Recognition of Heavy Metal Ions by Using E-5-((5-Isopropyl-3,8-Dimethylazulen-1-yl) Dyazenyl)-1H-Tetrazole Modified Electrodes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Electrochemical Study of L
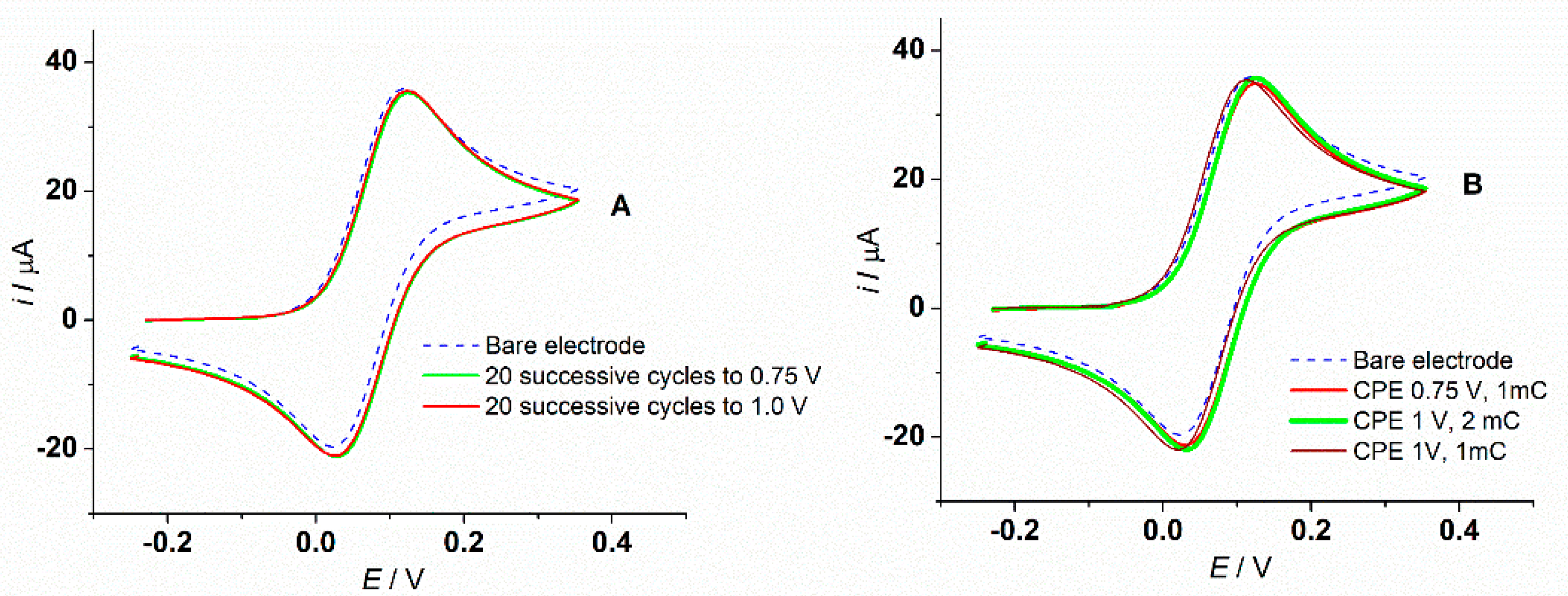
3.2. Preparation of L-CMEs
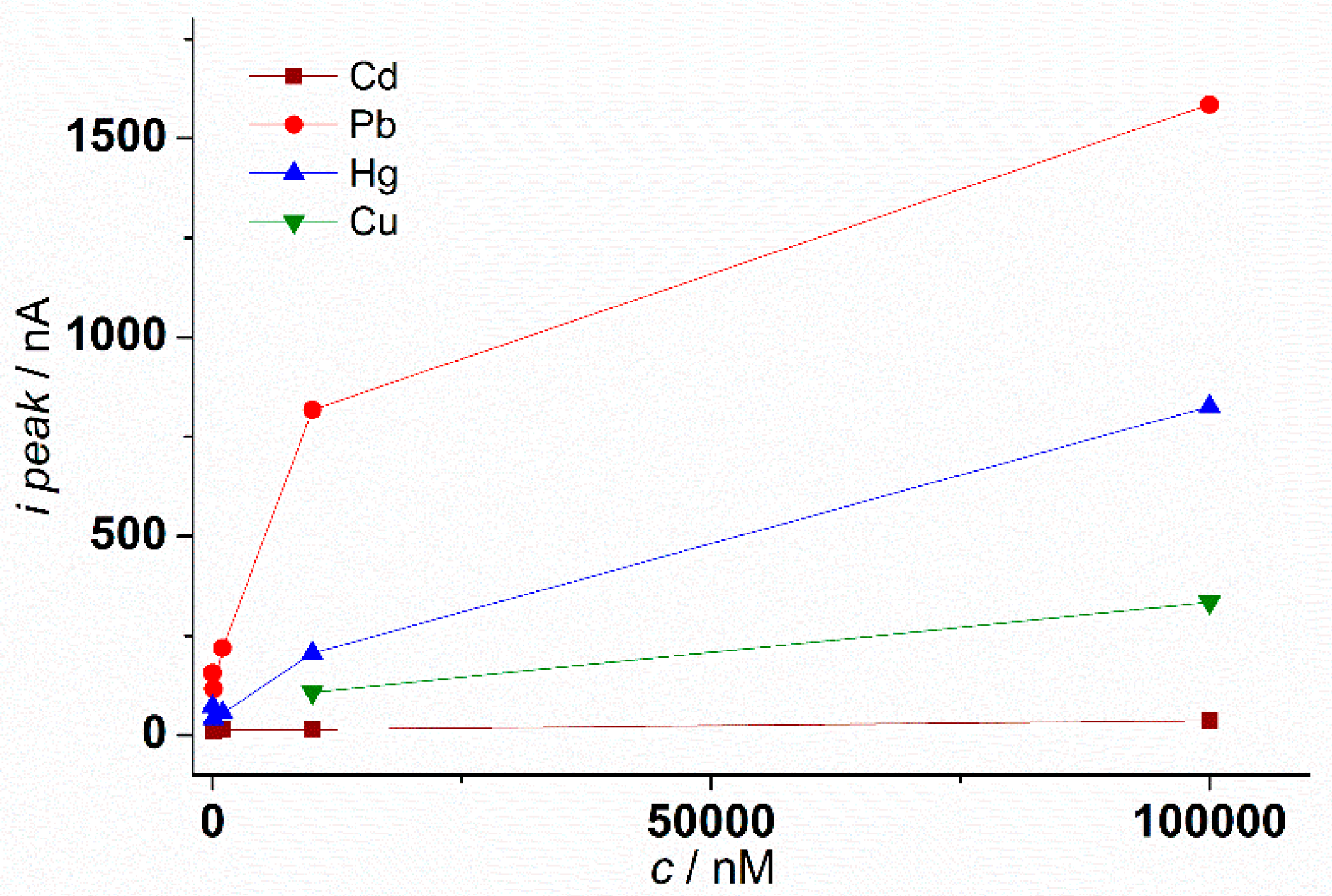
3.3. Recognition of Heavy Metal Ions Using Poly L
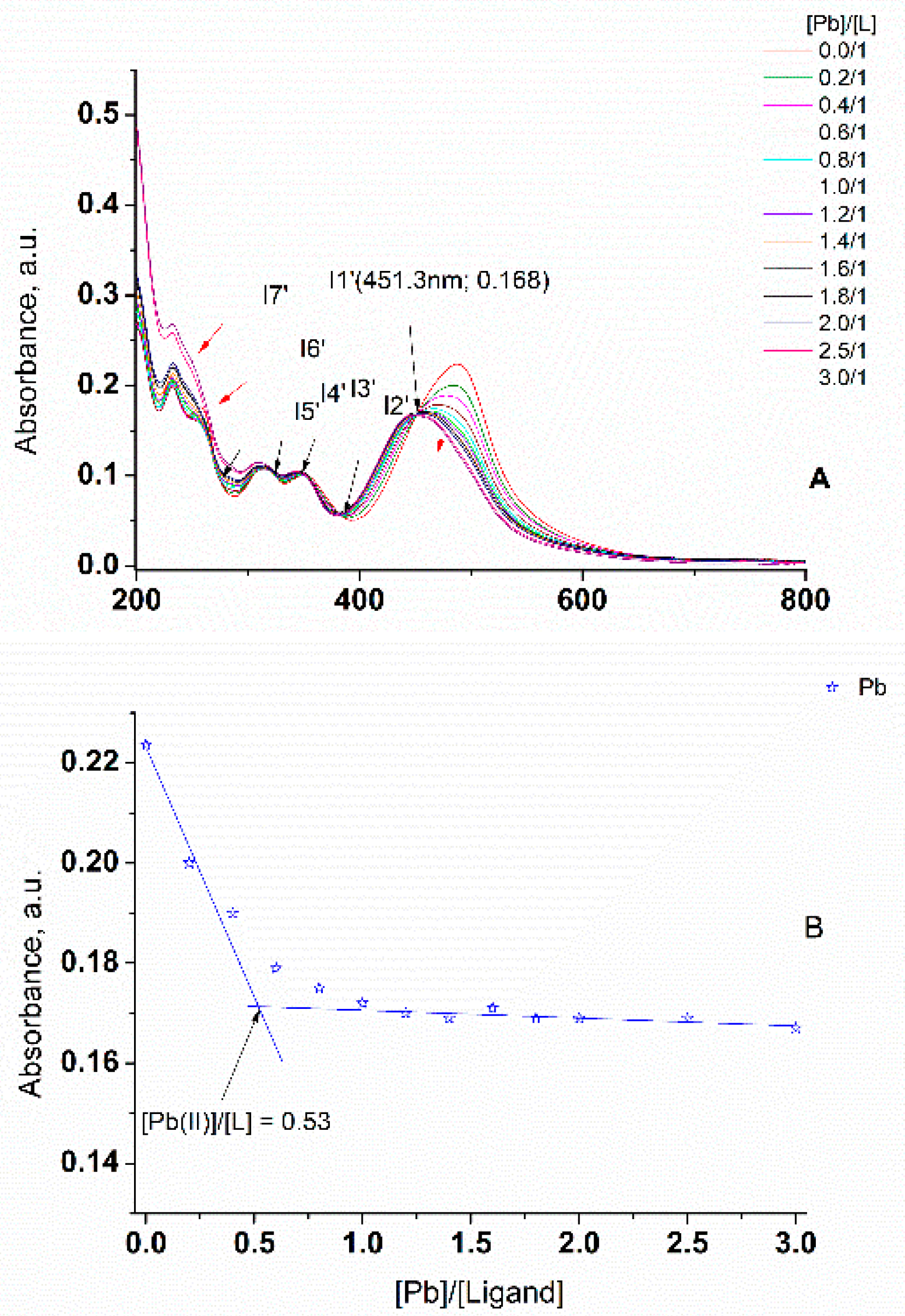
3.4. UV-Vis Study of Hg(II) and Pb(II) Metal ion Complexation Using L
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welz, B.; Sperling, M. Atomic Absorption Spectrometry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1999; pp. 335–475. [Google Scholar] [CrossRef]
- Evans, E.H.; Day, J.A.; Palmer, C.D.; Price, W.J.; Smith, C.M.M.; Tyson, J.F. Atomic spectrometry update. Advances in atomic emission, absorption and fluorescence spectrometry, and related techniques. J. Anal. At. Spectrom. 2005, 20, 562–590. [Google Scholar] [CrossRef]
- Zhang, Y.; Adeloju, S.B. Coupling of non-selective adsorption with selective elution for novel in-line separation and detection of cadmium by vapour generation atomic absorption spectrometry. Talanta 2015, 137, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Montes-Bayón, M.; DeNicola, K.; Caruso, J.A. Liquid chromatography–inductively coupled plasma mass spectrometry. J. Chromatogr. A 2003, 1000, 457–476. [Google Scholar] [CrossRef]
- Philips, M.F.; Gopalan, A.I.; Lee, K.P. Development of a novel cyano group containing electrochemically deposited polymer film for ultrasensitive simultaneous detection of trace level cadmium and lead. J. Hazard. Mater. 2012, 237, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, C.; Sun, C.; Yang, X. Simultaneously determination of trace Cd2+ and Pb2+ based on L-cysteine/graphene modified glassy carbon electrode. Food Chem. 2016, 192, 351–357. [Google Scholar] [CrossRef] [PubMed]
- March, G.; Nguyen, T.D.; Piro, B. Modified Electrodes Used for Electrochemical Detection of Metal Ions in Environmental Analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Buică, G.O.; Bucher, C.; Moutet, J.C.; Royal, G.; Saint-Aman, E.; Ungureanu, E.M. Voltammetric Sensing of Mercury and Copper Cations at Poly(EDTA-like) Film Modified Electrode. Electroanalysis 2009, 21, 77–86. [Google Scholar] [CrossRef]
- Arsene, P.; Marinescu, C. Organic Chemistry; E.D.P.: Bucharest, Romania, 2016; pp. 253–254. ISBN 9786063103285. [Google Scholar]
- Buică, G.O.; Ungureanu, E.M.; Birzan, L.; Răzus, A.C.; Popescu, L.R. Voltammetric sensing of lead and cadmium using poly(4-azulen-1-yl-2,6-bis(2-thienyl)pyridine) complexing films. J. Electroanal. Chem. 2013, 693, 67–72. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Tecuceanu, V.; Hanganu, A.; Ungureanu, E.M.; Răzus, A.C. 5-(Azulen-1-yldiazenyl)tetrazoles; Syntheses and Properties. Rev. Chim. 2020, 71, 251–264. [Google Scholar] [CrossRef]
- Buica, G.O.; Lazar, I.G.; Birzan, L.; Lete, C.; Prodana, M.; Enachescu, M.; Tecuceanu, V.; Stoian, A.B.; Ungureanu, E.-M. Azulene-ethylenediaminetetraacetic acid: A versatile molecule for colorimetric and electrochemical sensors for metal ions. Electrochim. Acta 2018, 263, 382–390. [Google Scholar] [CrossRef]
- Enache, L.B.; Anăstăsoaie, V.; Birzan, L.; Ungureanu, E.M.; Diao, P.; Enăchescu, M. Polyazulene-Based Materials for Heavy Metal Ion Detection. 2. (E)-5-(azulen-1-yldiazenyl)-1H-Tetrazole-Modified Electrodes for Heavy Metal Sensing. Coatings 2020, 10, 869. [Google Scholar] [CrossRef]
- Cordos, E.; Frentiu, T.; Ponta, M.; Rusu, A.; Darvasi, E. Analiza Prin Spectroscopie de Absorbție Moleculară in Ultraviolet-Vizibil; Institutul National de Optoelectronică: București, Romania, 2001; pp. 199–200. [Google Scholar]


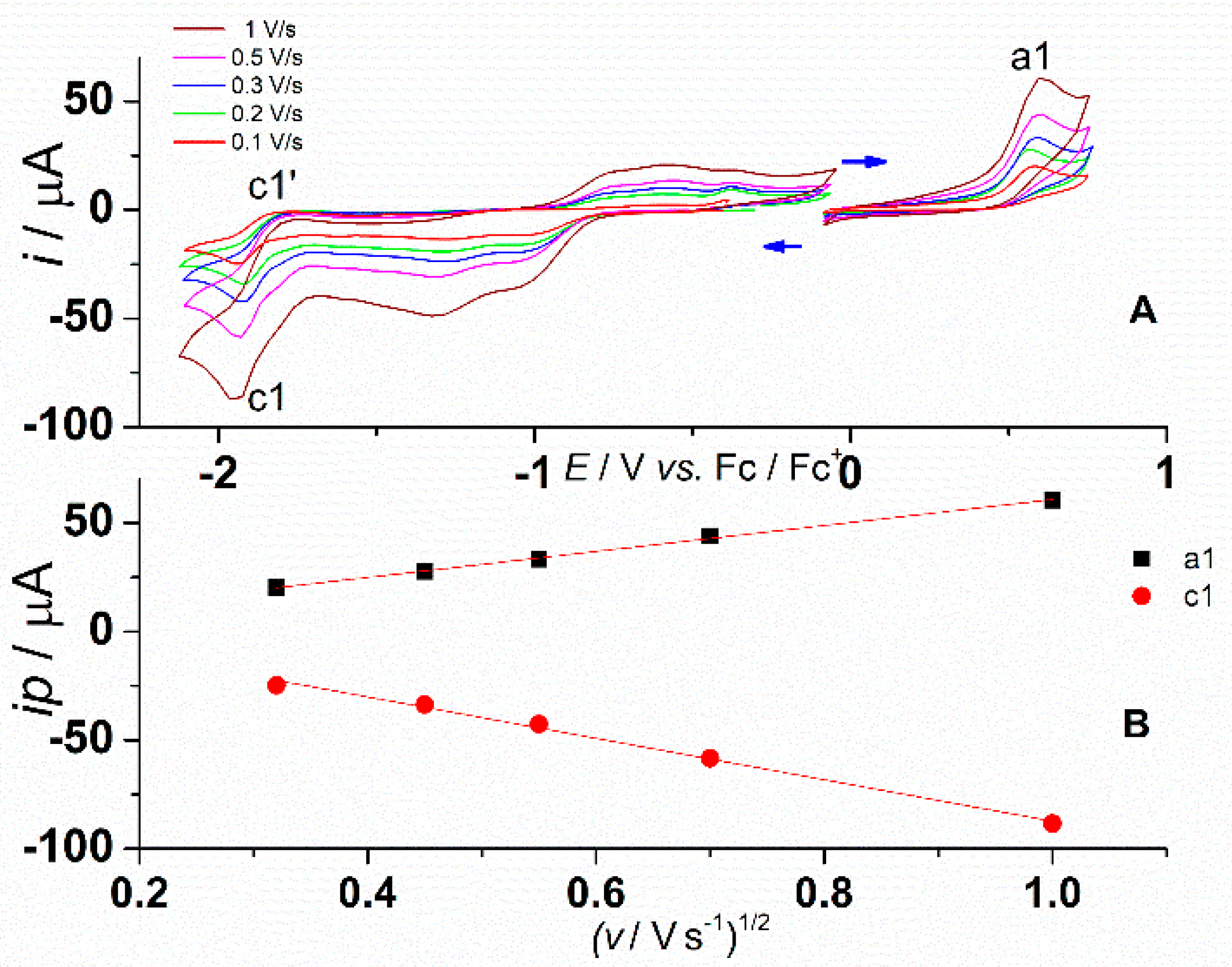
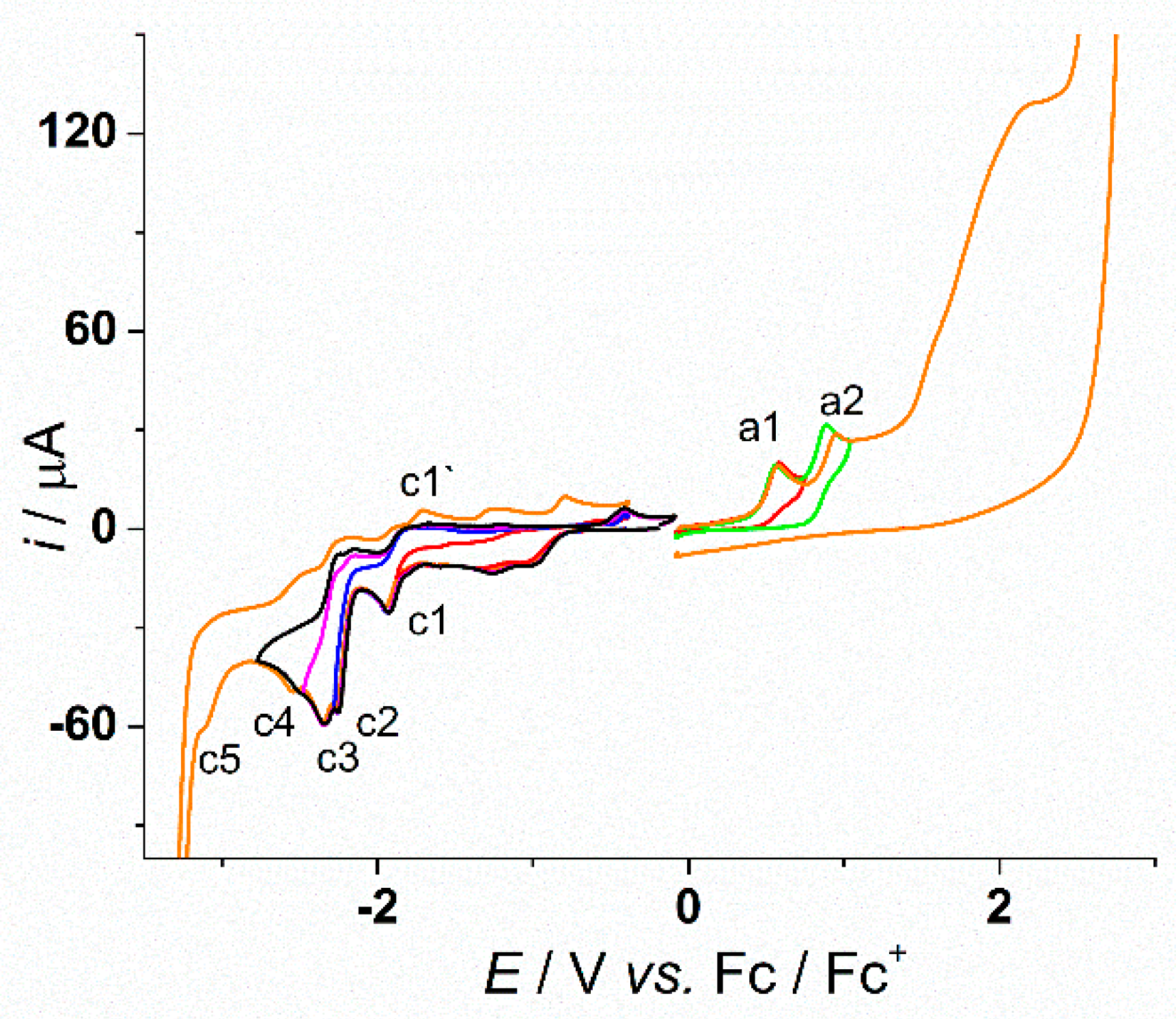




| Peak | Method | Process Characteristics | ||
|---|---|---|---|---|
| CV | DPV | RDE (E1/2) | ||
| a1 | 0.57 | 0.55 | Irreversible | |
| a2 | 0.93 | 0.90 | Quasireversible | |
| c1 | −1.95 | −1.91 | −1.867 (500 rpm) −1.888 (1000 rpm) −1.906 (1500 rpm) | Quasireversible |
| C’1 | −1.70 | - | ||
| c2 | −2.26 | −2.23 | Irreversible | |
| c3 | −2.34 | −2.32 | −2.288 (500 rpm) −2.292 (1000 rpm) −2.330 (1500 rpm) | Quasireversible |
| c4 | −2.57 | −2.55 | Quasireversible | |
| c5 | −3.11 | −3.05 | Irreversible | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păun, A.-M.; Matica, O.-T.; Anăstăsoaie, V.; Enache, L.-B.; Diacu, E.; Ungureanu, E.-M. Recognition of Heavy Metal Ions by Using E-5-((5-Isopropyl-3,8-Dimethylazulen-1-yl) Dyazenyl)-1H-Tetrazole Modified Electrodes. Symmetry 2021, 13, 644. https://doi.org/10.3390/sym13040644
Păun A-M, Matica O-T, Anăstăsoaie V, Enache L-B, Diacu E, Ungureanu E-M. Recognition of Heavy Metal Ions by Using E-5-((5-Isopropyl-3,8-Dimethylazulen-1-yl) Dyazenyl)-1H-Tetrazole Modified Electrodes. Symmetry. 2021; 13(4):644. https://doi.org/10.3390/sym13040644
Chicago/Turabian StylePăun, Adina-Maria, Ovidiu-Teodor Matica, Veronica Anăstăsoaie, Laura-Bianca Enache, Elena Diacu, and Eleonora-Mihaela Ungureanu. 2021. "Recognition of Heavy Metal Ions by Using E-5-((5-Isopropyl-3,8-Dimethylazulen-1-yl) Dyazenyl)-1H-Tetrazole Modified Electrodes" Symmetry 13, no. 4: 644. https://doi.org/10.3390/sym13040644
APA StylePăun, A.-M., Matica, O.-T., Anăstăsoaie, V., Enache, L.-B., Diacu, E., & Ungureanu, E.-M. (2021). Recognition of Heavy Metal Ions by Using E-5-((5-Isopropyl-3,8-Dimethylazulen-1-yl) Dyazenyl)-1H-Tetrazole Modified Electrodes. Symmetry, 13(4), 644. https://doi.org/10.3390/sym13040644







