Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications
Abstract
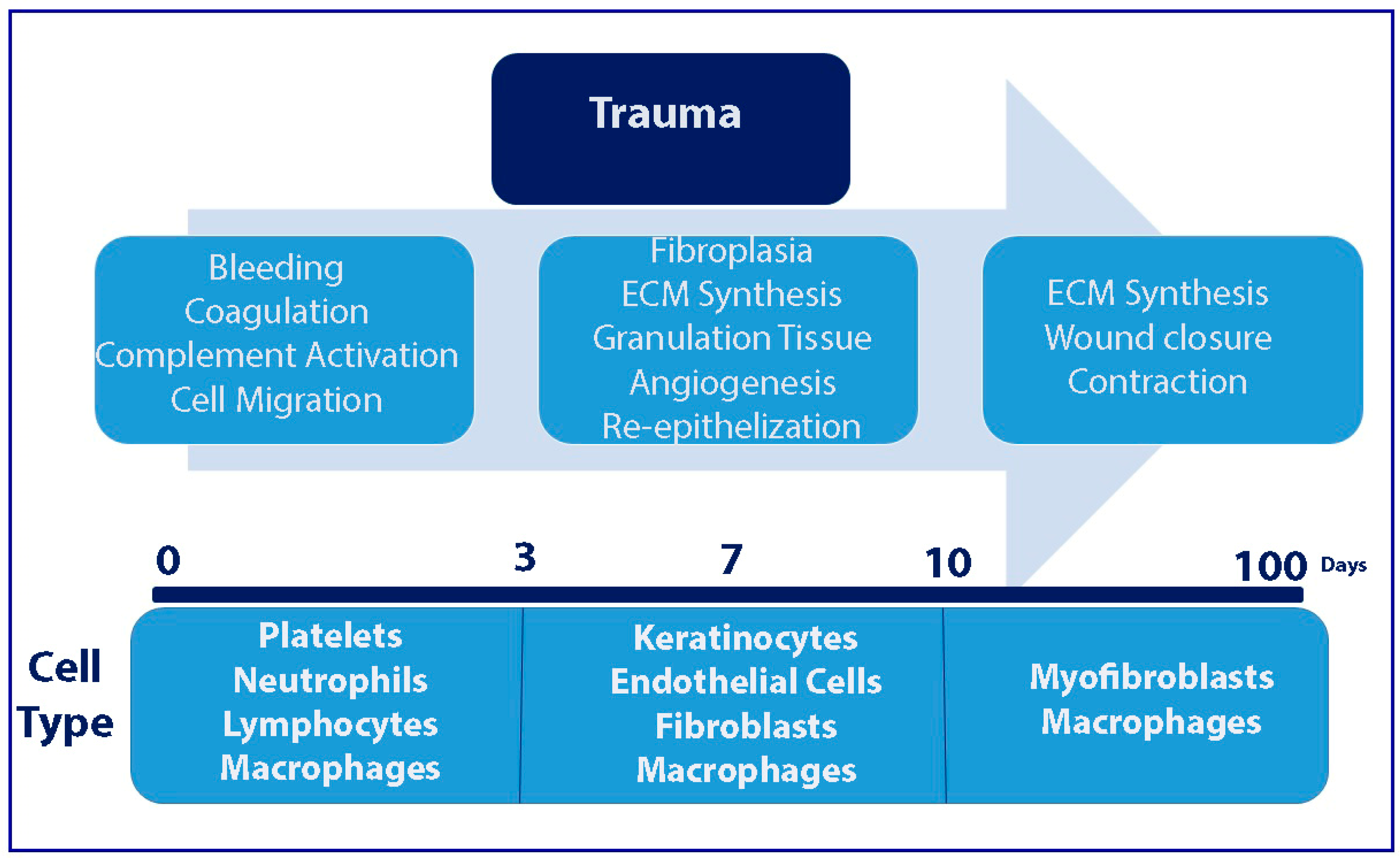
1. Introduction
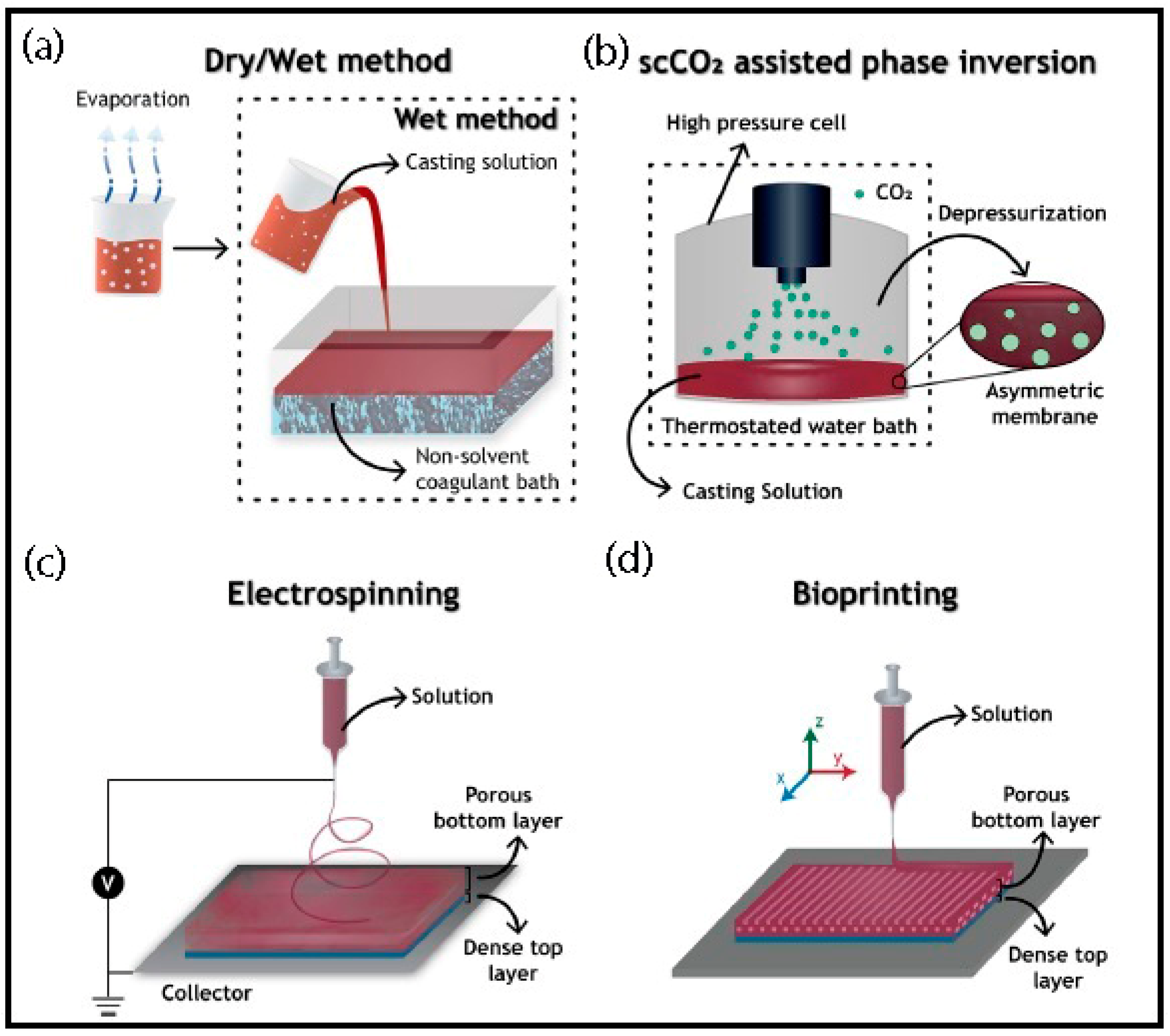
2. Production Methods and General Properties
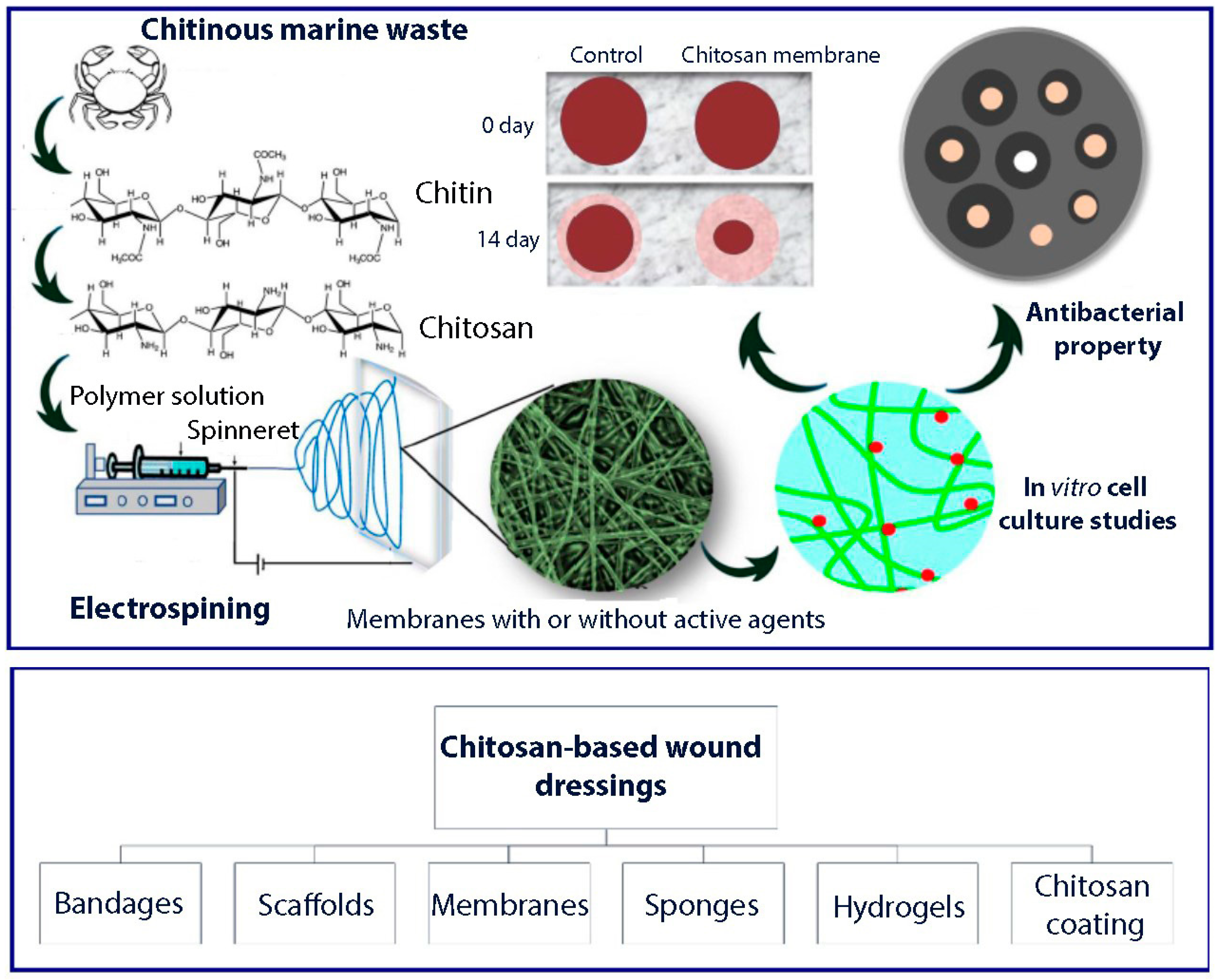
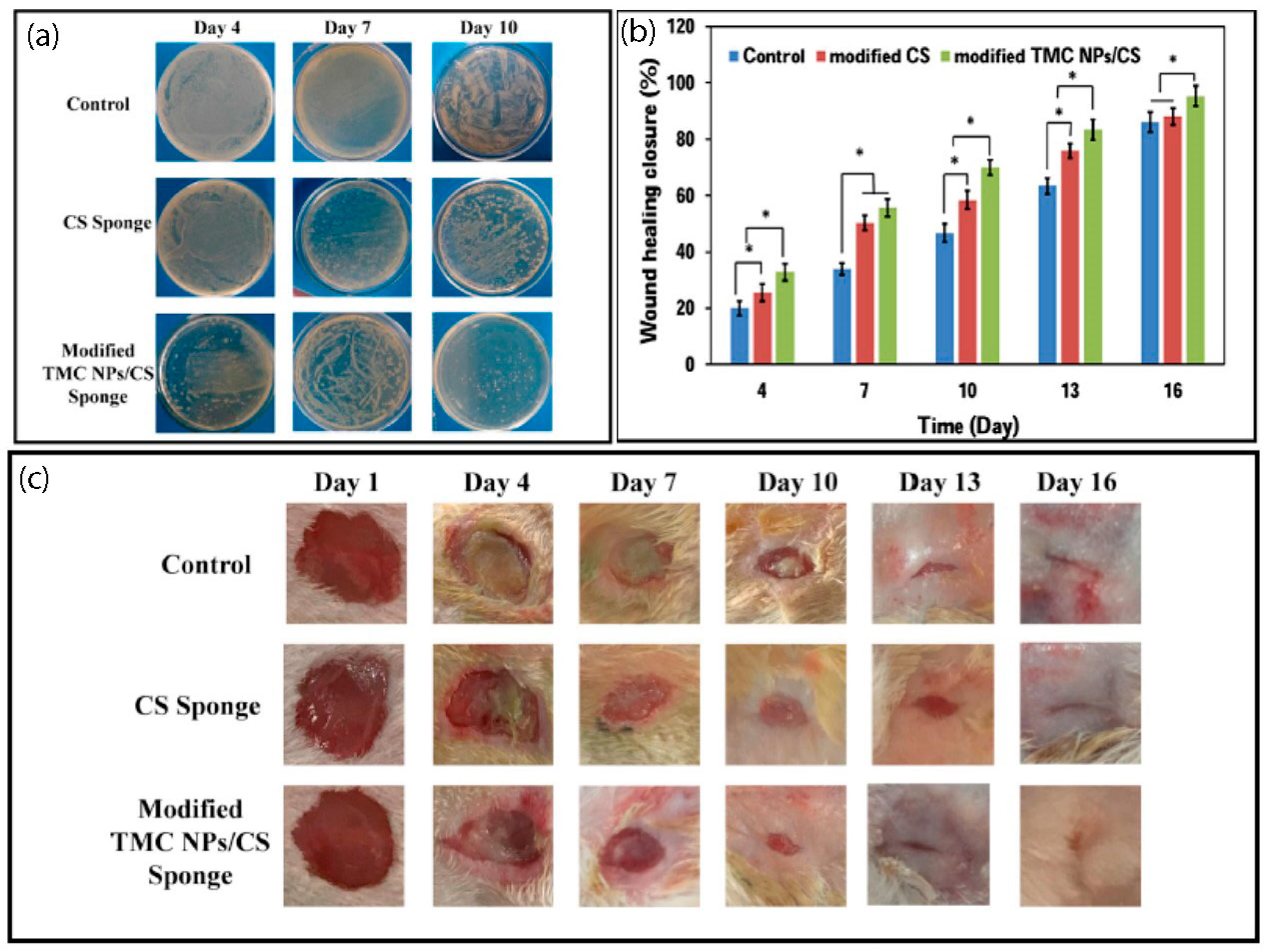
3. Chitosan Based Asymmetric Membranes
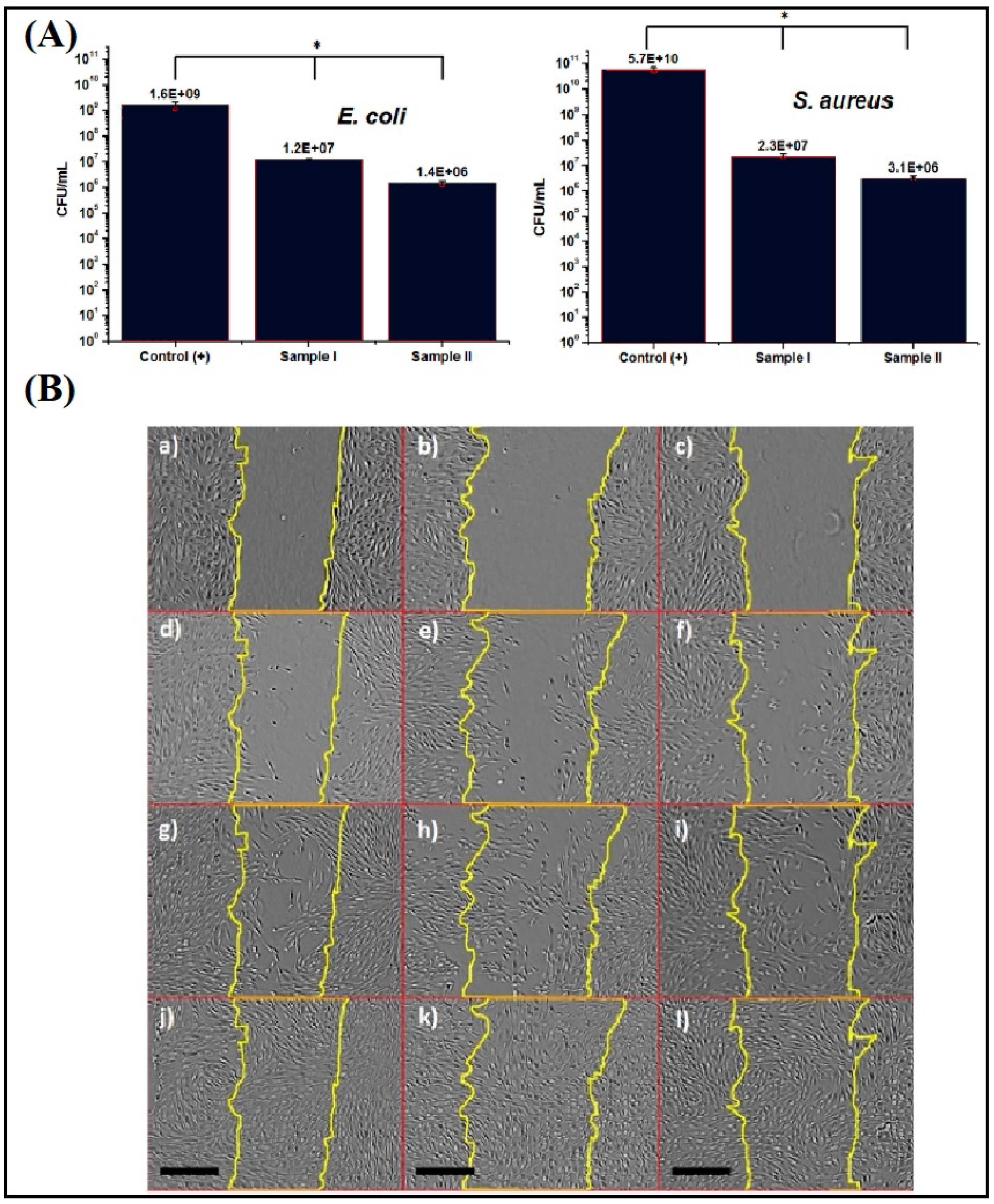
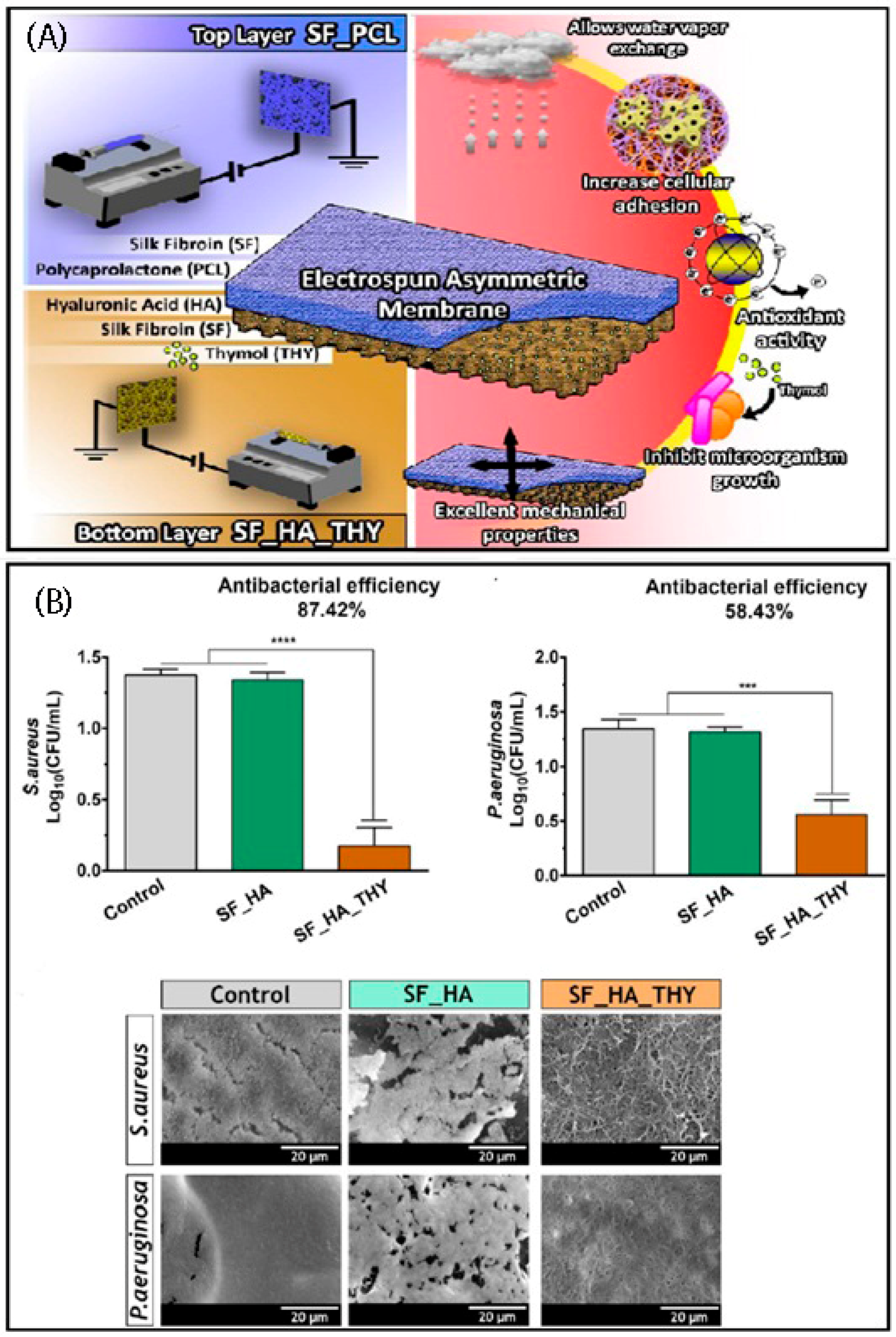
4. Electrospun Asymmetric Membranes
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Peck, M.D. Epidemiology of burns throughout the World. Part II: Intentional burns in adults. Burns 2012, 38, 630–637. [Google Scholar] [CrossRef]
- Madaghiele, M.; Sannino, A.; Ambrosio, L.; Demitri, C. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burn. Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Nagarajan, S.; Bechelany, M.; Kalkura, S.N. Collagen Based Biomaterials for Tissue Engineering Applications: A Review. In Lecture Notes in Earth System Sciences; Springer Science and Business Media LLC: Berlin, Germany, 2019; pp. 3–22. [Google Scholar]
- Yildirimer, L.; Thanh, N.T.; Seifalian, A. Skin regeneration scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012, 30, 638–648. [Google Scholar] [CrossRef]
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burn. Trauma 2018, 6, 4. [Google Scholar] [CrossRef]
- Clark, R.A.F.; Ghosh, K.; Tonnesen, M.G. Tissue Engineering for Cutaneous Wounds. J. Investig. Derm. 2007, 127, 1018–1029. [Google Scholar] [CrossRef]
- Ma, B. Treatment of Skin Aging and Photoaging with Innovative Oral Dosage Forms of Non-Hydrolized Carnosine and Carcinine. Int. J. Clin. Derm. Res. 2017, 5, 116–143. [Google Scholar] [CrossRef]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2009, 7, 229–258. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M.W. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Boil. 2017, 61, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Alemdaroğlu, C.; Deĝim, Z.; Celebi, N.; Zor, F.; Ozturk, S.; Erdogan, D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 2006, 32, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.; Stevens, H.N.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; A Wynn, T.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Catalano, E.; Cochis, A.; Varoni, E.M.; Rimondini, L.; Azzimonti, B. Tissue-engineered skin substitutes: An overview. J. Artif. Organs 2013, 16, 397–403. [Google Scholar] [CrossRef]
- Pereira, R.F.; Barrias, C.C.; Granja, P.L.; Bartolo, P.J. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 2013, 8, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Nyame, T.T.; Chiang, H.; Orgill, D. Clinical Applications of Skin Substitutes. Surg. Clin. North Am. 2014, 94, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, W.L.J.; Lommen, E.J.C.M.P.; Wildevuur, C.R.H.; Feijen, J. Fabrication and characterization of an asymmetric polyurethane membrane for use as a wound dressing. J. Appl. Biomater. 1992, 3, 287–303. [Google Scholar] [CrossRef]
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J. Membr. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Priya, S.G.; Gupta, A.; Jain, E.; Sarkar, J.; Damania, A.; Jagdale, P.R.; Chaudhari, B.P.; Gupta, K.C.; Kumar, A. Bilayer Cryogel Wound Dressing and Skin Regeneration Grafts for the Treatment of Acute Skin Wounds. ACS Appl. Mater. Interfaces 2016, 8, 15145–15159. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.; Tu, Y.; Ay, B.; Sun, X.; Li, Y.; Wang, X.; Chen, X.; Zhang, L. Chitosan-based asymmetric topological membranes with cell-like features for healthcare applications. J. Mater. Chem. B 2019, 7, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Shaulsky, E.; Karanikola, V.; Straub, A.P.; Deshmukh, A.; Zucker, I.; Elimelech, M. Asymmetric membranes for membrane distillation and thermo-osmotic energy conversion. Desalination 2019, 452, 141–148. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Xue, J.; Zhuang, L.; Wang, H. Asymmetric membrane structure: An efficient approach to enhance hydrogen separation performance. Sep. Purif. Technol. 2018, 207, 363–369. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, J.; Chen, L.; Xue, J.; Chen, X.; Wang, H. Metalloid phosphorus cation doping: An effective strategy to improve permeability and stability through the hydrogen permeable membranes. Sep. Purif. Technol. 2019, 210, 320–326. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Zhou, Y.; You, P.; Wang, M.; Tang, L.; Deng, Y. Development of a novel extracellular matrix membrane with an asymmetric structure for guided bone regeneration. Mater. Lett. 2020, 127926. [Google Scholar] [CrossRef]
- Simon, S.; Espuche, E. Effect of different metal in situ growing routes on the morphology and gas separation properties of polyetherimide/palladium nanocomposite asymmetric membranes. Sep. Purif. Technol. 2014, 129, 41–49. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, J.; Li, Z.; Yang, K.; Guo, J.; Zhang, S.; Sherazi, T.A.; Li, S. Bio-inspired fabrication of asymmetric wettability Janus porous membrane for secure F-oil infused F-free-membrane filtration. J. Membr. Sci. 2018, 566, 161–167. [Google Scholar] [CrossRef]
- Zulhairun, A.; Fachrurrazi, Z.; Izwanne, M.N.; Ismail, A. Asymmetric hollow fiber membrane coated with polydimethylsiloxane–metal organic framework hybrid layer for gas separation. Sep. Purif. Technol. 2015, 146, 85–93. [Google Scholar] [CrossRef]
- Apel, P.Y.; Blonskaya, I.; Dmitriev, S.; Orelovich, O.; Sartowska, B. Ion track symmetric and asymmetric nanopores in polyethylene terephthalate foils for versatile applications. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2015, 365, 409–413. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Lih, E.; Lee, J.-S.; Park, K.M.; Thi, T.T.H. Rapidly curable chitosan–PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef]
- Kunio, N.R.; Riha, G.M.; Watson, K.M.; Differding, J.A.; Schreiber, M.A.; Watters, J.M. Chitosan based advanced hemostatic dressing is associated with decreased blood loss in a swine uncontrolled hemorrhage model. Am. J. Surg. 2013, 205, 505–510. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane; American Chemical Society (ACS): Washington, DC, USA, 1963; Volume 38, pp. 117–132. [Google Scholar]
- Loeb, S. The Loeb-Sourirajan Membrane: How It Came about; American Chemical Society (ACS): Washington, DC, USA, 1981; Volume 153, pp. 1–9. [Google Scholar]
- Wang, Z.-G.; Wan, L.-S.; Xu, Z.-K. Surface engineerings of polyacrylonitrile-based asymmetric membranes towards biomedical applications: An overview. J. Membr. Sci. 2007, 304, 8–23. [Google Scholar] [CrossRef]
- Watenabe, K.; Yuasa, M.; Kida, T.; Teraoka, Y.; Yamazoe, N.; Shimanoe, K. High-Performance Oxygen-Permeable Membranes with an Asymmetric Structure Using Ba0. 95La0. 05FeO3− δ Perovskite-Type Oxide. Adv. Mater. 2010, 22, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.-S.; Lai, J.-Y.; Chung, T.-S. Evolution of polymeric hollow fibers as sustainable technologies: Past, present, and future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.-S.; Lai, J.-Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef]
- VijayaVenkataRaman, S.; Lu, W.F.; Fuh, J.Y.H. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [Google Scholar] [CrossRef]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Skin bioprinting: Impending reality or fantasy? Trends Biotechnol. 2016, 34, 689–699. [Google Scholar] [CrossRef]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burn. Trauma 2018, 6, 5. [Google Scholar] [CrossRef]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar-Ricardo, A. Poly (vinyl alcohol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Membr. Sci. 2014, 469, 262–271. [Google Scholar] [CrossRef]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Modification of polypropylene-starch blend by eggshell nano-particle, EVA and maleic anhydride to improve biodegradability and thermal properties. Int. J. Chem. Sci. 2017, 15, 2017. [Google Scholar]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Ahmadi-Aghkand, F.; Aziz, S.G.-G.; Panahi, Y.; Daraee, H.; Gorjikhah, F.; Aziz, S.G.-G.; Hsanzadeh, A.; Akbarzadeh, A. Recent prospective of nanofiber scaffolds fabrication approaches for skin regeneration. Artif. CellsNanomed. Biotechnol. 2015, 44, 1–7. [Google Scholar] [CrossRef]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A.; et al. Emerging Biofabrication Strategies for Engineering Complex Tissue Constructs. Adv. Mater. 2017, 29, 1606061. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Loboa, E. Nanofibrous Smart Bandages for Wound Care; Elsevier: Amsterdam, The Netherlands, 2016; pp. 483–499. [Google Scholar]
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun Nanofibrous Materials for Wound Healing. Adv. Fiber Mater. 2020, 1–16. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S. Electrospun Nanofibrous Membranes with Essential Oils for Wound Dressing Applications. Fibers Polym. 2020, 21, 999–1012. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, L.; Yuan, T.; Zhang, Q.; Fan, H. Asymmetric polyurethane membrane with in situ-generated nano-TiO2 as wound dressing. J. Appl. Polym. Sci. 2010, 119, 1532–1541. [Google Scholar] [CrossRef]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J.; et al. Spongy bilayer dressing composed of chitosan–Ag nanoparticles and chitosan–Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Fabrication of asymmetric nanostarch reinforced Chitosan/PVP membrane and its evaluation as an antibacterial patch for in vivo wound healing application. Int. J. Boil. Macromol. 2018, 114, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Boil. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Boil. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Otto, M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem. Boil. 2012, 19, 1503–1513. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Amani, A.M.; Babapoor, A. Nanosensors for Chemical and Biological and Medical Applications. Med. Chem. 2018, 8, 205–217. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.R. Polydopamine for Biomedical Application and Drug Delivery System. Med. Chem. 2018, 8, 218–229. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Babapoor, A.; Amani, A.M. A conceptual review of rhodanine: Current applications of antiviral drugs, anticancer and antimicrobial activities. Artif. CellsNanomed. Biotechnol. 2019, 47, 1132–1148. [Google Scholar] [CrossRef]
- Zonoubi, A.; Cn, P.; Perumal, D.V.; Mafibaniasadi, Z. In silico Analysis of Active Constituents of Silymarin as Αlpha-Glucosidase Enzyme Inhibitors in Type 2 Diabetes Mellitus. Asian J. Pharm. Clin. Res. 2019, 12, 225–229. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Bahrani, S.; Savardashtaki, A.; Esmaeili, H.; Lai, C.W.; Mazraedoost, S.; Abassi, M.; Ramavandi, B. Data on cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris. Data Brief 2020, 28, 104929. [Google Scholar] [CrossRef]
- Tech, J.E.T. Investigating the Activity of Antioxidants Activities Content in Apiaceae and to Study Antimicrobial and Insecticidal Activity of Antioxidant by using SPME Fiber Assembly Carboxen/Polydimethylsiloxane (CAR/PDMS). J. Environ. Treat. Tech. 2020, 8, 214–224. [Google Scholar]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A. Gold nanostars-diagnosis, bioimaging and biomedical applications. Drug Metab. Rev. 2020, 52, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Karuppuswamy, P.; Venugopal, J.R.; Navaneethan, B.; Laiva, A.L.; Sridhar, S.; Ramakrishna, S. Functionalized hybrid nanofibers to mimic native ECM for tissue engineering applications. Appl. Surf. Sci. 2014, 322, 162–168. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Development of biodegradable porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2001, 17, 63–69. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, S.S.; Zo, S.M.; Choi, S.M. Cellulose/poly-(m-phenylene isophthalamide) porous film as a tissue-engineered skin bioconstruct. Results Phys. 2018, 9, 113–120. [Google Scholar] [CrossRef]
- Zahouani, H.; Sohm, B.; Vargiolu, R.; Cenizo, V.; Debret, R.; Pailler-Mattei, C. Characterization of the mechanical properties of a dermal equivalent compared with human skinin vivoby indentation and static friction tests. Ski. Res. Technol. 2009, 15, 68–76. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Boil. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef]
- Carvalho, T.; Guedes, G.; Sousa, F.L.; Freire, C.S.R.; Santos, H.A. Latest Advances on Bacterial Cellulose-Based Materials for Wound Healing, Delivery Systems, and Tissue Engineering. Biotechnol. J. 2019, 14, e1900059. [Google Scholar] [CrossRef]
- Sen, S.; Basak, P.; Sinha, B.P.; Maurye, P.; Jaiswal, K.K.; Das, P.; Mandal, T.K. Anti-inflammatory effect of epidermal growth factor conjugated silk fibroin immobilized polyurethane ameliorates diabetic burn wound healing. Int. J. Boil. Macromol. 2020, 143, 1009–1032. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Fang, Q.-Q.; Wang, X.-F.; Zhang, T.; Shi, B.-H.; Zheng, B.; Mm, D.Z.; Hu, Y.-Y.; Ma, L.; Tan, W.-Q.; et al. Chitosan-calcium alginate dressing promotes wound healing: A preliminary study. Wound Repair Regen. 2019, 28, 326–337. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Figueira, D.R.; Miguel, S.P.; De Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Boil. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Amiri, N.; Pishavar, E.; Kalalinia, F.; Movaffagh, J.; Hashemi, M.; Hashemi, M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, H.; Zhang, J.; Li, X.; Wu, Y.; Wei, Y.; Zhao, Q. Functional poly (ε-caprolactone)/chitosan dressings with nitric oxide-releasing property improve wound healing. Acta Biomater. 2017, 54, 128–137. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Liao, W.-J.; Kuo, S.-M.; Tsai, F.-J.; Chen, Y.-S.; Huang, C.-Y.; Yao, C.-H. Asymmetric Chitosan Membrane Containing Collagen I Nanospheres for Skin Tissue Engineering. Biomacromolecules 2009, 10, 1642–1649. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Silva, F.W.D.O.; Colque, J.T.; Da Silva, V.M.; Prieto, T.; Venancio, E.; Bonvent, J.J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef]
- Amini, F.; Semnani, D.; Karbasi, S.; Banitaba, S.N. A novel bilayer drug-loaded wound dressing of PVDF and PHB/Chitosan nanofibers applicable for post-surgical ulcers. Int. J. Polym. Mater. 2018, 68, 772–777. [Google Scholar] [CrossRef]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and in Vivo Evaluation. Acs Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Boil. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Xia, G.; Zhai, D.; Sun, Y.; Hou, L.; Guo, X.; Wang, L.; Li, Z.; Wang, F. Preparation of a novel asymmetric wettable chitosan-based sponge and its role in promoting chronic wound healing. Carbohydr. Polym. 2019, 227, 115296. [Google Scholar] [CrossRef]
- Georgescu, M.; Chifiriuc, M.; Marutescu, L.; Gheorghe, I.; Lazăr, V.; Bolocan, A.; Berteșteanu, Ș. Bioactive Wound Dressings for the Management of Chronic Wounds. Curr. Org. Chem. 2016, 21, 53–63. [Google Scholar] [CrossRef]
- García, A.D.P.; Cassini-Vieira, P.; Ribeiro, C.C.; Jensen, C.E.D.M.; Barcelos, L.D.S.; Cortés, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2016, 105, 1938–1949. [Google Scholar] [CrossRef]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993. [Google Scholar]
- Riyajan, S.-A.; Sakdapipanich, J.T. Encapsulated neem extract containing Azadiractin-A within hydrolyzed poly(vinyl acetate) for controlling its release and photodegradation stability. Chem. Eng. J. 2009, 152, 591–597. [Google Scholar] [CrossRef]
- Genevro, G.M.; Neto, R.J.G.; Paulo, L.D.A.; Lopes, P.S.; De Moraes, M.A.; Beppu, M.M. Glucomannan asymmetric membranes for wound dressing. J. Mater. Res. 2019, 34, 481–489. [Google Scholar] [CrossRef]
- Basar, A.O.; Castro, S.; Torres-Giner, S.; Lagaron, J.M.; Sasmazel, H.T. Novel poly (ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater. Sci. Eng. C 2017, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Altiok, D.; Altiok, E.; Tihminlioğlu, F.; Altıok, D.; Altıok, E. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Electron. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Aragón, J.; Costa, C.; Coelhoso, I.; Mendoza, G.; Aguiar-Ricardo, A.; Irusta, S. Electrospun asymmetric membranes for wound dressing applications. Mater. Sci. Eng. C 2019, 103, 109822. [Google Scholar] [CrossRef] [PubMed]

| Membrane Composition | Main Properties | Fabrication Method | Reference Number |
|---|---|---|---|
| Polycaprolactone- hyaluronic acid/chitosan- zein | High anti-inflammatory and antibacterial effect | Electrospinning | [76] |
| Chitosan/PVP/nanostarch | High re epithelialization and collagen formation rate | Coating by stearic acid and evaporation/casting techniques | [55] |
| Bromelain-loaded chitosan | Curing of burn wound | Electrospinning | [77] |
| poly(Ɛ-caprolactone)/chitosan | Treatment of chronic wound caused by the ischemia. | Electrospinning | [78] |
| Chitosan-collagen nanospheres | High swelling ratio and acceptable porosity | Freeze-drying | [79] |
| PVA/Chitosan | Extraordinary antibacterial effect and great cytocompatibility | Electrospinning | [80] |
| CS/Polyhydroxybutyrate/polyvinylidenefluoride | Treatment of post-surgical ulcer | Electrospinning | [81] |
| Chitosan/AgNPs | High Re-epithelialization rate with non- toxic effect | Stearic acid coating and freeze-drying | [82] |
| Chitosan/PVA/zinc oxide | Useful for diabetic wounds | Electrospinning | [83] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A.; Omidifar, N.; Shokripour, M. Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications. Symmetry 2020, 12, 1100. https://doi.org/10.3390/sym12071100
Mousavi SM, Zarei M, Hashemi SA, Ramakrishna S, Chiang W-H, Lai CW, Gholami A, Omidifar N, Shokripour M. Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications. Symmetry. 2020; 12(7):1100. https://doi.org/10.3390/sym12071100
Chicago/Turabian StyleMousavi, Seyyed Mojtaba, Maryam Zarei, Seyyed Alireza Hashemi, Seeram Ramakrishna, Wei-Hung Chiang, Chin Wei Lai, Ahmad Gholami, Navid Omidifar, and Mansoureh Shokripour. 2020. "Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications" Symmetry 12, no. 7: 1100. https://doi.org/10.3390/sym12071100
APA StyleMousavi, S. M., Zarei, M., Hashemi, S. A., Ramakrishna, S., Chiang, W.-H., Lai, C. W., Gholami, A., Omidifar, N., & Shokripour, M. (2020). Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications. Symmetry, 12(7), 1100. https://doi.org/10.3390/sym12071100










