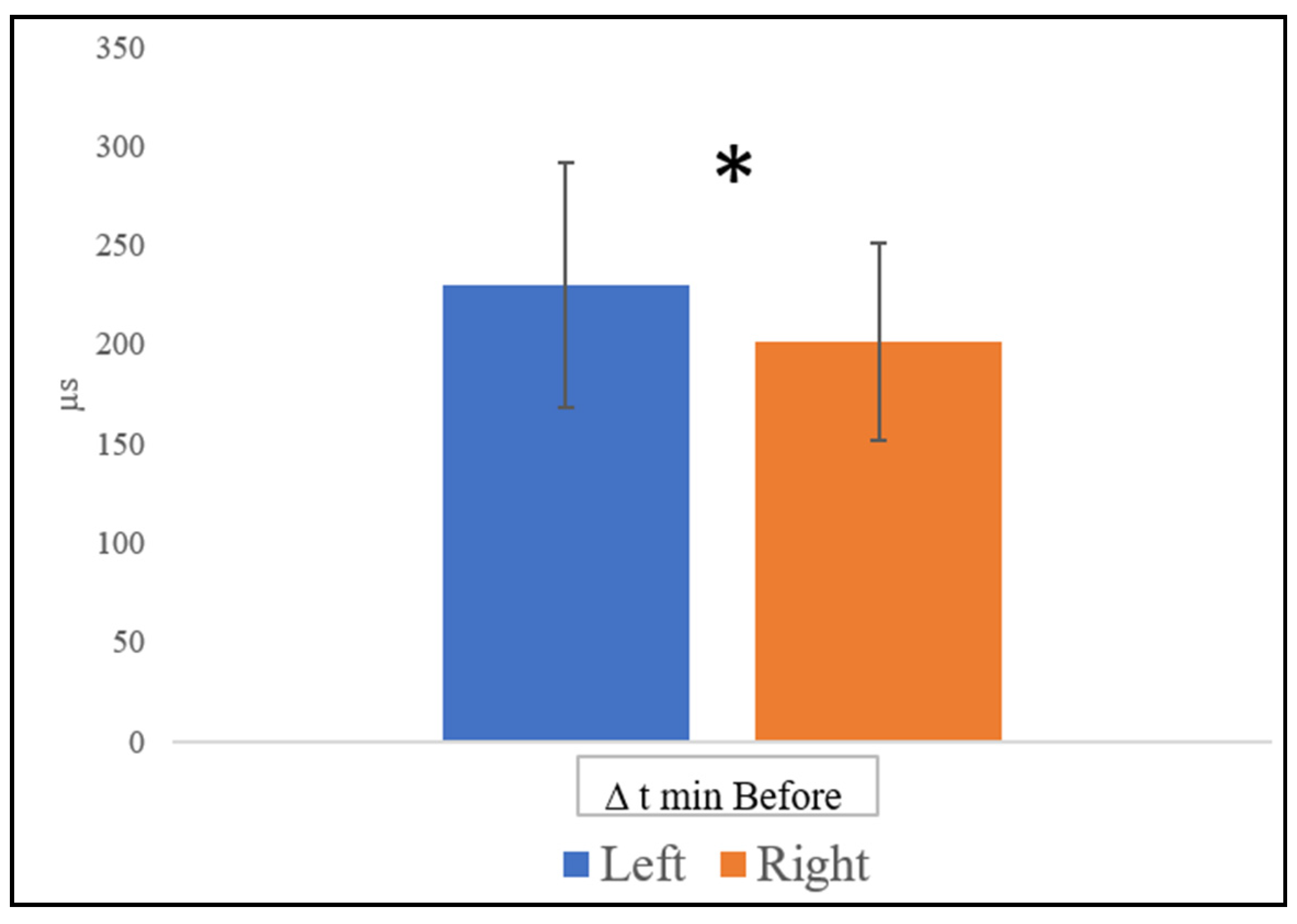
3.1. Functional Hemispheric Asymmetry Dynamics
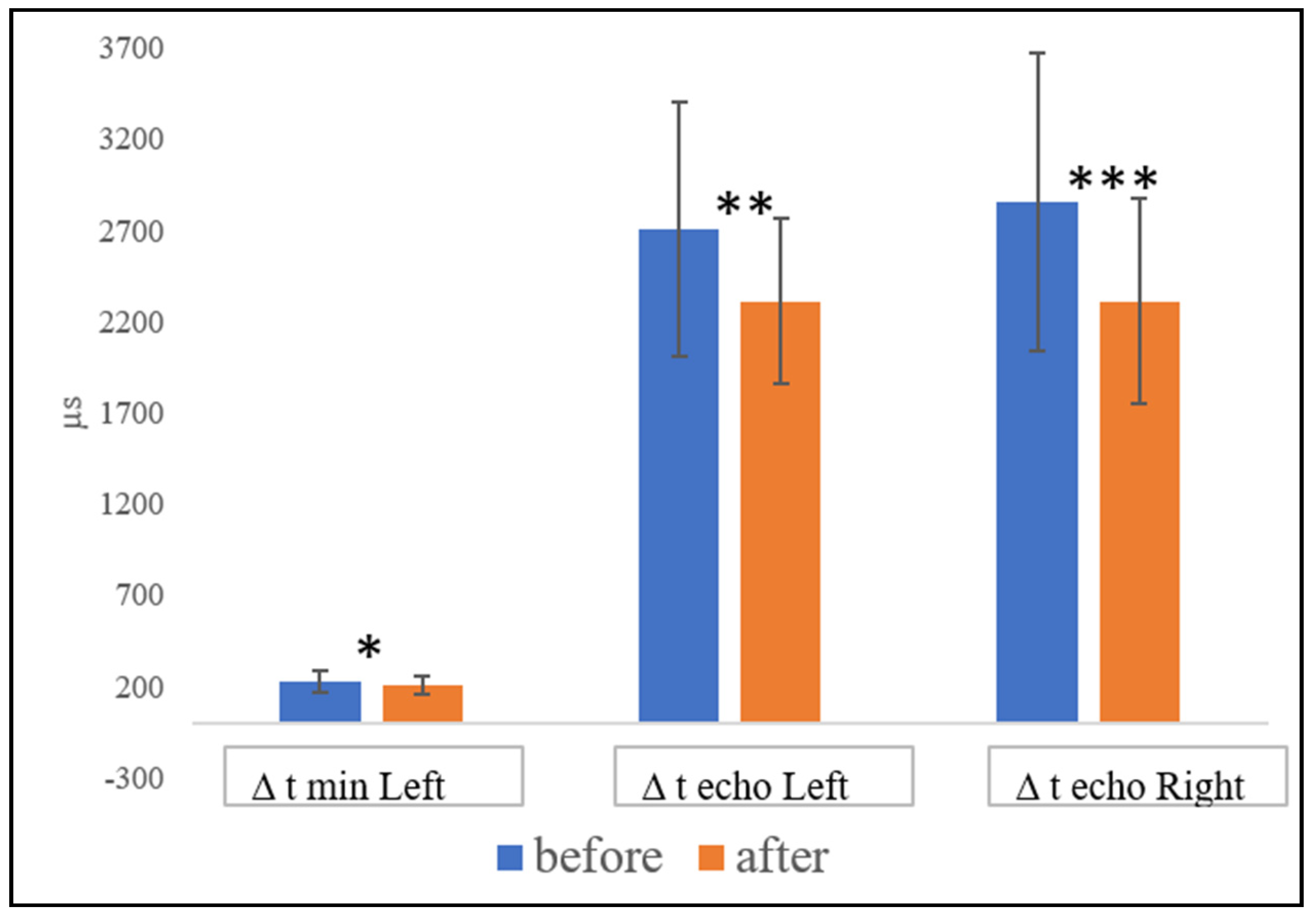
3.2. Functional Hemispheric Activity Dynamics
3.3. Gender Effects in Functional Hemispheric Asymmetry and Activity
4. Conclusions
- Double biofeedback did not alter functional brain asymmetry on any of its characteristics—lability, excitability, or stability.
- An increase in the right hemisphere lability and a decrease in the stability of both hemispheres were evidenced after double biofeedback training.
- Further research on double biofeedback is needed with different control groups, varying experimental conditions in treatment groups, as well as controlling for gender, age, and time of testing.
- In the future, we plan to apply the results of the study on the effect of double biofeedback to determine optimal brain states for foreign language acquisition. Since functional hemispheric activity is related to the success of Russian-speaking students in mastering English [76], and since the pilot study revealed variability in this activity before and after double biofeedback, going forward, we will be able to select such double biofeedback protocols, which will be optimal for language acquisition.
- Computer laterometry itself is a useful and convenient tool for studying functional hemispheric asymmetry and activity. It seems important to conduct a separate study on brain activity during computer laterometry. It can be achieved with modern quantitative EEG.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matitaishvili, T.; Domianidze, T.; Burdjanadze, G.; Nadareishvili, D.; Khananashvili, M. Informational stress as a depression inducing factor (Experimental study). Georgian Med. News 2017, 262, 106–111. [Google Scholar]
- Misra, S.; Roberts, P.; Rhodes, M. Information overload, stress, and emergency managerial thinking. Int. J. Disaster Risk Reduct. 2020, 51, 101762. [Google Scholar] [CrossRef]
- Floegel, M.; Kell, C.A. Functional hemispheric asymmetries during the planning and manual control of virtual avatar movements. PLoS ONE 2017, 12, e0185152. [Google Scholar] [CrossRef]
- Fokin, V.F.; Ponomareva, N.V.; Gorodenskij, N.G.; Ivashchenko, E.I.; Razygraev, I.I. Functional interhemispheric asymmetry and asymmetry of interhemispheric relations. Sist. Podhod Fiziol. 2004, 12, 111–127. (In Russian) [Google Scholar]
- Watson, C.E.; Gotts, S.J.; Martin, A.; Buxbaum, L.J. Bilateral functional connectivity at rest predicts apraxic symptoms after left hemisphere stroke. NeuroImage Clin. 2018, 21, 101526. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lim, J.; Kwok, K.; Bezerianos, A. Functional cortical connectivity analysis of mental fatigue unmasks hemispheric asymmetry and changes in small-world networks. Brain Cogn. 2014, 85, 220–230. [Google Scholar] [CrossRef]
- Stanković, M.; Nešić, M. Functional brain asymmetry for emotions: Psychological stress-induced reversed hemispheric asymmetry in emotional face perception. Exp. Brain Res. 2020, 238, 2641–2651. [Google Scholar] [CrossRef]
- Cao, R.; Shi, H.; Wang, X.; Huo, S.; Hao, Y.; Wang, B.; Guo, H.; Xiang, J. Hemispheric Asymmetry of Functional Brain Networks under Different Emotions Using EEG Data. Entropy 2020, 22, 939. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Güntürkün, O. Hemispheric asymmetries: The comparative view. Front. Psychol. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Fink, G.R. Spatial cognition: Where we were and where we are. Neuroimage 2001, 14, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Olulade, O.A.; Seydell-Greenwald, A.; Chambers, C.E.; Turkeltaub, P.E.; Dromerick, A.W.; Berl, M.M.; Gaillard, W.D.; Newport, E.L. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. USA 2020, 117, 23477–23483. [Google Scholar] [CrossRef]
- O’Regan, L.; Serrien, D.J. Individual Differences and Hemispheric Asymmetries for Language and Spatial Attention. Front. Hum. Neurosci. 2018, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Development and function of lateralization in the avian brain. Brain Res. Bull. 2008, 76, 235–244. [Google Scholar] [CrossRef]
- Facchin, L.; Duboué, E.R.; Halpern, M.E. Disruption of Epithalamic Left-Right Asymmetry Increases Anxiety in Zebrafish. J. Neurosci. 2015, 35, 15847–15859. [Google Scholar] [CrossRef]
- Goto, K.; Kurashima, R.; Gokan, H.; Inoue, N.; Itō, I.; Watanabe, S. Left−Right Asymmetry Defect in the Hippocampal Circuitry Impairs Spatial Learning and Working Memory in iv Mice. PLoS ONE 2010, 5, e15468. [Google Scholar] [CrossRef]
- Leonova, A.B. The concept of human functional state in Russian applied Psychology. Psychol. Russ. State Art 2009, 2, 517–538. [Google Scholar] [CrossRef]
- Burdakov, D.S. Self-regulation of individuals with different types of functional brain asymmetry and mental strain. Exp. Psychol. 2010, 3, 123–134. [Google Scholar]
- Rotenberg, V.S. The peculiarity of the right-hemisphere function in depression: Solving the paradoxes. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2004, 28, 1–13. [Google Scholar] [CrossRef]
- Adolph, D.; Margraf, J. The differential relationship between trait anxiety, depression, and resting frontal α-asymmetry. J. Neural Transm. 2016, 124, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Suckling, J.; Feng, L. Asymmetry of Hemispheric Network Topology Reveals Dissociable Processes between Functional and Structural Brain Connectome in Community-Living Elders. Front. Aging Neurosci. 2017, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.A. Categories of manual asymmetry and their variation with advancing age. Cortex J. Devoted Study Nerv. Syst. Behav. 2008, 44, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Reiss, M.; Reiss, G. ZurUntersuchung der motorischen Asymmetrien [Motor assymetry]. Fortschr. Neurol. Psychiatr. 2000, 68, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Schrammen, E. Response inhibition to emotional faces is modulated by functional hemispheric asymmetries linked to handedness. Brain Cogn. 2020, 145, 105629. [Google Scholar] [CrossRef]
- Paszkiel, S.; Szpulak, P. Methods of Acquisition, Archiving and Biomedical Data Analysis of Brain Functioning. In Biomedical Engineering and Neuroscience. BCI 2018. Advances in Intelligent Systems and Computing; Hunek, W., Paszkiel, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 720. [Google Scholar] [CrossRef]
- Westerhausen, R. A primer on dichotic listening as a paradigm for the assessment of hemispheric asymmetry. Laterality 2019, 24, 740–771. [Google Scholar] [CrossRef] [PubMed]
- Antonets, V.A.; Polevaya, S.A.; Kazakov, V.V. Handtracking: The study of human primary cognitive functions by their motor manifestations. In Modern Experimental Psychology; Barabanshchikova, V.A., Ed.; Izd-vo IP RAN: Moscow, Russian, 2011; Volume 2, pp. 39–54. [Google Scholar]
- Tollin, D.J.; Yin, T.C. Psychophysical investigation of an auditory spatial illusion in cats: The precedence effect. J. Neurophysiol. 2003, 90, 2149–2162. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.D.; Stecker, G.C.; Tollin, D.J. The Precedence Effect in Sound Localization. J. Assoc. Res. Otolaryngol. 2015, 16, 1–28. [Google Scholar] [CrossRef]
- Shcherbakov, V.I.; Kosyuga, Y.I. On the functional significance of uncrossed auditory pathways. Uspekhi Fiziol. Nauk 1994, 1, 98. (in Russian). [Google Scholar]
- Parenko, M.K. Psihofiziologicheskie Mekhanizmy Formirovaniya Prostranstvennogo Obraza Zvuka i Subektivnogo Zvukovogo Polya. Ph.D. Thesis, Lobachevsky State University of Nizhny Novgorod, Nizhny Novgorod, Russia, 2009. (In Russian). [Google Scholar]
- Paszkiel, S. Characteristics of Question of Blind Source Separation Using Moore-Penrose Pseudoinversion for Reconstruction of EEG Signal. In Automation 2017. ICA 2017. Advances in Intelligent Systems and Computing; Szewczyk, R., Zieliński, C., Kaliczyńska, M., Eds.; Springer: Cham, Switzerland, 2017; Volume 550. [Google Scholar] [CrossRef]
- Balconi, M.; Crivelli, D.; Fronda, G.; Venturella, I. Neuro-Rehabilitation and Neuro-Empowerment by Wearable Devices. Applications to Well-Being and Stress Management. In Converging Clinical and Engineering Research on Neurorehabilitation III; Masia, L., Micera, S., Akay, M., Pons, J., Eds.; Springer International Publishing: Cambridge, UK, 2018; pp. 963–966. [Google Scholar] [CrossRef]
- McAllister, B.T.; Hitchcock, E.R. Investigating the use of traditional and spectral biofeedback approaches to intervention for /r/ misarticulation. Am. J. Psychol. 2012, 21, 207–221. [Google Scholar] [CrossRef]
- Adler-Bock, M.; Bernhardt, B.M.; Gick, B.; Bacsfalvi, P. The Use of Ultrasound in Remediation of North American English /r/ in 2 Adolescents. Am. J. Speech Lang. Pathol. 2007, 16, 128–139. [Google Scholar] [CrossRef]
- McAllister, B.T. Efficacy of visual–acoustic biofeedback intervention for residual rhotic errors: A single-subject randomization study. J. Speech Lang. Hear. Res. 2017, 60, 1175–1193. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.L.; Leaman, M. Ultrasound visual feedback for acquired apraxia of speech: A case report. Aphasiology 2014, 28, 278–295. [Google Scholar] [CrossRef]
- Kartushina, N.; Hervais-Adelman, A.; Frauenfelder, U.H.; Golestani, N. The effect of phonetic production training with visual feedback on the perception and production of foreign speech sounds. J. Acoust. Soc. Am. 2015, 138, 817–832. [Google Scholar] [CrossRef]
- Levine, S.P.; Huggins, J.E.; BeMent, S.L.; Kushwaha, R.K.; Schuh, L.A.; Rohde, M.M. A direct brain interface based on event-related potentials. IEEE Trans. Rehabil. Eng. 2000, 8, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.; Benson, A.; LaDou, T. Clinical practice and observations of infralow neurofeedback as an adjunctive treatment within Camp Pendleton’s Deployment Health Center. In Proceedings of the Navi and Marine Corps Combat Operational Stress Conference, San Diego, CA, USA, 26–29 April 2011. [Google Scholar]
- Deiber, M.-P.; Hasler, R.; Colin, J.; Dayer, A.; Aubry, J.-M.; Baggio, S.; Perroud, N.; Ros, T. Linking alpha oscillations, attention and inhibitory control in adult ADHD with EEG neurofeedback. NeuroImage Clin. 2020, 25, 102145. [Google Scholar] [CrossRef] [PubMed]
- Niv, S. Clinical efficacy and potential mechanisms of neurofeedback. Personal. Individ. Differ. 2013, 54, 676–686. [Google Scholar] [CrossRef]
- Choi, S.W.; Chi, S.E.; Chung, S.Y.; Kim, J.W.; Ahn, C.Y.; Kim, H.T. Is Alpha Wave Neurofeedback Effective with Randomized Clinical Trials in Depression? A Pilot Study. Neuropsychobiology 2011, 63, 43–51. [Google Scholar] [CrossRef]
- Markiewcz, R. The use of EEG biofeedback / neurofeedback in psychiatric rehabilitation. Psychiatr. Pol. 2017, 51, 1095–1106. [Google Scholar] [CrossRef]
- Putman, J.A.; Othmer, S.F.; Othmer, S.; Pollock, V.E. TOVA results following inter-hemispheric bipolar EEG training. J. Neurother. 2005, 9, 37–52. [Google Scholar] [CrossRef]
- Kim, S.; Zemon, V.; Cavallo, M.M.; Rath, J.F.; McCraty, R.; Foley, F.W. Heart rate variability biofeedback, executive functioning and chronic brain injury. Brain Inj. 2013, 27, 209–222. [Google Scholar] [CrossRef]
- Landes, J.K.; Reid, C.L.; Arns, M.; Badcock, N.A.; Ros, T.; Enriquez-Geppert, S.; Bulsara, M.K.; Brini, S.; Rabipour, S.; Mason, M.; et al. EEG neurofeedback for executive functions in children with neurodevelopmental challenges. Cochrane Database Syst. Rev. 2017, 1–22. [Google Scholar] [CrossRef]
- Kleinman, K.M. The role of reinforcement and motivation in biofeedback performance. Physiol. Behav. 1981, 26, 921–925. [Google Scholar] [CrossRef]
- Sano, H.; Harano, K. Effects of motivation and biofeedback upon voluntary control of heart rate increase. Jpn. J. Biofeedback Res. 1984, 11, 34–39. [Google Scholar]
- Keefe, F.J.; Gardner, E.T. Learned control of skin temperature: Effects of short- and long-term biofeedback training. Behav. Ther. 1979, 10, 202–210. [Google Scholar] [CrossRef]
- Pastor, C.M.; Menéndez, J.F.; Sanz, M.T. The Influence of Respiration on Biofeedback Techniques. Appl. Psychophysiol. Biofeedback 2008, 33, 49–54. [Google Scholar] [CrossRef]
- Suter, S. Individual differences in biofeedback performance: EEG alpha, skin resistance and EMG. Percept. Mot. Ski. 1979, 48, 586. [Google Scholar] [CrossRef]
- Li, J.J.; Ayala, S.; Harel, D.; Shiller, D.M.; McAllister, T. Individual predictors of response to biofeedback training for second-language production. J. Acoust. Soc. Am. 2019, 146, 4625. [Google Scholar] [CrossRef]
- Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Rodrigues, J.P.; Ma, J.; Qu, X.; Wan, F.; Mak, P.I.; Mak, P.U.; Vai, M.I.; Rosa, A. Individual alpha neurofeedback training effect on short term memory. Int. J. Psychophysiol. 2012, 86, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, J.; Ridderinkhof, K.R.; Cohen, M.X. Not All Errors Are Alike: Theta and Alpha EEG Dynamics Relate to Differences in Error-Processing Dynamics. J. Neurosci. 2012, 32, 16795–16806. [Google Scholar] [CrossRef]
- Fink, A.; Benedek, M. EEG alpha power and creative ideation. Neurosci. Biobehav. Rev. 2014, 44, 111–123. [Google Scholar] [CrossRef]
- Bauer, M.; Kennett, S.; Driver, J. Attentional selection of location and modality in vision and touch modulates low-frequency activity in associated sensory cortices. J. Neurophysiol. 2012, 107, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P. Efficacy of Alfa EEG wave biofeedback in the management of anxiety. Ind. Psychiatry J. 2010, 19, 111–114. [Google Scholar] [CrossRef]
- Fedotchev, A.I. Human Electroencephalogram-Controlled Effects of Photostimulation. Biophysics 2019, 64, 268–271. [Google Scholar] [CrossRef]
- Fedotchev, A.I.; Bondar’, A.T.; Semenov, V.S. Efficiency of photostimulation controlled by subject’s EEG decreases under the conditions of feedback delay. Hum. Physiol. 2016, 42, 381–384. [Google Scholar] [CrossRef]
- Moore, W.H. Hemispheric alpha asymmetries during an electromyographic biofeedback procedure for stuttering: A single-subject experimental design. J. Fluen. Disord. 1984, 9, 143–162. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Ge, Y. Asymmetric hemisphere activation in tenderness: Evidence from EEG signals. Sci. Rep. 2018, 8, 8029. [Google Scholar] [CrossRef]
- Dziembowska, I.; Izdebski, P.; Rasmus, A.; Brudny, J.; Grzelczak, M.; Cysewski, P. Effects of Heart Rate Variability Biofeedback on EEG Alpha Asymmetry and Anxiety Symptoms in Male Athletes: A Pilot Study. Appl. Psychophysiol. Biofeedback 2016, 41, 141–150. [Google Scholar] [CrossRef]
- Allen, J.J.; Harmon-Jones, E.; Cavender, J.H. Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology 2001, 38, 685–693. [Google Scholar] [CrossRef]
- Rockstroh, B.; Elbert, T.; Birbaumer, N.; Lutzenberger, W. Biofeedback-produced hemispheric asymmetry of slow cortical potentials and its behavioural effects. Int. J. Psychophysiol. 1990, 9, 151–165. [Google Scholar] [CrossRef]
- Fokin, V.F. Stationary and dynamic organization of functional interhemispheric asymmetry. In Functional Hemispheric Asymmetry Guide; Nauchnyj mir: Moscow, Russia, 2009; pp. 389–428. (In Russian) [Google Scholar]
- Shcherbakov, V.I.; Kosyuga, Y.I. Physiological mechanisms of spatial hearing. Zhurnal Vyss. Nervn. Deyatel’nosti. I.P. Pavlov. 1980, 30, 288. (In Russian) [Google Scholar]
- Shcherbakov, V.I.; Parenko, M.K.; Sheromova, N.N.; Polevaya, S.A. Sposob Issledovaniya Mezhpolusharnoj Sensornoj Asimmetrii [The Method of Research of Interhemispheric Sensory Asymmetry]. Patent RF No. 2207041, 27 June 2003. [Google Scholar]
- Shcherbakov, V.I.; Sheromova, N.N.; Parenko, M.K.; Polevaya, S.A. Sposob Issledovaniya Mezhpolusharnoj Sensornoj Asimmetrii [The Method of Research of Interhemispheric Sensory Asymmetry]. Patent RF No. 2198589, 20 February 2003. [Google Scholar]
- Shcherbakov, V.I.; Parenko, M.K.; Ageeva, E.L.; Kuznecova, I.A.; Egorov, A.A.; Antipenko, E.A. Sposob Issledovaniya Mezhpolusharnoj Sluhovoj Asimmetrii [The Method of Research of Interhemispheric Auditory Asymmetry]. Patent RF No. 2318430, 10 March 2008. [Google Scholar]
- Musiek, F.E.; Baran, J.A. The Auditory System, Anatomy, Physiology and Clinical Correlates; Allyn & Bacon: Boston, MA, USA, 2007. [Google Scholar]
- Polevaya, S.A. Integraciya Endogennyh Faktorov v Sistemu Obrabotki Eksteroceptivnyh Signalov. Ph.D. Thesis, Lobachevsky State University of Nizhny Novgorod, Nizhny Novgorod, Russia, 2009. (In Russian). [Google Scholar]
- Blauert, J.; Butler, R.A. Spatial Hearing: The Psychophysics of Human Sound Localization by Jens Blauert. J. Acoust. Soc. Am. 1985, 77, 334–335. [Google Scholar] [CrossRef]
- Litovsky, R.Y.; Colburn, H.S.; Yost, W.A.; Guzman, S.J. The precedence effect. J. Acoust. Soc. Am. 1999, 106, 1633–1654. [Google Scholar] [CrossRef]
- Hammond, D.C. What is Neurofeedback: An Update. J. Neurother. 2011, 15, 305–336. [Google Scholar] [CrossRef]
- Demareva, V.; Polevaya, S. Behavioral and cognitive correlates of foreign language proficiency. Int. J. Psychophysiol. 2014, 94, 208. [Google Scholar] [CrossRef]
- Hirnstein, M.; Hugdahl, K.; Hausmann, M. Cognitive sex differences and hemispheric asymmetry: A critical review of 40 years of research. Laterality Asymmetries Body Brain Cogn. 2019, 24, 204–252. [Google Scholar] [CrossRef]


| Precedence Effect Components | Characteristics of Cognitive and Neural Representations | Auditory System Regions | |
|---|---|---|---|
| Quantity/ Localization of Virtual Auditory Space Stimuli | The Components of the Auditory Evoked Potential | ||
| Δt min | One/ vertex | wave V of the short-latency complex | Stem regions |
| Δt max | One/ maximum lateralization on the advance signal side | N1 of the long-latency complex | Auditory cortex |
| Δt echo | Two/ maximum lateralization on Right/Left side | N1, P2 and late response of the long-latency complex | Frontal, parietal, occipital |
| Mean | SD | t | p | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| K min (lability) | −0.064 | −0.018 | 0.136 | 0.136 | −1.3 | 0.197 |
| K max (excitability) | −0.011 | 0.011 | 0.098 | 0.099 | −0.9 | 0.372 |
| K echo (stability) | −0.020 | 0.005 | 0.125 | 0.096 | −0.9 | 0.373 |
| K all | 0.203 | 0.179 | 0.078 | 0.070 | 1.1 | 0.281 |
| Δt min (Lability) | Δt max (Excitability) | Δt echo (Stability) | |||
|---|---|---|---|---|---|
| Left -> Right | Right -> Left | Left -> Right | Right -> Left | Left -> Right | Right -> Left |
| 7 | 3 | 8 | 5 | 7 | 6 |
| Zero -> Right | Zero -> Left | Zero -> Right | Zero -> Left | Zero -> Right | Zero -> Left |
| 2 | 2 | 1 | 1 | 0 | 0 |
| Change to Right | Change to Left | Change to Right | Change to Left | Change to Right | Change to Left |
| 9 | 5 | 9 | 6 | 7 | 6 |
| No change | No change | No change | |||
| 16 | 15 | 17 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demareva, V.; Mukhina, E.; Bobro, T.; Abitov, I. Does Double Biofeedback Affect Functional Hemispheric Asymmetry and Activity? A Pilot Study. Symmetry 2021, 13, 937. https://doi.org/10.3390/sym13060937
Demareva V, Mukhina E, Bobro T, Abitov I. Does Double Biofeedback Affect Functional Hemispheric Asymmetry and Activity? A Pilot Study. Symmetry. 2021; 13(6):937. https://doi.org/10.3390/sym13060937
Chicago/Turabian StyleDemareva, Valeriia, Elena Mukhina, Tatiana Bobro, and Ildar Abitov. 2021. "Does Double Biofeedback Affect Functional Hemispheric Asymmetry and Activity? A Pilot Study" Symmetry 13, no. 6: 937. https://doi.org/10.3390/sym13060937
APA StyleDemareva, V., Mukhina, E., Bobro, T., & Abitov, I. (2021). Does Double Biofeedback Affect Functional Hemispheric Asymmetry and Activity? A Pilot Study. Symmetry, 13(6), 937. https://doi.org/10.3390/sym13060937





