Development of Perceptual Inhibition in Adolescents—A Critical Period?
Abstract
1. Introduction
2. Method
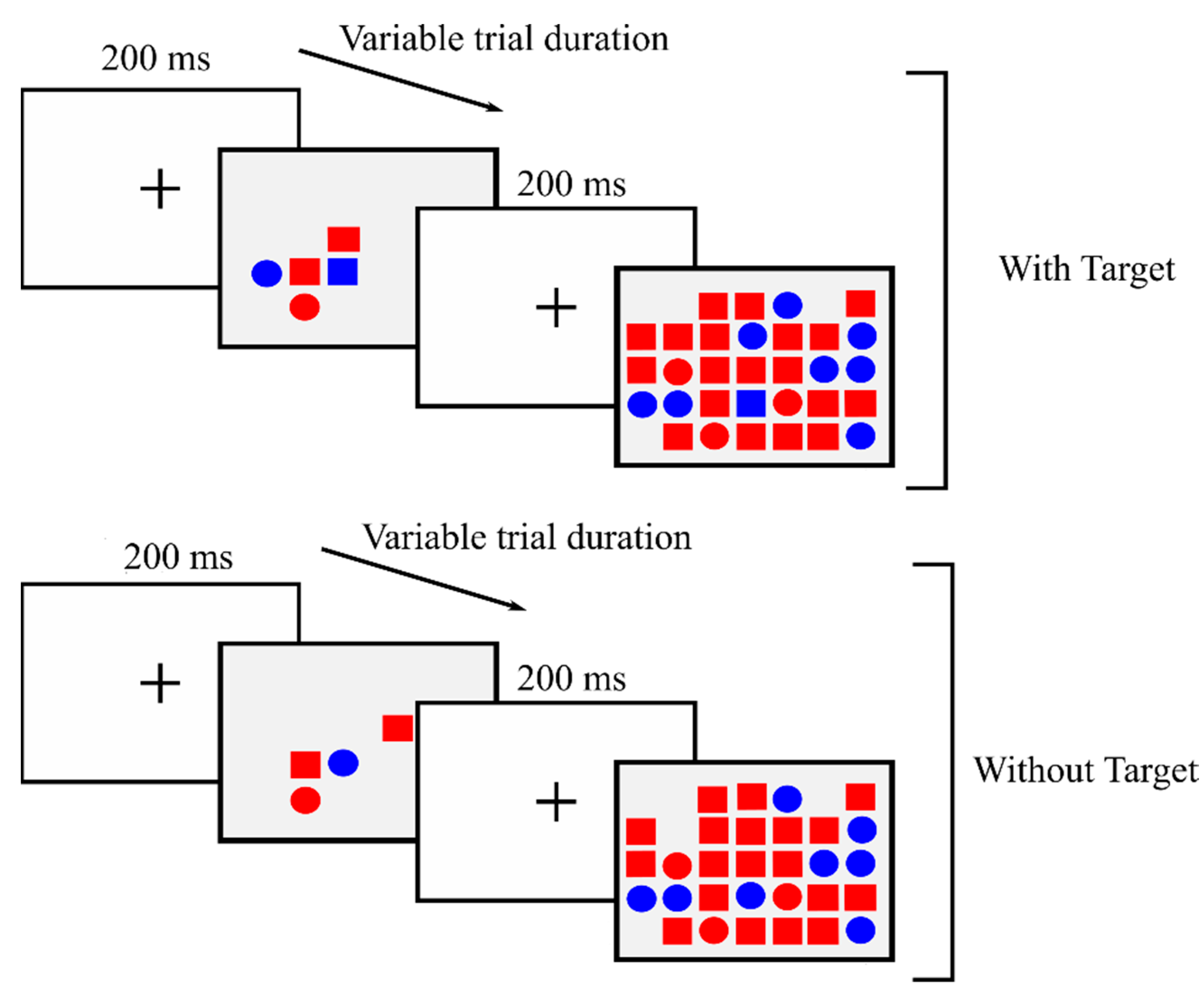
2.1. Design, Participants, and Procedure
2.2. Ethical Considerations
2.3. Instruments
3. Data Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hofmann, W.; Schmeichel, B.J.; Baddeley, A.D. Executive functions and self-regulation. Trends Cogn. Sci. 2012, 16, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rothbart, M.K.; Sheese, B.E.; Rueda, M.R.; Posner, M.I. Developing mechanisms of self-regulation in early life. Emot. Rev. 2011, 3, 207–213. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Schmeichel, B.J.; Tang, D. Individual Differences in Executive Functioning and Their Relationship to Emotional Processes and Responses. Curr. Dir. Psychol. Sci. 2015, 24, 93–98. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Blair, C.B.; Willoughby, M.T. Executive Function: Implications for Education; (NCER 2017–2000); National Center for Education Research, Institute of Education Sciences, U.S. Department of Education: Washington, DC, USA, 2016. Available online: http://ies.ed.gov (accessed on 9 October 2020).
- Diamond, A. Why improving and assessing executive functions early in life is critical. In Executive Functions in Pre-School Age-Children. Integrating Measurement, Neurodevelopment and Translational Research; Griffin, J., McCardle, P., Freund, L., Eds.; American Psychological Association: Washington, DC, USA, 2016; pp. 11–44. [Google Scholar] [CrossRef]
- Friedman, N.P.; Miyake, A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex 2017, 86, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, N.; Smith, C.; Spinrad, T. Effortful Control Relations with Emotion Regulation, Adjustment, and Socialization in Childhood. In Handbook of Self-Regulation. Research, Theory, and Applications, 3rd ed.; Vohs, K.D., Baumeister, R.F., Eds.; Guilford Press: New York, NY, USA, 2016; pp. 458–478. [Google Scholar]
- Sharifian, N.; Sol, K.; Zahodne, L.B.; Antonucci, T.C. Social relationships and adaptation in later life. Ref. Mod. Neurosci. Biobehav. Psych. 2020. [Google Scholar] [CrossRef]
- Brock, L.L.; Rimm-Kaufman, S.E.; Nathanson, L.; Grimm, K.J. The contributions of ‘hot’ and ‘cool’ executive function to children’s academic achievement, learning-related behaviors, and engagement in kindergarten. Early Child. Res. Q. 2009, 24, 337–349. [Google Scholar] [CrossRef]
- Jacobson, L.A.; Williford, A.P.; Pianta, R.C. The role of executive function in children’s competent adjustment to middle school. Child Neuropsychol. 2011, 17, 255–280. [Google Scholar] [CrossRef]
- Orbach, L.; Herzog, M.; Fritz, A. Relation of attention deficit hyperactivity disorder (ADHD) to basic number skills and arithmetic fact retrieval in children. Res. Dev. Disabil. 2020, 103, 103697. [Google Scholar] [CrossRef]
- Allan, J.L.; McMinn, D.; Daly, M.A. Bidirectional Relationship between Executive Function and Health Behavior: Evidence, Implications, and Future Directions. Front. Neurosci. 2016, 10, 386. [Google Scholar] [CrossRef]
- Jabłoński, S. Inhibitory control and literacy development among 3- to 5-year-old children. L1-Educ. Stud. Lang. Lit. 2013, 13, 1–25. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Nigg, J.T. Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry 2017, 58, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Goldman-Rakic, P. Comparison of human infants and rhesus monkeys on Piaget’s A-not-B task: Evidence for dependence on dorsolateral prefrontal cortex. Exp. Brain Res. 1989, 74, 24–40. [Google Scholar] [CrossRef]
- Klatt, L.; Getzmann, S.; Wascher, E.; Schneider, D. Searching for auditory targets in external space and in working memory: Electrophysiological mechanisms underlying perceptual and retroactive spatial attention. Behav. Brain Res. 2018, 353, 98–107. [Google Scholar] [CrossRef]
- Hommel, B.; Li, K.Z.H.; Li, S.C. Visual search across the life span. Dev. Psychol. 2004, 40, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Lobaugh, N.J.; Cole, S.; Rovet, J.F. Visual search for features and conjunctions in development. Can. J. Exp. 1998, 52, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Trick, L.M.; Enns, J.T. Lifespan changes in attention: The visual search task. Cogn. Dev. 1998, 13, 369–386. [Google Scholar] [CrossRef]
- Jacques, S.; Marcovitch, S. Development of executive function across the life span. In The Handbook of Life-span Development; Overton, W.F., Lerner, R.M., Eds.; Wiley: Hoboken, NJ, USA, 2010; Volume 1, pp. 431–466. [Google Scholar]
- Zelazo, P.D.; Craik, F.I.M.; Booth, L. Executive function across the life span. Acta Psychol. 2004, 115, 167–184. [Google Scholar] [CrossRef]
- Carlson, S. Developmentally Sensitive Measures of Executive Function in Preschool Children. Dev. Neuropsychol. 2005, 28, 595–616. [Google Scholar] [CrossRef]
- León-Carrión, J.; García Orza, J.; Pérez-Santamaría, F.J. Development of the inhibitory component of the executive functions in children and adolescents. Int. J. Neurosci. 2004, 114, 1291–1311. [Google Scholar] [CrossRef] [PubMed]
- Mezzacappa, E. Alerting, orienting, and executive attention: Developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Dev. 2004, 75, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Rueda, M.R.; Fan, J.; McCandliss, B.D.; Halparin, J.D.; Gruber, D.B.; Lercari, L.P.; Posner, M.I. Development of attentional networks in childhood. Neuropsychologia 2004, 42, 1029–1040. [Google Scholar] [CrossRef]
- Rueda, M.R.; Conejero, A. Developing attention and self-regulation in infancy and childhood. In Neural Circuit and Cognitive Development, 2nd ed.; Rubenstein, J., Rakic, P., Chen, B., Kwan, K.Y., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 505–522. [Google Scholar] [CrossRef]
- Poon, K. Hot and Cool Executive Functions in Adolescence: Development and Contributions to Important Developmental Outcomes. Front. Psychol. 2018, 8, 2311. [Google Scholar] [CrossRef]
- Blakemore, S.-J.; Frith, U. The learning brain: Lessons for education: A precis. Dev. Sci. 2005, 8, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ruotsalainen, I.; Gorbach, T.; Perkola, J.; Renvall, V.; Syväoja, H.J.; Tammelin, T.H.; Parviainen, T. Physical activity, aerobic fitness, and brain white matter: Their role for executive functions in adolescence. Dev. Cogn. Neurosci. 2020, 42, 100765. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.; Mackiewicz Seghete, K.; Claus, E.; Burgess, G.; Ruzic, L.; Banich, M. Cognitive Control in Adolescence: Neural Underpinnings and Relation to Self-Report Behaviors. PLoS ONE 2011, 6, e21598. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.J.; Choudhury, S. Development of the adolescent brain: Implications for executive function and social cognition. J. Child Psychol. Psychiatry 2006, 47, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Casey, B. Beyond Simple Models of Self-Control to Circuit-Based Accounts of Adolescent Behavior. Annu. Rev. Psychol. 2015, 66, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.; Getza, S.; Galván, A. The adolescent brain. Dev. Rev. 2008, 28, 62–77. [Google Scholar] [CrossRef]
- Giedd, J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Hallquist, M.N.; Luna, B. Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: A longitudinal fMRI study. Neuroimage 2017, 157, 695–704. [Google Scholar] [CrossRef]
- McGivern, R.F.; Andersen, J.; Byrd, D.A.; Mutter, K.A.; Reilly, J.S. Cognitive efficiency on a match to sample task decreases at the onset of puberty in childrem. Brain Cog. 2002, 50, 73–89. [Google Scholar] [CrossRef]
- Pauls, F.; Macha, T.; Petermann, F. U-shaped development: An old but unsolved problem. Front. Psychol. 2013, 4, 301. [Google Scholar] [CrossRef]
- Andreson, P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002, 8, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Mackinlay, R.; Charman, T.; Karmiloff-Smith, A. High functioning children with autism spectrum disorder: A novel test of multitasking. Brain Cog. 2006, 61, 14–24. [Google Scholar] [CrossRef]
- Anderson, V.; Anderson, P.; Northam, E.; Jacobs, R.; Catroppa, C. Development of executive functions through late childhood and adolescence in an Australian sample. Dev. Neuropsychol. 2001, 20, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Burnett Heyes, S.; Lau, J.Y.F.; Holmes, E.A. Mental imagery, emotion and psychopathology across child and adolescent development. Dev. Cogn. Neurosci. 2013, 5, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Best, J.R.; Miller, P.H. A developmental perspective on executive function. Child Dev. 2010, 81, 1641–1660. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Miyake, A.; Robinson, J.L.; Hewitt, J.K. Developmental trajectories in toddlers’ self-restraint predict individual differences in executive functions 14 years later: A behavioral genetic analysis. Dev. Psychol. 2011, 47, 1410–1430. [Google Scholar] [CrossRef]
- Bodison, S.C.; Colby, J.B.; Sowell, E.R. Structural Brain Development: Birth through Adolescence, 1st ed.; Rubenstein, J., Rakic, P., Chen, B., Kwan, K.Y., Eds.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef]
- Giedd, J.N.; Snell, J.W.; Lange, N.; Rajapakse, J.C.; Kaysen, D.; Vaituzis, A.C.; Vauss, Y.C.; Hamburger, S.D.; Kozuch, P.L.; Rapoport, J.L. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb. Cortex 1996, 6, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Paus, T.; Zijdenbos, A.; Worsley, K.; Collins, D.L.; Blumenthal, J.; Giedd, J.N.; Rapoport, J.L.; Evans, A.C. Structural maturation of neural pathways in children and adolescents: In vivo study. Science 1999, 283, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Stramba-Badiale, C.; Mancuso, V.; Cavedoni, S.; Pedroli, E.; Cipresso, P.; Riva, G. Transcranial Magnetic Stimulation Meets Virtual Reality: The Potential of Integrating Brain Stimulation With a Simulative Technology for Food Addiction. Front. Neurosci. 2020, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Sowell, E.R.; Peterson, B.S.; Thompson, P.M.; Welcome, S.E.; Henkenius, A.L.; Toga, A.W. Mapping cortical change across the life span. Nat. Neurosci. 2003, 6, 309–315. [Google Scholar] [CrossRef]
- Bourgeois, J.P.; Goldman-Rakic, P.S.; Rakic, P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex 1994, 4, 78–96. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Tessner, K.D.; Toga, A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J. Neurosci. 2001, 21, 8819–8829. [Google Scholar] [CrossRef]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.M.; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef]
- Huttenlocher, P.R. Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Res. 1979, 163, 195–205. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Dabholkar, A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997, 387, 167–178. [Google Scholar] [CrossRef]
- Ólafsdóttir, I.M.; Gestsdóttir, S.; Kristjánsson, Á. Age differences in foraging and executive functions: A cross-sectional study. J. Exp. Child Psychol. 2020, 198, 104910. [Google Scholar] [CrossRef] [PubMed]
- Laviola, G.; Marco, E.M. Passing the knife edge in adolescence: Brain pruning and specification of individual lines of development. Neurosci. Biobehav. R. 2011, 35, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, N.J.; Kramer, A.F.; Gonzalez de Sather, J. Changes in executive control across the life span: Examination of task-switching performance. Dev. Psychol. 2001, 37, 715–730. [Google Scholar] [CrossRef]
- De Luca, C.; Wood, S.; Anderson, V.; Buchanan, J.; Proffitt, T.; Mahony, K.; Pantelis, C. Normative Data From the Cantab. I: Development of Executive Function Over the Lifespan. J. Clin. Exp. Neuropsychol. 2003, 25, 242–254. [Google Scholar] [CrossRef]
- Hernández Sampieri, R.; Fernández Collado, C.; Baptista Lucio, P. Metodología de la Investigación, 6th ed.; McGraw-Hill: México DF, México, 2014. [Google Scholar]
- Darowski, E.S.; Helder, E.; Zacks, R.T.; Hasher, L.; Hambrick, D.Z. Age-related differences in cognition: The role of distraction control. Neuropsychology 2008, 22, 638–644. [Google Scholar] [CrossRef]
- Huizinga, M.; Dolan, C.V.; Van der Molen, M.W. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia 2006, 44, 2017–2036. [Google Scholar] [CrossRef]
- McAuley, T.; White, D.A. A latent variables examination of processing speed, response inhibition, and working memory during typical development. J. Exp. Child Psychol. 2011, 108, 453–468. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- American Psychological Association (APA). Ethical Principles of Psychologists and Code of Conduct; APA: Whashington DC, USA, 2017; Available online: www.apa.org/ethics/code/ethics-code-2017.pdf (accessed on 9 October 2020).
- Congress of the Argentine Nation. Argentine National Law on the Protection of Personal Data; No. 25.326; Buenos Aires, Argentina, 2000. Available online: https://www.argentina.gob.ar/normativa/nacional/ley-25326-64790 (accessed on 9 October 2020).
- National Executive Government. Personal Data Protection Act; No. 1558/2001; Buenos Aires (Argentina), 2001. Available online: http://servicios.infoleg.gob.ar/infolegInternet/anexos/70000-74999/70368/norma.htm (accessed on 9 October 2020).
- Canet-Juric, L.; Stelzer, F.; Andrés, M.L.; Vernucci, S.; Introzzi, I.; Burin, D. Evidencias de validez de una tarea computarizada de memoria de trabajo verbal y viso-espacial para niños. Interam. J. Psychol. 2018, 52, 112–128. [Google Scholar]
- Introzzi, I.M.; Canet-Juric, L.; Aydmune, Y.; Stelzer, F. Perspectivas teóricas y evidencia empírica sobre la inhibición. Rev. Colomb. Psicol. 2016, 25, 351–368. [Google Scholar] [CrossRef]
- Richard’s, M.; Introzzi, I.; Zamora, E.; Vernucci, S. Analysis of Internal and External Validity Criteria for a Computerized Visual Search Task. A pilot study. Appl. Neuropsychol. Child 2017, 6, 110–119. [Google Scholar] [CrossRef]
- Treisman, A.M.; Gelade, G. A feature-integration theory of attention. Cogn. Psychol. 1980, 12, 97–136. [Google Scholar] [CrossRef]
- Klein, R.M.; Christie, J.J.; Ivanoff, J. Graphical and other methods for representing the speed and accuracy of performance. In Proceedings of the 45th Annual Meeting of the Psychonomic Society, Minneapolis, MN, USA, 18–21 November 2004; Abstract Number 7. Volume 9, p. 1. Available online: https://cdn.ymaws.com/www.psychonomic.org/resource/resmgr/Annual_Meeting/Past_and_Future_Meetings/Abstracts04.pdf (accessed on 9 October 2020).
- Townsend, J.T.; Ashby, F.G. Stochastic Modeling of Elementary Psychological Processes, 1st ed.; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Christie, J.; Klein, R. Familiarity and attention: Does what we know affect what we notice? Mem. Cogn. 1995, 23, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Castro-Palacio, J.C.; Fernández-de-Córdoba, P.; Isidro, J.M.; Navarro-Pardo, E.; Selvas Aguilar, R. Percentile Study of χ Distribution. Application to Response Time Data. Mathematics 2020, 8, 514. [Google Scholar] [CrossRef]
- Introzzi, I.; Aydmune, Y.; Zamora, E.V.; Vernucci, S.; Ledesma, R. Mecanismos de desarrollo de la atención selectiva en población infantil (The development mechanisms of selective attention in child population). Rev. CES Psicol. 2019, 12, 105–118. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: Boston, MA, USA, 2013. [Google Scholar]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Q. Appl. Math. 1944, 2, 164–168. Available online: http://www.jstor.org/stable/43633451 (accessed on 9 October 2020). [CrossRef]
- Marquardt, D. An algorithm for least-squares estimation of nonlinear parameters. J. SIAM 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Bardikoff, N.; Sabbagh, M. The Differentiation of Executive Functioning Across Development: Insights from Developmental Cognitive Neuroscience. In New Perspectives on Human Development, 1st ed.; Budwig, N., Turiel, E., Zelazo, P., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 27–46. [Google Scholar] [CrossRef]
- Bull, R.; Lee, K. Executive functioning and mathematics achievement. Child Dev. Perspect. 2014, 8, 36–41. [Google Scholar] [CrossRef]
- Cragg, L.; Keeble, S.; Richardson, S.; Roome, H.E.; Gilmore, C. Direct and indirect influences of executive functions on mathematics achievement. Cognition 2017, 162, 12–26. [Google Scholar] [CrossRef]
- Borella, E.; De Ribaupierre, A. The role of working memory, inhibition, and processing speed in text comprehension in children. Learn. Indiv. Differ. 2014, 34, 86–92. [Google Scholar] [CrossRef]
- Arán Filippetti, V.; Krumm, G.; Raimondi, W. Funciones Ejecutivas y sus correlatos con Inteligencia Cristalizada y Fluida: Un estudio en Niños y Adolescentes. Rev. Neuropsi. Lat. 2015, 7, 24–33. [Google Scholar] [CrossRef]
- Hasher, L.; Lustig, C.; Zacks, R. Inhibitory mechanisms and the control of attention. In Variation in Working Memory; Conway, A., Jarrold, C., Kane, M., Miyake, A., Towse, J., Eds.; Oxford University Press: New York, NY, USA, 2007; pp. 227–249. [Google Scholar] [CrossRef]
- Friedman, N.P.; Miyake, A. The relations among inhibition and interference control functions: A latent-variable analysis. J. Exp. Psychol. 2004, 133, 101–135. [Google Scholar] [CrossRef] [PubMed]
- Bausela Herreras, E. Desarrollo evolutivo de la función ejecutiva. Rev. Galego Port. Psicoloxía Educ. 2005, 10, 85–93. [Google Scholar]
- Flores-Lázaro, J.; Castillo-Preciado, R.; Jiménez-Miramonte, N. Desarrollo de funciones ejecutivas, de la niñez a la juventud. An. Psicol. 2014, 30, 463–473. [Google Scholar] [CrossRef]
- Luciana, M.; Conklin, H.; Hooper, C.; Yarger, R. The Development of Nonverbal Working Memory and Executive Control Processes in Adolescents. Child Dev. 2005, 76, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Romine, C.B.; Reynolds, C.R. A model of the development of frontal lobe functioning: Findings from a meta-analysis. Appl. Neuropsychol. 2005, 12, 190–201. [Google Scholar] [CrossRef]
- Baltes, P.B. On the incomplete architecture of human ontogeny. Am. Psychol. 1997, 52, 366–380. [Google Scholar] [CrossRef]
- Baltes, P.B.; Lindenberger, U.; Staudinger, U.M. Life-span theory in Developmental Psychology. In Handbook of Child Psychology, 6th ed.; Damon, W., Lerner, R.M., Eds.; Wiley: New York, NY, USA, 2006; Volume 1, pp. 569–664. [Google Scholar] [CrossRef]
- Introzzi, I.; Zamora, E.; Aydmune, Y.; Canet-Juric, L.; López, S. El rol de la inhibición en la Teoría de Integración de las Características. Cuad. Neuropsicol. 2017, 11, 135–150. [Google Scholar] [CrossRef]
- Mullane, J.C.; Corkum, P.V.; Klein, R.M.; McLaughlin, E. Interference control in children with and without ADHD: A systematic review of Flanker and Simon task performance. Child Neuropsychol. 2009, 15, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Best, J.R.; Miller, P.H.; Jones, L.L. Executive Functions after Age 5: Changes and Correlates. Dev. Rev. 2009, 29, 180–200. [Google Scholar] [CrossRef] [PubMed]
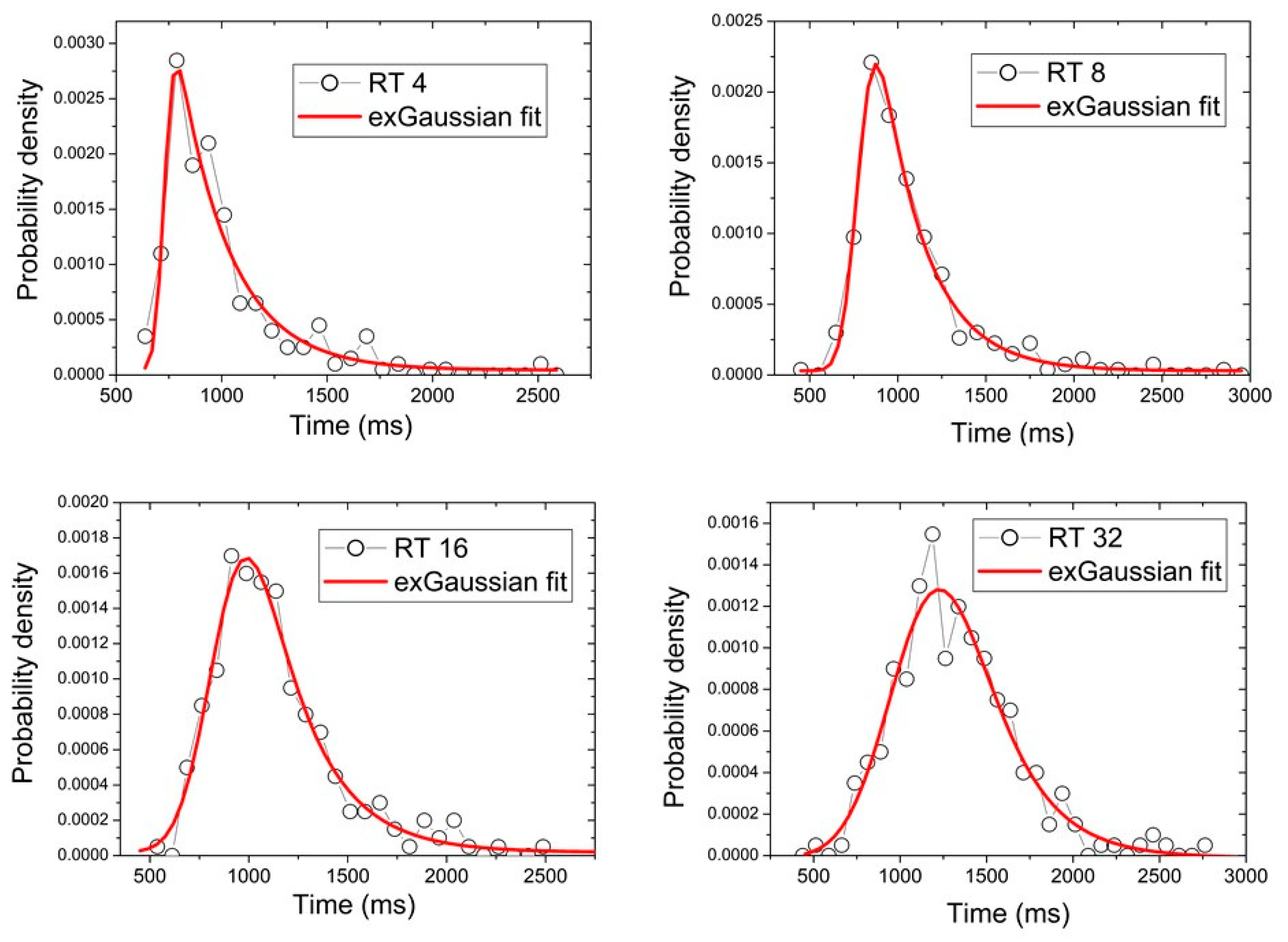
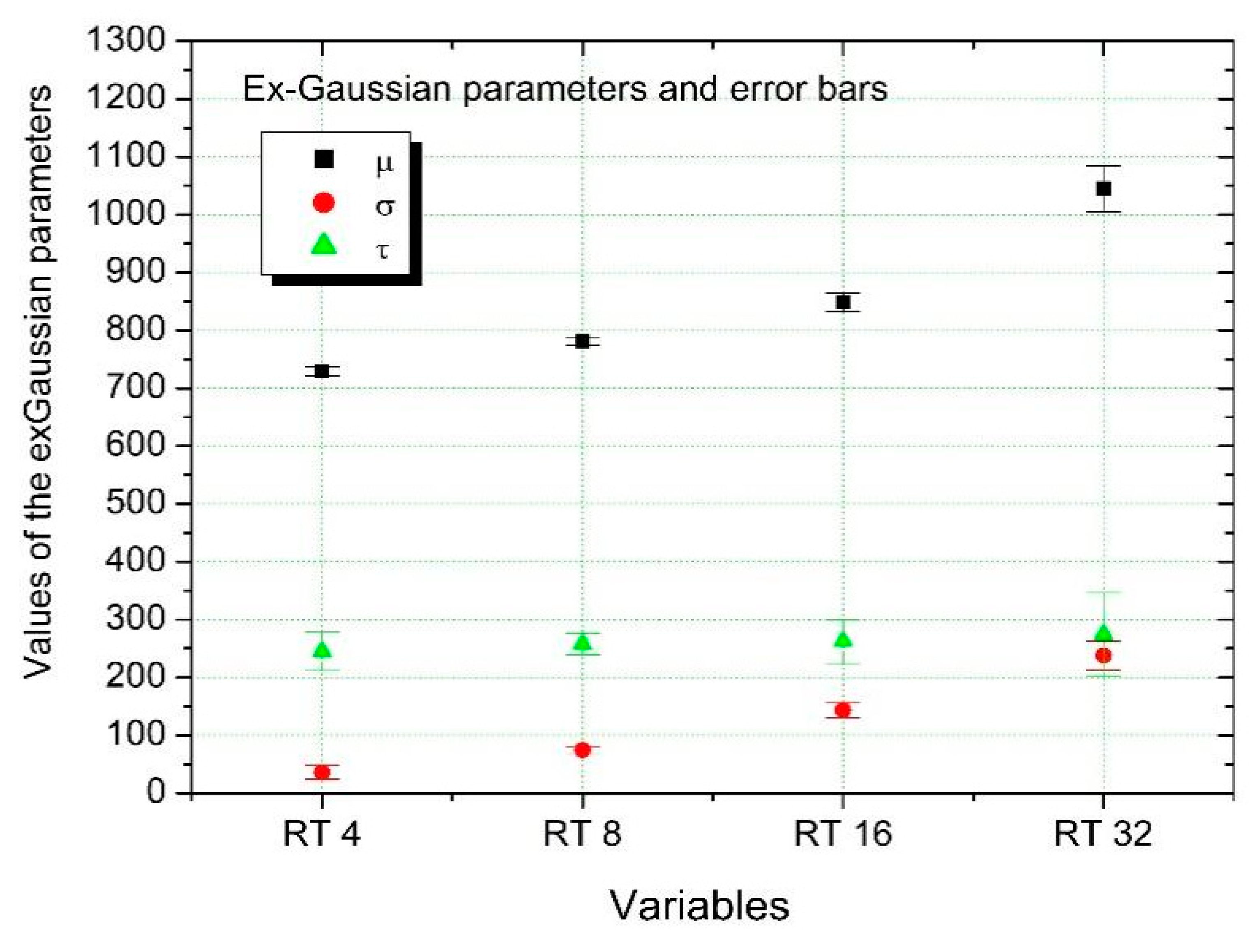
| µ | Δµ | σ | Δσ | τ | Δτ | R2 | |
|---|---|---|---|---|---|---|---|
| RT 4 | 729.54 | 8.04 | 35.98 | 11.65 | 244.98 | 33.08 | 0.95 |
| RT 8 | 780.75 | 5.86 | 74.55 | 6.25 | 257.49 | 18.49 | 0.99 |
| RT 16 | 848.45 | 16.24 | 143.63 | 12.77 | 262.28 | 38.12 | 0.97 |
| RT 32 | 1045.05 | 39.68 | 238.00 | 25.01 | 274.53 | 71.96 | 0.95 |
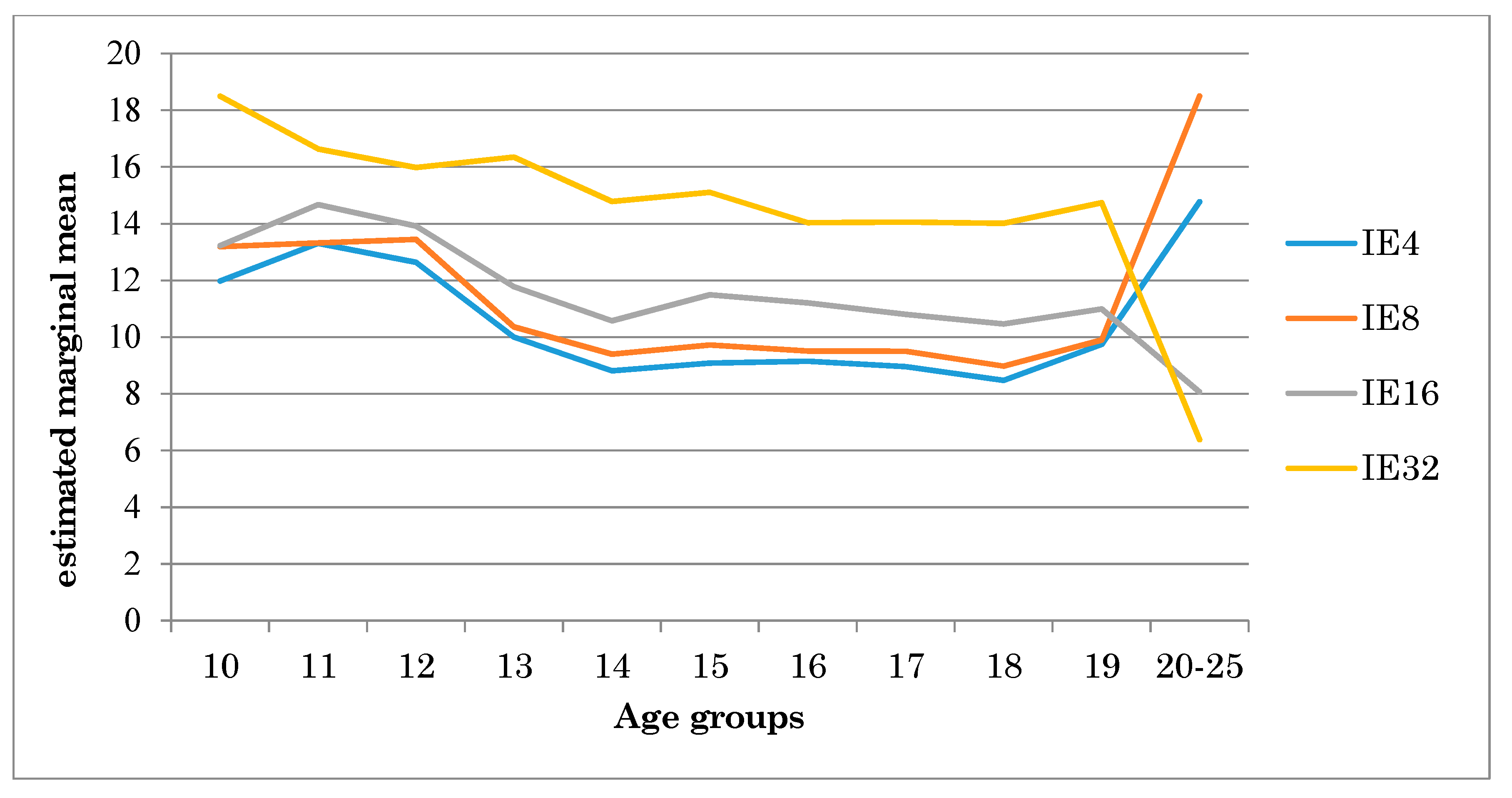
| EI4 | EI8 | EI16 | EI32 | EI without Distractors | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Age groups | 10 | 13.25 | 3.12 | 14.48 | 3.91 | 15.00 | 2.49 | 19.83 | 3.96 | 9.84 | 1.28 |
| 11 | 15.04 | 4.29 | 15.08 | 3.15 | 17.10 | 4.09 | 18.45 | 4.91 | 10.49 | 1.81 | |
| 12 | 13.47 | 3.99 | 14.29 | 5.27 | 15.08 | 4.32 | 16.85 | 3.35 | 9.22 | 1.48 | |
| 13 | 10.56 | 1.58 | 10.93 | 1.57 | 12.56 | 1.80 | 16.93 | 3.06 | 8.84 | 1.50 | |
| 14 | 9.16 | 1.67 | 9.75 | 1.50 | 11.06 | 1.51 | 15.15 | 3.29 | 8.54 | 1.70 | |
| 15 | 9.65 | 2.99 | 10.30 | 4.00 | 12.29 | 4.64 | 15.70 | 3.06 | 8.85 | 1.27 | |
| 16 | 8.49 | 1.72 | 8.87 | 1.37 | 10.38 | 1.90 | 13.65 | 3.22 | 7.34 | 1.10 | |
| 17 | 8.78 | 1.32 | 9.32 | 1.37 | 10.55 | 1.99 | 13.87 | 2.62 | 7.80 | 0.93 | |
| 18 | 8.33 | 1.59 | 8.83 | 1.59 | 10.26 | 1.63 | 13.86 | 2.64 | 7.85 | 1.61 | |
| 19 | 9.47 | 2.08 | 9.62 | 1.90 | 10.61 | 1.82 | 14.45 | 3.15 | 7.67 | 1.51 | |
| 20 | 9.82 | 1.96 | 13.35 | 3.23 | 1.62 | 2.09 | 1.69 | 2.39 | 1.28 | 1.31 | |
| Comparisons between Age Groups | Interference Conditions | ||||
|---|---|---|---|---|---|
| EI4 | EI8 | EI16 | EI32 | ||
| 10 | 11 | NS | NS | NS | NS |
| 12 | NS | NS | NS | NS | |
| 13 | NS | p = 0.022 | NS | NS | |
| 14 | p < 0.001 | p < 0.001 | p = 0.009 | p = 0.003 | |
| 15 | p = 0.002 | p = 0.001 | NS | p = 0.011 | |
| 16 | p = 0.012 | p = 0.001 | NS | p < 0.001 | |
| 17 | p =0.002 | p = 0.001 | p = 0.049 | p < 0.001 | |
| 18 | p < 0.001 | p < 0.001 | p = 0.011 | p < 0.001 | |
| 19 | NS | p = 0.004 | NS | p = 0.004 | |
| 20–25 | NS | p = 0.003 | p = 0.001 | p < 0.001 | |
| 11 | 12 | NS | NS | NS | NS |
| 13 | p < 0.001 | p < 0.14 | p = 0.003 | NS | |
| 14 | p < 0.001 | p < 0.001 | p < 0.001 | NS | |
| 15 | p < 0.001 | p = 0.001 | p = 0.001 | NS | |
| 16 | p < 0.001 | p = 0.001 | p = 0.001 | NS | |
| 17 | p < 0.001 | p < 0.001 | p < 0.001 | NS | |
| 18 | p < 0.001 | p < 0.001 | p < 0.001 | NS | |
| 19 | p < 0.001 | p = 0.004 | p < 0.001 | NS | |
| 20–25 | NS | p = 0.009 | p < 0.001 | p < 0.001 | |
| 12 | 13 | p = 0.007 | p = 0.005 | NS | NS |
| 14 | p < 0.001 | p < 0.001 | p < 0.001 | NS | |
| 15 | p < 0.001 | p < 0.001 | p = 0.025 | NS | |
| 16 | p < 0.001 | p < 0.001 | p = 0.014 | NS | |
| 17 | p < 0.001 | p < 0.001 | p = 0.001 | NS | |
| 18 | p < 0.001 | p < 0.001 | p < 0.001 | NS | |
| 19 | p = 0.002 | p = 0.001 | p = 0.002 | NS | |
| 20–25 | NS | p = 0.003 | p < 0.001 | p < 0.001 | |
| 13 | 14 | NS | NS | NS | NS |
| 15 | NS | NS | NS | NS | |
| 16 | NS | NS | NS | NS | |
| 17 | NS | NS | NS | NS | |
| 18 | NS | NS | NS | NS | |
| 19 | NS | NS | NS | NS | |
| 20–25 | p = 0.001 | p < 0.001 | p = 0.030 | p < 0.001 | |
| 14 | 15 | NS | NS | NS | NS |
| 16 | NS | NS | NS | NS | |
| 17 | NS | NS | NS | NS | |
| 18 | NS | NS | NS | NS | |
| 19 | NS | NS | NS | NS | |
| 20–25 | p < 0.001 | p < 0.001 | NS | p < 0.001 | |
| 15 | 16 | NS | NS | NS | NS |
| 17 | NS | NS | NS | NS | |
| 18 | NS | NS | NS | NS | |
| 19 | NS | NS | NS | NS | |
| 20–25 | p < 0.001 | p < 0.001 | NS | p < 0.001 | |
| 16 | 17 | NS | NS | NS | NS |
| 18 | NS | NS | NS | NS | |
| 19 | NS | NS | NS | NS | |
| 20–25 | p < 0.001 | p < 0.001 | NS | p < 0.001 | |
| 17 | 18 | NS | NS | NS | NS |
| 19 | NS | NS | NS | NS | |
| 20–25 | p < 0.001 | p < 0.001 | NS | p < 0.001 | |
| 18 | 19 | NS | NS | NS | NS |
| 20–25 | p < 0.001 | p < 0.001 | NS | p < 0.001 | |
| 19 | 20–25 | p < 0.001 | p < 0.001 | NS | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Introzzi, I.M.; Richard’s, M.M.; Aydmune, Y.; Zamora, E.V.; Stelzer, F.; García Coni, A.; Lopez-Ramon, M.F.; Navarro-Pardo, E. Development of Perceptual Inhibition in Adolescents—A Critical Period? Symmetry 2021, 13, 457. https://doi.org/10.3390/sym13030457
Introzzi IM, Richard’s MM, Aydmune Y, Zamora EV, Stelzer F, García Coni A, Lopez-Ramon MF, Navarro-Pardo E. Development of Perceptual Inhibition in Adolescents—A Critical Period? Symmetry. 2021; 13(3):457. https://doi.org/10.3390/sym13030457
Chicago/Turabian StyleIntrozzi, Isabel María, María Marta Richard’s, Yesica Aydmune, Eliana Vanesa Zamora, Florencia Stelzer, Ana García Coni, María Fernanda Lopez-Ramon, and Esperanza Navarro-Pardo. 2021. "Development of Perceptual Inhibition in Adolescents—A Critical Period?" Symmetry 13, no. 3: 457. https://doi.org/10.3390/sym13030457
APA StyleIntrozzi, I. M., Richard’s, M. M., Aydmune, Y., Zamora, E. V., Stelzer, F., García Coni, A., Lopez-Ramon, M. F., & Navarro-Pardo, E. (2021). Development of Perceptual Inhibition in Adolescents—A Critical Period? Symmetry, 13(3), 457. https://doi.org/10.3390/sym13030457








