Asymmetric Behavior in Ptyodactylus guttatus: Can a Digit Ratio Reflect Brain Laterality?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Housing
2.2. Sex and Age
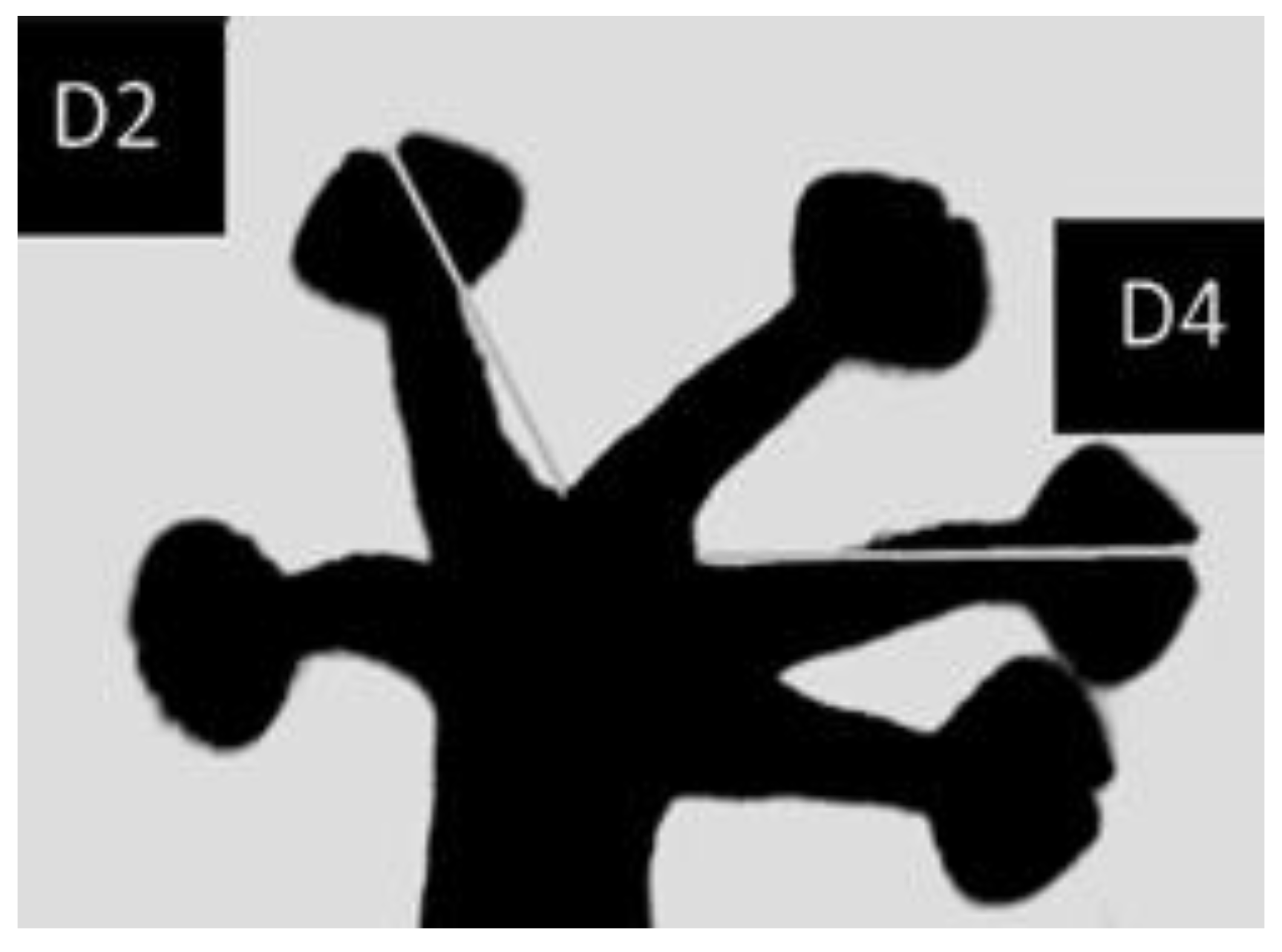
2.3. Measurements
2.4. Risk-Taking Strategy
2.5. Laboratory Conditions
2.6. Foot Preference
2.7. Detour Test
2.8. Data Analysis
3. Results
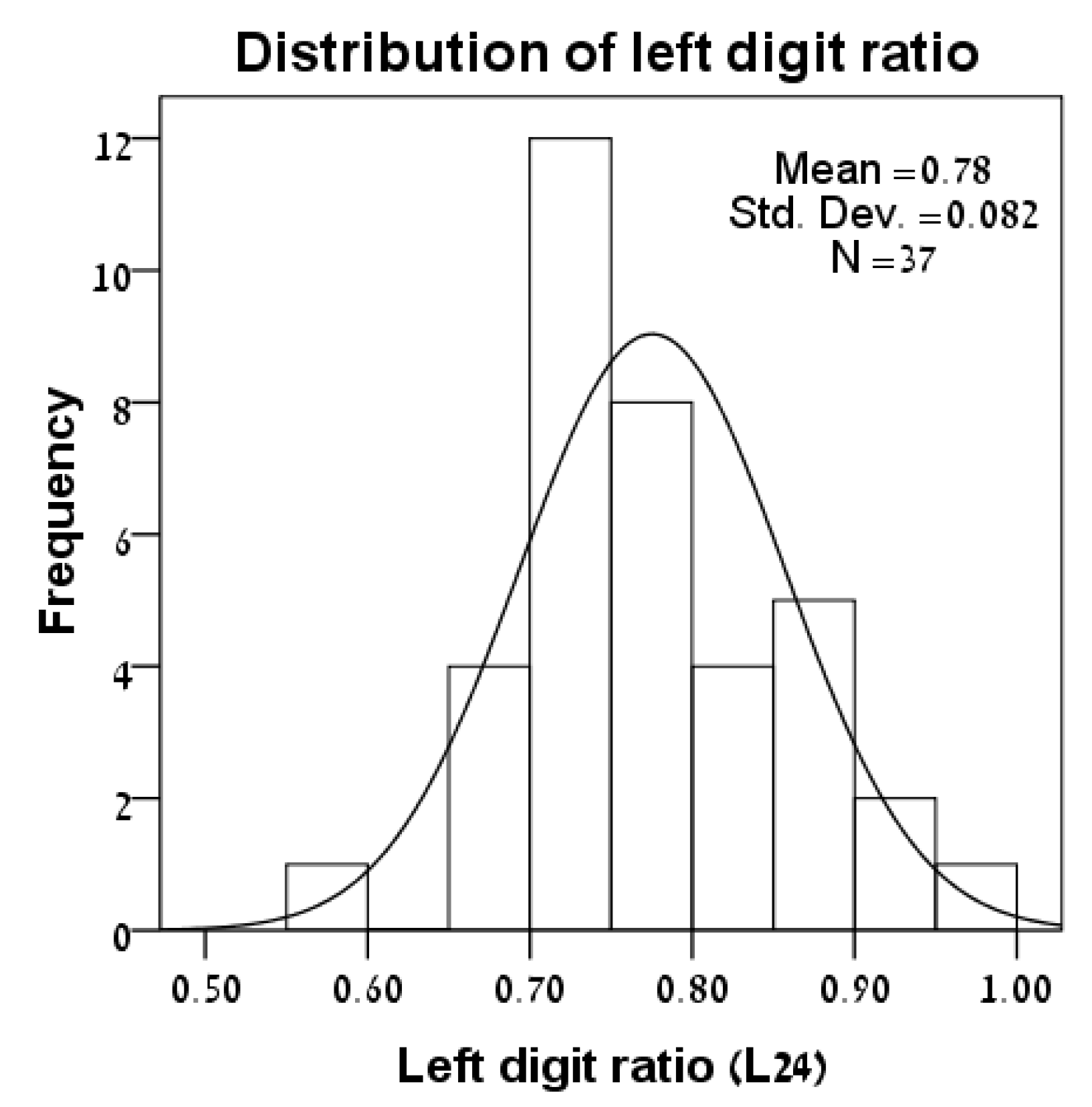
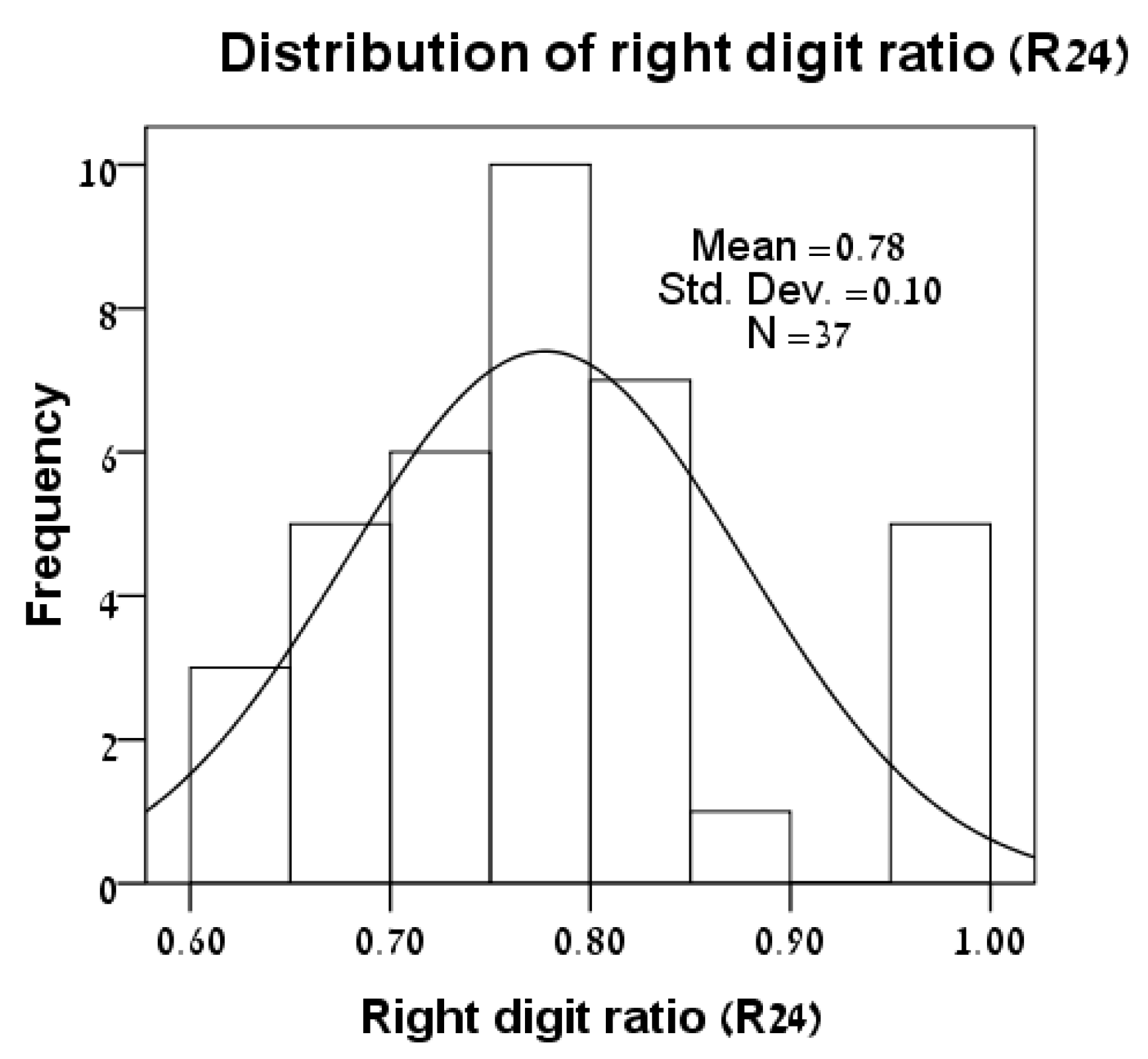
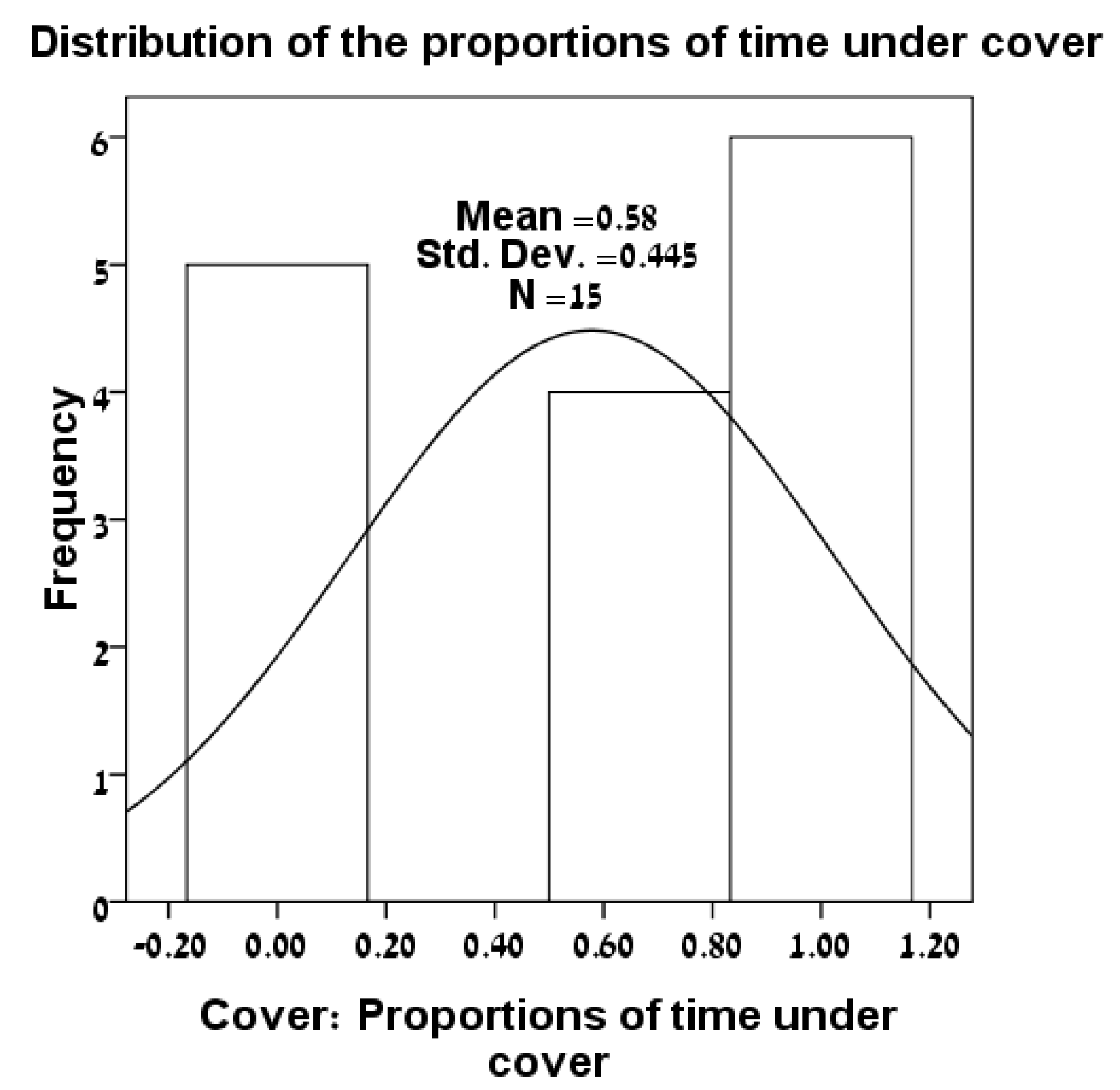
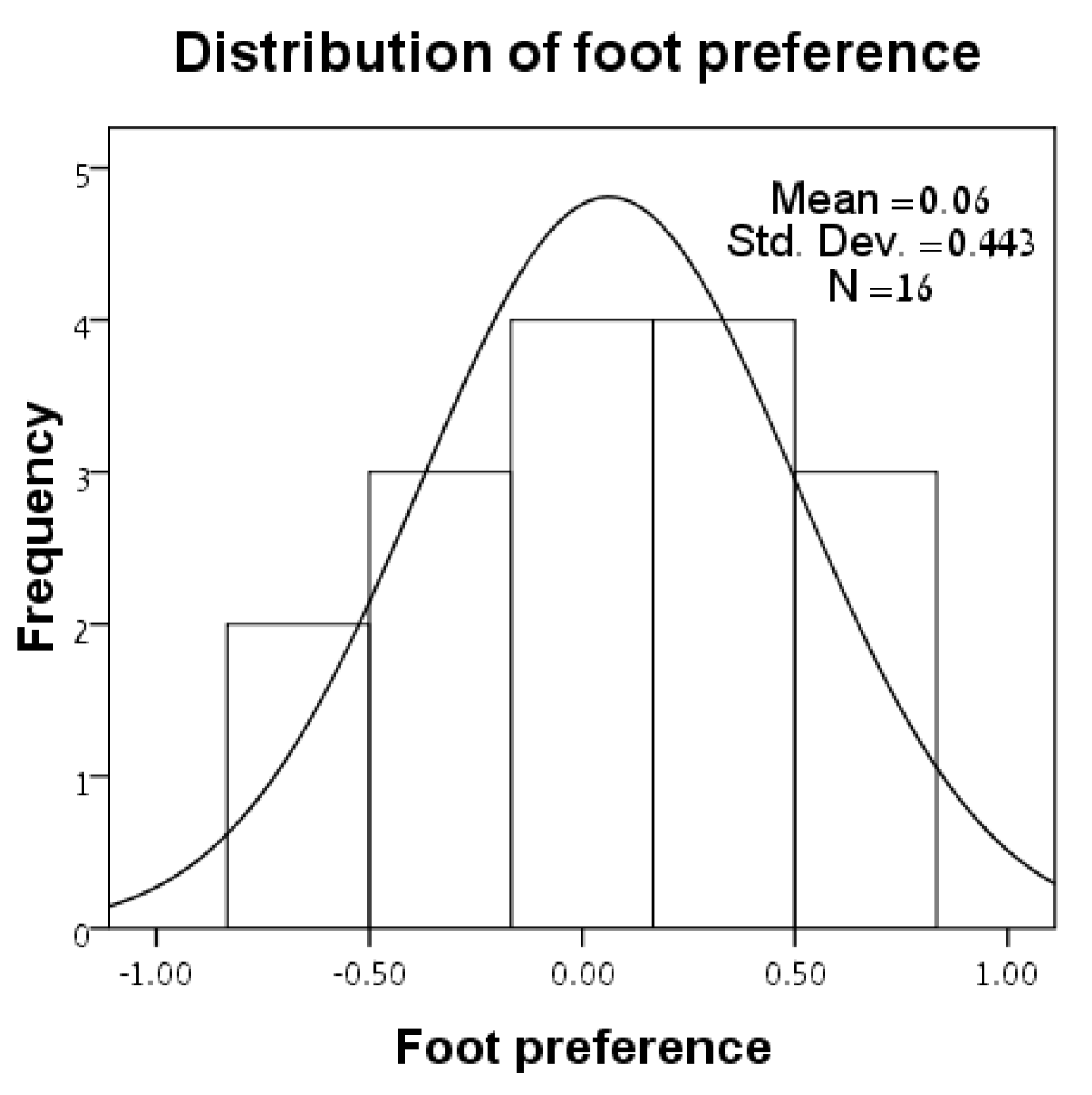
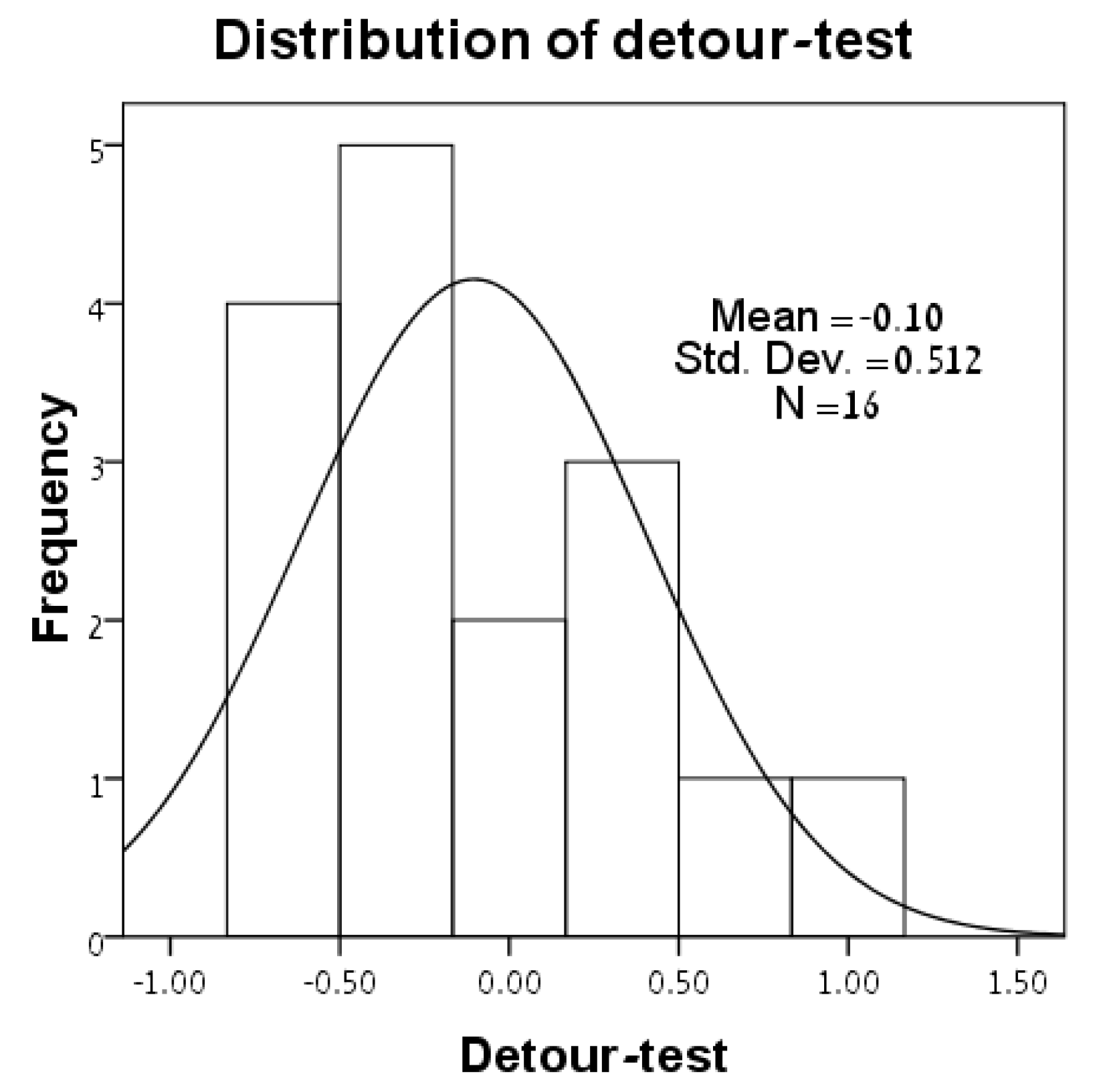
3.1. Distributions of Variables
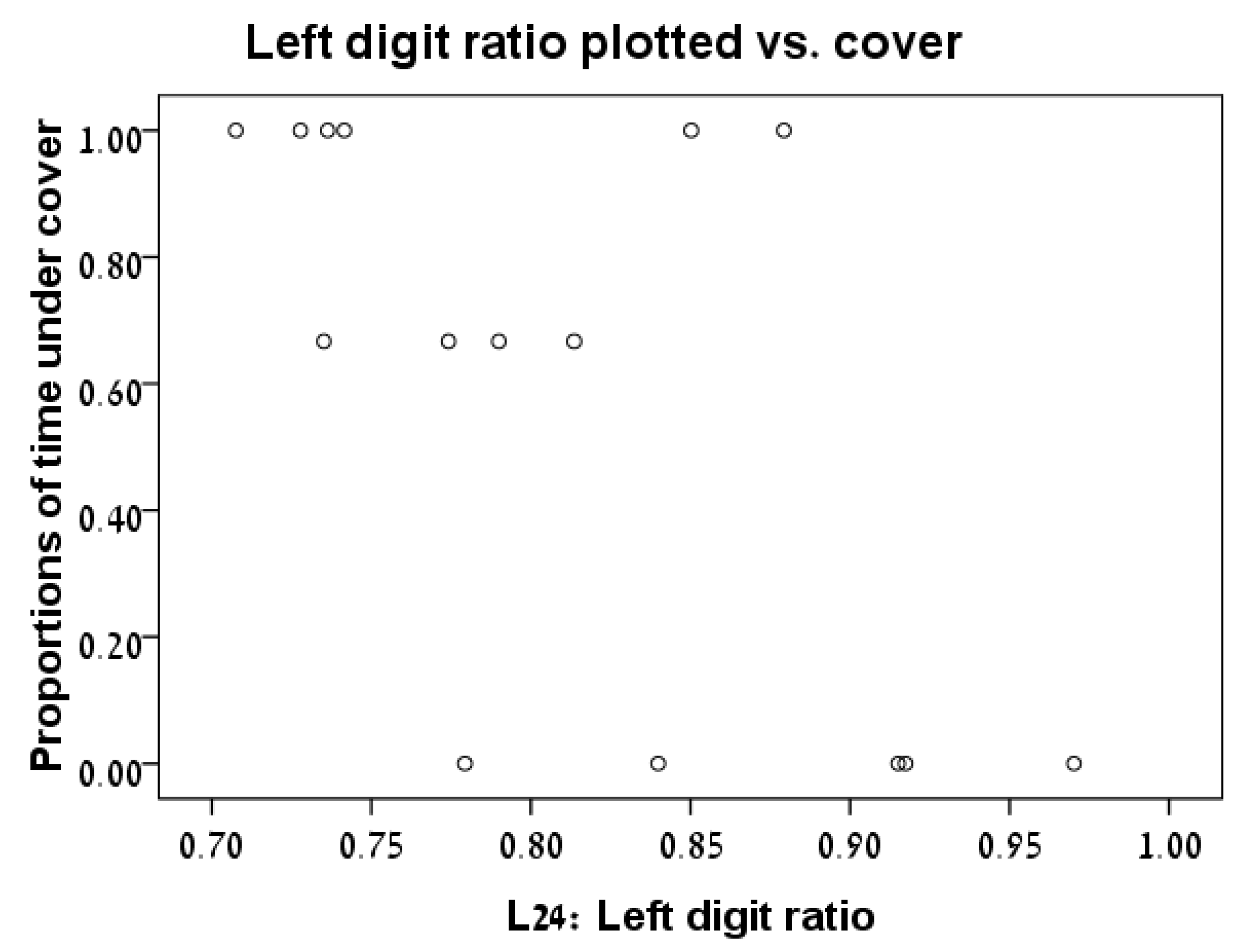
3.2. Digit Ratio vs. Time under Cover
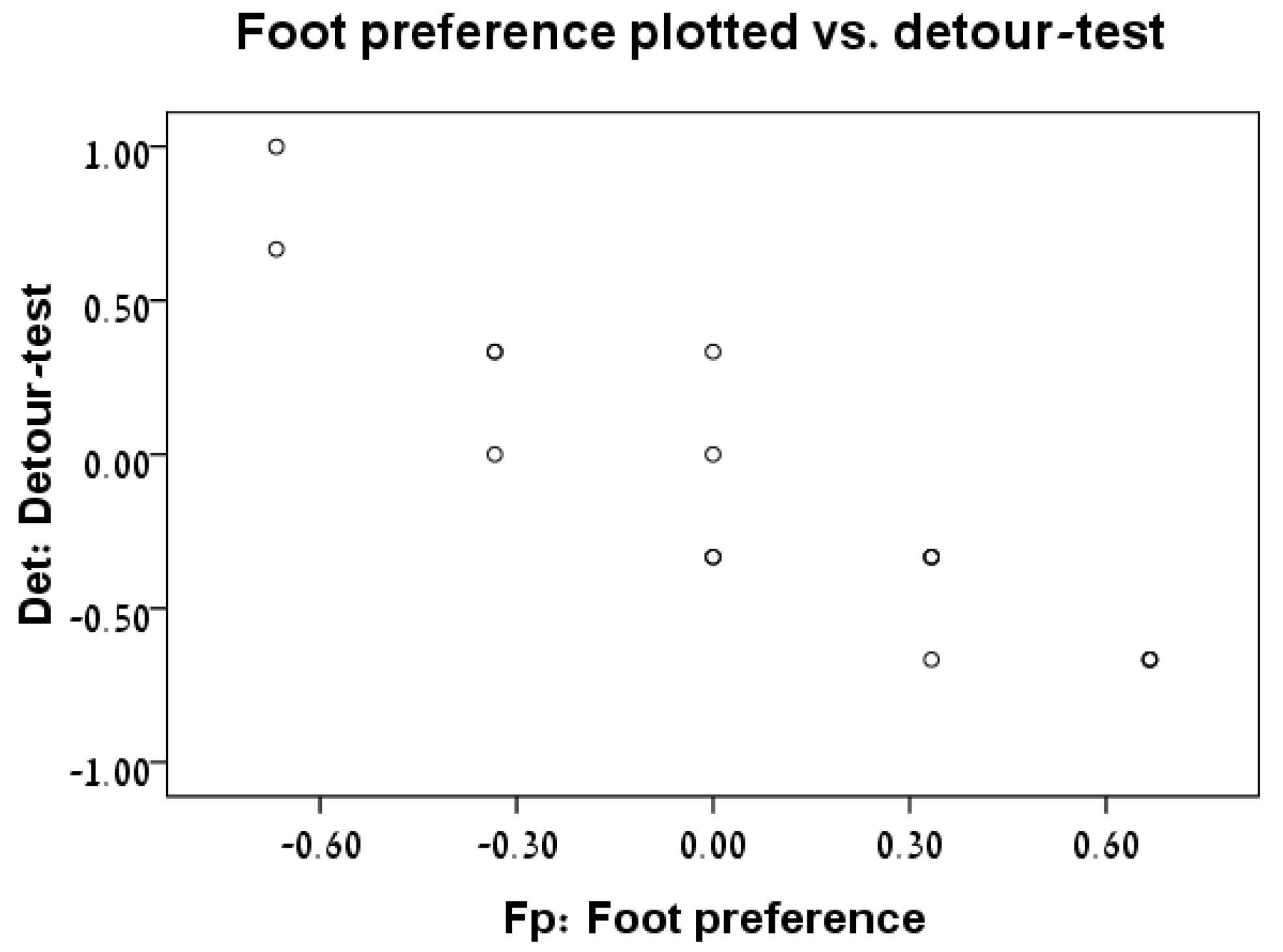
3.3. Foot Preference vs. Detour Test
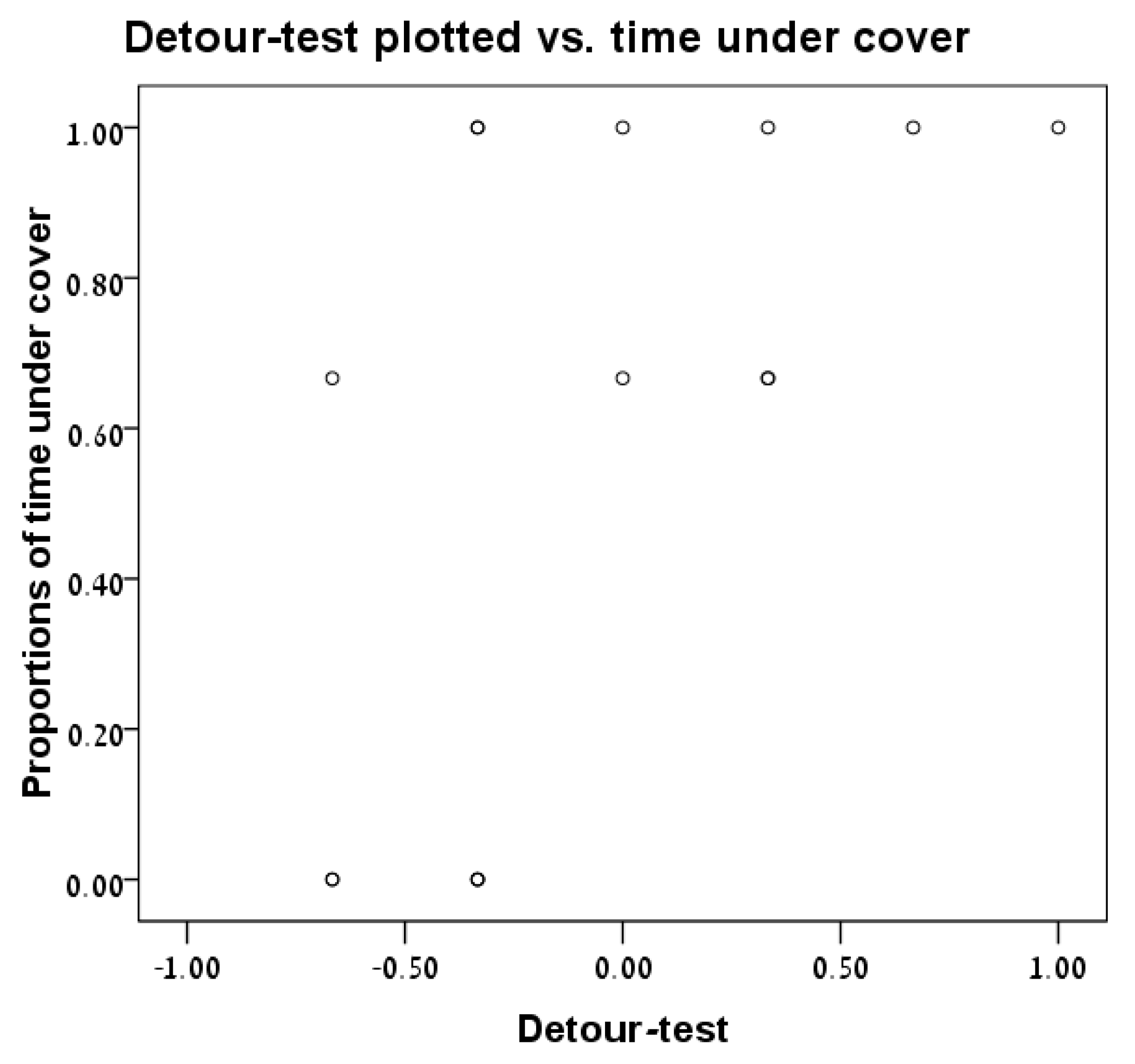
3.4. Detour Test vs. Time under Cover
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vallortigara, G. Comparative Neuropsychology of the Dual Brain: A Stroll through Animals’ Left and Right Perceptual Worlds. Brain Lang. 2000, 73, 189–219. [Google Scholar] [CrossRef]
- Rogers, L.J.; Anson, J.M. Lateralization of function in the chicken forebrain. Pharmacol. Biochem. Behav. 1979, 10, 679–686. [Google Scholar] [CrossRef]
- Rogers, L.J. Advantages and disadvantages of lateralization. In Comparative Vertebrate Lateralization; Rogers, L.J., Andrew, R.J., Eds.; Cambridge University Press: Cambridge, MA, USA, 2002; pp. 126–153. [Google Scholar]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Ocklenburg, S.; Gunturkun, O. The Lateralized Brain: The Neuroscience and Evolution of Hemispheric Asymmetries; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Deckel, A.W. Laterality of aggressive responses in Anolis. J. Exp. Zool. 1995, 272, 194–200. [Google Scholar] [CrossRef]
- Deckel, A.W.; Fuqua, L. Effects of serotonergic drugs on lateralised aggression and aggressive displays in Anolis carolinensis. Behav. Brain Res. 1998, 95, 227–232. [Google Scholar] [CrossRef]
- Hews, D.K.; Castellano, M.; Hara, E. Aggression in females is also lateralized: Left-eye bias during aggressive courtship rejection in lizards. Anim. Behav. 2004, 68, 1201–1207. [Google Scholar] [CrossRef]
- Mench, J.A.; Andrew, R.J. Lateralization of a food search task in the domestic chick. Behav. Neural Biol. 1986, 46, 107–114. [Google Scholar] [CrossRef]
- Rogers, L.J. Evolution of hemispheric specialisation: Advantages and disadvantages. Brain Lang. 2000, 73, 236–253. [Google Scholar] [CrossRef]
- Rogers, L.J.; Zucca, P.; Vallortigara, G. Advantages of having a lateralized brain. Proc. R. Soc. B Biol. Sci. 2004, 271 (Suppl. S6), S420–S422. [Google Scholar] [CrossRef]
- Rhodes, R.D.; Harrison, D.W.; Demaree, H.A. Hostility as a Moderator of Physical Reactivity and Recovery to Stress. Int. J. Neurosci. 2002, 112, 167–186. [Google Scholar] [CrossRef]
- Alves, N.T.; Aznar-Casanova, J.A.; Fukusima, S.S. Patterns of brain asymmetry in the perception of positive and negative facial expressions. Laterality Asymmetries Body Brain Cogn. 2009, 14, 256–272. [Google Scholar] [CrossRef]
- Preibisch, C.; Lanfermann, H.; Wallenhorst, T.; Walter, H.; Erk, S. Neuroanatomical correlates of visual field bias: A sensitive system for detecting potential threats? Brain Res. 2009, 1263, 69–77. [Google Scholar] [CrossRef]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Bertino, D.; D’Ingeo, S.; Quaranta, A. Relationship between Motor Laterality and Aggressive Behavior in Sheepdogs. Symmetry 2019, 11, 233. [Google Scholar] [CrossRef]
- Siniscalchi, M.; D’Ingeo, S.; Fornelli, S.; Quaranta, A. Relationship between visuospatial attention and paw preference in dogs. Sci. Rep. 2016, 6, 31682. [Google Scholar] [CrossRef]
- Miklósi, Á.; Andrew, R.J. Right eye use associated with decision to bite in zebrafish. Behav. Brain Res. 1999, 105, 199–205. [Google Scholar] [CrossRef]
- Bisazza, A.; de Santi, A. Lateralization of aggression in fish. Behav. Brain Res. 2003, 141, 131–136. [Google Scholar] [CrossRef]
- Andrew, R.J. Lateralization of Emotional and Cognitive Function in Higher Vertebrates, with Special Reference to the Domestic Chick. In Advances in Vertebrate Neuroethology; Ewert, J.-P., Capranica, R.R., Ingle, D.J., Eds.; Springer Science and Business Media LLC: Berlin, Germany, 1983; pp. 477–509. [Google Scholar]
- Anderson, R.; Fleming, D.; Rhees, R.; Kinghorn, E. Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res. 1986, 370, 1–10. [Google Scholar] [CrossRef]
- Denenberg, V.; Garbanati, J.; Sherman, D.; Yutzey, D.; Kaplan, R. Infantile stimulation induces brain lateralization in rats. Science 1978, 201, 1150–1152. [Google Scholar] [CrossRef]
- Fitch, R.; Brown, C.; Oconnor, K.; Tallal, P. Functional lateralization for auditory temporal processing in male and female rats. Behav. Neurosci. 1993, 107, 844–850. [Google Scholar] [CrossRef]
- Tang, A.C.; Verstynen, T. Early life environment modulates ‘handedness’ in rats. Behav. Brain Res. 2002, 131, 1–7. [Google Scholar] [CrossRef]
- Yan, R.H.; Malisch, J.L.; Hannon, R.M.; Hurd, P.L.; Garland, T. Selective Breeding for a Behavioral Trait Changes Digit Ratio. PLoS ONE 2008, 3, e3216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hews, D.K.; Worthington, R.A. Fighting from the Right Side of the Brain: Left Visual Field Preference during Aggression in Free-Ranging Male Tree Lizards (Urosaurus ornatus). Brain Behav. Evol. 2001, 58, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Lustig, A.; Ketter-Katz, H.; Katzir, G. Lateralization of visually guided detour behavior in the common chameleon, (Chamaeleo chameleon): A reptile with highly independent eye movements. Behav. Process. 2013, 100, 110–115. [Google Scholar] [CrossRef]
- Sion, G. Foot preference underlies bite-scar asymmetry in the gecko Ptyodactylus guttatus. Laterality Asymmetries Body Brain Cogn. 2017, 23, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Sion, G. Inter-Relations among Behavior, Physiology, Morphology and Directional Asymmetry in the Gecko Ptyodactylus guttatus. Ph.D. Thesis, The Hebrew University, Jerusalem, Israel, 2015. [Google Scholar]
- Seligmann, H. Evidence that minor directional asymmetry is functional in lizard hindlimbs. J. Zool. 1998, 245, 205–208. [Google Scholar] [CrossRef]
- Seligmann, H. Evolution and ecology of developmental processes and of the resulting morphology: Directional asymmetry in hindlimbs of Agamidae and Lacertidae (Reptilia: Lacertilia). Biol. J. Linn. Soc. 2000, 69, 461–481. [Google Scholar] [CrossRef][Green Version]
- Seligmann, H.; Beiles, A.; Werner, Y.L. More injuries in left-footed individual lizards and Sphenodon. J. Zool. 2003, 260, 129–144. [Google Scholar] [CrossRef]
- Seligmann, H.; Beiles, A.; Werner, Y.L. Avoiding injury and surviving injury: Two coexisting evolutionary strategies in lizards. Biol. J. Linn. Soc. 2003, 78, 307–324. [Google Scholar] [CrossRef]
- Seligmann, H.; Moravec, J.; Werner, Y.L. Morphological, functional and evolutionary aspects of tail autotomy and regeneration in the ‘living fossil’ Sphenodon (Reptilia: Rhynchocephalia). Biol. J. Linn. Soc. 2008, 93, 721–743. [Google Scholar] [CrossRef]
- Shacham, B. Tail injury linked to morphological asymmetry in a polymorphic snake. Isr. J. Zool. 2005, 51, 77–78. [Google Scholar]
- Razzetti, E.; Faiman, R.; Werner, Y.L. Directional asymmetry and correlation of tail injury with left-side dominance occur in Serpentes (Sauropsida). Zoomorphology 2007, 126, 31–43. [Google Scholar] [CrossRef]
- Sion, G. Digit asymmetry and digit ratio (2:4) derived from brain laterality: The lizard Ptyodactylus guttatus as a model. In Proceedings of the 10th Meeting of the Lacertid Lizards of the Mediterranean Basin, Tel-Aviv, Israel, 18–21 June 2018. [Google Scholar]
- Tang, A.C. A.C. A hippocampal theory of cerebral lateralization. In The Asymmetrical Brain; Davidson, R., Hugdahl, K., Eds.; MIT Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Tang, A.C.; Reeb, B.C.; Romeo, R.D.; McEwen, B.S. Modification of Social Memory, Hypothalamic-Pituitary-Adrenal Axis, and Brain Asymmetry by Neonatal Novelty Exposure. J. Neurosci. 2003, 23, 8254–8260. [Google Scholar] [CrossRef]
- Siman-Tov, T.; Papo, D.; Gadoth, N.; Schonberg, T.; Mendelsohn, A.; Perry, D.; Hendler, T. Mind Your Left: Spatial Bias in Subcortical Fear Processing. J. Cogn. Neurosci. 2009, 21, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Coyne, S.M.; Manning, J.T.; Ringer, L.; Bailey, L. Directional asymmetry (right–left differences) in digit ratio (2D:4D) predict indirect aggression in women. Pers. Individ. Differ. 2007, 43, 865–872. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Wilson, D.H.; Gazzaniga, M.S. Manipulo-spatial aspects of cerebral lateralization: Clues to the origin of lateralization. Neuropsychol. 1977, 15, 743–750. [Google Scholar] [CrossRef]
- Woldorff, M.G.; Tempelmann, C.; Fell, J.; Tegeler, C.; Gaschler-Markefski, B.; Hinrichs, H.; Heinze, H.J.; Scheich, H. Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Hum. Brain Mapp. 1999, 7, 49–66. [Google Scholar] [CrossRef]
- Manning, J.T.; Scutt, D.; Wilson, J.; Lewis-Jones, D.I. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. 1998, 13, 3000–3004. [Google Scholar] [CrossRef]
- Manning, J.T. Resolving the role of prenatal sex steroids in the development of digit ratio. Proc. Natl. Acad. Sci. USA 2011, 108, 16143–16144. [Google Scholar] [CrossRef]
- Zheng, Z.; Cohn, M.J. Developmental basis of sexually dimorphic digit ratios. Proc. Natl. Acad. Sci. USA 2011, 108, 16289–16294. [Google Scholar] [CrossRef]
- Manning, J.; Kilduff, L.; Cook, C.; Crewther, B.; Fink, B. Digit Ratio (2D:4D): A Biomarker for Prenatal Sex Steroids and Adult Sex Steroids in Challenge Situations. Front. Endocrinol. 2014, 5, 9. [Google Scholar] [CrossRef]
- DiRenzo, G.V.; Stynoski, J.L. Patterns of Second-to-Fourth Digit Length Ratios (2D:4D) in Two Species of Frogs and Two Species of Lizards at La Selva, Costa Rica. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Apicella, C.L.; Dreber, A.; Campbell, B.; Gray, P.B.; Hoffman, M.; Little, A.C. Testosterone and financial risk preferences. Evol. Hum. Behav. 2008, 29, 384–390. [Google Scholar] [CrossRef]
- Marler, C.A.; Moore, M.C. Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behav. Ecol. Sociobiol. 1988, 23, 21–26. [Google Scholar] [CrossRef]
- Huyghe, K.; Husak, J.F.; Herrel, A.; Tadić, Z.; Moore, I.T.; Van Damme, R.; Vanhooydonck, B. Relationships between hormones, physiological performance and immunocompetence in a colour-polymorphic lizard species, Podarcis melisellensis. Horm. Behav. 2009, 55, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.M.; Hodgson, J.C. Is digit ratio (2D:4D) a reliable pointer to speech laterality? Behav. Brain Res. 2016, 301, 258–261. [Google Scholar] [CrossRef]
- Valla, J.M.; Ceci, S.J. Can Sex Differences in Science Be Tied to the Long Reach of Prenatal Hormones? Brain Organization Theory, Digit Ratio (2D/4D), and Sex Differences in Preferences and Cognition. Perspect. Psychol. Sci. 2011, 6, 134–146. [Google Scholar] [CrossRef][Green Version]
- Wong, W.I.; Hines, M. Interpreting digit ratio (2D:4D)–behavior correlations: 2D:4D sex difference, stability, and behavioral correlates and their replicability in young children. Horm. Behav. 2016, 78, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Witelson, S.F. Neural sexual mosaicism: Sexual differentiation of the human temporo-parietal region for functional asymmetry. Psychoneuroendocrinology 1991, 16, 131–153. [Google Scholar] [CrossRef]
- Beaton, A.A.; Rudling, N.; Kissling, C.; Taurines, R.; Thome, J. Digit ratio (2D:4D), salivary testosterone, and handedness. Laterality Asymmetries Body Brain Cogn. 2011, 16, 136–155. [Google Scholar] [CrossRef] [PubMed]
- Lust, J.M.; Geuze, R.H.; Van De Beek, C.; Cohen-Kettenis, P.T.; Bouma, A.; Groothuis, T.G. Differential effects of prenatal testosterone on lateralization of handedness and language. Neuropsychology 2011, 25, 581–589. [Google Scholar] [CrossRef]
- Geschwind, N.; Galaburda, A.M. Cerebral Lateralization: Biological Mechanisms, Associations, and Pathology; MIT Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Jamison, C.S.; Meier, R.J.; Campbell, B.C. Dermatoglyphic asymmetry and testosterone levels in normal males. Am. J. Phys. Anthr. 1993, 90, 185–198. [Google Scholar] [CrossRef]
- Daisley, J.N.; Bromundt, V.; Möstl, E.; Kotrschal, K. Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks Coturnix japonica. Horm. Behav. 2005, 47, 185–194. [Google Scholar] [CrossRef]
- Mhlanga, M. Investigating the Role of Testosterone on Risk-Taking Behavior in Male Sprague Dawley Rats. Ph.D. Thesis, Lake Forest College, Lake Forest, IL, USA, 2012. [Google Scholar]
- Hönekopp, J. Relationships between digit ratio 2D:4D and self-reported aggression and risk taking in an online study. Pers. Individ. Differ. 2011, 51, 77–80. [Google Scholar] [CrossRef]
- Coates, J.M.; Gurnell, M.; Rustichini, A. Second-to-fourth digit ratio predicts success among high-frequency financial traders. Proc. Natl. Acad. Sci. USA 2009, 106, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Blázi, G.; Hegyi, G.; Torok, J. Side-specific effect of yolk testosterone elevation on second-to-fourth digit ratio in a wild passerine. Naturwissenschaften 2016, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Cantalupo, C.; Taglialatela, J. Handedness Is Associated with Asymmetries in Gyrification of the Cerebral Cortex of Chimpanzees. Cereb. Cortex 2007, 17, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.; Landis, T.; Bracha, H.S.; Brugger, P. Opposite Turning Behavior in Right-Handers and Non-Right-Handers Suggests a Link between Handedness and Cerebral Dopamine Asymmetries. Behav. Neurosci. 2003, 117, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Dopamine signals for reward value and risk: Basic and recent data. Behav. Brain Funct. 2010, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, E.; Milman, T.; Sion, G.; Werner, Y.L. Predatory behavior of the gekkonid lizards, Ptyodactylus spp., toward the scorpion Leiurus quinquestriatus hebraeus, and their tolerance of its venom. J. Nat. Hist. 2003, 37, 641–646. [Google Scholar] [CrossRef]
- Sion, G. Foraging Strategy and Social Hierarchy are State Dependent in the Gecko Ptyodactylus guttatus. Master’s Thesis, Ben-Gurion University of the Negev, Be’er Sheva, Israel, 2006. [Google Scholar]
- Werner, Y.L. Uber die israelischen geckos der gattung Ptyodactylus und ihre biologie. Salamandra 1965, 1, 15–25. [Google Scholar]
- Werner, Y.L. Ecology of eggs and laying sites of Ptyodactylus geckos. Stud. Herpetol. 1986, 441, 444. [Google Scholar]
- Werner, Y.L. Reptile Life in the Land of Israel, with Comments on Adjacent Regions; Chimaira: Frankfurt, Germany, 2016; 494p. [Google Scholar]
- Werner, Y.L.; Sivan, N. Systematics and zoogeography of Ptyodactylus (Reptilia: Sauria: Gekkonidae) in the Levant: 2, Taxonomy, with a review of ecology and zoogeography. Rev. Española Herpetol. 1994, 8, 105–122. [Google Scholar]
- Johnston, G.; Bouskila, A. Sexual Dimorphism and Ecology of The Gecko, Ptyodactylus Guttatus. S. Am. J. Herpetol. 2007, 41, 506–513. [Google Scholar] [CrossRef]
- Meiri, S. Traits of lizards of the world: Variation around a successful evolutionary design. Glob. Ecol. Biogeogr. 2018, 27, 1168–1172. [Google Scholar] [CrossRef]
- Moussaffi, O.; Sion, G. The curious incidence of the gecko at winter-time: A rational explanation for a seemingly irrational behavior of the sub-adults of Ptyodactylus guttatus. In Proceedings of the 48th Meeting of the Zoological Society of Israel, Tel Aviv, Israel, 25 December 2011; p. 45. [Google Scholar]
- Bar, A.; Haimovitch, G. A Field Guide to Reptiles and Amphibians of Israel; Pazbar Limited: Herzliya, Israel, 2011. [Google Scholar]
- Smith, R.J. Statistics of sexual size dimorphism. J. Hum. Evol. 1999, 36, 423–458. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.A.; Al-Deen, A.; Al-Shareef, M.F.; Rashed, A.A. Ecology of some sympatric reptilian species from Egypt with special reference to helminthic parasites: Ptyodactylus guttatus and Tarentola annularis. J. Egypt. Soc. Parasitol. 1995, 25, 395–406. [Google Scholar] [PubMed]
- D’Ingeo, S.; Quaranta, A.; Siniscalchi, M.; Stomp, M.; Coste, C.; Bagnard, C.; Hausberger, M.; Cousillas, H. Horses associate individual human voices with the valence of past interactions: A behavioural and electrophysiological study. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Robins, A.; Chen, P.; Beazley, L.D.; Dunlop, S.A. Lateralized predatory responses in the ornate dragon lizard (Ctenophorus ornatus). NeuroReport 2005, 16, 849–852. [Google Scholar] [CrossRef]
- Talarovičová, A.; Krsková, L.; Blažeková, J. Testosterone enhancement during pregnancy influences the 2D:4D ratio and open field motor activity of rat siblings in adulthood. Horm. Behav. 2009, 55, 235–239. [Google Scholar] [CrossRef]
- Manning, J.T. Digit ratio (2D:4D), sex differences, allometry, and finger length of 12–30 year olds: Evidence from the British Broadcasting Corporation (BBC) internet study. Am. J. Hum. Biol. 2010, 22, 604–608. [Google Scholar] [CrossRef]
- Manning, J.T.; Fink, B.; Neave, N.; Szwed, A. The second to fourth digit ratio and asymmetry. Ann. Hum. Biol. 2006, 33, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Regolin, L.; Pagni, P. Detour behaviour, imprinting and visual lateralization in the domestic chick. Cogn. Brain Res. 1999, 7, 307–320. [Google Scholar] [CrossRef]
- Clark, C.W. Antipredator behavior and the asset-protection principle. Behav. Ecol. 1994, 5, 159–170. [Google Scholar] [CrossRef]
- Reinhardt, U.G.; Healey, M.C. Season- and size-dependent risk taking in juvenile coho salmon: Experimental evaluation of asset protection. Anim. Behav. 1999, 57, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Coren, S. Left-handedness and accident-related injury risk. Am. J. Public Heal. 1989, 79, 1040–1041. [Google Scholar] [CrossRef]
- Zverev, Y.; Adeloye, A. Left-handedness as a risk factor for head injuries. East Afr. Med J. 2001, 78, 22–24. [Google Scholar] [CrossRef][Green Version]
- Huyghe, K.; Husak, J.F.; Moore, I.T.; Vanhooydonck, B.; Van Damme, R.; Molina-Borja, M.; Herrel, A. Effects of testosterone on morphology, performance and muscle mass in a lizard. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313, 9–16. [Google Scholar] [CrossRef]
- Leal, M.; Powell, B.J. Behavioural flexibility and problem-solving in a tropical lizard. Biol. Lett. 2011, 8, 28–30. [Google Scholar] [CrossRef]
- Carazo, P.; Noble, D.W.; Chandrasoma, D.; Whiting, M.J. Sex and boldness explain individual differences in spatial learning in a lizard. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133275. [Google Scholar] [CrossRef]
- Seligmann, H. Behavioural and morphological asymmetries in hindlimbs of Hoplodactylus duvaucelii (Lacertilia: Gekkonomorpha: Gekkota: Diplodactylinae). Laterality Asymmetries Body Brain Cogn. 2002, 7, 277–283. [Google Scholar] [CrossRef]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal). Wiley Interdiscip. Rev. Cogn. Sci. 2010, 2, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Versace, E. Laterality at the neural, cognitive and behavioural levels. In APA Handbook of Comparative Psychology; Burghardt, G., Pepperberg, I., Call, J., Eds.; American Psychological Association Press: Washington, DC, USA, 2017; pp. 557–577. [Google Scholar]

| Lizard | Sex | SVL | L24 | R24 | Foot-Preference |
|---|---|---|---|---|---|
| 1 | Female | 68.3 | 0.598 | 0.972 | 0 |
| 2 | Male | 66.8 | 0.651 | 0.753 | 0.333 |
| 3 | Female | 66.5 | 0.657 | 0.783 | −0.2 |
| 4 | Female | 64.4 | 0.672 | 0.62 | 0.5 |
| 5 | Male | 64.1 | 0.682 | 0.682 | −1 |
| 6 | Female | 61.9 | 0.702 | 0.606 | −0.6 |
| 7 | Female | 64.6 | 0.72 | 0.792 | 0.5 |
| 8 | Female | 65 | 0.731 | 0.8 | 0.143 |
| 9 | Male | 76.3 | 0.731 | 0.642 | −0.5 |
| 10 | Male | 71.5 | 0.735 | 0.681 | −1 |
| 11 | Male | 69 | 0.747 | 0.729 | −0.2 |
| 12 | Female | 66.8 | 0.747 | 0.762 | −1 |
| 13 | Female | 65 | 0.753 | 0.829 | 0.6 |
| 14 | Female | 50 | 0.758 | 0.805 | −0.667 |
| 15 | Female | 69.3 | 0.773 | 0.651 | −1 |
| 16 | Male | 66.6 | 0.783 | 0.688 | −0.333 |
| 17 | Juvenile | 45.6 | 0.796 | 0.964 | 0.5 |
| 18 | Female | 49.9 | 0.826 | 0.814 | 0.6 |
| 19 | Female | 61.7 | 0.847 | 0.667 | −0.6 |
| 20 | Female | 67.2 | 0.854 | 0.738 | 0 |
| 21 | Female | 61.1 | 0.87 | 0.708 | 0.6 |
| 22 | Female | 68.8 | 0.872 | 0.812 | 0.2 |
| Lizard | Sex | SVL | L24 | R24 | Foot-Preference | Detour-Test | Cover |
|---|---|---|---|---|---|---|---|
| 1 | Female | 41.03 | 0.917 | 0.831 | 0 | −0.333 | 0 |
| 2 | Female | 38.89 | 0.789 | 0.805 | −0.333 | 0.333 | 0.667 |
| 3 | Male | 48.85 | 0.707 | 0.884 | 0 | 0.333 | 1 |
| 4 | Male | 36.35 | 0.879 | 0.72 | 0.333 | −0.333 | 1 |
| 5 | Female | 37.09 | 0.97 | 0.959 | 0.333 | −0.333 | 0 |
| 6 | Female | 43.44 | 0.85 | 0.721 | 0.333 | −0.333 | 1 |
| 7 * | Female | 47.17 | 1.306 | 0.824 | 0.667 | −0.667 | 0 |
| 8 | Female | 48.46 | 0.735 | 0.805 | 0.667 | −0.667 | 0.667 |
| 9 | Female | 48.38 | 0.915 | 0.973 | 0 | −0.333 | 0 |
| 10 | Female | 34.06 | 0.741 | 0.779 | −0.667 | 0.667 | 1 |
| 11 | Male | 51.07 | 0.839 | 0.711 | 0.333 | −0.667 | 0 |
| 12 | Female | 38.79 | 0.736 | 0.785 | −0.333 | 0 | 1 |
| 13 | Juvenile | 35.45 | 0.779 | 0.798 | 0.667 | −0.667 | 0 |
| 14 | Female | 46.57 | 0.727 | 0.981 | −0.667 | 1 | 1 |
| 15 | Male | 27.68 | 0.813 | 0.752 | −0.333 | 0.333 | 0.667 |
| 16 | Female | 46.73 | 0.774 | 0.752 | 0 | 0 | 0.667 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sion, G.; Tal, R.; Meiri, S. Asymmetric Behavior in Ptyodactylus guttatus: Can a Digit Ratio Reflect Brain Laterality? Symmetry 2020, 12, 1490. https://doi.org/10.3390/sym12091490
Sion G, Tal R, Meiri S. Asymmetric Behavior in Ptyodactylus guttatus: Can a Digit Ratio Reflect Brain Laterality? Symmetry. 2020; 12(9):1490. https://doi.org/10.3390/sym12091490
Chicago/Turabian StyleSion, Guy, Rahav Tal, and Shai Meiri. 2020. "Asymmetric Behavior in Ptyodactylus guttatus: Can a Digit Ratio Reflect Brain Laterality?" Symmetry 12, no. 9: 1490. https://doi.org/10.3390/sym12091490
APA StyleSion, G., Tal, R., & Meiri, S. (2020). Asymmetric Behavior in Ptyodactylus guttatus: Can a Digit Ratio Reflect Brain Laterality? Symmetry, 12(9), 1490. https://doi.org/10.3390/sym12091490







