Abstract
In this work, we prepared new biocompatible N-alkyl cholinium-based ionic liquids to be used as cosolvents to improve the solubility of poorly water-soluble drugs, namely, sodium diclofenac and paracetamol. In this set of ionic liquids, we intend to understand the effect of increasing the asymmetry of the ionic liquid cation/anion by growing the length of one of the alkyl chains attached to the nitrogen center/sulfonate center on the dissolution capacity of the ionic liquid. The addition of these new ionic liquids to water increased the dissolution capacity of the drugs up to four-times that in water, and improved the pharmacodynamic properties of these drugs, especially the case of sodium diclofenac. The intermolecular interactions between the drugs and ionic liquids were investigated by NMR. Two-dimensional 1H/1H nuclear overhauser effect spectroscopy (NOESY) revealed an interaction between sodium diclofenac and the alaninate anion from the [C2Ch]2[SucAla]. In the case of paracetamol and [C4Ch][C2SO3], it was possible to observe two intermolecular interactions between the hydroxyl group of paracetamol and two protons from the cation [C4Ch]+. Interestingly, the ionic liquid bearing a succinyl-DL-alaninate anion, [SucAla]2−, and a N-ethyl cholinium cation, [C2Ch]+, which presented the highest ability to dissolve sodium diclofenac, showed no cytotoxicity up to 500 mM. Therefore, this ionic liquid is a potential candidate for drug delivery applications.
1. Introduction
Almost 60–70% of drug molecules are insufficiently soluble in aqueous media and/or have very low permeability to allow their adequate and reproducible absorption from the gastrointestinal tract [1].
Ionic liquids (ILs) are able to dissolve both polar and nonpolar species, and their use can notably enhance the pharmacokinetic and pharmacodynamic properties of drugs. Lately, it was found that ILs are very powerful in dissolving some important drug molecules that are very slightly soluble in water and most of the pharmaceutically accepted organic solvents [2]. One of the most common techniques to increase the water solubility of poorly water-soluble drugs is to transform them in the anion and/or cation of an ionic liquid. Some examples include diclofenac, ibuprofen, ketoprofen, naproxen, sulfadiazine, sulfamethoxazole, and tolbutamide [3,4,5,6,7,8,9]. In most of the reported literature, no cytotoxicity values for the API-ILs are presented. Only recently, M.M. Santos et al. [10] synthesized several [Ibu]-ILs and studied their cytotoxicity in human normal dermal fibroblasts and in human ovarian carcinoma cell line A2780. With exception of [C16Pyr][Ibu], these ILs did not present cytotoxicity at 1 mM for human dermal fibroblasts and 100 µM for A2780 cells.
From a distinct perspective, T.E. Sintra et al. [11] studied the capacity of imidazolium, pyridinium, piperidinium, pyrrolidinium, phosphonium, ammonium, and cholinium ionic liquids to improve the solubility of ibuprofen in water. They studied the solubility of ibuprofen in ionic liquid aqueous solutions with concentrations between 0.02 and 1.3 M. The results indicate that for ibuprofen there is an exceptional increase in the solubility due to the formation of ionic liquids-drug aggregates.
In the reported literature, to the best of our knowledge, there are almost no data available for the solubility enhancement of sodium diclofenac mediated by ionic liquids. Formerly, our group showed that N-acetyl amino acid N-alkyl cholinium-based ILs were able to significantly increase the water solubility of two poorly water-soluble drugs, paracetamol and sodium diclofenac [12].
Cholinium-based ionic liquids proved to constitute a more sustainable alternative to the imidazolium-based ones, due to their very low toxicity, biocompatibility, easy availability, and cost-effectiveness [13,14,15,16,17]. Examples of hydrophilic ionic liquids that present very low (eco)toxicity in aqueous solutions in comparison to other types of ionic liquids include choline saccaharinate and choline acesulfamate [18]. Moreover, organic anions based on acetate, sulphate, and phosphate groups are considered readily biodegradable.
The discovery of more biocompatible and biodegradable ILs would promote their application in the field of pharmaceuticals, including drug formulation and drug delivery technologies [19].
In this work, we designed new ILs combining the high ability to increase the aqueous solubility of distinct drugs with a very low cytotoxicity profile. In literature, sulfonate anions are often reported to improve biocompatibility [20] and biodegradability of many molecules, which led us to choose these anions for this study. Moreover, the succinyl alaninate anion is a precursor for a variety of biological oligopeptides and, therefore biocompatible [21].
Hence, we synthesized 12 new ILs bearing a N-alkyl cholinium cation (Figure 1a) and an alkyl sulfonate anion (Figure 1b). Four new dianionic ILs containing a succinyl-DL-alaninate anion keeping some N-alkyl cholinium cation were also synthesized (Figure 1c).
Figure 1.
(a) N-alkyl cholinium-based cations; (b) alkyl sulfonate anions; (c) N-succinyl-DL-alaninate anion.
Our research group already reported that dianionic ionic liquids based on ammonium cations and carboxylic and/or phosphonate anions are able to increase the solubility of ibuprofen and piroxicam, but all of them presented an IC50 between 55–120 mM [22,23].
Excipients are very important because they deliver the active drug to the site in the body where the drug employs its action and keep it from being released too early in the absorption task [24,25]. The study of these new cholinium-based ionic liquids as green excipients and their influence on the solubility of the poorly water drugs, paracetamol and sodium diclofenac, was evaluated. The increment on solubility was compared with common excipients such as sodium bicarbonate, lactose, and tartaric acid.
2. Materials and Methods
A detailed analysis of data on all the synthesized ionic liquids is given in the ESI.
Butane sulfonyl chloride (98%), methane sulfonyl chloride (98%), triethylamine (99%), succinic anhydride (99%), DL-alanine (99%), and D-lactose (98%) were purchased from Alfa-Aesar and used as received. Ethane sulfonyl chloride (99%), N,N-dimethylethanolamine (≥99.5%), sodium bicarbonate (≥99.7%), and tartaric acid (99%) were purchased from Aldrich and used as received. Glacial acetic acid (100%) was purchased from Merck and used without further purification. Toluene (≥99.9%), dichloromethane (≥99.9%), ethyl acetate (≥99.5%), hexane (≥99.9%), and diethyl ether (≥99.9 %) were purchased from Aldrich.
General procedure for the synthesis of alkyl sulfonate esters. Alkyl sulfonate esters were prepared as described elsewhere [26]. Shortly, alkan-1-ol (1.1 equiv.) and triethylamine (1.1 equiv.) were dissolved in dichloromethane (50 mL), placed in an ice-bath and stirred vigorously. Alkane sulfonyl chloride (1.0 mmol) dissolved in dichloromethane (10 mL) was added dropwise to keep the reaction temperature close to 0 ºC. Then, the temperature could increase until room temperature, and the reaction was maintained under stirring for several hours until the formation of a white precipitate. The solid was removed by filtration and the solvent was evaporated in rotavapor. The purity of alkyl sulfonate esters was confirmed by 1H-NMR.
General procedure for the synthesis of N-alkyl cholinium N-alkyl sulfonate ionic liquids. Alkyl sulfonate ester (3 g) and N,N-dimethyl ethanolamine (1.05 equiv.) were dissolved in ethyl acetate (30 mL) and stirred overnight at 80–90 °C. After cooling, the ionic liquid was washed with acetone or diethyl ether and dried in high-vacuum overnight. 1H and 13C NMR was used to evaluate the purity of these ionic liquids.
Synthesis of N-succinyl-DL-alanine. DL-Alanine (0.1 mol) and succinic anhydride (1.05 equiv.) were dissolved in glacial acetic acid (100 mL). The reaction was stirred for 90 min at 70 °C until total dissolution of the reactants. Then, toluene was added, and the acetic acid/toluene mixture was removed by evaporation. The white solid formed was washed with toluene and dried in high vacuum overnight. The purity of target compound was estimated by 1H-NMR.
General procedure for the synthesis of N-alkyl cholinium N-succinyl-DL-alanine ionic liquids. N-alkyl cholinium bromide ionic liquids were prepared as previously [27]. An aqueous solution of N-alkyl cholinium bromide (10 mmol) was passed slowly through an anion exchange column AmberliteTM IRN-78. Then, the corresponding hydroxide solution was slowly added to an aqueous solution of N-succinyl-DL-alanine (0.5 equiv.). The reaction mixture stirred at room temperature for 2–3 h prior water removal by evaporation. Ionic liquids were dried in high vacuum for at least 24 h. The purity of ILs was assessed by 1H and 13C NMR experiments (Figure S1).
Solubility assays. A calibration curve was prepared for both drugs in water. The maximum absorbance wavelength for paracetamol is 243 nm for paracetamol, and 277 nm for sodium diclofenac, at 25 °C. The absorbance values were always kept below 1.
General procedure for the determination of the solubility limit of Paracetamol and Sodium Diclofenac in IL/water solutions. The solubility limit for paracetamol and sodium diclofenac were determined as described previously [12]. Briefly, aqueous solutions (5 mL) of ionic liquids (1 mol% or 0.2 mol%) were prepared, and paracetamol or sodium diclofenac (325 mg or 500 mg) was added to each sample (at this concentration we are above the solubility limit for all the samples). The solutions were stirred for 24 h to ensure that the solubility limit is achieved, and temperature of assay was 37 °C. Triplicates were prepared for each sample. The solubility of both drugs in the presence of each ionic liquid was calculated from the calibration curve. The pH was checked during assay and no variation was observed.
General procedure for the determination of partition coefficient and log p. To determine the Kow and log p values of paracetamol and sodium diclofenac, in the presence of ionic liquids was used the shake-flask method as previously described [12]. Triplicates were performed.
Octanol and water layers were separated and water phase was centrifuged for 30 min at 2000 rpm and analysed in a UV-Vis VWR® spectrophotometer (UV-6300PC, Radnor, PA, USA), using a previously prepared calibration curve (Figure S2). Samples were diluted until the absorbance was smaller than 1.
The drug concentration in the octanol-rich phase was directly calculated by subtracting the amount in the water-rich phase to the initial amount dissolved.
Statistical analysis. Graphpad Prism 7 was used for statistical analysis. Statistical significant differences were calculated through One-Way Analysis of Variance (ANOVA) by Tukey’s multiple comparisons tests and a p-value < 0.05 was considered significant. Data are expressed as average ± standard deviation from at least three independent experiments.
Cell Culture. Primary dermal normal fibroblasts (ATCCP® PCS-201-010, ATCC, Manassas, VA, USA) were cultured in DMEM, Dulbecco’s modified Eagle’s medium, supplemented with 10% (v/v) FBS, Fetal Bovine Serum, 100 U/mL penicillin, and 100 mg/mL streptomycin (all from Thermofisher Scientific, Waltham, MA, USA) at 37 °C in an atmosphere with 5% (v/v) CO and 99% (v/v) relative humidity [12,28].
Viability assays. Cell viability was evaluated via the MTS method as previously described [12,26]. Briefly, 96-well plates (SPL Life Sciences, Co., Ltd. Naechon-myeon, Korea) were used to incubate fibroblasts (7500 cells per well), for 48 hours with different concentrations of selected ionic liquids. The CellTiter 96® Aqueous One Solution Cell Proliferation Assay System (Promega, Fitchburg, WI, USA) was used, according to the manufacturer’s instructions, to evaluate cell viability. Formazan is produced by viable cells by the reduction of the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS). The formazan product can be quantified by measuring the absorbance at 490 nm in an Infinite M200 microplate reader (Tecan, Mannedorf, Switzerland).
Statistical analysis was performed using the GraphPad Prism v7.00 (GraphPad Software Inc, La Jolla, CA, USA). Data were expressed as mean ± standard deviation from at least 3 independent biological experiments.
3. Results and Discussion
3.1. Solubility Assays
We studied the enhancement of water solubility of two commercial drugs, paracetamol and sodium diclofenac in aqueous solutions containing a small percentage (0.2–1.0 mol%) of different ionic liquids. We tested 16 new ionic liquids containing a cation from the N-alkyl cholinium family and different anions.
The values 15.0 mg/mL and 20.4 mg/mL obtained for the solubility of paracetamol and sodium diclofenac in water at 25 °C were obtained previously [12]. These values closely agree with the ones reported in literature, 14.9 mg/mL and 19.4 mg/mL, respectively [29].
In this study, all the measurements were performed at 37 °C to mimic the body temperature. Therefore, at this temperature the experimental results obtained for the maximum solubility of paracetamol and sodium diclofenac in pure water was 18.0 mg/mL and 34.5 mg/mL, respectively. We also measured the maximum solubility of each drug at the same temperature in aqueous solution of ionic liquids. Figure 2 shows the maximum solubility of paracetamol in 1.0 mol% ionic liquids aqueous solutions and pure water, for comparison purposes. It was possible to observe that all the ionic liquids upgrade a higher solubility (28–57 mg/mL). The ionic liquids that presented a larger enhancement of the drug solubility were [C4Ch][C4SO3] and [C5Ch]2[SucAla], showing solubility values higher than 50.0 mg/mL.
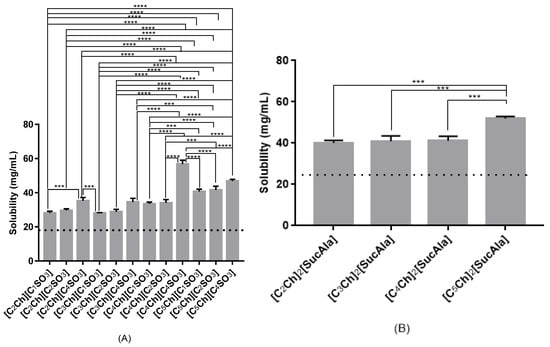
Figure 2.
Solubility of paracetamol in aqueous solutions (1.0 mol%) of distinct monoanionic ionic liquids (A) and dianionic ionic liquids (B). Dashed line represents solubility limit of paracetamol in water and is use for comparison purposes. Only most significant statistical differences are represented in asterisks: **** p < 0.0001 and *** p = 0.0007.
It was also possible to observe that in general, ILs with longer alkyl chains in the cation led to higher values of solubility for this drug. The exception was the IL [C5Ch][C4SO3], in which the solubility is slightly lower than the one in [C4Ch][C4SO3]. Moreover, it was also visible that the longer the chain in the anion moiety the highest solubility of paracetamol.
Furthermore, we decided to evaluate the solubility of paracetamol in aqueous solutions with lower ionic liquid concentration (0.5 mol%) to understand the importance of the ionic liquid concentration and to decrease a potential toxicity effect. Hence, we selected the 3 ionic liquids that afforded the highest solubility capacity in the first experiments. The results are presented in Figure 3, and it is visible that higher ionic liquid concentration implies higher drug dissolution capacity, except for [C3Ch]2[SucAla] aqueous solution, in which the drug solubility is the same at both IL concentrations.
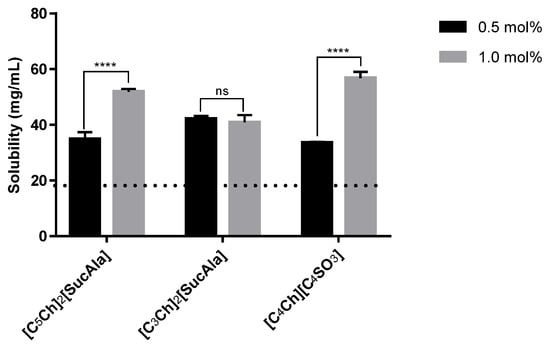
Figure 3.
Solubility of paracetamol in 0.5 mol% vs. 1.0 mol% IL aqueous solutions Horizontal line represents solubility of the drug in pure water. Statistical significant differences are represented in asterisks: **** p < 0.0001 and ns—non significant.
Moreover, we also compared the amount of paracetamol dissolved in the 1.0 mol% aqueous solution of ionic liquids after 6 h and 24 h (Figure 4). We chose two representative ionic liquids for this purpose and the results are showed in Figure 4. More than 6 hours are necessary to achieve the maximum solubility of the drug in these systems. Paracetamol solubility tests were also performed for 48 h, and the values were very similar to those obtained at 24 h.
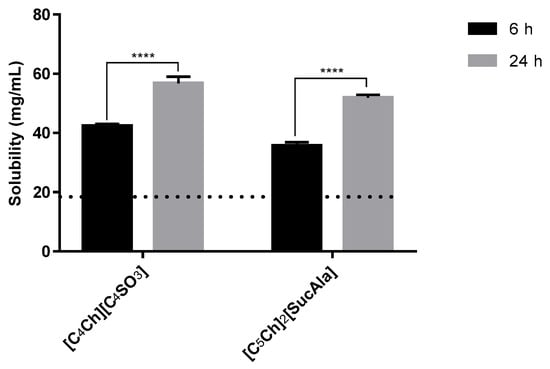
Figure 4.
Solubility of paracetamol after 6 h vs. 24 h in 1.0 mol% IL aqueous solutions Horizontal line represents maximum solubility in water. Statistical significant differences are represented in asterisks: **** p < 0.0001.
To test the maximum solubility of sodium diclofenac in aqueous solutions of the same 16 ionic liquids, we performed preliminary tests that showed that using only 0.2 mol% of these ILs is enough to significantly improve the maximum solubility of this drug, which could be useful in a pharmaceutical context. In Figure 5, we present the solubility limit of sodium diclofenac in aqueous solutions of ionic liquids at 0.2 mol%. The solubility data ranged from 76–99 mg/mL, which is significantly higher than that in water (34.5 mg/mL).
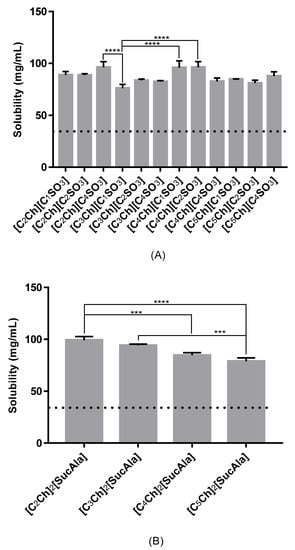
Figure 5.
Solubility of sodium diclofenac in and aqueous solutions (0.2 mol%) of distinct monoanionic ionic liquids (A) and dianionic ionic liquids (B). Dashed horizontal line represents solubility limit of drug in water. Only most significant statistical differences are represented in asterisks: **** p < 0.0001 and *** p = 0.0007.
In contrast with the previous example, in this case it was not possible to observe any correlation between the length of the cation/anion moiety and the solubility limit for ionic liquids bearing an alkyl sulfonate anion.
Regarding the ionic liquids containing a succinyl-DL-alaninate anion ([SucAla]) it is showed in Figure 5 that the highest values of solubility were achieved in the presence of the IL with the shortest N-alkyl chain in the cation moiety. The increase in the lipophilicity of the IL due to the existence of two cation moieties may explain the results obtained since the increase of the N-alkyl chain leads to a poorer dissolution of the drug.
The three best ionic liquids were used to evaluate their ability to improve the water solubility of sodium diclofenac in only 0.1 mol% aqueous solution of these ionic liquids (Figure 6). Interestingly, the ionic liquid [C3Ch]2[SucAla] afforded similar results at both concentrations. On the other hand, for the other two ILs, it is required at least 0.2 mol% to achieve considerably high values of solubility. Nevertheless, 0.1% mol of any of these ILs can improve the solubility of the drug, if compared with water alone.
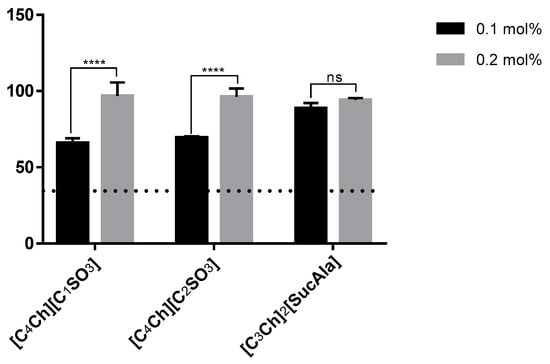
Figure 6.
Solubility of sodium diclofenac at 0.1 mol% vs. 0.2 mol% IL (horizontal line represents solubility in water). Statistical significant differences are represented in asterisks: **** p < 0.0001 and ns—nonsignificant.
The effect of the dissolution time of sodium diclofenac in the aqueous solution of some ionic liquids (0.2 mol%) was evaluated. In Figure 7 are presented the results and, it is evident that after 24 h it was possible to dissolve ca. 10 mg/mL more than after 6 h, in both cases.
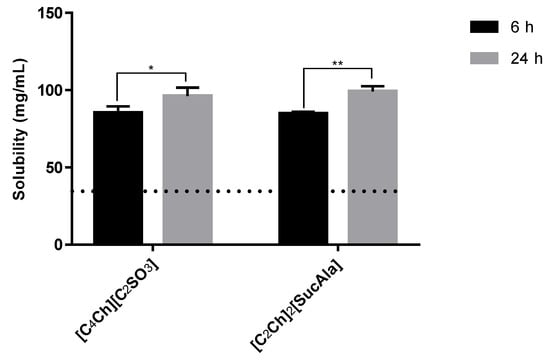
Figure 7.
Solubility of sodium diclofenac at 6 h vs. 24 h (horizontal line represents maximum solubility in water). Statistical significant differences are represented in asterisks: * p = 0.0356 and ** p = 0.0078.
For comparison purposes, common excipients like tartaric acid and sodium bicarbonate were tested as solubility enhancers of paracetamol with the same molar concentration of the ILs used in this study, and lactose and sodium bicarbonate were tested for sodium diclofenac (Figure S3). These compounds are used in commercial formulations, but they do not increase the solubility of any of these drugs, which suggests that these new ILs are potential candidates as excipients for new drug formulations.
3.2. Two Dimensional 1H/1H NOESY
A sample of paracetamol with [C4Ch][C2SO3] was prepared to observe the intermolecular interaction between drug and ionic liquid, using a 2D 1H-1H NOESY experiment in DMSO-d6. The molar concentrations used mimic the conditions used in solubility assays. The spectrum obtained is showed in Figure 8.
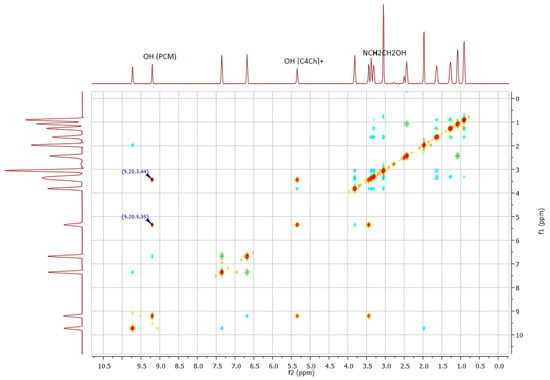
Figure 8.
2D 1H-1H NOESY NMR data of mixture of paracetamol with [C4Ch][C2SO3].
Clearly, there is an intermolecular interaction between the OH group from paracetamol (δ9.20 ppm) and OH group from the cation [C4Ch]+ (δ5.35 ppm). There is also an intermolecular interaction between the OH group from paracetamol at (δ9.20 ppm) and methylene group connected to N atom from the cation (NCH2CH2OH) at (δ3.44 ppm). These interactions may justify the improvement on the solubility of this drug in the aqueous solution of this IL.
Analogously, a NOESY experiment of sodium diclofenac with dianionic IL [C2Ch]2[SucAla] was also performed in DMSO-d6, as presented in Figure 9. The molar concentrations used mimic the conditions in solubility assay.
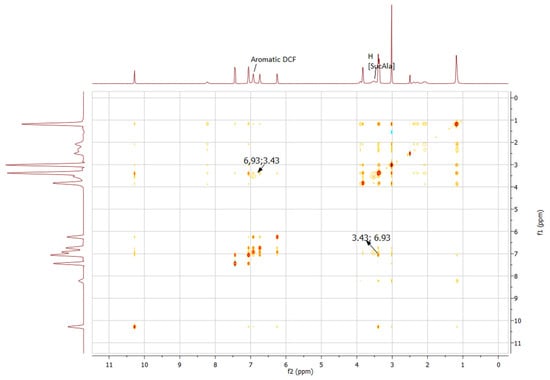
Figure 9.
2D 1H-1H NOESY NMR spectrum of sodium diclofenac with IL [C2Ch]2[SucAla].
From the NMR data, it was possible to observe the hydrophobic intermolecular interaction between the aromatic protons of sodium diclofenac (δ6.93 ppm) and proton from the anion [SucAla] (δ3.43 ppm), with suppression of methyl group at (δ1.09 ppm).
3.3. Octanol-Water Partition Coefficient and Log p
Neither of paracetamol or sodium diclofenac possess an optimal lipophilicity parameter; paracetamol has a log p = 0.3–0.56[30] and sodium diclofenac has a log p > 4 [31]. A drug must have a log p value between 1 and 3 to have a good balance between permeability and solubility.
In this study, we proved that these new 16 ionic liquids are able to improve water solubility of both drugs; however, we still need to prove that it is also possible to improve their pharmacological properties. For that purpose, we have determined the log p of both drugs in water/IL systems (Table 1).

Table 1.
Log p values for paracetamol and sodium diclofenac.
For each drug, we selected the ionic liquids that promoted the highest solubility values. Log p of sodium diclofenac in water was not determined since the separation of octanol-water phases was not possible [32].
Analysing the results for paracetamol, it is visible, as expected, that the presence of the small percentage of IL slightly decrease the log p value, and the values are quite similar independently of the IL type. Nevertheless, it was possible with these systems to increase up to 3.2-times its solubility without drastically changing its lipophilicity.
On the other hand, the ability of sodium diclofenac to move to the aqueous phase is highly affected by the presence of ionic liquids in solution. However, the results are dependent on the type of IL. For instance, the two ILs with an alkane sulfonate anion gave very similar values (1.5–1.7), but slightly different of those with a dianionic anion (1.0–2.2). Moreover, even within the same family of dianionic ILs, the size of the cation moiety has a great influence in the log p values.
In summary, the presence of an IL not only significantly improves the solubility of these drugs but also meliorates the pharmacodynamic properties of each drug, especially for sodium diclofenac, which presented the most outstanding results in this study.
3.4. Cell Viability Evaluation
Considering the previous results, the ILs showing an improvement in the solubility of these drugs were selected to study their effect in normal fibroblasts viability. Cell viability was assessed via the MTS method, in which a tetrazolium salt is reduced into formazan by viable cells (Figure 10 and Table 2) [12,26].
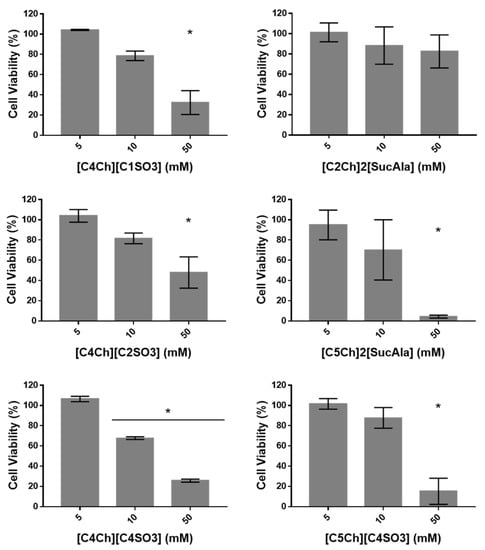
Figure 10.
Cell viability in normal human dermal fibroblasts after 48 h incubation with different concentrations of each ILs. Results are expressed as mean ± SD from at least three independent assays. Symbol * indicates that p < 0.05.

Table 2.
Values of IC50 (mM) in human normal dermal fibroblasts.
For concentrations up to 5 mM of ILs no loss in cell viability was observed (Figure 10). However, there is a concentration dependency effect on cell viability induced by ILs for concentrations higher than 10 mM except for [C2Ch]2[SucAla] (Figure 10). For this IL, no significant cytotoxicity was observed up to 500 mM (Figure 11).
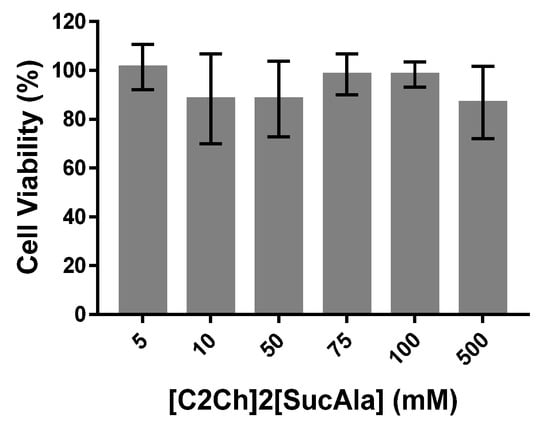
Figure 11.
Cell viability in normal human dermal fibroblasts after 48 h incubation with different concentrations of [C2Ch]2[SucAla]. Results are expressed as mean ± SD from at least three independent assays.
Interestingly, the increase in the length of the N-alkyl chain of the cholinium cation leads to a higher loss of cell viability, which is visible observing the ILs with succinyl-DL-alaninate anions. Also, the results suggested that the longer N-alkyl [C4SO3]− anion may increase the cytotoxic effect of the C4 cholinium cation, displaying a lower cellular viability than the other [C4Ch]+ ILs tested at 50 mM (Figure 10 and Table 2). These effects may be due to the ability of these ILs to permeate cell membranes, as already observed in a previous study reported by our group [12]. Moreover, this lower permeability in cell membranes might explain the lower cytotoxicity of [C2Ch]2[SucAla] compared to that of longer alkyl chains of the cholinium cation.
4. Conclusions
In this study, we showed that the presence of an ionic liquid has a relevant effect on the solubility of both paracetamol and sodium diclofenac in aqueous media, with values 2- to 4-fold higher than their solubility in pure water. It was possible to demonstrate the existence of intermolecular interactions between the drugs and the ionic liquids by NMR, which may contribute to the improvement in the solubility. NMR suggests that the increase in the solubility of paracetamol may be due to the interaction between its OH group and the two protons from cation moiety of the IL, while in the case of sodium diclofenac it can be explained by the interaction between its aromatic protons and the alaninate anion of the IL.
Paracetamol as intermolecular interaction between OH group and two protons from cation moiety of IL and sodium diclofenac between the aromatic protons and alalinate anion from [SucAla]. Moreover, we showed that these new ionic liquids are able to meliorate the pharmacodynamic properties of sodium diclofenac by increasing its affinity to aqueous systems. Additionally, they are biologically safe. The increase in the cation length directly affects their cytotoxic profile, and herein, this effect is even more pronounced when combined with a succinyl-DL-alaninate anion. Nevertheless, one IL was found to be an excellent enhancer for drug delivery showing a very low cytotoxicity, [C2Ch]2[SucAla], which makes it a potential excipient to be used in new formulations for oral drug delivery.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/sym13112053/s1, Figure S1: Characterization data for ionic liquids; Figure S2: Calibration Curves of Paracetamol and Sodium Diclofenac in octanol-saturated water; Figure S3: Solubility of Paracetamol and Sodium Diclofenac with common excipients in aqueous solutions at 37 °C after 24 h.
Author Contributions
Conceptualization, A.R.J., L.R.R. and M.R.C.S.; writing—original draft preparation, P.M.R., A.R.J. and D.A.S.A.; writing—review and editing, P.M.R., J.M.S.S.E., A.R.F. and P.V.B.; supervision, P.M.R. and A.R.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank Fundação para a Ciência e Tecnologia, FCT/MCTES (Portugal) for financial support through investigator contract (IF/00621/2015—P.M. Reis) and project IF/00621/2015. This work was also supported by the Associate Laboratory for Green Chemistry—LAQV (UIDB/50006/2020 and UIDP/50006/2020) and by projects UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences-UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy-i4HB, which are financed by national funds from FCT/MCTES. The NMR spectrometers at FCT-NOVA are part of Rede Nacional de RMN (PTNMR), supported by FCT-MCTES (ROTEIRO/0031/2013—PINFRA/22161/2016) (cofinanced by FEDER through COMPETE 2020, POCI and PORL and FCT through PIDDAC). LRR acknowledges FCT/MCTES project PTDC/CVT-EPI/6685/2014.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, S.; Kesarla, R.; Omri, A. Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs with Special Emphasis on Self-Emulsifying Systems. ISRN Pharm. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Balk, A.; Wiest, J.; Widmer, T.; Galli, B.; Holzgrabe, U.; Meinel, L. Transformation of acidic poorly water soluble drugs into ionic liquids. Eur. J. Pharm. Biopharm. 2015, 94, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Viau, L.; Tourné-Péteilh, C.; Devoisselle, J.-M.; Vioux, A. Ionogels as drug delivery system: One-step sol–gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 2009, 46, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Balk, A.; Holzgrabe, U.; Meinel, L. ‘Pro et contra’ ionic liquid drugs-Challenges and opportunities for pharmaceutical translation. Eur. J. Pharm. Biopharm. 2015, 94, 291–304. [Google Scholar] [CrossRef]
- Balk, A.; Widmer, T.; Wiest, J.; Bruhn, H.; Rybak, J.-C.; Matthes, P.; Müller-Buschbaum, K.; Sakalis, A.; Lühmann, T.; Berghausen, J.; et al. Ionic Liquid Versus Prodrug Strategy to Address Formulation Challenges. Pharm. Res. 2015, 32, 2154–2167. [Google Scholar] [CrossRef]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic liquids for addressing unmet needs in healthcare. Bioeng. Transl. Med. 2018, 3, 7–25. [Google Scholar] [CrossRef] [Green Version]
- Sahbaz, Y.; Nguyen, T.-H.; Ford, L.; McEvoy, C.L.; Williams, H.D.; Scammells, P.J.; Porter, C.J.H. Ionic Liquid Forms of Weakly Acidic Drugs in Oral Lipid Formulations: Preparation, Characterization, in Vitro Digestion, and in Vivo Absorption Studies. Mol. Pharm. 2017, 14, 3669–3683. [Google Scholar] [CrossRef]
- Santos, M.; Raposo, L.; Carrera, G.; Costa, A.; Dionísio, M.; Baptista, P.; Fernandes, A.; Branco, L.C. Ionic Liquids and Salts from Ibuprofen as Promising Innovative Formulations of an Old Drug. ChemMedChem 2019, 14, 907–911. [Google Scholar] [CrossRef]
- Sintra, T.E.; Shimizu, K.; Ventura, S.P.M.; Lopes, J.N.C.; Coutinho, J.A.P. Enhanced dissolution of ibuprofen using ionic liquids as catanionic hydrotropes. Phys. Chem. Chem. Phys. 2018, 20, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.R.; Soromenho, M.R.C.; Raposo, L.R.; Esperança, J.M.S.S.; Baptista, P.V.; Fernandes, A.R.; Reis, P.M. Enhancement of water solubility of poorly water-soluble drugs by new biocompatible N-acetyl amino acid N-alkyl cholinium-based ionic liquids. Eur. J. Pharm. Biopharm. 2019, 137, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Gadilohar, B.; Shankarling, G. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liq. 2017, 227, 234–261. [Google Scholar] [CrossRef]
- Ventura, S.; Silva, F.; Gonçalves, A.M.; Pereira, J.; Gonçalves, F.J.M.; Coutinho, J.A. Ecotoxicity analysis of cholinium-based ionic liquids to Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; de Almeida, T.S.; Araújo, M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-D.; Liu, Q.-P.; Smith, T.; Li, N.; Zong, M.-H. Evaluation of Toxicity and Biodegradability of Cholinium Amino Acids Ionic Liquids. PLoS ONE 2013, 8, e59145. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.Q.N.; Ferreira, R.; Leitão, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatible cholinium-based ionic liquids-toxicity and biodegradability. Green Chem. 2010, 12, 643–649. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Driesen, K.; Janssen, C.; Van Hecke, K.; Van Meervelt, L.; Kossmann, S.; Kirchner, B.; Binnemans, K. Choline Saccharinate and Choline Acesulfamate: Ionic Liquids with Low Toxicities. J. Phys. Chem. B 2007, 111, 5254–5263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Moulik, S.P. Biocompatible Microemulsions and Their Prospective Uses in Drug Delivery. J. Pharm. Sci. 2008, 97, 22–45. [Google Scholar] [CrossRef]
- He, Q.-X.; Tang, L.; Fu, T.; Shi, Y.-Q.; Wang, X.-L.; Wang, Y.-Z. Novel phosphorus-containing halogen-free ionic liquids: Effect of sulfonate anion size on physical properties, biocompatibility, and flame retardancy. RSC Adv. 2016, 6, 52485–52494. [Google Scholar] [CrossRef]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef]
- Agostinho, D.A.S.; Jesus, A.R.; Silva, A.B.P.; Esperança, J.M.S.S.; Paiva, A.; Duarte, A.R.C.; Reis, P.M. Improvement of New Dianionic Ionic Liquids vs Monoanionic in Solubility of Poorly Water-Soluble Drugs. J. Pharm. Sci. 2021, 110, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, D.A.S.; Santos, F.; Esperança, J.M.S.S.; Duarte, A.R.C.; Reis, P.M. New non-toxic biocompatible dianionic ionic liquids that enhance solubility of oral drugs from BCS class II. J. Ionic Liq. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.R.; Araújo, M.E.M.; Fernandes, A.S.; Costa, J.G.; De Almeida, T.S. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Júlio, A.; Antunes, C.; Mineiro, R.; Raposo, M.; Caparica, R.; Araújo, M.E.; Rosado, C.; Fonte, P.; De Almeida, T.S. Influence of two choline-based ionic liquids on the solubility of caffeine. J. Biomed. Biopharm. Res. 2018, 15, 96–102. [Google Scholar] [CrossRef]
- Blesic, M.; Swadźba-Kwaśny, M.; Belhocine, T.; Gunaratne, H.Q.N.; Lopes, J.N.C.; Gomes, M.F.C.; Pádua, A.A.H.; Seddon, K.R.; Rebelo, L.P.N. 1-Alkyl-3-methylimidazolium alkanesulfonate ionic liquids, [CnH2n+1mim][CkH2k+1SO3]: Synthesis and physicochemical properties. Phys. Chem. Chem. Phys. 2009, 11, 8939–8948. [Google Scholar] [CrossRef]
- Costa, A.J.L.; Soromenho, M.R.C.; Shimizu, K.; Marrucho, I.M.; Esperança, J.M.S.S.; Lopes, J.N.C.; Rebelo, L.P.N. Density, Thermal Expansion and Viscosity of Cholinium-Derived Ionic Liquids. ChemPhysChem 2012, 13, 1902–1909. [Google Scholar] [CrossRef]
- Maroń, A.; Czerwińska, K.; Machura, B.; Raposo, L.; Roma-Rodrigues, C.; Fernandes, A.R.; Małecki, J.G.; Szlapa-Kula, A.; Kula, S.; Krompiec, S. Spectroscopy, electrochemistry and antiproliferative properties of Au(iii), Pt(ii) and Cu(ii) complexes bearing modified 2,2′:6′,2′′-terpyridine ligands. Dalton Trans. 2018, 47, 6444–6463. [Google Scholar] [CrossRef] [PubMed]
- Žilnik, L.; Jazbinšek, A.; Hvala, A.; Vrečer, F.; Klamt, A. Solubility of sodium diclofenac in different solvents. Fluid Phase Equilibria 2007, 261, 140–145. [Google Scholar] [CrossRef]
- Somani, A.A.; Thelen, K.; Zheng, S.; Trame, M.N.; Coboeken, K.; Meyer, M.; Schnizler, K.; Ince, I.; Willmann, S.; Schmidt, S. Evaluation of changes in oral drug absorption in preterm and term neonates for Biopharmaceutics Classification System (BCS) class I and II compounds. Br. J. Clin. Pharmacol. 2015, 81, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Chuasuwan, B.; Binjesoh, V.; Polli, J.; Zhang, H.; Amidon, G.; Junginger, H.; Midha, K.; Shah, V.; Stavchansky, S.; Dressman, J.; et al. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Diclofenac Sodium and Diclofenac Potassium. J. Pharm. Sci. 2009, 98, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A.; Box, K.J.; Comer, J.E.A.; Hibbert, C.; Tam, K. pH-Metric logP 10. Determination of Liposomal Membrane-Water Partition Coefficients of lonizable Drugs. Pharm. Res. 1998, 15, 209–215. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).