Graphene Oxide Hybridised TiO2 for Visible Light Photocatalytic Degradation of Phenol
Abstract
1. Introduction
2. Experimental Setup
2.1. Materials and Reagents
2.2. Synthesis of TiO2 Nanoparticles
2.3. Synthesis of TiO2/rGO Nanocomposites
2.4. Characterisation
2.5. Photoelectrochemical Analysis
2.6. Photocatalytic Test
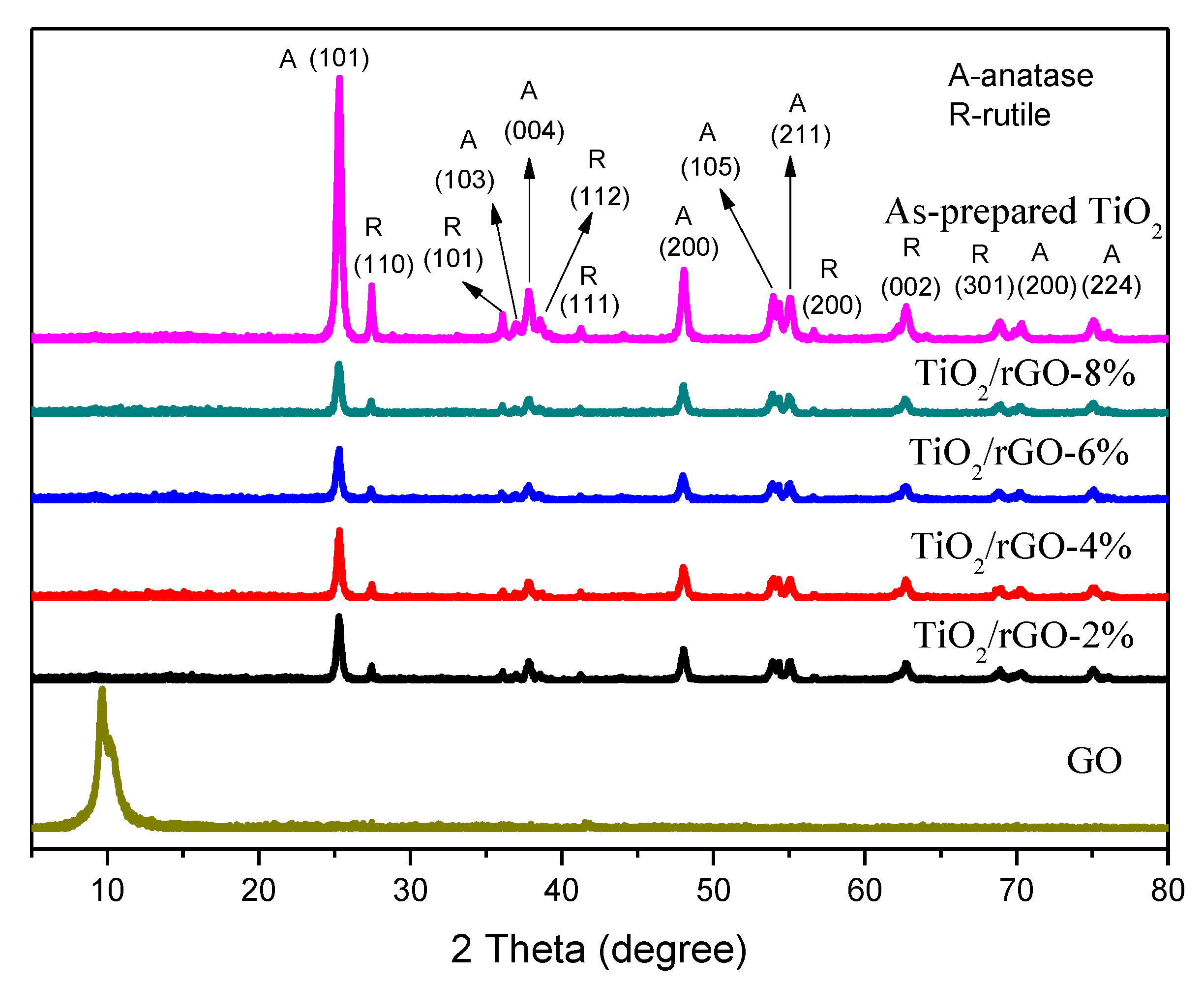
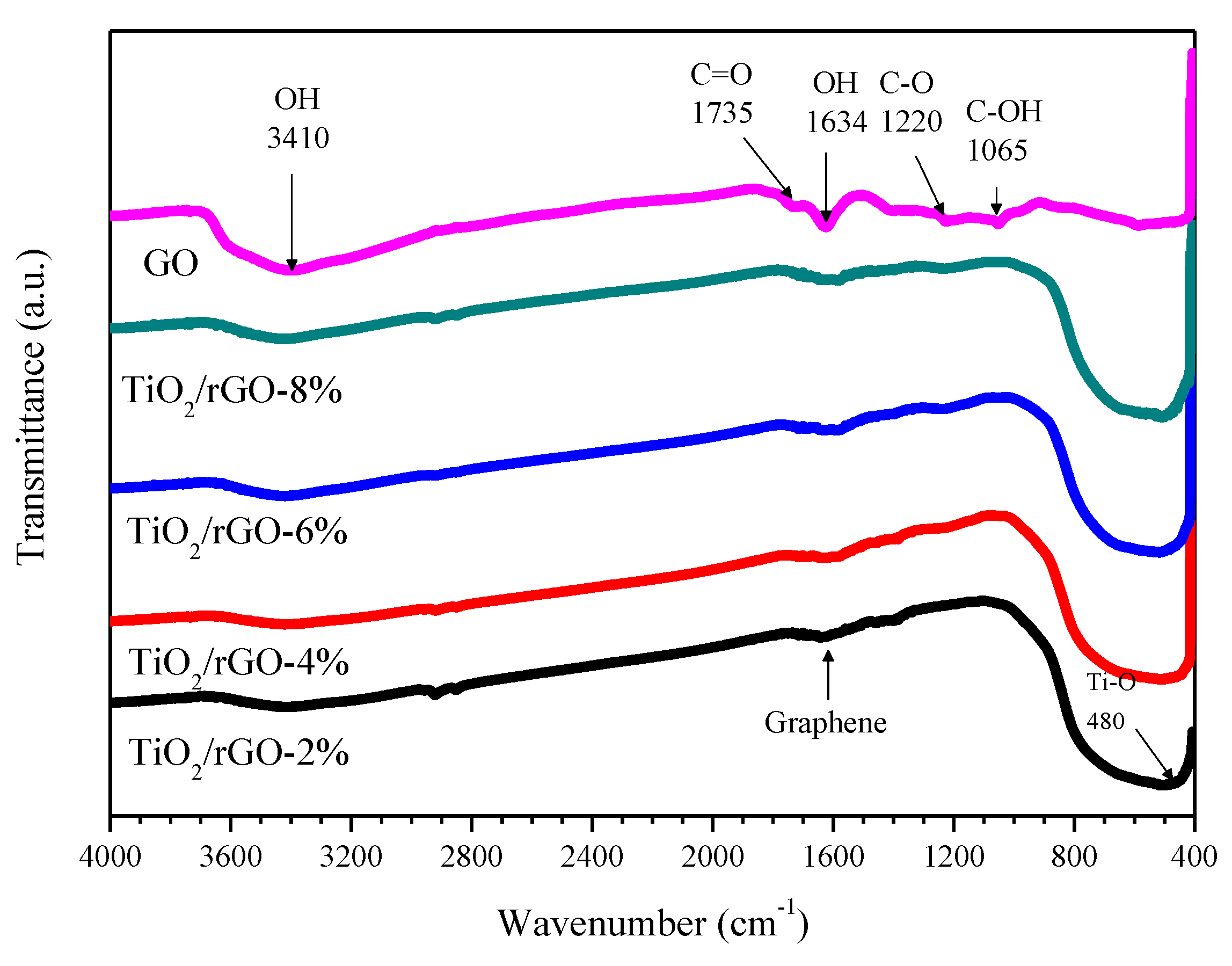
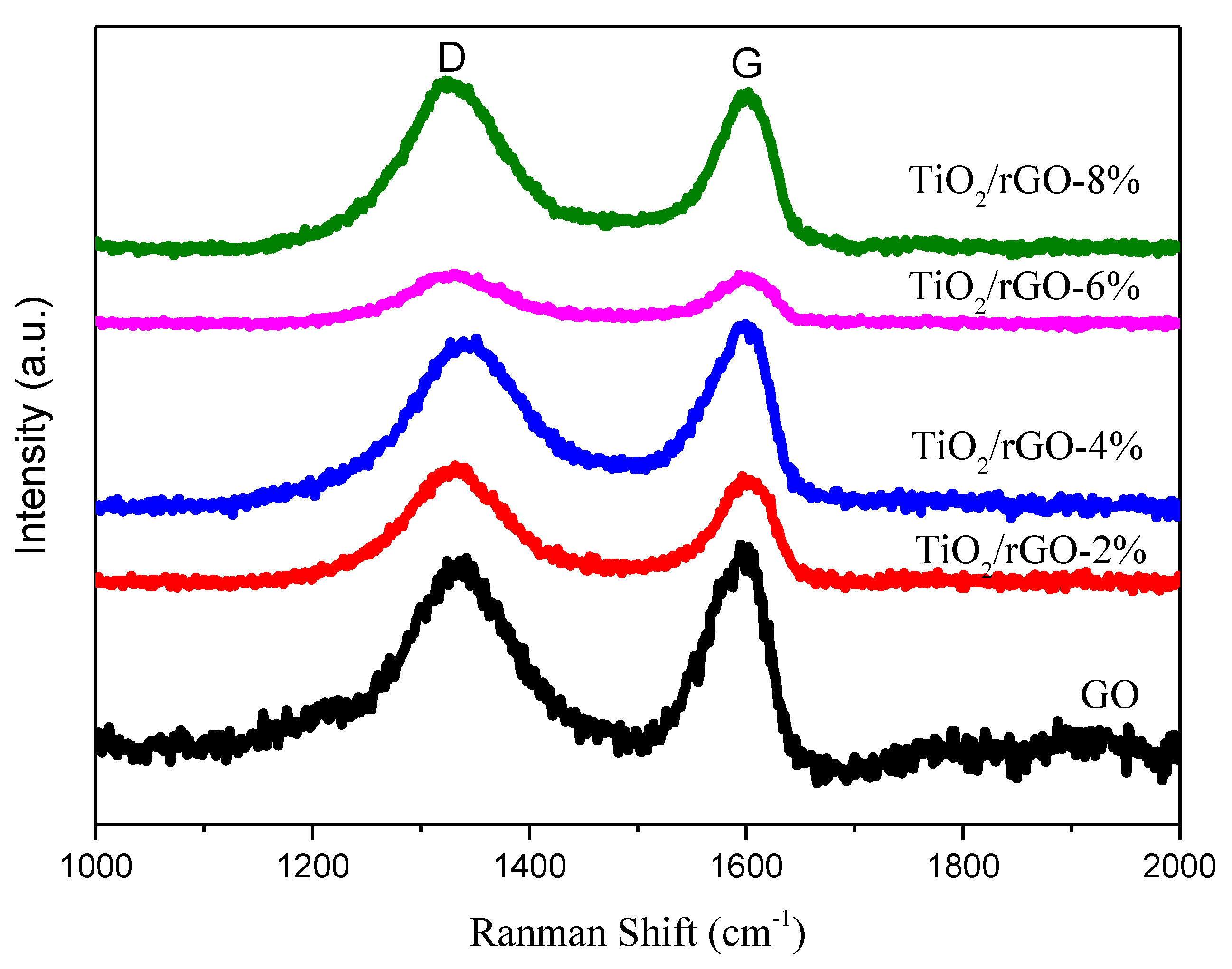
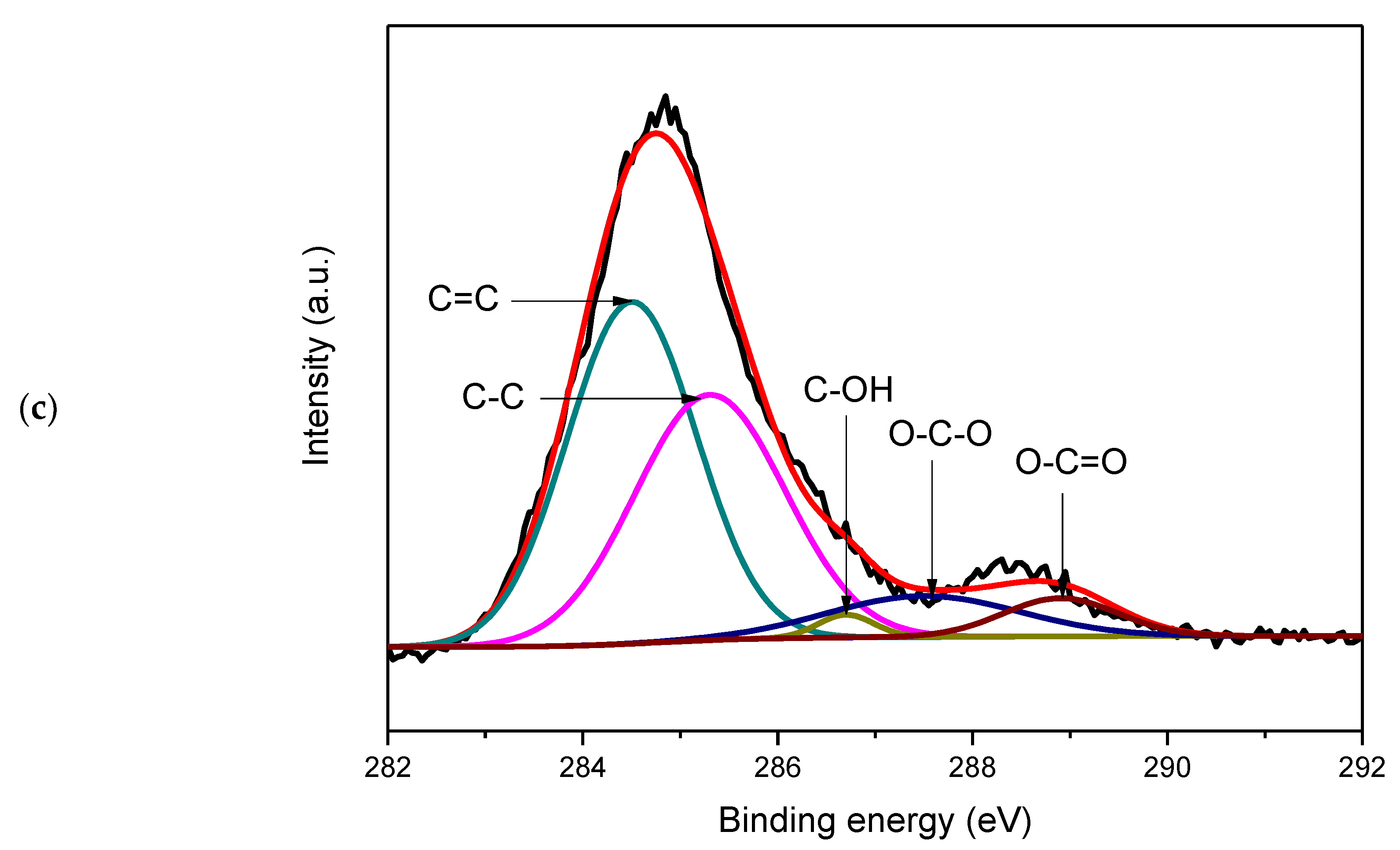
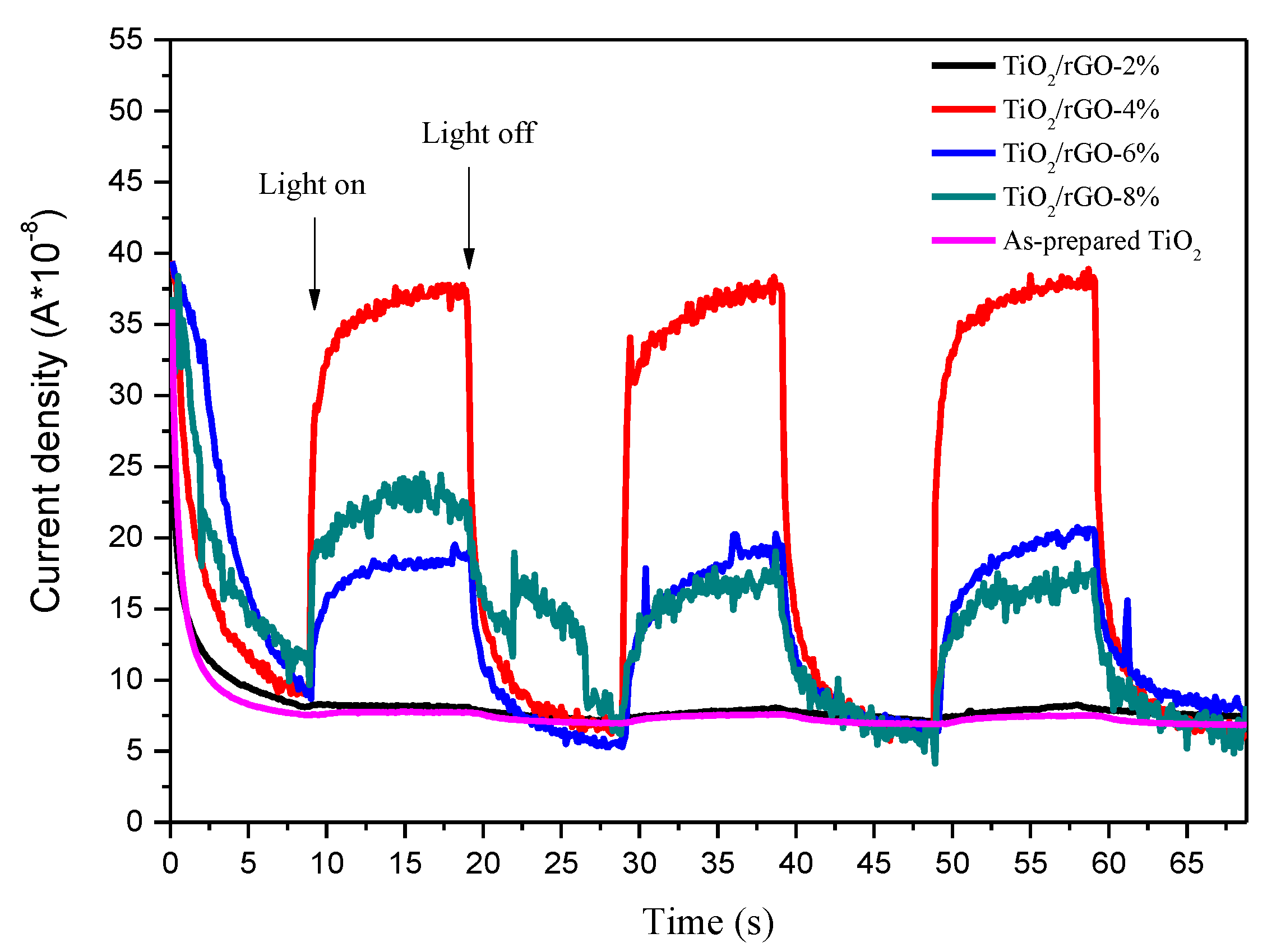
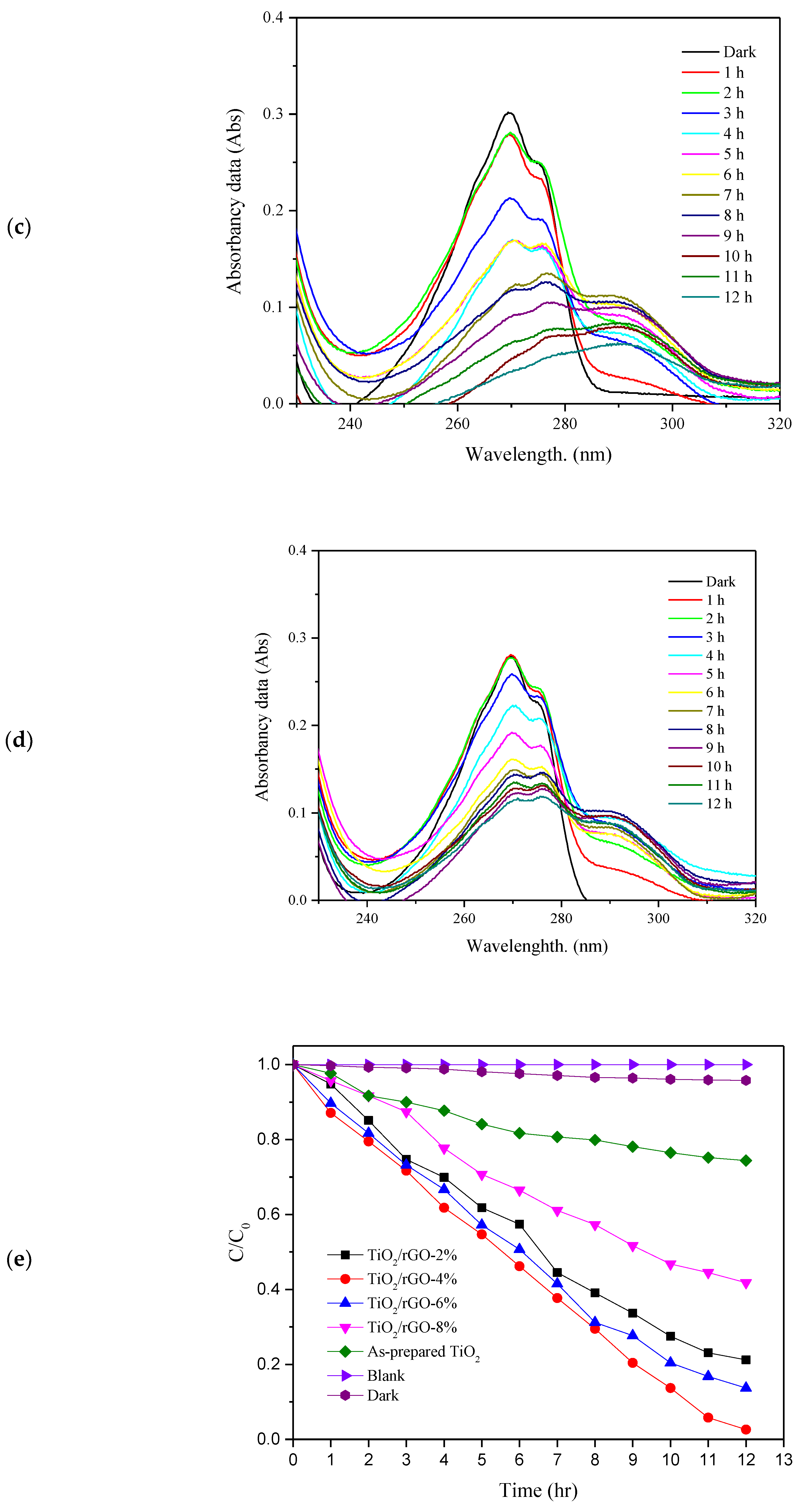
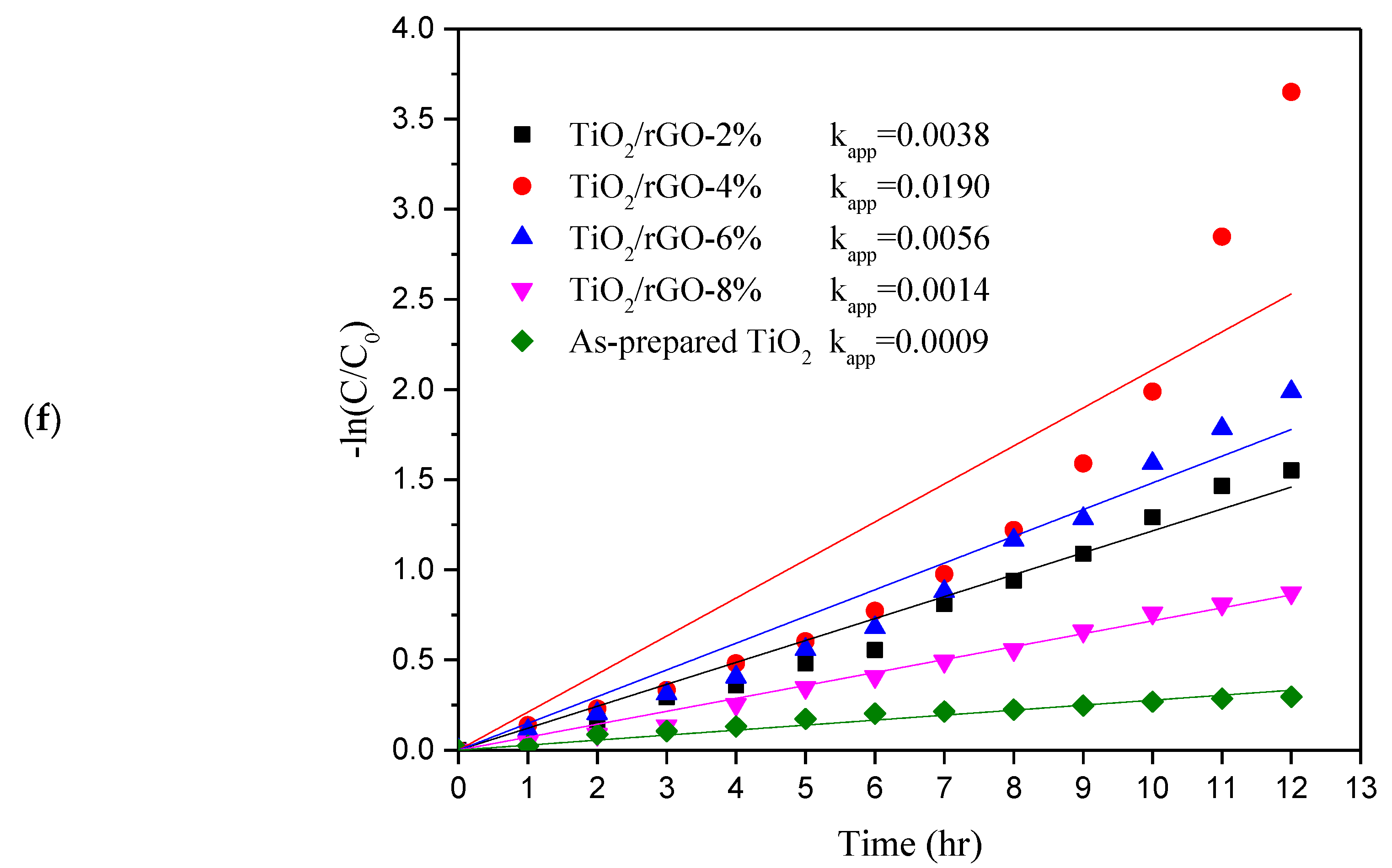
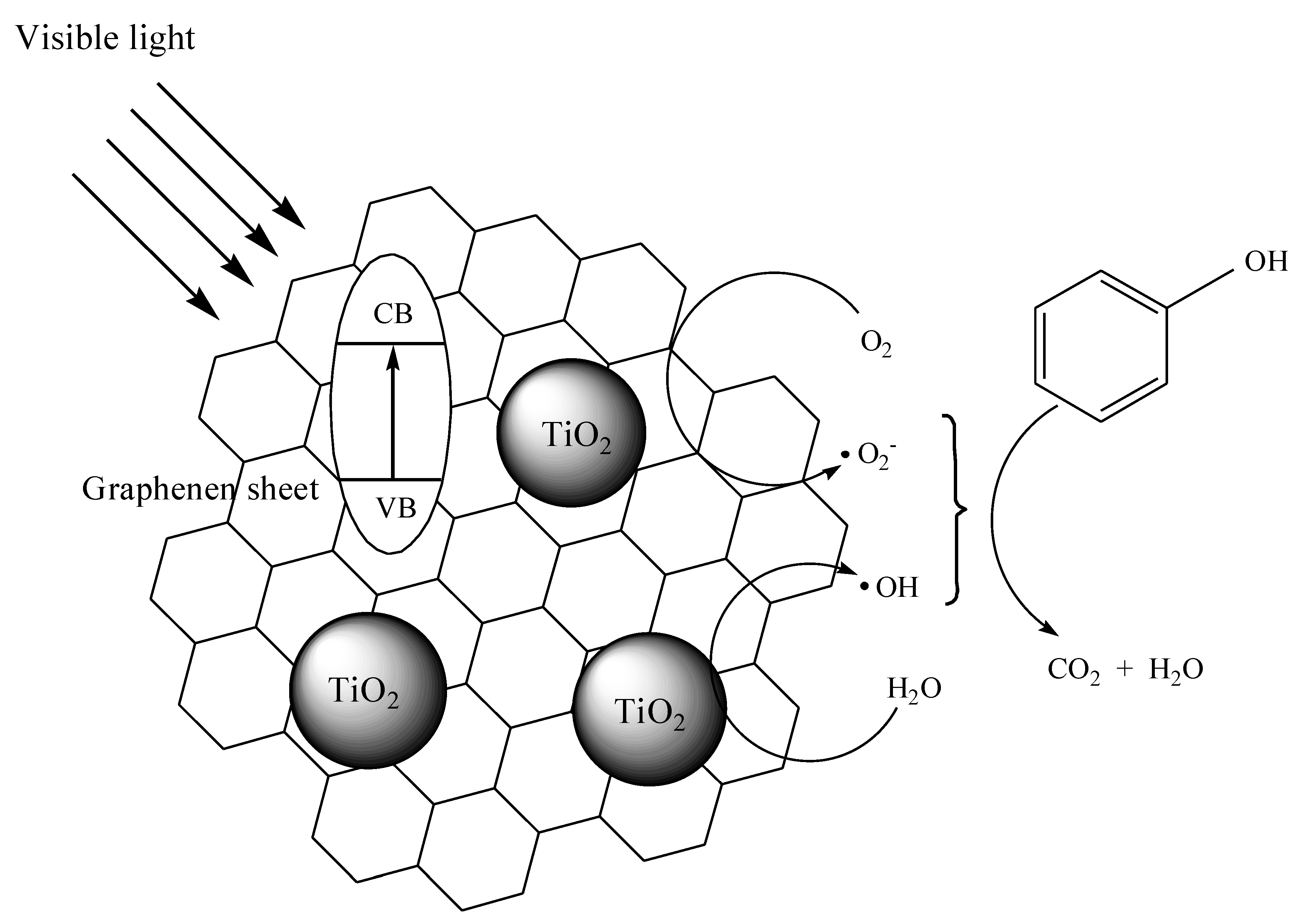
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.L.; Yue, X.P.; Wang, G.Y. Simultaneous heterotrophic nitrification and aerobic denitrification at high initial phenol concentration by isolated bacterium Diaphorobacter sp PD-7. Chin. J. Chem. Eng. 2015, 23, 835–841. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Karagianni, P.; Giannakas, A. Photocatalytic degradation of phenol by char/N-TiO2 and char/N-F-TiO2 composite photocatalysts. Catal. Today 2017, 280, 114–121. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Ling, H.J.; Kyungduk, K.; Liu, Z.W. Photocatalytic degradation of phenol in water on as-prepared and surface modified TiO2 nanoparticles. Catal. Today 2015, 258, 96–102. [Google Scholar] [CrossRef]
- Eiroa, M.; Vilar, A.; Kennes, C. Effect of phenol on the biological treatment of wastewaters from a resin producing industry. Bioresour. Technol. 2008, 99, 3507–3512. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.D.; Zhou, S.J.; Yang, F. Degradation of phenol in industrial wastewater over the F-Fe/TiO2 photocatalysts under visible light illumination. Chin. J. Chem. Eng. 2016, 24, 1712–1718. [Google Scholar] [CrossRef]
- Liu, Y.D.; Zhou, S.J.; Yang, F. Highly Efficient Oxidation of Gaseous Benzene on Novel Ag3VO4/TiO2 Nanocomposite Photocatalysts under Visible and Simulated Solar Light Irradiation. J. Phys. Chem. C 2012, 116, 13935–13943. [Google Scholar]
- Lim, J.; Monllor-Satoca, D.; Jang, J.S. Visible light photocatalysis of fullerol-complexed TiO2 enhanced by Nb doping. Appl. Catal. B-Environ. 2014, 152, 233–240. [Google Scholar] [CrossRef]
- Li, B.Z.; Sun, K.Q.; Guo, Y.B. Adsorption kinetics of phenol from water on Fe/AC. Fuel 2013, 110, 99–106. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Dobrosz-Gomez, I.; Gomez-Garcia, M.A.; Lopez Zamora, S.M.; Gilpavas, E.; Bojarska, J.; Kozanecki, M.; Rynkowski, J.M. Transition metal loaded TiO2 for phenol photo-degradation. Comptes Rendus Chim. 2015, 18, 1170–1182. [Google Scholar] [CrossRef]
- Ali, I.; Kim, S.R.; Kim, S.P.; Kim, J.O. Andodization of bismuth doped TiO2 nanotubes composite for photocatalytic degradation of phenol in visible light. Catal. Today 2017, 282, 31. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Miyauchi, M. Nanoporous-walled tungsten oxide nanotubes as highly active visible-light-driven photocatalysts. Angew. Chem. Int. Ed. 2008, 47, 7051–7055. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burda, C. Photoelectron investigation of nitrogen-doped titania nanoparticles. Phys. Chem. B 2004, 108, 15446–15449. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation photocatalytic activity, and mechanism of nano-TiO2 co-doped with nitrogen and iron (III). Phy. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.X.; Ma, Y.; Jia, X.L.; Ye, G.T.; Guan, S.K. Ehanced photocatalytic degradation properties of nitrogen-doped titania nanotube arrays. Trans. Nonferrous Met. Soc. China 2009, 19, 1583–1587. [Google Scholar] [CrossRef]
- Nawi, M.A.; Zain, S.M. Enhancing the surface properties of the immobilised Degussa P-25 TiO2 for the efficient photocatalytic removal of methylene blue from aqueous solution. Appl. Surf. Sci. 2012, 258, 6148–6157. [Google Scholar] [CrossRef]
- Chen, X.; Mao, X.X. Titanium dioxide nanomaterials: Synthesis, properties, modification, and applications. Chen. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.Q.; Liu, S.; Sun, Y.; Xu, Y.J. Waltzing with the versatile platform of graphene to synthesis composite photocatalysts. Chen. Rev. 2015, 115, 10307–103717. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activitiy. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Bell, N.J.; Ng, Y.H.; Du, A.; Coster, H.; Smith, S.C.; Amal, R. Understanding the enhancement in photoelectrochemical properties of photocatalytically prepared TiO2-reduced graphene oxide composite. J. Phys. Chem. C 2011, 115, 6004–6009. [Google Scholar] [CrossRef]
- Shao, X.; Lu, W.; Zahng, R.; Pan, F. Enhanced photocatalytic activity of TiO2-C hybrid aerogels for methylene blue degradation. Sci. Rep. 2013, 3, 3018. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hidalgo, M.C.; Navio, J.A.; Arana, J.; Dona-Rodriguez, J.M. Study of the phenol photocatalytic degradation over TiO2 modified by sulfation, fluorination, and platinum nanoparticles photodeposition. Appl. Catal. B Environ. 2015, 179, 305–312. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Al-Thani, N.J.; Tawbi, K.; Al-Kandari, H. Carbon/nitrogen-doped TiO2: New synthesis route, characterisation and application for phenol degradation. Arab. J. Chem. 2016, 9, 229–237. [Google Scholar] [CrossRef]
- Sohrabi, S.; Akhlaghian, F. Modeling and optimisation of phenol degradation over copper-doped titanium dioxide photocatalyst using response surface methodology. Process Saf. Environ. Prot. 2016, 99, 120–128. [Google Scholar] [CrossRef]
- De Almeida, M.F.; Bellato, C.R.; Mounteer, A.H.; Ferreira, S.O.; Milagres, J.L.; Miranda, L.D.L. Enhanced photocatalytic activity of TiO2-impregnated with MgZnAl mixed oxides obtained from layered double hydroxides for phenol degradation. Appl. Surf. Sci. 2015, 357, 1765–1775. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, J. Preparation and enhanced daylight-induced photocatalytic activity of C, N, S-tridoped titanium dioxide powders. Hazard. Mater. 2008, 152, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Williiams, K.J.; Nelson, C.A.; Yan, X.; Li, L.S.; Zhu, X. Hot electon injection from graphene quantum dots to TiO2. ACS Nano 2013, 7, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Malekshoar, G.; Pal, K.; He, Q.; Yu, A.; Ray, A.K. Enhanced Solar Photocatalytic Degrdation of Phenol with Coupled Graphene-Based Titanium Dioxide and Zinc Oxide. Ind. Eng. Chem. Res. 2014, 53, 18824–18832. [Google Scholar]
- Farzan, H.; Ali, A.I.; Moslem, F.; Bagher, A.; Sahand, J. Photocatalytic decontamination of phenol and petrochemical wastwater through ZnO/TiO2 decorated on reduced graphene oxide nanocomposite: Influential operating factors, mechanism, and electrical energy consumption. RSC Adv. 2018, 8, 40035. [Google Scholar]
- Ezzat, R.; Elham, N.; Ali, A.Z.; Hadis, Z. Photocatalytic degradation of phenol using a new developed TiO2/graphene/heteropoly acid nanocomposite: Synthesis, characterization and process optimization. RSC Adv. 2016, 6, 96554–96562. [Google Scholar]
- Chen, C.; Cai, W.; Long, M.; Zhou, B.; Wu, Y.; Wu, D.; Feng, Y. Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction. ACS Nano 2010, 4, 6425–6432. [Google Scholar] [CrossRef]
- Patil, G.P.; Bagal, V.S.; Mahajian, C.R.; Chaudhari, V.R.; Suryawanshi, S.R.; More, M.A.; Chavan, P.G. Observation of low turn-on field emmission from nanocomposites of GO/TiO2 and RGO/TiO2. Vacuum 2016, 123, 167–174. [Google Scholar] [CrossRef]
- Agrawal, Y.; Kedawat, G.; Kumar, P.; Dwivedi, J.; Singh, V.N.; Gupta, R.K.; Gupta, B.K. High-performance stable field emission with ultralow turn on voltage from rGO conformal coated TiO2 nanotubes 3D arrays. Sci. Rep. 2015, 5, 11612–11623. [Google Scholar] [CrossRef]
- Huang, S.C.; Chen, Y.J. Enhanced field emission properties of titled graphene nanoribbons on aggregated TiO2, nanotube arrays. Mater. Res. Bull. 2016, 79, 115–120. [Google Scholar] [CrossRef]
- Zhu, W.D.; Wang, C.W.; Chen, J.B.; Yan, L.; Wang, J. Enhance field emission from Ti3+ self doped TiO2 nanotube arrays synthesised by a facile cathodic reduction process. Appl. Surf. Sci. 2014, 301, 525–529. [Google Scholar] [CrossRef]
- Peter, L.M. Band-edge tuning in self-assembled layers of BI2S3 nanoparticles used to photosensitise nanocrystalline TiO2. J. Phys. Chem. B 2003, 107, 8378–8381. [Google Scholar] [CrossRef]
- Du, J. Hierarchically ordered macro-mesoporous TiO2-graphenen composite films: Improved mass transfer, reduced charge recombination, and their enhanced photocatalytic activities. ACS Nano 2010, 5, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. A facile one-step synthesis of TiO2/graphene composites for photodegradation of methyl orange. Nano Res. 2011, 4, 274–283. [Google Scholar] [CrossRef]
- Wu, Z.S. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2013, 1, 110–116. [Google Scholar] [CrossRef]
- Wang, D.; Choi, D.; Li, J.; Yang, Z.; Nie, Z.; Kou, R. Self assembled TiO2-graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 2009, 3, 907–914. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Cui, X.; Jiang, Z. A green and facile synthesis of TiO2/graphene nanocomposites and their photocatalytic for hydrogen evolution. Int. J. Hydrogen Energy X 2011, 37, 1–15. [Google Scholar] [CrossRef]
- Xiang, Q.; Wu, J.; Jaroniec, M. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, H.; Chen, Q.; Li, J.; Wang, P. Preparation of graphene film decorated TiO2 nano-tube array photoelectrode and its enhanced visible light photocatalytic mechanism. Carbon 2014, 70, 249–257. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Guo, W.; Xu, D.; Liu, D.; Qin, M. Graphene Oxide Hybridised TiO2 for Visible Light Photocatalytic Degradation of Phenol. Symmetry 2020, 12, 1420. https://doi.org/10.3390/sym12091420
Wang G, Guo W, Xu D, Liu D, Qin M. Graphene Oxide Hybridised TiO2 for Visible Light Photocatalytic Degradation of Phenol. Symmetry. 2020; 12(9):1420. https://doi.org/10.3390/sym12091420
Chicago/Turabian StyleWang, Guanyu, Weijie Guo, Deping Xu, Di Liu, and Mengtao Qin. 2020. "Graphene Oxide Hybridised TiO2 for Visible Light Photocatalytic Degradation of Phenol" Symmetry 12, no. 9: 1420. https://doi.org/10.3390/sym12091420
APA StyleWang, G., Guo, W., Xu, D., Liu, D., & Qin, M. (2020). Graphene Oxide Hybridised TiO2 for Visible Light Photocatalytic Degradation of Phenol. Symmetry, 12(9), 1420. https://doi.org/10.3390/sym12091420



