Abstract
The synthesis of 2-pyridyltellurenyl bromide via Br2 oxidative cleavage of the Te–Te bond of dipyridylditelluride is reported. Single-crystal X-ray diffraction analysis of 2-pyridyltellurenyl bromide demonstrated that the Te atom of 2-pyridyltellurenyl bromide was involved in four different noncovalent contacts: Te⋯Te interactions, two Te⋯Br ChB, and one Te⋯N ChB contact forming 3D supramolecular symmetrical framework. In contrast to 2-pyridylselenenyl halides, the Te congener does not react with nitriles furnishing cyclization products. 2-Pyridylselenenyl chloride was demonstrated to easily form the corresponding adduct with benzonitrile. The cyclization product was studied by the single-crystal X-ray diffraction analysis, which revealed that in contrast to earlier studied cationic 1,2,4-selenadiazoles, here we observed that the adduct with benzonitrile formed supramolecular dimers via Se⋯Se interactions in the solid state, which were never observed before for 1,2,4-selenadiazoles.
1. Introduction
The field of noncovalent interactions has experienced rapid growth and constitutes one of the most intensely studied areas of current chemistry. Noncovalent interactions allow the design and construction of supramolecular materials and control of their ultimate architectures and symmetry [1,2]. Importantly, the properties of supramolecular aggregates are different from the sum of the constituent molecules [1,3,4,5,6,7,8,9]. Recently, chalcogen bonding (ChB) has emerged as a powerful tool for the creation of such materials. In contrast to halogen bonding (XB) or hydrogen bonding (HB), usage of ChB in crystal engineering, preparative chemistry, sensing, etc., is still emerging [10].
We have recently showcased that the addition of 2-pyridylselenenyl halides to a triple CN bond of unactivated nitriles resulted in the formation of novel cationic 1,2,4-selenadiazoles [11]. Moreover, we showed that the Se atom in the adducts of 2-pyridylselenenyl halides and nitriles could provide two σ-holes and act as a donor ChB (Figure 1).
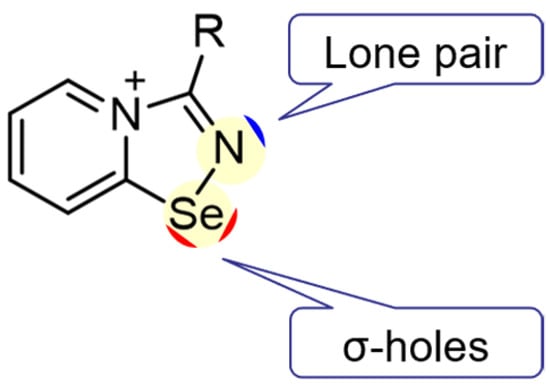
Figure 1.
Schematic representation of adducts of 2-pyridylselenenyl halides and nitriles showing the position of the Se-centered σ-holes and the N atom lone pair.
It is worth mentioning that chalcogen diazoles are appealing research objects due to their utilization in the preparation of soft materials with tunable physical parameters [12,13,14,15]. ChB allows modulation of the self-assembly and, therefore, fine-tuning of charge transport within these heterocycles.
Here we describe the synthesis and crystal structure of 2-pyridyltellurenyl bromide, compare its self-assembly in the solid-state with structurally similar selenium congener, 2-pyridylselenenyl chloride, and compare the reactivity of these two 2-pyridylchalcogenenyl halides towards benzonitrile. Interestingly, while 2-pyridylselenenyl chloride readily forms an adduct with PhCN, the Te analog does not react with benzonitrile or any other simple nitrile tested in the framework of the current study.
2. Materials and Methods
General remarks. All manipulations were carried out in air, unless specified. Unless specified, chemicals were purchased from the commercial sources. NMR data was obtained on a Bruker Avance neo 700; chemical shifts are given in ppm, coupling constants in Hz. C, H, S, and N elemental analyses were performed on Euro EA 3028HT CHNS/O. Py2Se2 was prepared as reported earlier [16].
X-ray crystal structure determination.
The single-crystal X-ray diffraction data for 15 and 16 were obtained on a three-circle Bruker D8 Venture(Kurnakov Institute of General and Inorganic Chemistry, RAS, Bremen, Germany) or Bruker D8 QUEST PHOTON-III CCD (Zelinsky Institute of Organic Chemistry, RAS, Bremen, Germany) diffractometers using φ and ω scan mode. The diffraction data were processed using the SAINT program [17] and an absorption correction based on equivalent reflections was applied with the SADABS program [18]. Crystal data, details of data collection, and results of structure refinement are summarized in Table S1. The structures were solved by the direct method and refined on F2 with anisotropic displacement parameters for non-hydrogen atoms. The hydrogen atoms in all compounds were placed in calculated positions and refined within the riding model with fixed isotropic displacement parameters (Uiso(H) = 1.5Ueq(C) for the CH3-groups and 1.2Ueq(C) for the other groups). All calculations were carried out using the SHELXTL program [19] and OLEX2 program package [20].
Crystallographic data for all investigated compounds have been deposited with the Cambridge Crystallographic Data Center, CCDC 2113480 and 2113481. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CHB2 1EZ, UK (Fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or www.ccdc.cam.ac.uk accessed on 2 October 2021).
Computational details.
The single point calculations based on the experimental X-ray geometries of 1, 15, and 16 have been carried out at the ωB97X-D3/Sapporo-DZP-2012 level of theory [21,22,23,24] with the help of the ORCA 4.2.1 program package [25]. The RIJCOSX approximation [26] has been utilized. The QTAIM analysis [27] has been performed by using the Multiwfn program (version 3.7) [28]. The Cartesian atomic coordinates for model supramolecular associates are presented in Table S1 and in attached xyz-files, Supplementary Materials.
Synthesis of 15. 2-Pyridylselenenyl chloride (1 eq, 234 µmol, 45 mg) and PhCN (4.14 eq, 970 µmol, 100 µL) were stirred in Et2O (3 mL) at ambient temperature for 3 h. White solid precipitated, which was decantated, quickly washed with CH2Cl2 (1 mL), Et2O (3 × 3 mL), and dried under vacuum. Yield: 60 mg (87%). Anal. Calcd for C12H9ClN2Se: C, 48.75; H, 3.07; N, 9.48. Found: C, 48.91; H, 3.16; N, 9.39. 1H NMR (700 MHz, D2O) δ 9.21 (1H, d, J = 6.8 Hz, H5), 8.86 (1H, d, J = 8.7 Hz, H8), 8.42 (1H, t, J = 7.9 Hz, H7), 7.92 (1H, t, J = 7.0 Hz, H6), 7.82 (1H, t, J = 7.4 Hz, H12), 7.79 (2H, d, J = 7.6 Hz, H13 & H14), 7.74 (2H, t, J = 7.6 Hz, H10 and H11). 13C {1H} NMR δ 168.25 (C3), 157.28 (C9), 139.73 (C5), 137.14 (C8), 132.61 (C15), 129.71 (C10 and C14), 129.63 (C11 and C13), 127.29 (C12), 125.80 (C7), 122.89 (C6).
Synthesis of 16. A solution of bromine (200 µmol, 32 mg) in CH2Cl2 (10 mL) was added to a solution of di(2-pyridyl)ditelluride (200 µmol, 82 mg) in CH2Cl2 (10 mL), and the mixture was stirred at room temperature for 30 min. Then the solvent was removed in vacuo, the precipitate was washed with Et2O (3 × 5 mL) and recrystallized from CH2Cl2. Yield: 112 mg (98%). Anal. Calcd for C5H4NTeBr: C, 20.99; H, 1.39; N, 4.88. Found: C, 21.02; H, 1.41; N, 4.90.
3. Results and Discussion
Recently we demonstrated that 2-pyridylselenenyl halides selectively react with a broad scope of nitriles to form adducts 3–14 in excellent yields (Scheme 1) [11,29].
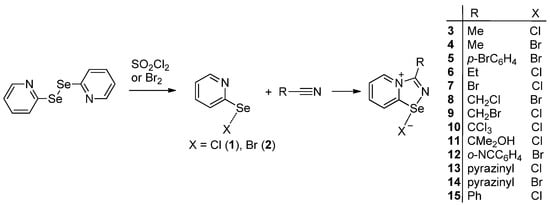
Scheme 1.
Synthesis of 3–15.
Within this work, following our interest in CN and NN triple bond activation [30,31,32,33,34,35,36,37,38,39,40,41,42,43], we attempted to prepare analogous Te derivatives. 2-Pyridyltellurenyl bromide (16) was easily synthesized by the oxidation of di-(2-pyridyl)-ditelluride with molecular bromine. Single crystals of 16 were grown from CH2Cl2 solution, and X-ray analysis demonstrated the formation of 2-pyridyltellurenyl bromide (Figure 2). Overall, metrical parameters for 16 are similar to those of earlier reported 2-pyridyltellurenyl halides [44,45].
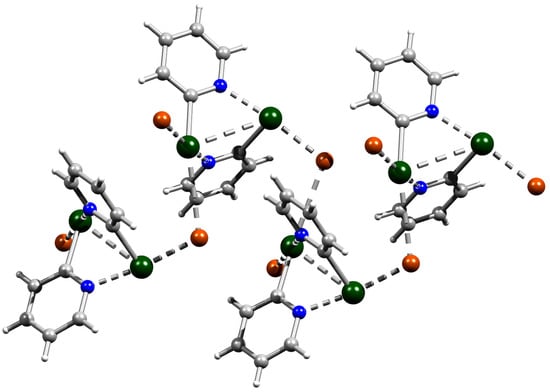
Figure 2.
Ball-and-stick representations of the crystal structures of 16, demonstrating symmetrical Te⋯Br and Te⋯N chalcogen bonding and Te⋯Te interactions. Grey and light-grey, blue, dark-green, and brown spheres represent carbon, hydrogen, nitrogen, tellurium, and bromine atoms, respectively.
Interestingly, in the crystal packing of 16 each Te atom is involved in four different noncovalent contacts: Te⋯Te interactions, two Te⋯Br ChB and one Te⋯N ChB contacts forming 3D supramolecular symmetrical framework.
In contrast, the Se analog 1, which we described earlier, does not exhibit Se⋯Se interactions in the crystal but features analogous Se⋯N ChB and terminal Se⋯Cl ChB forming supramolecular dimers (Figure 3) [46].
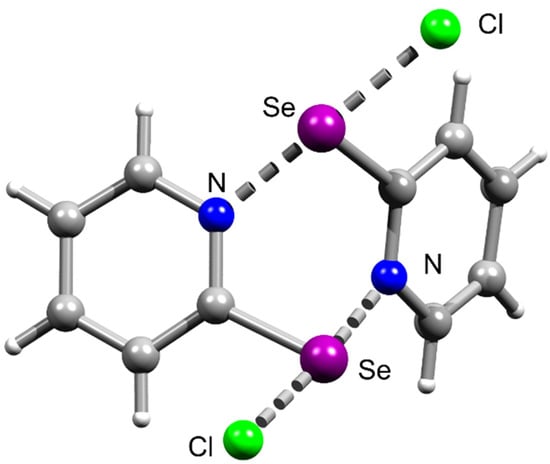
Figure 3.
Ball-and-stick representations of the crystal structures of 1, demonstrating symmetrical Se⋯Cl and Se⋯N chalcogen bonding. Grey and light-grey spheres represent carbon and hydrogen atoms, respectively.
Further, we were interested whether 2-pyridyltellurenyl bromide 16 would react with nitriles in a similar fashion as the Se analogs 1 or 2, which we showed to easily react with a broad scope of nitriles. Surprisingly, 16 turned to be inert towards nitriles. 16 did not react with PhCN, CCl3CN, or MeCN in CH2Cl2 at room temperature or at slight heating (60 °C).
Thus, switching from the Te to Se in 2-pyridyltellurenyl halides results in a dramatic impact on its reactivity towards nitriles.
In an extension of our earlier works, here we demonstrate that benzonitrile also easily reacts with 1 forming cationic 1,2,4-selenadiazole 15 in excellent yield (Scheme 1). Single crystals of 15 were obtained from CH2Cl2, and X-ray analysis pointed to the generation of the adduct with PhCN (Figure 4).
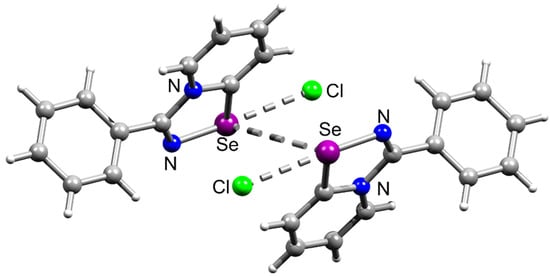
Figure 4.
Ball-and-stick representations of the crystal structures of 15, demonstrating symmetrical Se⋯Cl chalcogen bonding and Se⋯Se interactions. Grey and light-grey spheres represent carbon and hydrogen, respectively.
Interestingly, the adduct 15 formed supramolecular dimers via Se⋯Se interactions in the solid-state, which we never observed before. For the earlier studied cationic 1,2,4-selenadiazoles we observed supramolecular dimerization via four-center Se⋯N ChB, Se⋯Cl ChB and H⋯Cl interactions (Figure 5), but never via Se⋯Se contacts.
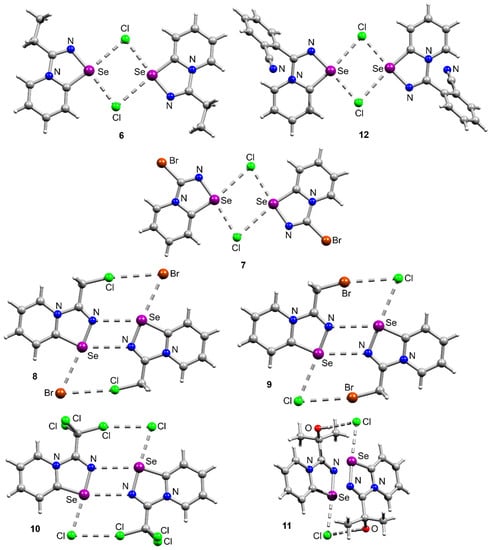
Figure 5.
Ball-and-stick representations of the crystal structures of 6–12, demonstrating supramolecular dimerization via ChB or HB. Grey and light-grey spheres represent carbon and hydrogen, respectively.
Inspection of the crystallographic data revealed the presence of several nontrivial noncovalent interactions in the crystal structures 1, 15, and 16. To understand the nature and quantify the strength of these noncovalent interactions, the quantum chemical calculations and QTAIM analysis [27] were carried out at the ωB97X-D3/Sapporo-DZP-2012 level of theory. For results of QTAIM analysis, see Table 1, and Figure 6, Figure 7 and Figure 8 shown diagrams of the Laplacian of electron density distribution ∇2ρ(r) as well as electron localization function (ELF) and reduced density gradient (RDG) analyses for these noncovalent interactions in the crystal structures of 1, 15 and 16.

Table 1.
Electron densities–ρ(r), electron density Laplacians–∇2ρ(r) and appropriate λ2 values, densities of energy–Hb, potential energy densities–V(r), and Lagrangian kinetic energies–G(r) (a.u.) at the bond critical points (3, −1), associated with various nontrivial noncovalent interactions in the model supramolecular associates 1, 15, and 16, and estimated strength for these contacts Eint (kcal/mol).
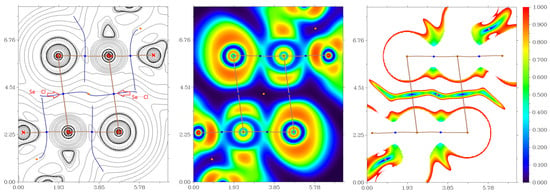
Figure 6.
Laplacian of electron density distribution ∇2ρ(r) (left panels), visualization of electron localization function (ELF, center panels) and reduced density gradient (RDG, right panels) analyses for noncovalent interactions Se⋯Cl in the crystal structure of 1. Bond critical points (3, −1) are shown in blue, the color scale for the ELF and RDG maps is presented in a.u., length units–Å.
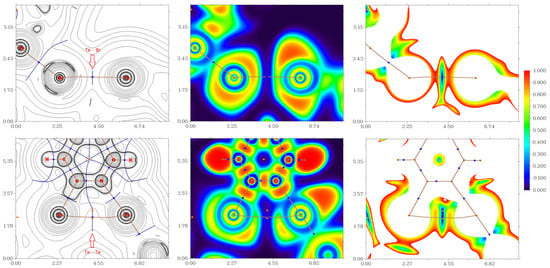
Figure 7.
Laplacians of electron density distribution ∇2ρ(r) (left panels), visualization of electron localization function (ELF, center panels) and reduced density gradient (RDG, right panels) analyses for noncovalent interactions Te⋯Br and Te⋯Te in the X-ray structure 16. Bond critical points (3, −1) are shown in blue, the color scale for the ELF and RDG maps is presented in a.u., length units–Å.
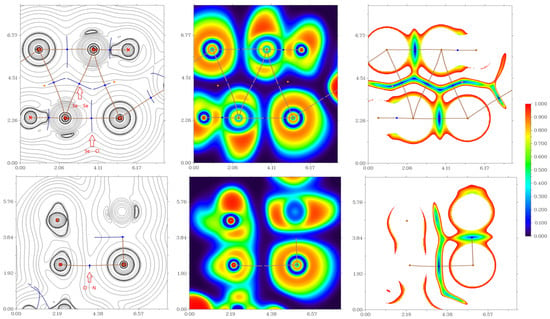
Figure 8.
Laplacians of electron density distribution ∇2ρ(r) (left panels), visualization of electron localization function (ELF, center panels) and reduced density gradient (RDG, right panels) analyses for noncovalent interactions Se⋯Se, Se⋯Cl, and Cl⋯N in the X-ray structure 15. Bond critical points (3, −1) are shown in blue, the color scale for the ELF and RDG maps is presented in a.u., length units–Å.
The QTAIM analysis of model supramolecular associates 1, 15, and 16 reveals the existence of critical bond points (3, −1) for noncovalent interactions listed in Table 1 and shown in Figure 6, Figure 7 and Figure 8. The low magnitude of the electron density (0.006–0.012 a.u.), positive values of the Laplacian of electron density (0.017–0.031 a.u.), and very close to zero energy density (0.000–0.001 a.u.) in appropriate bond critical points (3, −1) and energies for these short contacts (0.9–1.9 kcal/mol) are typical for weak noncovalent interactions involving halogen and chalcogen atoms in similar chemical systems [6,7,9,49,50,51,52]. The ratio –G(r)/V(r) > 1 at the bond critical points (3, −1) reveals that the nature of appropriate interaction is purely noncovalent [53]. The sign of λ2 can be used to distinguish bonding (λ2 < 0, attractive) weak contacts from nonbonding ones (λ2 > 0, repulsive) [54,55]. Thus, discussed weak noncovalent interactions in 1, 15 and 16 are attractive (Table 1).
In conclusion, we described the synthesis and characterization of 2-pyridyltellurenyl bromide and attempted to perform its cyclization with nitriles. In contrast to the Se analogs, 2-pyridyltellurenyl bromide does not react with nitriles. We also performed structural characterization of 2-pyridyltellurenyl bromide by the single-crystal X-ray diffraction. Interestingly, the Te atom of 2-pyridyltellurenyl bromide was involved in four different noncovalent contacts: Te⋯Te interactions, two Te⋯Br ChB and one Te⋯N ChB contacts forming 3D supramolecular symmetrical framework. In contrast, 1 did not exhibit Se⋯Se interactions in the crystal but featured similar Se⋯N ChB and terminal Se⋯Cl ChB forming supramolecular dimers. Within this study, we also prepared and performed a structural investigation of the adduct of 2-pyridylselenenyl chloride with benzonitrile. In contrast to earlier studied cationic 1,2,4-selenadiazoles here we observed that the adduct 15 formed supramolecular dimers via Se⋯Se interactions in the solid-state, which we never observed before.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/sym13122350/s1, Table S1: Cartesian atomic coordinates for model supramolecular associates, Table S2: Crystallographic parameters, data collection, and structure refinement details for 15 and 16.
Author Contributions
Conceptualization, A.G.T.; methodology, A.S.K. (Alexey S. Kubasov); software, A.S.N.; validation, Z.V.M. and A.V.B.; formal analysis, A.S.N. and A.S.K. (Andreii S. Kritchenkov); investigation, M.M.G. and J.M.L., I.V.B. and M.V.G.; resources, V.N.K.; data curation, I.V.B.; writing—original draft preparation, A.S.N.; writing—review and editing, A.G.T. and T.V.S.; visualization, A.S.N.; supervision, A.V.B. and A.A.K.; project administration, A.G.T.; funding acquisition, A.G.T. and T.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the RUDN University Strategic Academic Leadership Program. Funding for this research was provided by the Russian Foundation for Basic Research (project number 20-53-00006) and the Belarusian Foundation for Fundamental Research (grant X20P-066).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular Polymers: Historical Development, Preparation, Characterization, and Functions. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebov, A.G.; Novikov, A.S.; Odintsova, O.V.; Mikhaylov, V.N.; Sorokoumov, V.; Serebryanskaya, T.V.; Starova, G.L. Supramolecular polymers derived from the PtII and PdII schiff base complexes via C(sp2)–H … Hal hydrogen bonding: Combined experimental and theoretical study. J. Organomet. Chem. 2019, 886, 71–75. [Google Scholar] [CrossRef]
- Repina, O.V.; Novikov, A.S.; Khoroshilova, O.V.; Kritchenkov, A.S.; Vasin, A.A.; Tskhovrebov, A.G. Lasagna-like supramolecular polymers derived from the PdII osazone complexes via C(sp2)–H⋯Hal hydrogen bonding. Inorg. Chim. Acta 2020, 502, 119378. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Khrustalev, V.N. Identification of supramolecular dimers in the crystal structure of (Z)-1-(((5-fluoropyridin-2-yl)amino)methylene)naphthalen-2(1H)-one via C(sp2)–H⋯F HYDROGEN BONDING: A combined experimental and theoretical study. J. Struct. Chem. 2021, 62, 460–466. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Shikhaliyev, N.G.; Maharramov, A.M.; Bagirova, K.N.; Suleymanova, G.T.; Novikov, A.S.; Khrustalev, V.N.; Tskhovrebov, A.G. Halogenated Diazabutadiene Dyes: Synthesis, Structures, Supramolecular Features, and Theoretical Studies. Molecules 2020, 25, 5013. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Tupertsev, B.S.; Nazarov, A.A.; Antonets, A.A.; Astafiev, A.A.; Kritchenkov, A.S.; Kubasov, A.S.; Nenajdenko, V.G.; Khrustalev, V.N. Azoimidazole gold(III) complexes: Synthesis, structural characterization and self-assembly in the solid state. Inorg. Chim. Acta 2021, 522, 120373. [Google Scholar] [CrossRef]
- Shikhaliyev, N.G.; Maharramov, A.M.; Bagirova, K.N.; Suleymanova, G.T.; Tsyrenova, B.D.; Nenajdenko, V.G.; Novikov, A.S.; Khrustalev, V.N.; Tskhovrebov, A.G. Supramolecular organic frameworks derived from bromoaryl-substituted dichlorodiazabutadienes via Cl⋯Br halogen bonding. Mendeleev Commun. 2021, 31, 191–193. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Kritchenkov, A.S.; Khrustalev, V.N.; Haukka, M. Attractive halogen⋯halogen interactions in crystal structure of trans-dibromogold(III) complex. Z. Krist.-Cryst. Mater. 2020, 235, 477–480. [Google Scholar] [CrossRef]
- Ams, M.R.; Trapp, N.; Schwab, A.; Milić, J.V.; Diederich, F. Chalcogen Bonding “2S–2N Squares” versus Competing Interactions: Exploring the Recognition Properties of Sulfur. Chem.-A Eur. J. 2019, 25, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Khrustalev, V.N.; Grishina, M.M.; Matsulevich, Z.V.; Lukiyanova, J.M.; Borisova, G.N.; Osmanov, V.K.; Novikov, A.S.; Kirichuk, A.A.; Borisov, A.V.; Solari, E.; et al. Novel cationic 1,2,4-selenadiazoles: Synthesis via addition of 2-pyridylselenyl halides to unactivated nitriles, structures and four-center Se⋯N contacts. Dalton Trans. 2021, 50, 10689–10691. [Google Scholar] [CrossRef]
- Leitch, A.A.; Brusso, J.L.; Cvrkalj, K.; Reed, R.W.; Robertson, C.M.; Dube, P.A.; Oakley, R.T. Spin-canting in heavy atom heterocyclic radicals. Chem. Commun. 2007, 3368–3370. [Google Scholar] [CrossRef]
- Brusso, J.L.; Cvrkalj, K.; Leitch, A.A.; Oakley, R.T.; Reed, R.W.; Robertson, C.M. Resonance Stabilized Bisdiselenazolyls as Neutral Radical Conductors. J. Am. Chem. Soc. 2006, 128, 15080–15081. [Google Scholar] [CrossRef]
- Robertson, C.M.; Myles, D.J.T.; Leitch, A.A.; Reed, R.W.; Dooley, B.M.; Frank, N.L.; Dube, P.A.; Thompson, A.L.K.; Oakley, R.T. Ferromagnetism in a Heavy Atom Heterocyclic Radical Conductor. J. Am. Chem. Soc. 2007, 129, 12688–12689. [Google Scholar] [CrossRef]
- Lindner, B.D.; Coombs, B.A.; Schaffroth, M.; Engelhart, J.U.; Tverskoy, O.; Rominger, F.; Hamburger, M.; Bunz, U.H.F. From Thia- to Selenadiazoles: Changing Interaction Priority. Org. Lett. 2013, 15, 666–669. [Google Scholar] [CrossRef]
- Matsulevich, Z.V.; Lukiyanova, J.M.; Naumov, V.I.; Borisova, G.N.; Osmanov, V.K.; Borisov, A.V.; Grishina, M.; Khrustalev, V.N. Bromination of bis(pyridin-2-yl) diselenide in methylene chloride: The reaction mechanism and crystal structures of 1H-pyridine-2-selenenyl dibromide and its cycloadduct with cyclopentene (3aSR,9aRS)-2,3,3a,9a-tetrahydro-1H-cyclopenta[4,5][1,3]selenazolo[3,2-a]pyridinium bromide. Acta Cryst. Sect. E Cryst. Commun. 2019, 75, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Bruker, A.P.E.X.; Saint, A.X.S. Inc., Madison, WI, 2004 Search PubMed;(b) GM Sheldrick. Acta Cryst. Sect. A Fundam. Cryst. 2008, 64, 112. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Li, G.-D.; Mao, S.-P.; Chai, J.-D. Long-Range Corrected Hybrid Density Functionals with Improved Dispersion Corrections. J. Chem. Theory Comput. 2013, 9, 263–272. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Noro, T.; Sekiya, M.; Koga, T. Segmented contracted basis sets for atoms H through Xe: Sapporo-(DK)-nZP sets (n = D, T, Q). Theor. Chem. Acc. 2012, 131, 1124. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Grudova, M.V.; Khrustalev, V.N.; Kubasov, A.S.; Strashnov, P.V.; Matsulevich, Z.V.; Lukiyanova, J.M.; Borisova, G.N.; Kritchenkov, A.S.; Grishina, M.M.; Artemjev, A.A.; et al. Adducts of 2-Pyridylselenenyl Halides and Nitriles as Novel Supramolecular Building Blocks: Four-Center Se⋯N Chalcogen Bonding versus Other Weak Interactions. Cryst. Growth Des. 2021. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Tskhovrebov, A.G.; da Silva, M.F.C.G.; Haukka, M.; Pombeiro, A.J.L.; Kukushkin, V.Y. Metal-Mediated [2+3] Cycloaddition of Nitrones to Palladium-Bound Isonitriles. Chem.-A Eur. J. 2009, 15, 5969–5978. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Solari, E.; Wodrich, M.D.; Scopelliti, R.; Severin, K. Covalent Capture of Nitrous Oxide by N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2012, 51, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebov, A.G.; Vuichoud, B.; Solari, E.; Scopelliti, R.; Severin, K. Adducts of Nitrous Oxide and N-Heterocyclic Carbenes: Syntheses, Structures, and Reactivity. J. Am. Chem. Soc. 2013, 135, 9486–9492. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Solari, E.; Wodrich, M.D.; Scopelliti, R.; Severin, K. Sequential N–O and N–N Bond Cleavage of N-Heterocyclic Carbene-Activated Nitrous Oxide with a Vanadium Complex. J. Am. Chem. Soc. 2012, 134, 1471–1473. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Naested, L.C.E.; Solari, E.; Scopelliti, R.; Severin, K. Synthesis of Azoimidazolium Dyes with Nitrous Oxide. Angew. Chem. Int. Ed. 2015, 54, 1289–1292. [Google Scholar] [CrossRef]
- Eymann, L.Y.M.; Tskhovrebov, A.G.; Sienkiewicz, A.; Bila, J.L.; Živković, I.; Rønnow, H.M.; Wodrich, M.D.; Vannay, L.; Corminboeuf, C.; Pattison, P.; et al. Neutral Aminyl Radicals Derived from Azoimidazolium Dyes. J. Am. Chem. Soc. 2016, 138, 15126–15129. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Solari, E.; Scopelliti, R.; Severin, K. Insertion of Zerovalent Nickel into the N–N Bond of N-Heterocyclic-Carbene-Activated N2O. Inorg. Chem. 2013, 52, 11688–11690. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Haukka, M.; Kukushkin, V.Y. Synthesis and Characterization of cis-(RNC)2PtII Species Useful as Synthons for Generation of Various (Aminocarbene)PtII Complexes. J. Chem. Cryst. 2012, 42, 1170–1175. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Sorokoumov, V.N.; Liakhov, D.M.; Tskhovrebov, A.G.; Balova, I.A. Polystyrene-Supported Acyclic Diaminocarbene Palladium Complexes in Sonogashira Cross-Coupling: Stability vs. Catalytic Activity. Catalysts 2018, 8, 141. [Google Scholar] [CrossRef] [Green Version]
- Tskhovrebov, A.G.; Vasileva, A.A.; Goddard, R.; Riedel, T.; Dyson, P.J.; Mikhaylov, V.N.; Serebryanskaya, T.V.; Sorokoumov, V.N.; Haukka, M. Palladium(II)-Stabilized Pyridine-2-Diazotates: Synthesis, Structural Characterization, and Cytotoxicity Studies. Inorg. Chem. 2018, 57, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Varava, P.; Fabrizio, A.; Eymann, L.Y.M.; Tskhovrebov, A.G.; Planes, O.M.; Solari, E.; Fadaei-Tirani, F.; Scopelliti, R.; Sienkiewicz, A.; et al. Synthesis of aminyl biradicals by base-induced Csp3–Csp3 coupling of cationic azo dyes. Chem. Sci. 2019, 10, 5719–5724. [Google Scholar] [CrossRef] [Green Version]
- Astafiev, A.A.; Shakhov, A.M.; Kritchenkov, A.S.; Khrustalev, V.N.; Shepel, D.V.; Nadtochenko, V.A.; Tskhovrebov, A.G. Femtosecond laser synthesis of nitrogen-doped luminescent carbon dots from acetonitrile. Dye. Pigment. 2021, 188, 109176. [Google Scholar] [CrossRef]
- Astafiev, A.A.; Repina, O.V.; Tupertsev, B.S.; Nazarov, A.A.; Gonchar, M.R.; Vologzhanina, A.V.; Nenajdenko, V.G.; Kritchenkov, A.S.; Khrustalev, V.N.; Nadtochenko, V.N.; et al. Unprecedented Coordination-Induced Bright Red Emission from Group 12 Metal-Bound Triarylazoimidazoles. Molecules 2021, 26, 1739. [Google Scholar] [CrossRef]
- Tskhovrebov, A.; Haukka, M. cis-Dichloridobis(2-isocyanophenyl 4-methoxybenzoate)palladium(II) chloroform monosolvate. Acta Cryst. Sect. E Struct. Rep. Online 2012, 68, m1476–m1477. [Google Scholar] [CrossRef] [Green Version]
- Khrustalev, V.N.; Matsulevich, Z.V.; Lukiyanova, J.M.; Aysin, R.R.; Peregudov, A.S.; Leites, L.A.; Borisov, A.V. A Facile Route for Stabilizing Highly Reactive ArTeCl Species Through the Formation of T-Shaped Tellurenyl Chloride Adducts: Quasi-Planar Zwitterionic [HPy*]TeCl2 and [HPm*]TeCl2; Py* = 2-pyridyl, Pm* = 2-(4,6-dimethyl)pyrimidyl. Eur. J. Inorg. Chem. 2014, 2014, 3582–3586. [Google Scholar] [CrossRef]
- Da Silva, F.D.; Simões, C.A.D.P.; dos Santos, S.S.; Lang, E.S. Versatility of Bis(2-pyridyl)ditellane. ChemistrySelect 2017, 2, 2708–2712. [Google Scholar] [CrossRef]
- Khrustalev, V.N.; Matsulevich, Z.V.; Aysin, R.; Lukiyanova, J.M.; Fukin, G.K.; Zubavichus, Y.V.; Askerov, R.K.; Maharramov, A.M.; Borisov, A.V. An unusually stable pyridine-2-selenenyl chloride: Structure and reactivity. Struct. Chem. 2016, 27, 1733–1741. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii of Metals in Covalent Compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Khrustalev, V.N.; Savchenko, A.O.; Zhukova, A.I.; Chernikova, N.Y.; Kurykin, M.A.; Novikov, A.S.; Tskhovrebov, A.G. Attractive fluorine⋯fluorine interactions between perfluorinated alkyl chains: A case of perfluorinated Cu(II) diiminate Cu[C2F5–C(NH)–CF=C(NH)–CF3]2. Z. Krist.-Cryst. Mater. 2021, 236, 117–122. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Abramov, P.A.; Novikov, A.; Plyusnin, P.E.; Sokolov, M.N.; Fedin, V.P. Bromo- and Polybromoantimonates(V): Structural and Theoretical Studies of Hybrid Halogen-Rich Halometalate Frameworks. Chem.-A Eur. J. 2018, 24, 10165–10170. [Google Scholar] [CrossRef]
- Adonin, S.A.; Udalova, L.I.; Abramov, P.A.; Novikov, A.; Yushina, I.V.; Korolkov, I.V.; Semitut, E.Y.; Derzhavskaya, T.A.; Stevenson, K.; Troshin, P.A.; et al. A Novel Family of Polyiodo-Bromoantimonate(III) Complexes: Cation-Driven Self-Assembly of Photoconductive Metal-Polyhalide Frameworks. Chem.-A Eur. J. 2018, 24, 14707–14711. [Google Scholar] [CrossRef]
- Mikherdov, A.S.; Kinzhalov, M.A.; Novikov, A.S.; Boyarskiy, V.P.; Boyarskaya, I.A.; Dar’In, D.V.; Starova, G.L.; Kukushkin, V.Y. Difference in Energy between Two Distinct Types of Chalcogen Bonds Drives Regioisomerization of Binuclear (Diaminocarbene)PdII Complexes. J. Am. Chem. Soc. 2016, 138, 14129–14137. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-Garcia, J.; Cohen, A.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).