Specific Structure and Properties of Composite Membranes Based on the Torlon® (Polyamide-imide)/Layered Perovskite Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
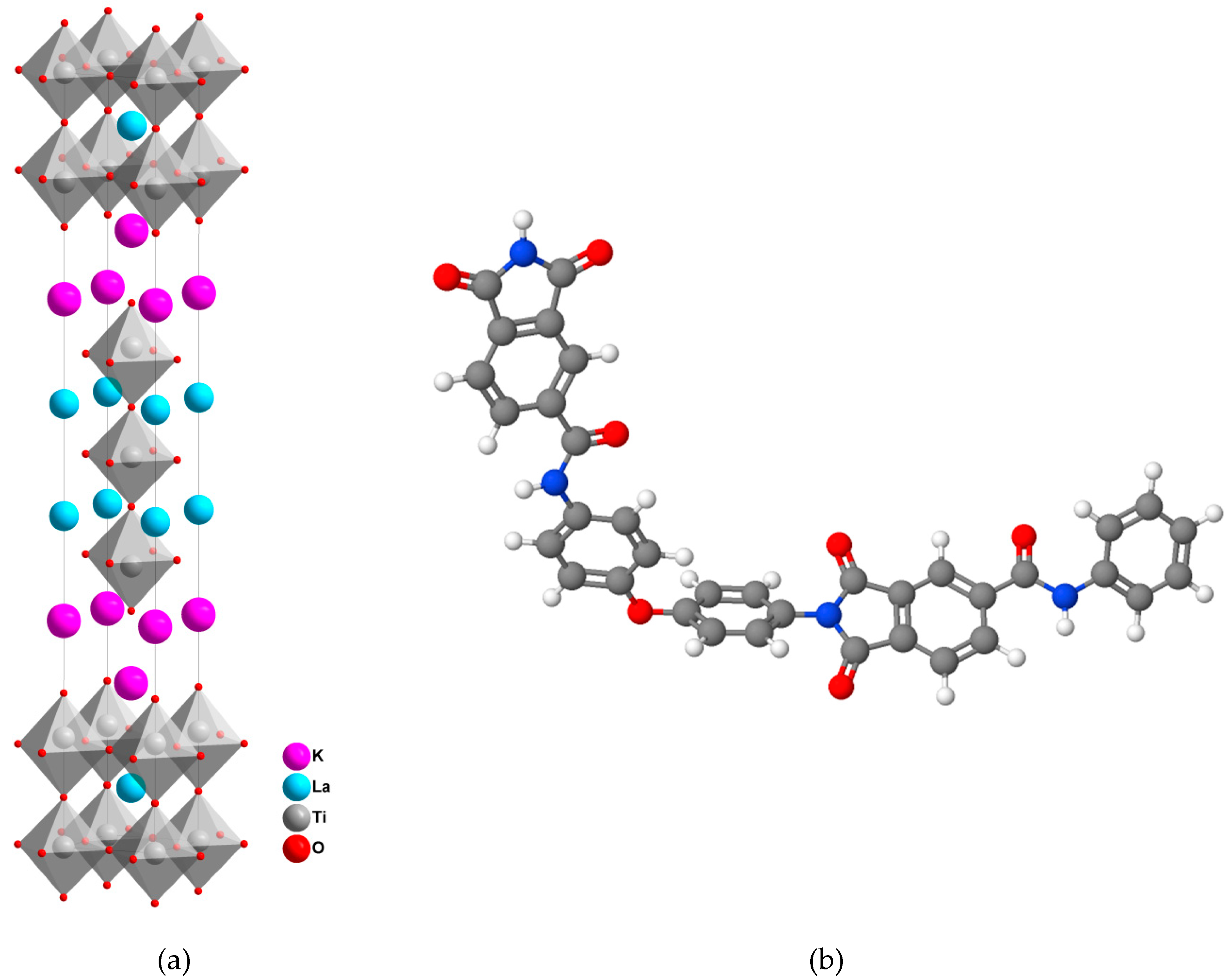
2.2. Synthesis of Perovskite
2.3. Perovskite Characterization
2.4. Membrane Preparation
2.5. Membrane Characterizations
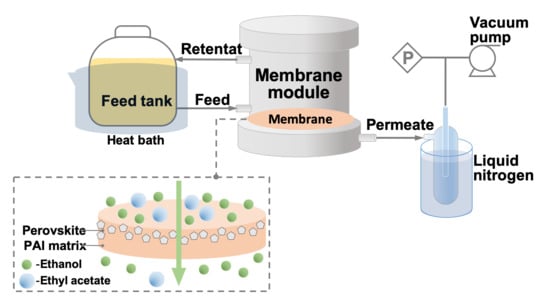
2.6. Pervaporation
3. Results
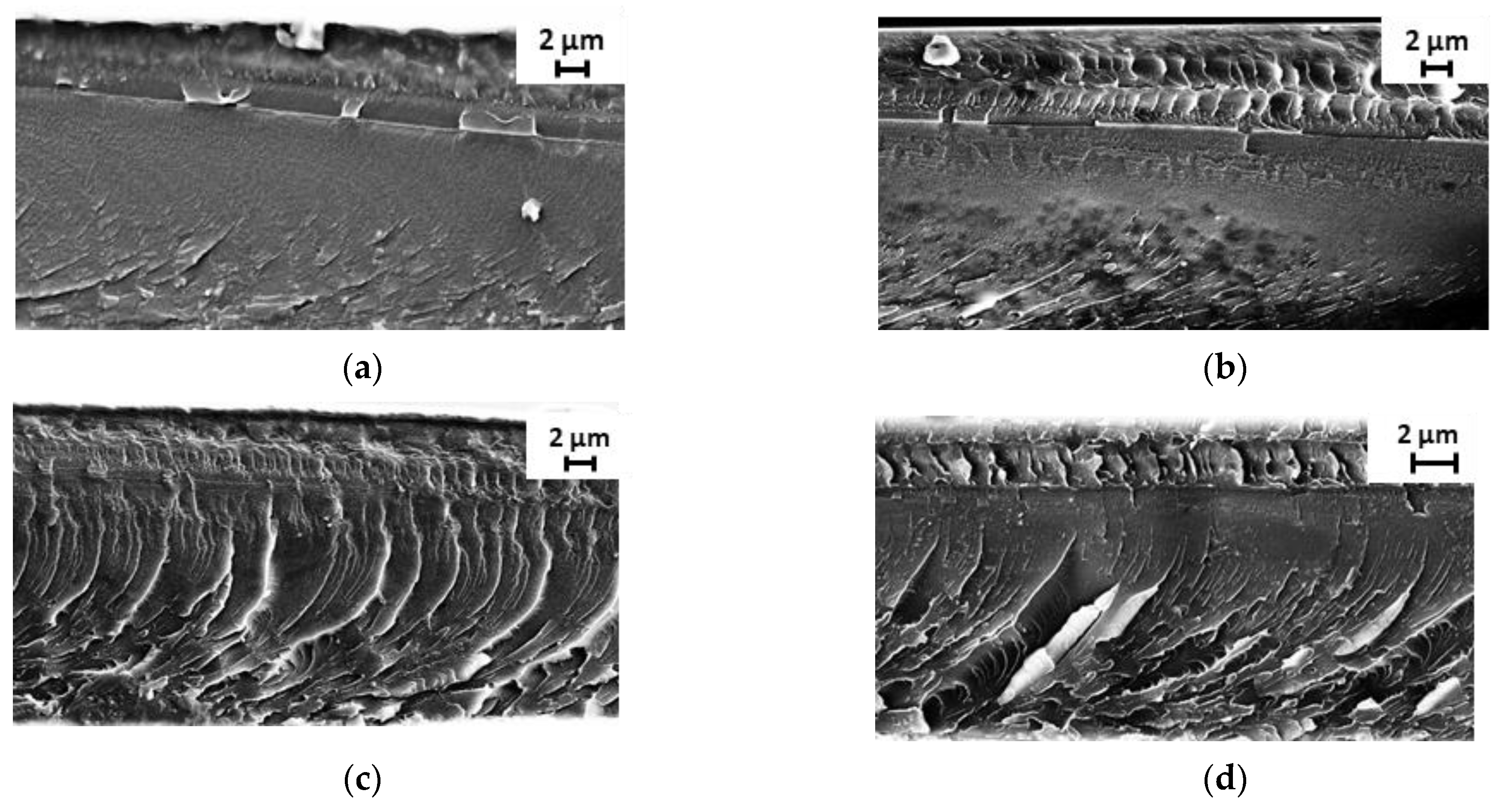
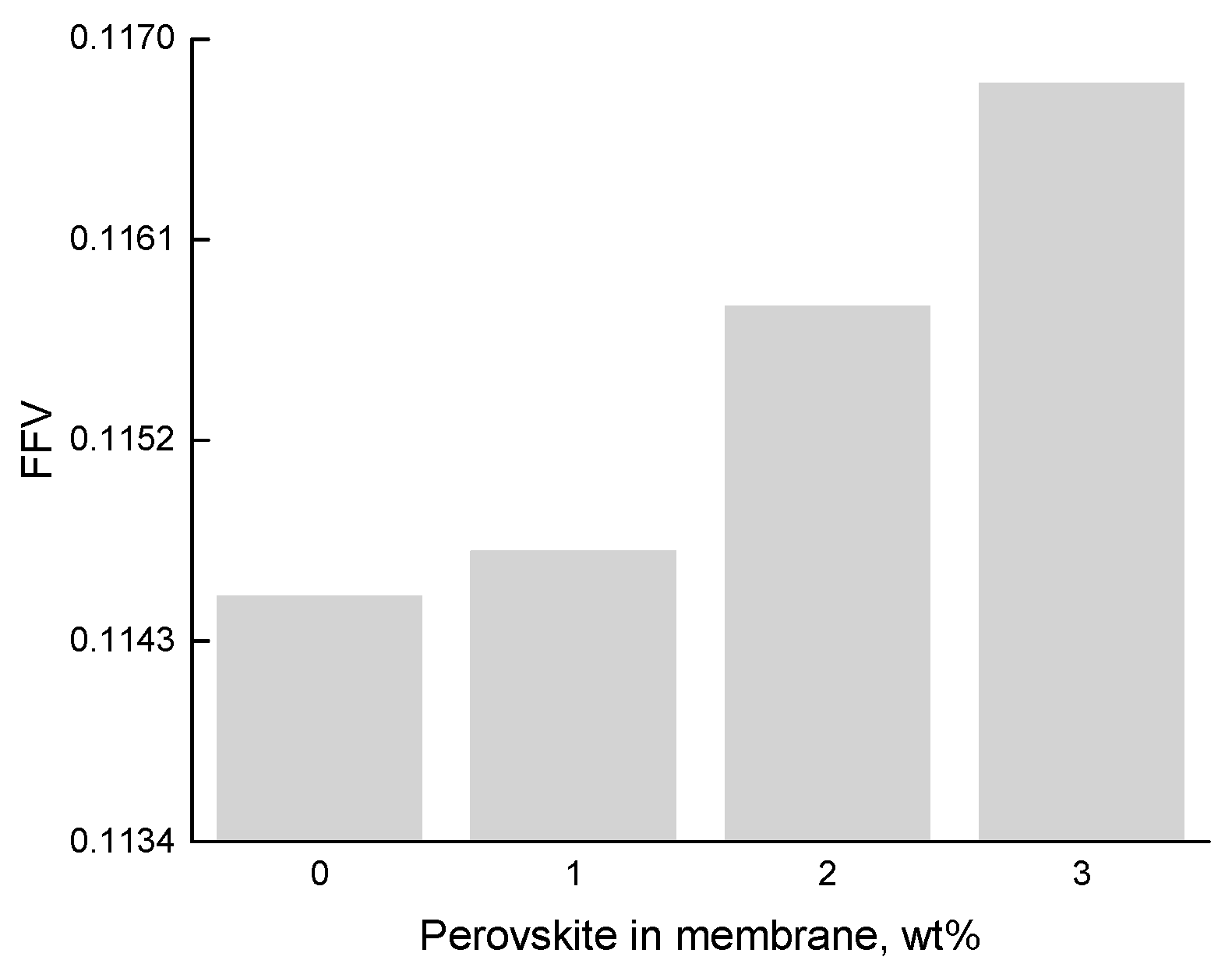
3.1. Membrane Structure
3.2. Surface Tension Properties
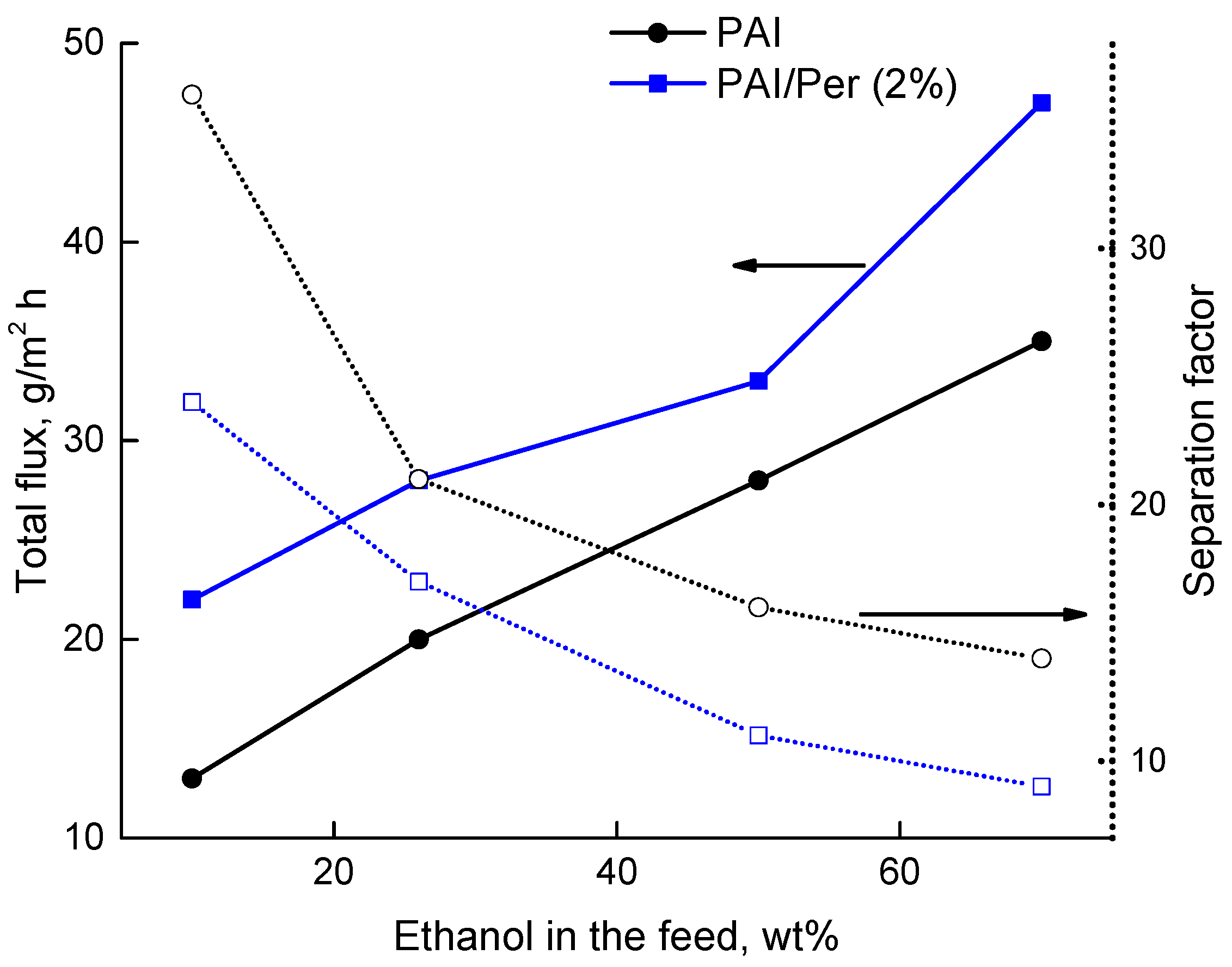
3.3. Transport Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; Wiley: Chichester, UK, 2012; ISBN 9780470743720. [Google Scholar]
- Genduso, G.; Luis, P.; Van der Bruggen, B. Pervaporation membrane reactors (PVMRs) for esterification. In Membrane Reactors for Energy Applications and Basic Chemical Production; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 565–603. ISBN 9781782422273. [Google Scholar]
- Buonomenna, M.G. Design Next Generation Membranes or Rethink the “Old” Asymmetric Membranes? Symmetry (Basel) 2020, 12, 270. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Energy Storage and Conversion Technologies: The Nanotechnology Role in their Advances. Recent Pat. Nanotechnol. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Pulyalina, A.Y.; Polotskaya, G.A.; Toikka, A.M. Membrane materials based on polyheteroarylenes and their application for pervaporation. Russ. Chem. Rev. 2016, 85. [Google Scholar] [CrossRef]
- Kreiter, R.; Wolfs, D.; Engelen, C.; Vanveen, H.; Vente, J. High-temperature pervaporation performance of ceramic-supported polyimide membranes in the dehydration of alcohols. J. Memb. Sci. 2008, 319, 126–132. [Google Scholar] [CrossRef]
- Pulyalina, A.Y.; Toikka, A.M.; Polotskaya, G.A. Investigation of pervaporation membranes based on polycarbamide: Effect of residual solvent. Pet. Chem. 2014, 54, 573–579. [Google Scholar] [CrossRef]
- Pulyalina, A.Y.; Polotskaya, G.A.; Kalyuzhnaya, L.M.; Sushchenko, I.G.; Meleshko, T.K.; Yakimanskii, A.V.; Chislov, M.V.; Toikka, A.M. Sorption and transport of aqueous isopropanol solutions in polyimide-poly(aniline-co-anthranilic acid) composites. Russ. J. Appl. Chem. 2011, 84, 840–846. [Google Scholar] [CrossRef]
- Sun, S.P.; Wang, K.Y.; Peng, N.; Hatton, T.A.; Chung, T.S. Novel polyamide-imide/cellulose acetate dual-layer hollow fiber membranes for nanofiltration. J. Memb. Sci. 2010, 363, 232–242. [Google Scholar] [CrossRef]
- Peng, N.; Chung, T.S. The effects of spinneret dimension and hollow fiber dimension on gas separation performance of ultra-thin defect-free Torlon® hollow fiber membranes. J. Memb. Sci. 2008, 310, 455–465. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Matsuura, T.; Chung, T.S.; Goh, S.H. Investigation of the fundamental differences between polyamide-imide (PAI) and polyetherimide (PEI) membranes for isopropanol dehydration via pervaporation. J. Memb. Sci. 2008, 318, 217–226. [Google Scholar] [CrossRef]
- Wang, Y.; Goh, S.H.; Chung, T.S.; Na, P. Polyamide-imide/polyetherimide dual-layer hollow fiber membranes for pervaporation dehydration of C1-C4 alcohols. J. Memb. Sci. 2009, 326, 222–233. [Google Scholar] [CrossRef]
- Teoh, M.M.; Chung, T.S.; Wang, K.Y.; Guiver, M.D. Exploring Torlon/P84 co-polyamide-imide blended hollow fibers and their chemical cross-linking modifications for pervaporation dehydration of isopropanol. Sep. Purif. Technol. 2008, 61, 404–413. [Google Scholar] [CrossRef][Green Version]
- Yoshikawa, M.; Higuchi, A.; Ishikawa, M.; Guiver, M.D.; Robertson, G.P. Vapor permeation of aqueous 2-propanol solutions through gelatin/Torlon® poly(amide imide) blended membranes. J. Memb. Sci. 2004, 243, 89–95. [Google Scholar] [CrossRef][Green Version]
- Higuchi, A.; Yoshikawa, M.; Guiver, M.D.; Robertson, G.P. Vapor permeation and pervaporation of aqueous 2-propanol solutions through the Torlon® poly(amide imide) membrane. Sep. Sci. Technol. 2005, 40, 2697–2707. [Google Scholar] [CrossRef][Green Version]
- Adoor, S.G.; Manjeshwar, L.S.; Bhat, S.D.; Aminabhavi, T.M. Aluminum-rich zeolite beta incorporated sodium alginate mixed matrix membranes for pervaporation dehydration and esterification of ethanol and acetic acid. J. Memb. Sci. 2008, 318, 233–246. [Google Scholar] [CrossRef]
- Nair, S.P.N.; Murugavel, P. Oxides: Their Properties and Uses. In Comprehensive Inorganic Chemistry II (Second Edition): From Elements to Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 4, pp. 47–72. ISBN 9780080965291. [Google Scholar]
- Uppuluri, R.; Sen Gupta, A.; Rosas, A.S.; Mallouk, T.E. Soft chemistry of ion-exchangeable layered metal oxides. Chem. Soc. Rev. 2018, 47, 2401–2430. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Perovskites by design: A toolbox of solid-state reactions. Chem. Mater. 2002, 14, 1455–1471. [Google Scholar] [CrossRef]
- Vente, J.F.; Haije, W.G.; Rak, Z.S. Performance of functional perovskite membranes for oxygen production. J. Memb. Sci. 2006, 276, 178–184. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Z.; Zhang, G.; Liu, Z.; Zhu, J.; Jin, W. Effects of polymer binders on separation performance of the perovskite-type 4-bore hollow fiber membranes. Sep. Purif. Technol. 2017, 187, 294–302. [Google Scholar] [CrossRef]
- Guironnet, L.; Geffroy, P.M.; Tessier-Doyen, N.; Boulle, A.; Richet, N.; Chartier, T. The surface roughness effect on electrochemical properties of La0.5Sr0.5Fe0.7Ga0.3O3-Δ perovskite for oxygen transport membranes. J. Memb. Sci. 2019, 588, 117199. [Google Scholar] [CrossRef]
- Prajongtat, P.; Sriprachuabwong, C.; Wongkanya, R.; Dechtrirat, D.; Sudchanham, J.; Srisamran, N.; Sangthong, W.; Chuysinuan, P.; Tuantranont, A.; Hannongbua, S.; et al. Moisture-Resistant Electrospun Polymer Membranes for Efficient and Stable Fully Printable Perovskite Solar Cells Prepared in Humid Air. ACS Appl. Mater. Interfaces 2019, 11, 27677–27685. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yamashita, H.; Saeki, D.; Yoshioka, T.; Shintani, T.; Kamio, E.; Kreissl, H.T.; Tsang, S.C.E.; Sugiyama, S.; Matsuyama, H. Niobate nanosheet membranes with enhanced stability for nanofiltration. Chem. Commun. 2017, 53, 7929–7932. [Google Scholar] [CrossRef] [PubMed]
- Avagimova, N.V.; Pulyalina, A.Y.; Toikka, A.M.; Suvorova, O.M.; Vilesov, A.D.; Polotskaya, G.A. Controlling the barrier properties of polymer composites containing montmorillonite. Pet. Chem. 2013, 53, 559–563. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, M.; Jiang, Z.; Liao, J.; Xie, X.; Huang, T.; Zhao, J.; Bai, J.; Pan, F. Preparation of a highly water-selective membrane for dehydration of acetone by incorporating potassium montmorillonite to construct ionized water channel. Chem. Eng. Sci. 2015, 135, 461–471. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Kariduraganavar, M.Y. Development of novel composite membranes using quaternized chitosan and Na+-MMT clay for the pervaporation dehydration of isopropanol. J. Colloid Interface Sci. 2009, 338, 111–120. [Google Scholar] [CrossRef]
- Roy, S.; Singha, N. Polymeric Nanocomposite Membranes for Next Generation Pervaporation Process: Strategies, Challenges and Future Prospects. Membranes (Basel) 2017, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sugimoto, K.; Nakane, T. Separation of ethanol/ethyl acetate mixture by pervaporation at 100–130 °C through NaY zeolite membrane for industrial purpose. Microporous Mesoporous Mater. 2008, 115, 170–175. [Google Scholar] [CrossRef]
- Hasanoǧlu, A.; Salt, Y.; Keleşer, S.; Özkan, S.; Dinçer, S. Pervaporation separation of ethyl acetate-ethanol binary mixtures using polydimethylsiloxane membranes. Chem. Eng. Process. Process Intensif. 2005, 44, 375–381. [Google Scholar] [CrossRef]
- Ong, Y.T.; Tan, S.H. Pervaporation separation of a ternary azeotrope containing ethyl acetate, ethanol and water using a buckypaper supported ionic liquid membrane. Chem. Eng. Res. Des. 2016, 109, 116–126. [Google Scholar] [CrossRef]
- PD. CRC Handbook of Chemistry and Physics. J. Mol. Struct. 1992, 268, 320. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, J.; Wei, Y.; Lin, J.; Huang, M. Hydrothermal synthesis of K2La2Ti3O10 and photocatalytic splitting of water. J. Alloys Compd. 2008, 456, 364–367. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Mechtaeva, E.V.; Burovikhina, A.A.; Silyukov, O.I.; Toikka, M.A.; Zvereva, I.A. Effect of protonation on the photocatalytic activity of the K2La2Ti3O10 layered oxide in the reaction of hydrogen production. Monatshefte für Chemie-Chem. Mon. 2018, 149, 475–482. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Boyer, R.F. Physical properties of molecular crystals, liquids and glasses. J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 2466. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Weber, S.G. Morphology and free volume of nanocomposite Teflon AF 2400 films and their relationship to transport behavior. J. Memb. Sci. 2013, 443, 115–123. [Google Scholar] [CrossRef]
- Askadskii, A.A. Computational Materials Science of Polymers; Cambridge International Science Publishing Conditions: Cambridge, UK, 2003; ISBN 1898326622. [Google Scholar]
- Pulyalina, A.; Tataurov, M.; Faykov, I.; Rostovtseva, V.; Polotskaya, G. Polyimide Asymmetric Membrane vs. Dense Film for Purification of MTBE Oxygenate by Pervaporation. Symmetry (Basel) 2020, 12, 436. [Google Scholar] [CrossRef]
- Belmares, M.; Blanco, M.; Goddard, W.A.; Ross, R.B.; Caldwell, G.; Chou, S.H.; Pham, J.; Olofson, P.M.; Thomas, C. Hildebrand and hansen solubility parameters from molecular dynamics with applications to electronic nose polymer sensors. J. Comput. Chem. 2004, 25, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.F.M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters; CRC Press: Boca Raton, FL, USA, 1991; ISBN 9780849301766. [Google Scholar]
- Knozowska, K.; Li, G.; Kujawski, W.; Kujawa, J. Novel heterogeneous membranes for enhanced separation in organic-organic pervaporation. J. Memb. Sci. 2020, 599, 117814. [Google Scholar] [CrossRef]
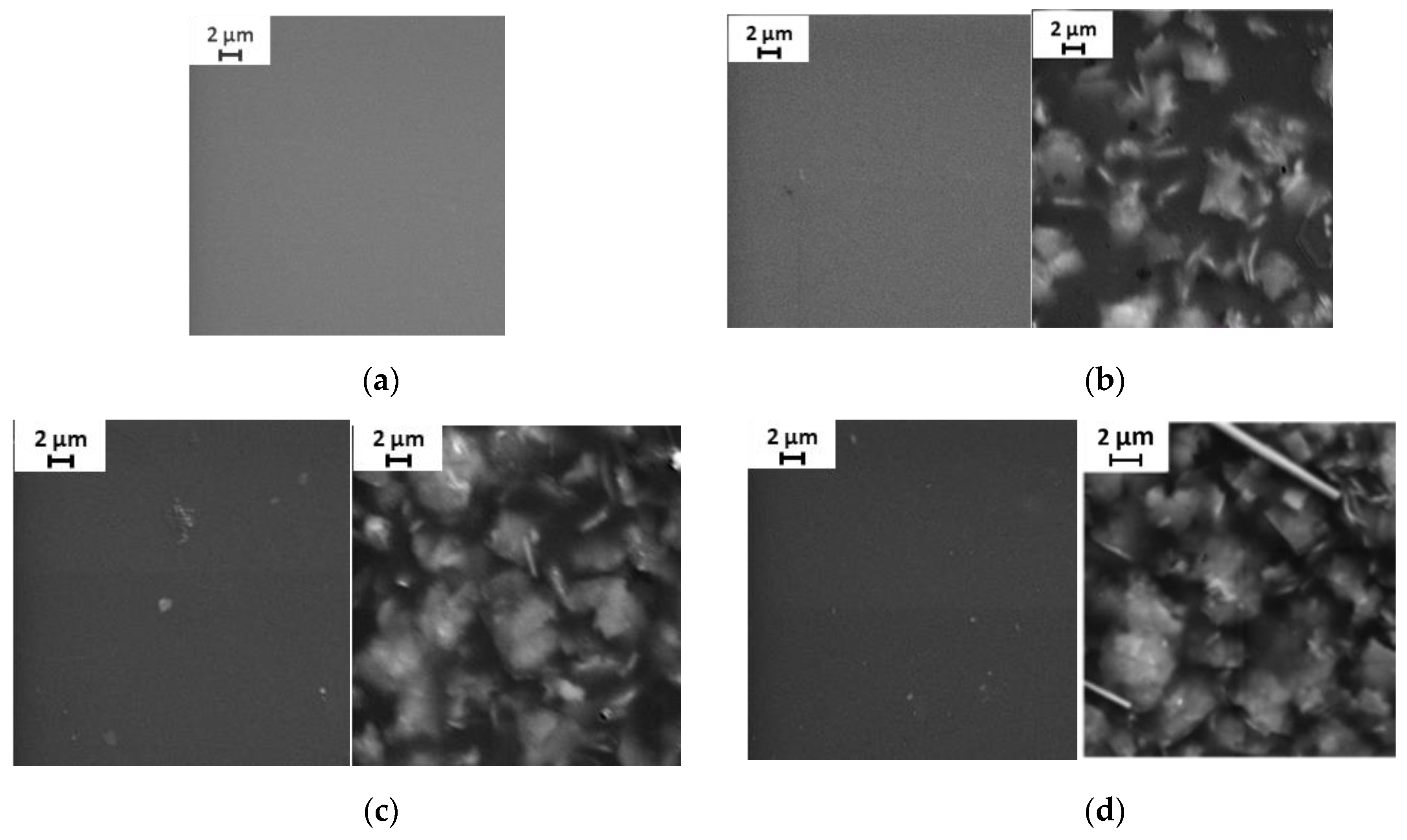





| Spectrum | C | N | O | K | Ti | La | Total |
|---|---|---|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | |
| Spectrum 1 (Top) | 77.01 | 8.08 | 14.91 | 0.00 | 0.00 | 0.00 | 100.0 |
| Spectrum 2 | 75.05 | 9.06 | 15.79 | 0.10 | 0.00 | 0.00 | 100.0 |
| Spectrum 3 | 74.49 | 9.07 | 15.66 | 0.17 | 0.00 | 0.60 | 100.0 |
| Spectrum 4 | 77.10 | 5.85 | 14.93 | 0.30 | 0.73 | 1.09 | 100.0 |
| Spectrum 5 (Bottom) | 73.39 | 5.93 | 16.63 | 0.42 | 1.21 | 2.42 | 100.0 |
| Membrane | Contact Angle, Degree | |
|---|---|---|
| Water | n-Propanol | |
| PAI | 79.4 ± 0.6 | 14.7 ± 0.3 |
| PAI/Per (1%) | 77.1 ± 0.7 | 13.2 ± 0.2 |
| PAI/Per (2%) | 76.8 ± 0.5 | 12.7 ± 0.2 |
| PAI/Per (3%) | 73.4 ± 0.4 | 11.5 ± 0.1 |
| Liquid | Mol. Weight, g/mol | Density, g/cm3 | Molar Volume, cm3/mol | Tboiling, °C | Hildebrand Solubility Parameter, δ (J/cm3)1/2 |
|---|---|---|---|---|---|
| Ethanol | 46.07 | 0.789 | 57.5 | 78.4 | 25.9 |
| Ethyl acetate | 88.11 | 0.901 | 97.8 | 77.1 | 18.5 |
| No | Membrane | Ethanol in the Feed, wt.% | Total Flux, g/m2h | Separation Factor | Ref. |
|---|---|---|---|---|---|
| 1 | PDMS | 20 | 4884 | 1.5 | [30] |
| 2 | BP-SILM-70 | 30 | 70 | 3 | [31] |
| 3 | PEBAX | 60 | 1.1 | [42] | |
| 4 | PDMS | 60 | 3.25 | [42] | |
| 5 | PERVAP 4060 | 60 | 2 | [42] | |
| 6 | PAI | 26 | 20 | 21 | Present work |
| 7 | PAI/Per(2%) | 26 | 28 | 17 | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulyalina, A.; Rostovtseva, V.; Minich, I.; Silyukov, O.; Toikka, M.; Saprykina, N.; Polotskaya, G. Specific Structure and Properties of Composite Membranes Based on the Torlon® (Polyamide-imide)/Layered Perovskite Oxide. Symmetry 2020, 12, 1142. https://doi.org/10.3390/sym12071142
Pulyalina A, Rostovtseva V, Minich I, Silyukov O, Toikka M, Saprykina N, Polotskaya G. Specific Structure and Properties of Composite Membranes Based on the Torlon® (Polyamide-imide)/Layered Perovskite Oxide. Symmetry. 2020; 12(7):1142. https://doi.org/10.3390/sym12071142
Chicago/Turabian StylePulyalina, Alexandra, Valeriia Rostovtseva, Iana Minich, Oleg Silyukov, Maria Toikka, Nataliia Saprykina, and Galina Polotskaya. 2020. "Specific Structure and Properties of Composite Membranes Based on the Torlon® (Polyamide-imide)/Layered Perovskite Oxide" Symmetry 12, no. 7: 1142. https://doi.org/10.3390/sym12071142
APA StylePulyalina, A., Rostovtseva, V., Minich, I., Silyukov, O., Toikka, M., Saprykina, N., & Polotskaya, G. (2020). Specific Structure and Properties of Composite Membranes Based on the Torlon® (Polyamide-imide)/Layered Perovskite Oxide. Symmetry, 12(7), 1142. https://doi.org/10.3390/sym12071142