Symmetry Breaking of Phospholipids †
Abstract
1. Introduction
2. The “Advantage” of Being Homochiral
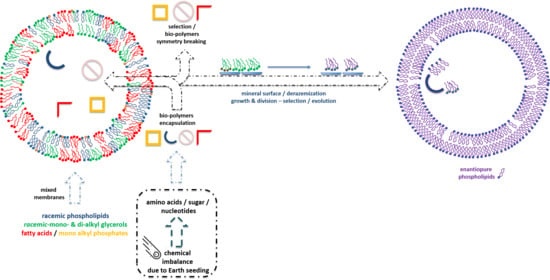
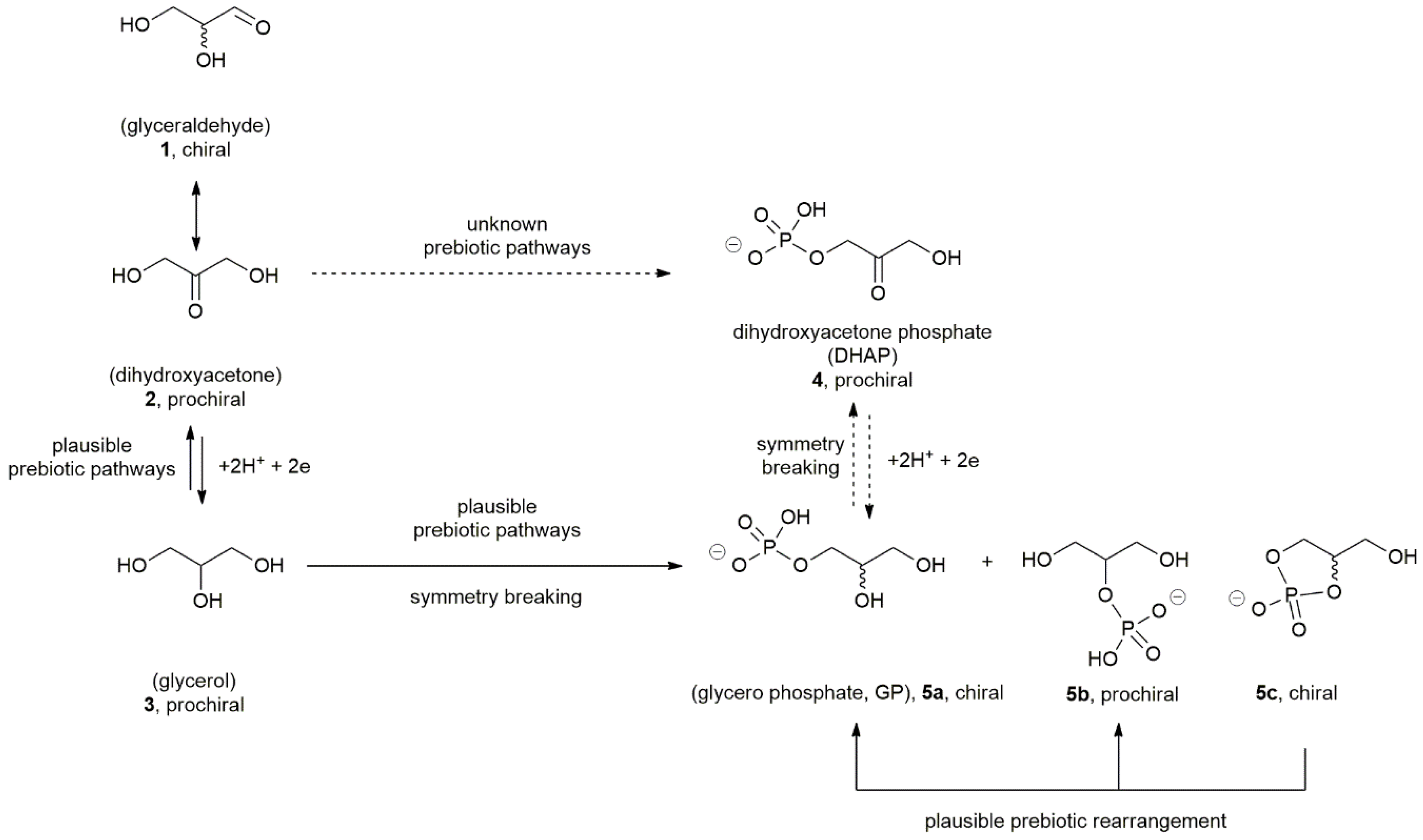
3. Prebiotic Scenarios for the Symmetry Imbalance of Phospholipids Precursors
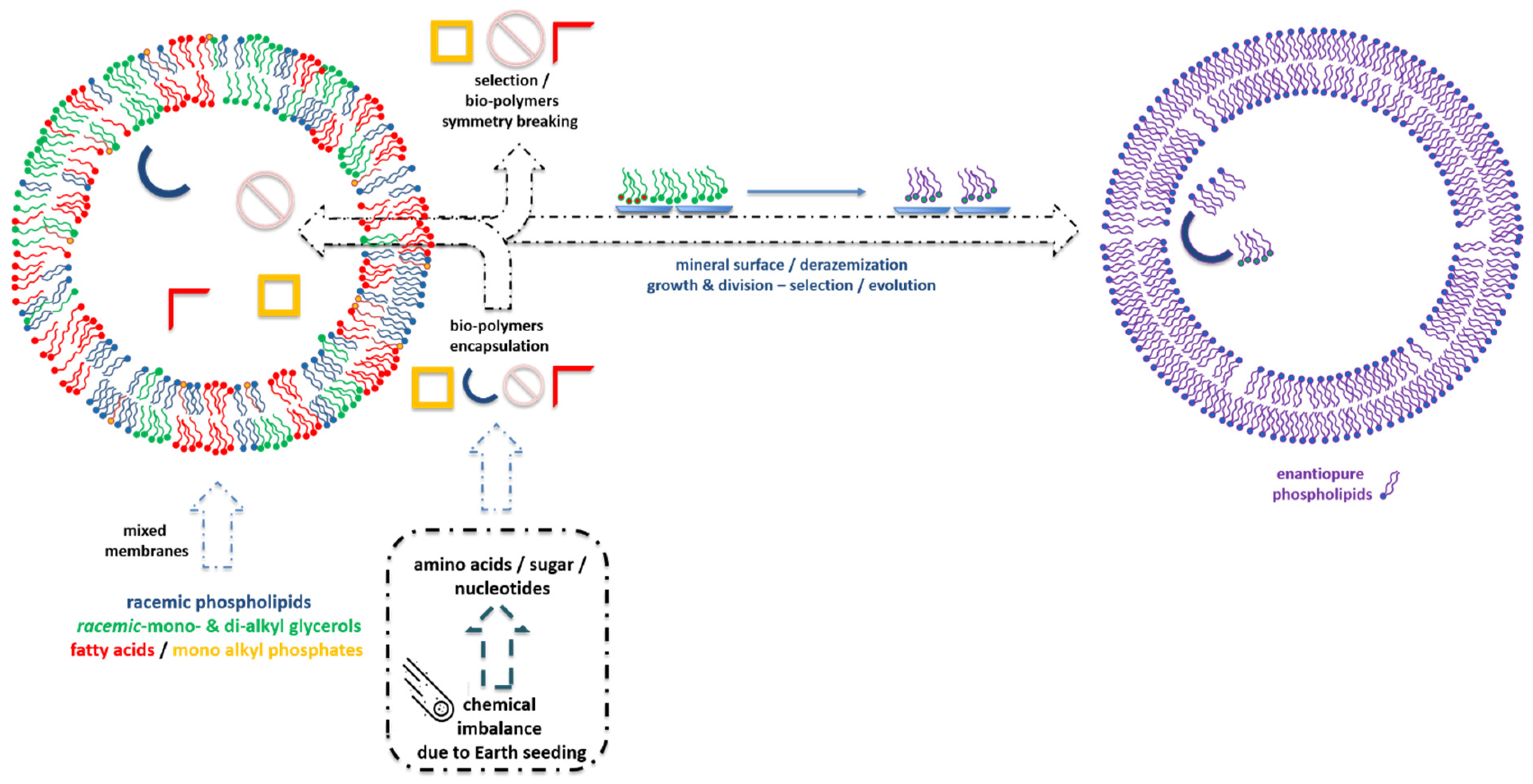
4. Achiral and Racemic Amphiphiles
4.1. Non-Chiral Amphiphiles
4.2. Racemic Amphiphiles and Their Precursors
5. Biological Synthesis in Archaea, Bacteria and Eukarya
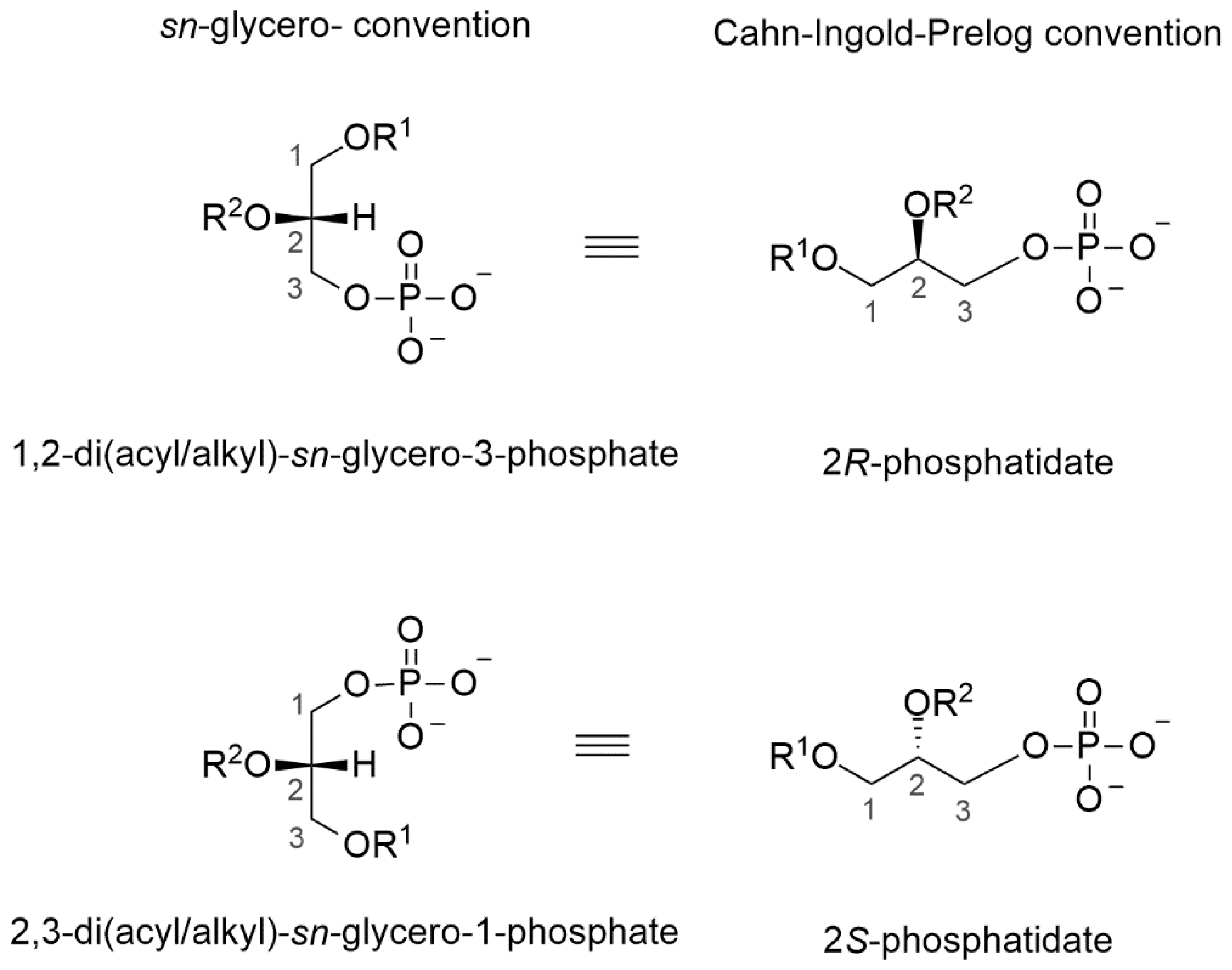
5.1. Lipid Characteristics in Archaea, Bacteria and Eukarya
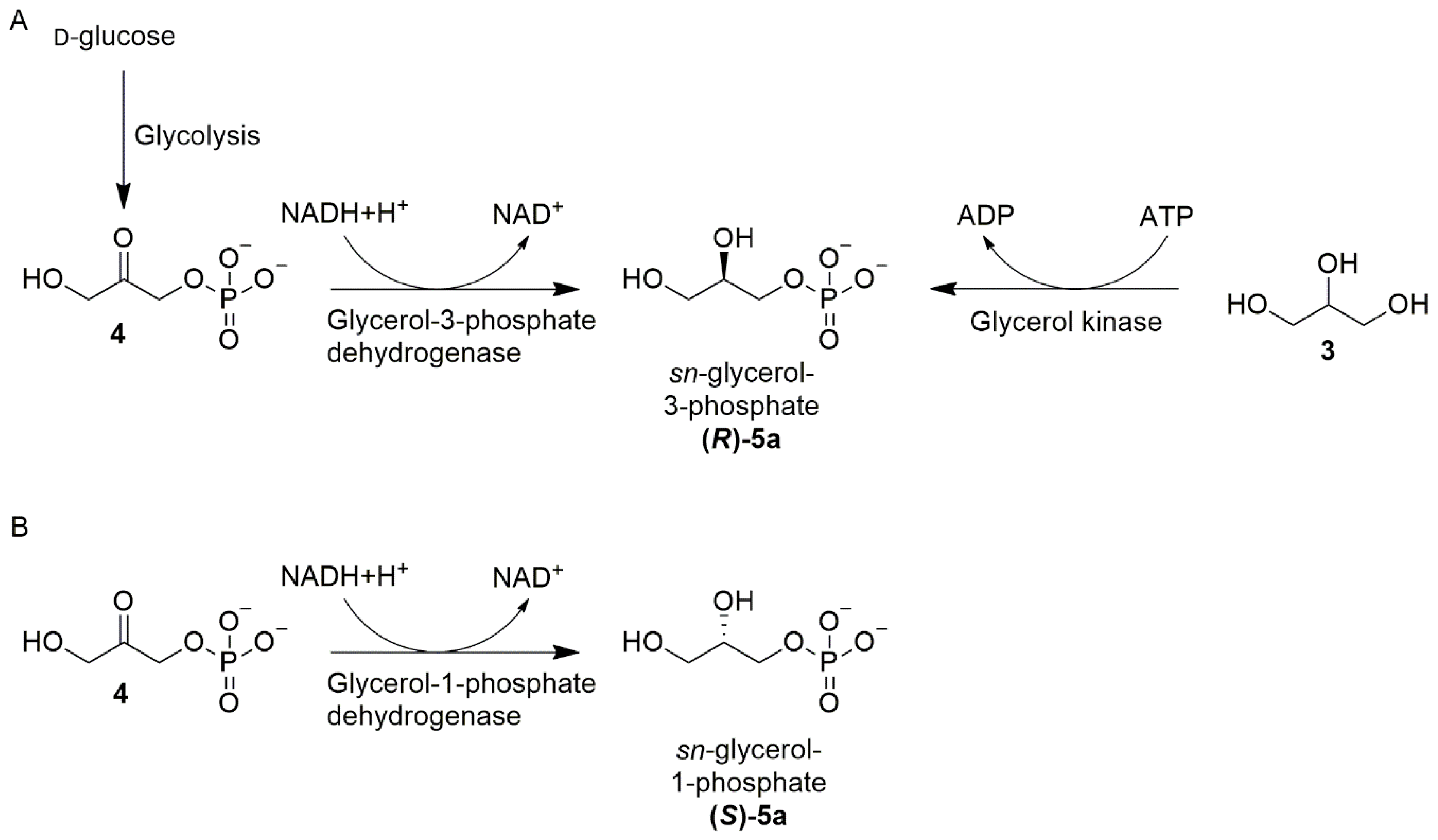
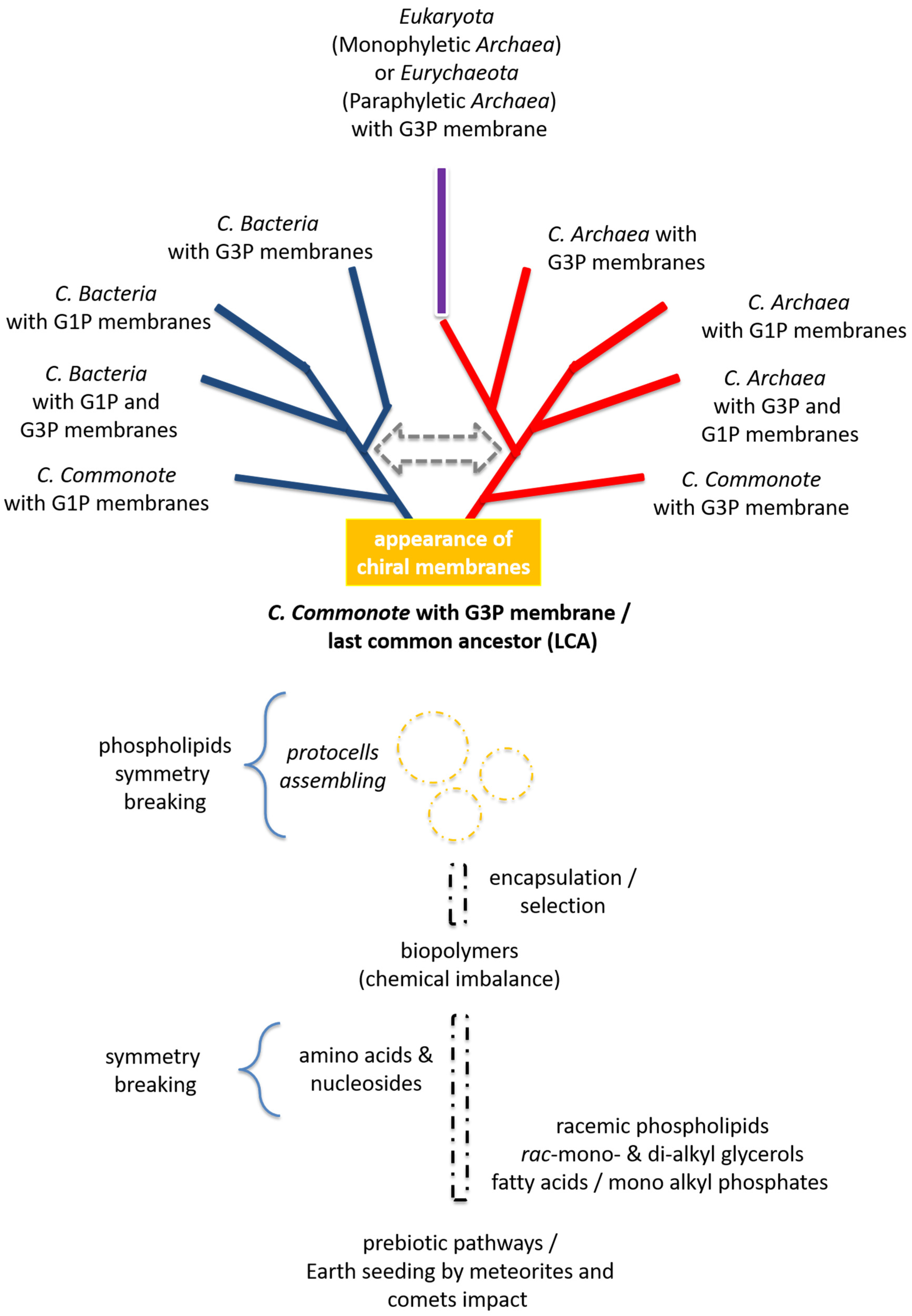
5.2. Appearance of Homochiral Membranes Based on Phylogenetic Analysis on Enzymes Forming sn-Glycerol-1-phosphate or sn-Glycerol-3-phosphate
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altamura, E.; Comte, A.; D’Onofrio, A.; Roussillon, C.; Fayolle, D.; Buchet, R.; Mavelli, F.; Stano, P.; Fiore, M.; Strazewski, P. Racemic Phospholipids for Origin of Life Studies. Symmetry 2020, 12, 1108. [Google Scholar] [CrossRef]
- Corbett, P.T.; Leclaire, J.; Vial, L.; West, K.R.; Wietor, J.-L.; Sanders, J.K.M.; Otto, S. Dynamic Combinatorial Chemistry. Chem. Rev. 2006, 106, 3652–3711. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Toward Self-Organization and Complex Matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; De La Escosura, A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef]
- Islam, S.; Powner, M.W. Prebiotic Systems Chemistry: Complexity Overcoming Clutter. Chem 2017, 2, 470–501. [Google Scholar] [CrossRef]
- Fiore, M.; Strazewski, P. Bringing Prebiotic Nucleosides and Nucleotides Down to Earth. Angew. Chem. 2016, 55, 13930–13933. [Google Scholar] [CrossRef]
- Fiore, M. The Origin and Early Evolution of Life: Prebiotic Chemistry. Life 2019, 9, 73. [Google Scholar] [CrossRef]
- Duim, H.; Otto, S. Towards open-ended evolution in self-replicating molecular systems. Beilstein J. Org. Chem. 2017, 13, 1189–1203. [Google Scholar] [CrossRef]
- Bissette, A.J.; Fletcher, S.P. Selecting complex behaviour. Nat. Chem. 2015, 7, 15–17. [Google Scholar] [CrossRef]
- Peretó, J. Out of fuzzy chemistry: From prebiotic chemistry to metabolic networks. Chem. Soc. Rev. 2012, 41, 5394–5403. [Google Scholar] [CrossRef] [PubMed]
- Dadon, Z.; Wagner, N.; Ashkenasy, G. The Road to Non-Enzymatic Molecular Networks. Angew. Chem. 2008, 47, 6128–6136. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Thoma, I.; Deutsch, A.; Gehrke, T.; Mayer, P.; Zipse, H.; Carell, T. A high-yielding, strictly regioselective prebiotic purine nucleoside formation pathway. Science 2016, 352, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Feldmann, J.; Wiedemann, S.; Okamura, H.; Schneider, C.; Iwan, K.; Crisp, A.; Rossa, M.; Amatov, T.; Carell, T. Unified prebiotically plausible synthesis of pyrimidine and purine RNA ribonucleotides. Science 2019, 366, 76–82. [Google Scholar] [CrossRef]
- Eghiaian, F.; Rico, F.; Colom, A.; Casuso, I.; Scheuring, S. High-speed atomic force microscopy: Imaging and force spectroscopy. FEBS Lett. 2014, 588, 3631–3638. [Google Scholar] [CrossRef]
- Böhm, C.; Möhwald, H.; Leiserowitz, L.; Als-Nielsen, J.; Kjaer, K. Influence of chirality on the structure of phospholipid monolayers. Biophys. J. 1993, 64, 553–559. [Google Scholar] [CrossRef][Green Version]
- Eschenmoser, A.; Loewenthal, E. Chemistry of potentially prebiological natural products. Chem. Soc. Rev. 1992, 21, 1. [Google Scholar] [CrossRef]
- Klabunovskii, E.I. Can Enantiomorphic Crystals like Quartz Play a Role in the Origin of Homochirality on Earth? Astrobiology 2001, 1, 127–131. [Google Scholar] [CrossRef]
- Joyce, G.F.; Schwartz, A.W.; Miller, S.L.; Orgel, L.E. The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc. Natl. Acad. Sci. USA 1987, 84, 4398–4402. [Google Scholar] [CrossRef]
- Subramanian, H.; Gatenby, R.A. Evolutionary advantage of directional symmetry breaking in self-replicating polymers. J. Theor. Biol. 2018, 446, 128–136. [Google Scholar] [CrossRef]
- Eghiaian, F. Lipid Chirality Revisited: A Change in Lipid Configuration Transforms Membrane-Bound Protein Domains. Biophys. J. 2015, 108, 2757–2758. [Google Scholar] [CrossRef]
- Altamura, E.; Stano, P.; Walde, P.; Mavelli, F. Giant vesicles as micro-sized enzymatic reactors: Perspectives and recent experimental advancements. Int. J. Unconv. Comput. 2015, 11, 5–21. [Google Scholar]
- Schreiber, A.; Huber, M.C.; Schiller, S.M. Prebiotic Protocell Model Based on Dynamic Protein Membranes Accommodating Anabolic Reactions. Langmuir 2019, 35, 9593–9610. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A.; Yorek, M.A. Membrane lipid composition and cellular function. J. Lipid Res. 1985, 26, 1015–1035. [Google Scholar] [PubMed]
- Koga, Y.; Kyuragi, T.; Nishihara, M.; Sone, N. Did Archaeal and Bacterial Cells Arise Independently from Noncellular Precursors? A Hypothesis Stating that the Advent of Membrane Phospholipid with Enantiomeric Glycerophosphate Backbones Caused the Separation of the Two Lines of Descent. J. Mol. Evol. 1998, 46, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, S.; Nakajima, Y.; Akanuma, S.; Yamagishi, A. Birth of Archaeal Cells: Molecular Phylogenetic Analyses of G1P Dehydrogenase, G3P Dehydrogenases, and Glycerol Kinase Suggest Derived Features of Archaeal Membranes Having G1P Polar Lipids. Archaea 2016, 2016, 1802675. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Williams, D.S.; Perriman, A.W.; Mann, S. Peptide-nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 2011, 3, 720–724. [Google Scholar] [CrossRef]
- Peretó, J.; López-García, P.; Moreira, D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 2004, 29, 469–477. [Google Scholar] [CrossRef]
- Lopez, A.; Fiore, M. Investigating prebiotic protocells for a comprehensive understanding of the origins of life: A prebiotic systems chemistry perspective. Life 2019, 9, 49. [Google Scholar] [CrossRef]
- Stano, P.; Altamura, E.; Mavelli, F. Novel directions in molecular systems design: The case of light-transducing synthetic cells. Commun. Integr. Biol. 2017, 10, 3837–3842. [Google Scholar] [CrossRef]
- Altamura, E.; Milano, F.; Tangorra, R.R.; Trotta, M.; Omar, O.H.; Stano, P.; Mavelli, F. Highly oriented photosynthetic reaction centers generate a proton gradient in synthetic protocells. Proc. Natl. Acad. Sci. USA 2017, 114, 3837–3842. [Google Scholar] [CrossRef] [PubMed]
- Altamura, E.; Albanese, P.; Marotta, R.; Milano, F.; Fiore, M.; Trotta, M.; Stano, P.; Mavelli, F. Light-driven ATP production promotes mRNA biosynthesis inside hybrid multi-compartment artificial protocells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems protobiology: Origin of life in lipid catalytic networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef] [PubMed]
- Strazewski, P. Low-digit and high-digit polymers in the origin of life. Life 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Blackmond, D.G. The origin of biological homochirality. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.; Hoffmann, S.V.; Cassam-Chenaï, P.; Evans, A.C.; Giri, C.; Nahon, L.; Meierhenrich, U.J. Photonenergy-Controlled Symmetry Breaking with Circularly Polarized Light. Angew. Chem. 2014, 53, 210–214. [Google Scholar] [CrossRef]
- Garcia, A.; Meinert, C.; Sugahara, H.; Jones, N.; Hoffmann, S.; Meierhenrich, U. The Astrophysical Formation of Asymmetric Molecules and the Emergence of a Chiral Bias. Life 2019, 9, 29. [Google Scholar] [CrossRef]
- Benner, S.A.; Kim, H.J.; Biondi, E. Prebiotic chemistry that could not not have happened. Life 2019, 9, 84. [Google Scholar] [CrossRef]
- Myrgorodska, I.; Meinert, C.; Martins, Z.; d’Hendecourt, L.L.S.; Meierhenrich, U.J. Molecular Chirality in Meteorites and Interstellar Ices, and the Chirality Experiment on Board the ESA Cometary Rosetta Mission. Angew. Chem. 2015, 54, 1402–1412. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef]
- Simonov, A.N.; Pestunova, O.P.; Matvienko, L.G.; Snytnikov, V.N.; Snytnikova, O.A.; Tsentalovich, Y.P.; Parmon, V.N. Possible prebiotic synthesis of monosaccharides from formaldehyde in presence of phosphates. Adv. Space Res. 2007, 40, 1634–1640. [Google Scholar] [CrossRef]
- Pasek, M.A. Thermodynamics of Prebiotic Phosphorylation. Chem. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.I.; Maity, S.; Jones, B.M. Synthesis of Prebiotic Glycerol in Interstellar Ices. Angew. Chem. 2015, 127, 197–202. [Google Scholar] [CrossRef]
- Wachtershauser, G. Origin of Life: Life as We Don’t Know It. Science 2000, 289, 1307–1308. [Google Scholar] [CrossRef]
- Sutherland, J.D. The Origin of Life-Out of the Blue. Angew. Chem. 2016, 55, 104–121. [Google Scholar] [CrossRef]
- Katagiri, A.; Yoshimura, S.; Yoshizawa, S. Formation Constant of the Tetracyanocuprate(II) Ion and the Mechanism of Its Decomposition. Inorg. Chem. 1981, 20, 4143–4147. [Google Scholar] [CrossRef]
- Shi, Q.; Ye, J. Deracemization Enabled by Visible-Light Photocatalysis. Angew. Chem. 2020, 59, 4998–5001. [Google Scholar] [CrossRef]
- Bonfio, C.; Godino, E.; Corsini, M.; de Biani, F.F.; Guella, G.; Mansy, S.S. Prebiotic iron-sulfur peptide catalysts generate a pH gradient across model membranes of late protocells. Nat. Catal. 2018, 1, 616–623. [Google Scholar] [CrossRef]
- Hall, D.O.; Cammak, R.; Rao, K.K.; Cammack, R.; Rao, K.K. Role for Ferredoxins in the Origin of Life and Biological Evolution. Nature 1971, 233, 136–138. [Google Scholar] [CrossRef]
- Johnson, A.P.; Cleaves, H.J.; Dworkin, J.P.; Glavin, D.P.; Lazcano, A.; Bada, J.L. The Miller Volcanic Spark Discharge Experiment. Science 2008, 322, 404. [Google Scholar] [CrossRef]
- Parker, E.T.; Zhou, M.; Burton, A.S.; Glavin, D.P.; Dworkin, J.P.; Krishnamurthy, R.; Fernández, F.M.; Bada, J.L. A Plausible Simultaneous Synthesis of Amino Acids and Simple Peptides on the Primordial Earth. Angew. Chem. 2014, 53, 8132–8136. [Google Scholar] [CrossRef] [PubMed]
- Piotto, S.; Sessa, L.; Piotto, A.; Nardiello, A.; Concilio, S. Plausible Emergence of Autocatalytic Cycles under Prebiotic Conditions. Life 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W.; Goodall, M.C.; Glickson, J.D.; Mayers, D.F. The Gramicidin A Transmembrane Channel: Characteristics of Head-to-Head Dimerized (L,D) Helices. Proc. Natl. Acad. Sci. USA 1971, 68, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Walde, P. Surfactant Assemblies and Their Various Possible Roles for the Origin(s) of Life. Orig. Life Evol. Biosph. 2006, 36, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Douliez, J.-P.; Gaillard, C. Self-assembly of fatty acids: From foams to protocell vesicles. New J. Chem. 2014, 38, 5142–5148. [Google Scholar] [CrossRef]
- Fiore, M.; Strazewski, P. Prebiotic lipidic amphiphiles and condensing agents on the early earth. Life 2016, 6, 17. [Google Scholar] [CrossRef]
- Hargreaves, W.R.; Deamer, D.W. Liposomes from Ionic, Single-Chain Amphiphiles. Biochemistry 1978, 17, 3759–3768. [Google Scholar] [CrossRef]
- Fayolle, D.; Altamura, E.; D’Onofrio, A.; Madanamothoo, W.; Fenet, B.; Mavelli, F.; Buchet, R.; Stano, P.; Fiore, M.; Strazewski, P. Crude phosphorylation mixtures containing racemic lipid amphiphiles self-assemble to give stable primitive compartments. Sci. Rep. 2017, 7, 18106. [Google Scholar] [CrossRef]
- Ourisson, G.; Nakatani, Y. The terpenoid theory of the origin of cellular life: The evolution of terpenoids to cholesterol. Chem. Biol. 1994, 1, 11–23. [Google Scholar] [CrossRef]
- Jordan, S.F.; Rammu, H.; Zheludev, I.N.; Hartley, A.M.; Maréchal, A.; Lane, N. Promotion of protocell self-assembly from mixed amphiphiles at the origin of life. Nat. Ecol. Evol. 2019, 3, 1705–1714. [Google Scholar] [CrossRef]
- Hargreaves, W.R.; Mulvil, S.J.; Deamer, D.W. Synthesis of phospholipids and membranes in prebiotic conditions. Nature 1977, 266, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Epps, D.E.; Sherwood, E.; Eichberg, J.; Oró, J. Cyanamide mediated syntheses under plausible primitive earth conditions. J. Mol. Evol. 1978, 11, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Eichberg, J.; Oró, J. Synthesis of phosphatidylcholine under possible primitive Earth conditions. J. Mol. Evol. 1982, 18, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Eichberg, J.; Oró, J. Synthesis of phosphatidylethanolamine under possible primitive earth conditions. J. Mol. Evol. 1987, 25, 1–6. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoneit, B.R.T.T. Abiotic condensation synthesis of glyceride lipids and wax esters under simulated hydrothermal conditions. Orig. Life Evol. Biosph. 2006, 36, 93–108. [Google Scholar] [CrossRef]
- Maheen, G.; Tian, G.; Wang, Y.; He, C.; Shi, Z.; Yuan, H.; Feng, S. Resolving the enigma of prebiotic C-O-P bond formation: Prebiotic hydrothermal synthesis of important biological phosphate esters. Heteroat. Chem. 2010. [Google Scholar] [CrossRef]
- Bonfio, C.; Caumes, C.; Duffy, C.D.; Patel, B.H.; Percivalle, C.; Tsanakopoulou, M.; Sutherland, J.D. Length-Selective Synthesis of Acylglycerol-Phosphates through Energy-Dissipative Cycling. J. Am. Chem. Soc. 2019, 141, 3934–3939. [Google Scholar] [CrossRef]
- Pasek, M.A.; Gull, M.; Herschy, B. Phosphorylation on the early earth. Chem. Geol. 2017, 475, 149–170. [Google Scholar] [CrossRef]
- Xu, J.; Green, N.J.; Gibard, C.; Krishnamurthy, R.; Sutherland, J.D. Prebiotic phosphorylation of 2-thiouridine provides either nucleotides or DNA building blocks via photoreduction. Nat. Chem. 2019, 11, 457–462. [Google Scholar] [CrossRef]
- Pross, A. Toward a general theory of evolution: Extending Darwinian theory to inanimate matter. J. Syst. Chem. 2011, 2, 1. [Google Scholar] [CrossRef]
- Toparlak, D.; Karki, M.; Egas Ortuno, V.; Krishnamurthy, R.; Mansy, S.S. Cyclophospholipids Increase Protocellular Stability to Metal Ions. Small 2020, 16, 1903381. [Google Scholar] [CrossRef] [PubMed]
- Gibard, C.; Bhowmik, S.; Karki, M.; Kim, E.-K.K.; Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2018, 10, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Burcar, B.; Pasek, M.; Gull, M.; Cafferty, B.J.; Velasco, F.; Hud, N.V.; Menor-Salván, C. Darwin’s Warm Little Pond: A One-Pot Reaction for Prebiotic Phosphorylation and the Mobilization of Phosphate from Minerals in a Urea-Based Solvent. Angew. Chem. 2016, 55, 13249–13253. [Google Scholar] [CrossRef] [PubMed]
- Menor-Salván, C.; Marín-Yaseli, M.R. Prebiotic chemistry in eutectic solutions at the water-ice matrix. Chem. Soc. Rev. 2012, 41, 5404–5415. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Johnston, W.K. RNA-Catalyzed RNA Polymerization: Accurate and General RNA-Templated Primer Extension. Science 2001, 292, 1319–1325. [Google Scholar] [CrossRef]
- Szostak, J.W. Systems chemistry on early Earth. Nature 2009, 459, 171–172. [Google Scholar] [CrossRef]
- IUPAC-IUD Commission on Biochemical Nomenclature. The nomenclature of lipids (Recommendations 1976) IUPAC-IUB Commission on Biochemical Nomenclature. Biochem. J. 1978, 171, 21–35. Available online: https://portlandpress.com/biochemj/article-abstract/171/1/21/11772/The-nomenclature-of-lipids-Recommendations-1976?redirectedFrom=fulltext (accessed on 22 July 2020). [CrossRef]
- Kennedy, E.P. The Biological Synthesis of Phosholipids. Can. J. Biochem. Physiol. 1956, 34, 334–348. [Google Scholar] [CrossRef]
- Weis, R.M.; McConnell, H.M. Two-dimensional chiral crystals of phospholipid. Nature 1984, 310, 47–49. [Google Scholar] [CrossRef]
- Lombard, J.; López-García, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. Microbiol. 2012, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, A.; Kon, T.; Takahashi, G.; Oshima, T. From the Common Ancestor of All Living Organisms to Protoeukaryotic Cell. In Thermophyles: The Keys to Molecular Evolution and the Origin of Life? Wiegel, J., Adams, M.W.W., Eds.; Rootledge Taylor & Francis: Abingdon, UK, 1998. [Google Scholar]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Glansdorff, N.; Xu, Y.; Labedan, B. The Last Universal Common Ancestor: Emergence, constitution and genetic legacy of an elusive forerunner. Biol. Direct 2008, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Hydrogen sulfide, reactive sulfur species and coping with reactive oxygen species. Free Radic. Biol. Med. 2019, 140, 74–83. [Google Scholar] [CrossRef]
- Kasting, J.F.; Zahnle, K.J.; Pinto, J.P.; Young, A.T. Sulfur, ultraviolet radiation, and the early evolution of life. Orig. Life Evol. Biosph. 1989, 19, 252–253. [Google Scholar] [CrossRef]
- Catling, D.C.; Zahnle, K.J. The Archean atmosphere. Sci. Adv. 2020, 6, eaax1420. [Google Scholar] [CrossRef]
- Guy, L.; Ettema, T.J.G. The archaeal ‘TACK’ superphylum and the origin of eukaryotes. Trends Microbiol. 2011, 19, 580–587. [Google Scholar] [CrossRef]
- Eme, L.; Doolittle, W.F. Archaea. Curr. Biol. 2015, 25, R851–R855. [Google Scholar] [CrossRef]
- Eme, L.; Spang, A.; Lombard, J.; Stairs, C.W.; Ettema, T.J.G. Archaea and the origin of eukaryotes. Nat. Rev. Microbiol. 2017, 15, 711–723. [Google Scholar] [CrossRef]
- Akanuma, S.; Yokobori, S.; Yamagishi, A. Comparative Genomics of Thermophilic Bacteria and Archaea. In Thermophilic Microbes in Environmental and Industrial Biotechnology; Springer: Dordrecht, The Netherlands, 2013; pp. 331–349. [Google Scholar]
- Williams, T.A.; Foster, P.G.; Cox, C.J.; Embley, T.M. An archaeal origin of eukaryotes supports only two primary domains of life. Nature 2013, 504, 231–236. [Google Scholar] [CrossRef]
- Lake, J.A.; Sinsheimer, J.S. The Deep Roots of the Rings of Life. Genome Biol. Evol. 2013, 5, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Daiyasu, H.; Hiroike, T.; Koga, Y.; Toh, H. Analysis of membrane stereochemistry with homology modeling of sn-glycerol-1-phosphate dehydrogenase. Protein Eng. Des. Sel. 2002, 15, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Carbone, V.; Schofield, L.R.; Zhang, Y.; Sang, C.; Dey, D.; Hannus, I.M.; Martin, W.F.; Sutherland-Smith, A.J.; Ronimus, R.S. Structure and Evolution of the Archaeal Lipid Synthesis Enzyme sn-Glycerol-1-phosphate Dehydrogenase. J. Biol. Chem. 2015, 290, 21690–21704. [Google Scholar] [CrossRef]
- Wächtershäuser, G. From pre-cells to Eukarya—A tale of two lipids. Mol. Microbiol. 2003, 47, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kandler, O. Cell wall biochemistry in Archaea and its phylogenetic implications. J. Biol. Phys. 1995, 20, 165–169. [Google Scholar] [CrossRef]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef]
- Saw, J.H.; Spang, A.; Zaremba-Niedzwiedzka, K.; Juzokaite, L.; Dodsworth, J.A.; Murugapiran, S.K.; Colman, D.R.; Takacs-Vesbach, C.; Hedlund, B.P.; Guy, L.; et al. Exploring microbial dark matter to resolve the deep archaeal ancestry of eukaryotes. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140328. [Google Scholar] [CrossRef]
- Spang, A.; Saw, J.H.; Jørgensen, S.L.; Zaremba-Niedzwiedzka, K.; Martijn, J.; Lind, A.E.; van Eijk, R.; Schleper, C.; Guy, L.; Ettema, T.J.G. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 2015, 521, 173–179. [Google Scholar] [CrossRef]
- Daubin, V.; Szöllősi, G.J. Horizontal Gene Transfer and the History of Life. Cold Spring Harb. Perspect. Biol. 2016, 8, a018036. [Google Scholar] [CrossRef]
- Carter, C.W. What RNA world? Why a peptide/rna partnership merits renewed experimental attention. Life 2015, 5, 294–320. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W. An Alternative to the RNA World. Nat. Hist. 2016, 125, 28–33. [Google Scholar] [PubMed]
- Carter, C.W.; Wills, P.R. Interdependence, Reflexivity, Fidelity, Impedance Matching, and the Evolution of Genetic Coding. Mol. Biol. Evol. 2018, 35, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; El-Hachemi, Z.; Díez-Pérez, I.; Crusats, J.; Ribó, J.M. Formation of an epitaxial monolayer on graphite upon short-time surface contact with highly diluted aqueous solutions of 1-monostearoylglycerol. Thin Solid Films 2007, 515, 5391–5394. [Google Scholar] [CrossRef]
- Fiore, M.; Maniti, O.; Girard-Egrot, A.; Monnard, P.-A.A.; Strazewski, P. Glass Microsphere-Supported Giant Vesicles for the Observation of Self-Reproduction of Lipid Boundaries. Angew. Chem. 2018, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Fayolle, D.; Fiore, M.; Strazewski, P. Chemical Analysis of Lipid Boundaries after Consecutive Growth of Supported Giant Vesicles. iScience 2020. accepted. [Google Scholar]
- Hanczyc, M.M.; Szostak, J.W. Replicating vesicles as models of primitive cell growth and division. Curr. Opin. Chem. Biol. 2004, 8, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, H.; Nishimura, K.; Suzuki, H.; Matsuura, T.; Yomo, T. Coupling of the fusion and budding of giant phospholipid vesicles containing macromolecules. Proc. Natl. Acad. Sci. USA 2012, 109, 5942–5947. [Google Scholar] [CrossRef]
- Miele, Y.; Medveczky, Z.; Holló, G.; Tegze, B.; Derényi, I.; Hórvölgyi, Z.; Altamura, E.; Lagzi, I.; Rossi, F. Self-division of giant vesicles driven by an internal enzymatic reaction. Chem. Sci. 2020, 11, 3228–3235. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M.; Buchet, R. Symmetry Breaking of Phospholipids. Symmetry 2020, 12, 1488. https://doi.org/10.3390/sym12091488
Fiore M, Buchet R. Symmetry Breaking of Phospholipids. Symmetry. 2020; 12(9):1488. https://doi.org/10.3390/sym12091488
Chicago/Turabian StyleFiore, Michele, and René Buchet. 2020. "Symmetry Breaking of Phospholipids" Symmetry 12, no. 9: 1488. https://doi.org/10.3390/sym12091488
APA StyleFiore, M., & Buchet, R. (2020). Symmetry Breaking of Phospholipids. Symmetry, 12(9), 1488. https://doi.org/10.3390/sym12091488