Investigating on the Iconic Gas Compositions Produced by Low-Temperature Heating Cotton
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Heating Cotton and Gas Collection
2.3. Gas Composition Analysis
3. Results and Discussions
3.1. Iconic Gas Compositions
3.2. Generation of the Iconic Gas Compositions
3.3. Discussions
4. Conclusions
- (1)
- The alkanes, furans, alkenes, aldehydes, hydrazines, and acids were produced during the heating process, while they could not be regarded as iconic gas compositions because of their little proportion. The methane has the highest proportion being nearly 99% in the organic gas compositions. The produced inorganic gas compositions contained a little hydrogen and carbon monoxide.
- (2)
- Methane was produced continuously during the heating process. At 95 °C, a small quantity of hydrogen occurred as a mid-product. The acetone was produced at 125 °C with the generation of hydrogen. A tiny amount of carbon monoxide is produced at 145 °C, showing the smoldering was at the early stage.
- (3)
- The joint detection of the methane and hydrogen could be used to predict if the smoldering happened. The produced acetone and carbon monoxide could be used to confirm the smoldering stage. Therefore, this study on the iconic gas compositions will provide a significant base for the prevention of cotton fires.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, C.; Lu, Z.; Zhang, F.; Zhu, P.; Wang, P.; Che, Y.; Sui, S. Combustion behaviors of cotton fabrics treated by a novel nitrogen- and phosphorus-containing polysiloxane flame retardant. J. Therm. Anal. Calorim. 2015, 123, 535–544. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Zheng, X.; Wang, K.; Cui, F.; Wang, R.; Zhu, J. Using a modified DNDC biogeochemical model to optimize field management of a multi-crop (cotton, wheat, and maize) system: A site-scale case study in northern China. Biogeosciences 2019, 16, 2905–2922. [Google Scholar]
- Hagen, B.C.; Frette, V.; Kleppe, G.; Arntzen, B.J. Onset of smoldering in cotton: Effects of density. Fire Saf. J. 2011, 46, 73–80. [Google Scholar]
- Hagen, B.C.; Frette, V.; Kleppe, G.; Arntzen, B.J. Effects of heat flux scenarios on smoldering in cotton. Fire Saf. J. 2013, 61, 144–159. [Google Scholar]
- Madsen, D.; Azeem, H.A.; Sandahl, M.; van Hees, P.; Husted, B. Levoglucosan as a tracer for smouldering fire. Fire Technol. 2018, 54, 1871–1885. [Google Scholar] [CrossRef]
- Travers, E.B.; Olsen, N.F. Effect of air permeability on smoldering characteristics of cotton upholstery fabrics. Text. Res. J. 1982, 52, 598–604. [Google Scholar]
- Wakelyn, P.J.; Hughs, S.E. Evaluation of the flammability of cotton bales. Fire Mater. 2002, 26, 183–189. [Google Scholar]
- Donaldson, D.J.; Yeadon, D.A.; Harper, R.J. Smoldering phenomenon associated with cotton. Text. Res. J. 1983, 53, 160–164. [Google Scholar] [CrossRef]
- Kellogg, D.S.; Waymack, B.E.; McRae, D.D. The Initiation of Smoldering Combustion in Cellulosic Fabrics. J. Fire Sci. 1998, 16, 90–104. [Google Scholar] [CrossRef]
- Li, H.W.; Yue, Y.T.; Li, Y.X.; Zhang, H. The determination of the heat release rate on cotton smoldering fire and polyurethane fire. Adv. Mater. Res. 2013, 668, 915–919. [Google Scholar]
- Li, W.; Liu, W.; Ni, Z. An experimental investigation of the smoldering combustion of cotton with different moisture contents. AEIC Academic Exchange Information Centre (China). In Proceedings of the 2nd International Workshop on Advances in Energy Science and Environment Engineering (AESEE 2018) (Advances in Energy Science and Environment Engineering II), AEIC Academic Exchange Information Centre (China), International Conference on Humanities and Social Science Research, Zhuhai, China, 2–4 February 2018; pp. 447–456. [Google Scholar]
- Hagen, B.C.; Frette, V.; Kleppe, G.; Arntzen, B.J. Transition from smoldering to flaming fire in short cotton samples with asymmetrical boundary conditions. Fire Saf. J. 2015, 71, 69–78. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Z.; Lin, S.; Qu, Y.; Huang, X. Smoldering fire of high-density cotton bale under concurrent wind. Fire Technol. 2020. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, S.; Tang, Z.; Cai, J.; Zhong, Y.; Zhou, B. Free radical and functional group reaction and index gas CO emission during coal spontaneous combustion. Combust. Sci. Technol. 2018, 190, 834–848. [Google Scholar] [CrossRef]
- Su, H.; Zhou, F.; Li, J.; Qi, H. Effects of oxygen supply on low-temperature oxidation of coal: A case study of Jurassic coal in Yima, China. Fuel 2017, 202, 446–454. [Google Scholar] [CrossRef]
- Su, H.; Ji, H.; Chen, X. Model simplification of coal combustion kinetics: A case study of Weihuliang coal in Urumchi, China. Combust. Theory Model. 2019, 23, 1071–1089. [Google Scholar] [CrossRef]
- Feng, K.K. Spontaneous combustion of Canadian coals. CIM Bull. 1985, 78, 71–75. [Google Scholar]
- Hu, X.; Yang, S.; Zhou, X.; Yu, Z.; Hu, C. Coal spontaneous combustion prediction in gob using chaos analysis on gas indicators from upper tunnel. J. Nat. Gas Sci. Eng. 2015, 26, 461–469. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Price, D.; Akalin, M. FTIR analysis of gases evolved from cotton and flame retarded cotton fabrics pyrolysed in air. Polym. Degrad. Stab. 1996, 52, 205–213. [Google Scholar] [CrossRef]
- Furneaux, R.H.; Gainsford, G.J.; Shafizadeh, F.; Stevenson, T.T. Synthesis and thermal chemistry of isolevoglucosenone. Carbohydr. Res. 1986, 146, 113–128. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Furneaux, R.H.; Stevenson, T.T. Some reactions of levoglucosenone. Carbohydr. Res. 1979, 71, 169–191. [Google Scholar] [CrossRef]
- Halpern, Y.; Riffer, R.; Broido, A. Levoglucosenone (1,6-anhydro-3,4-dideoxy-.DELTA.3-.beta.-D-pyranosen-2-one). Major product of the acid-catalyzed pyrolysis of cellulose and related carbohydrates. J. Org. Chem. 1973, 38, 204–209. [Google Scholar] [CrossRef]
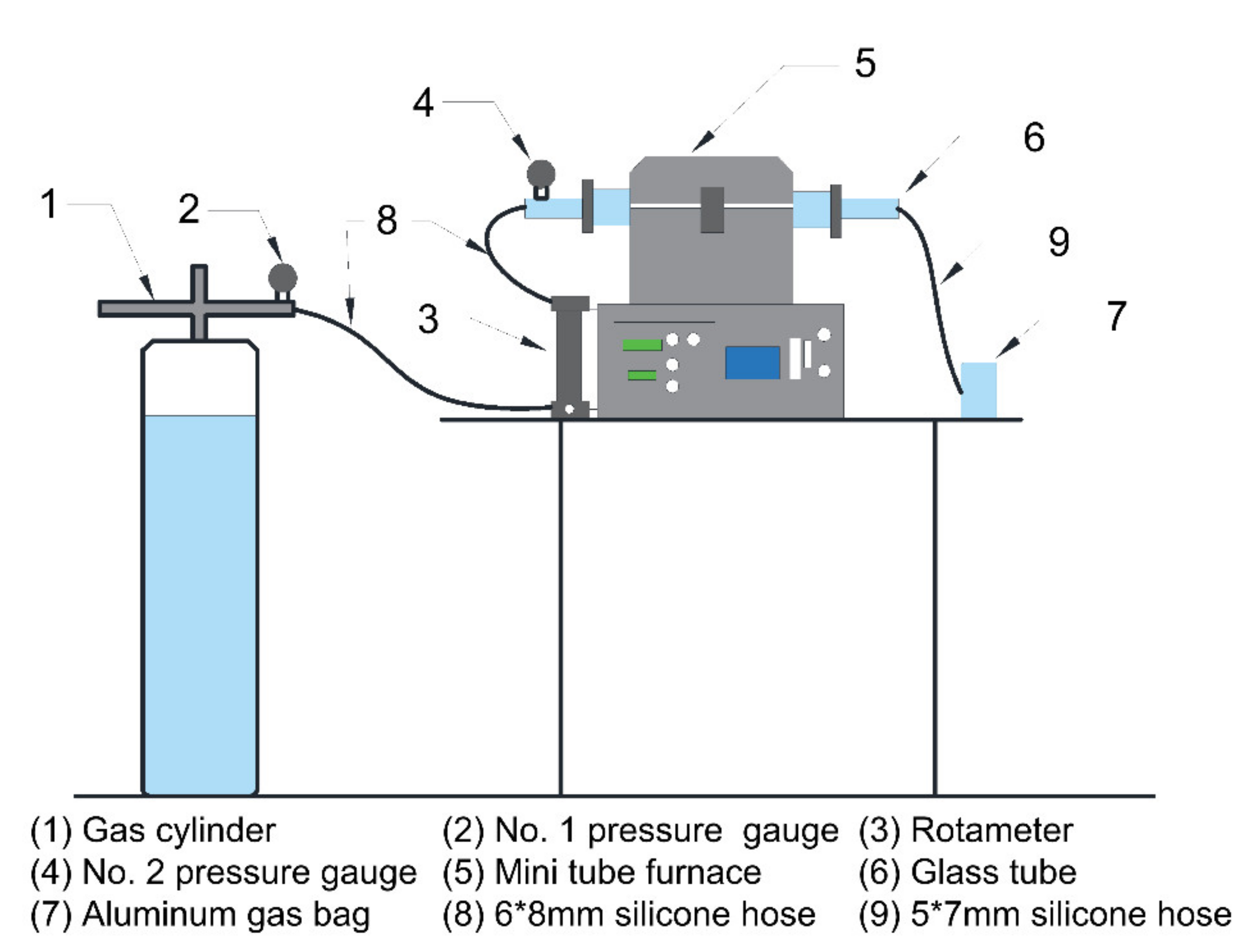
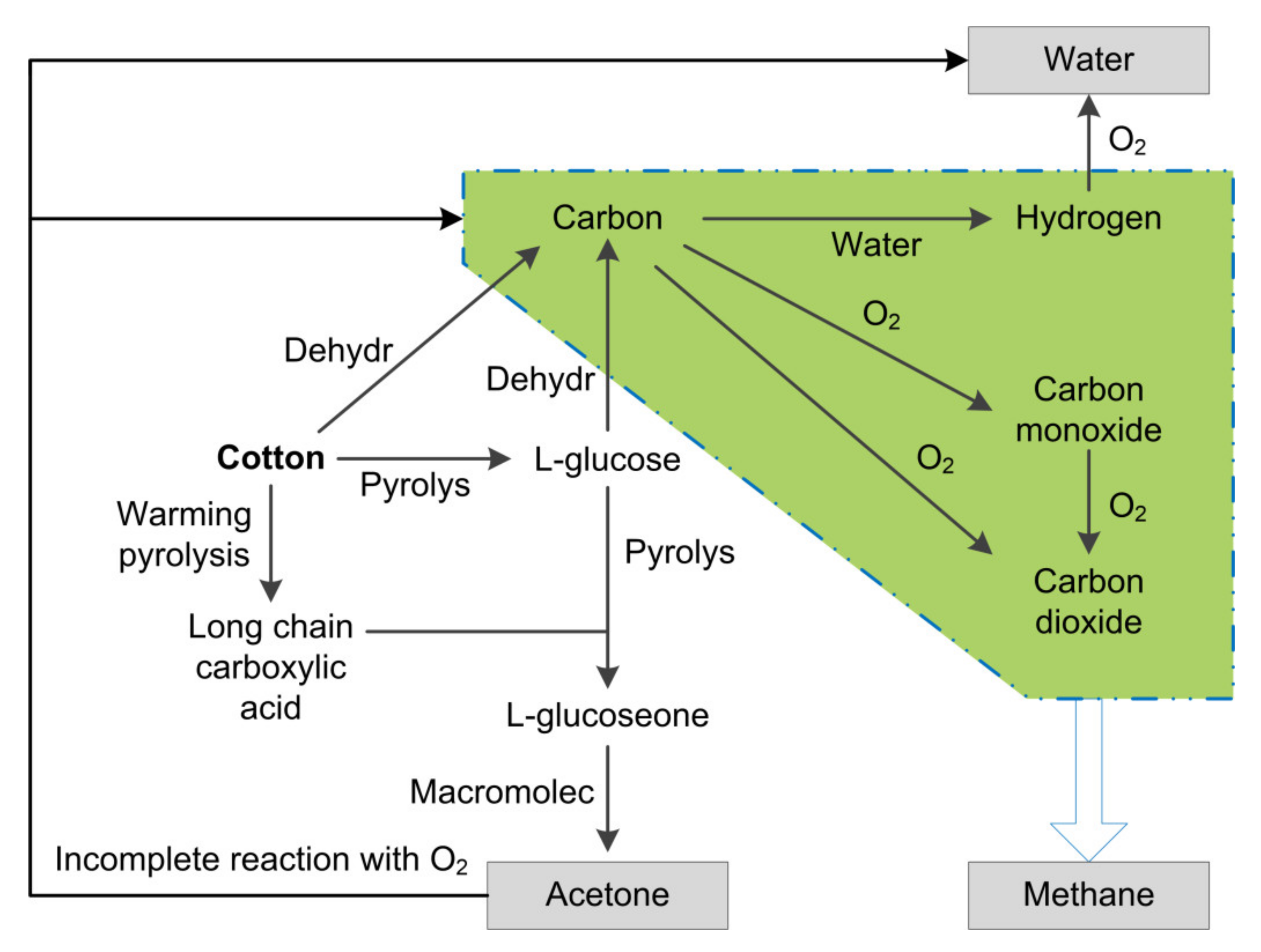
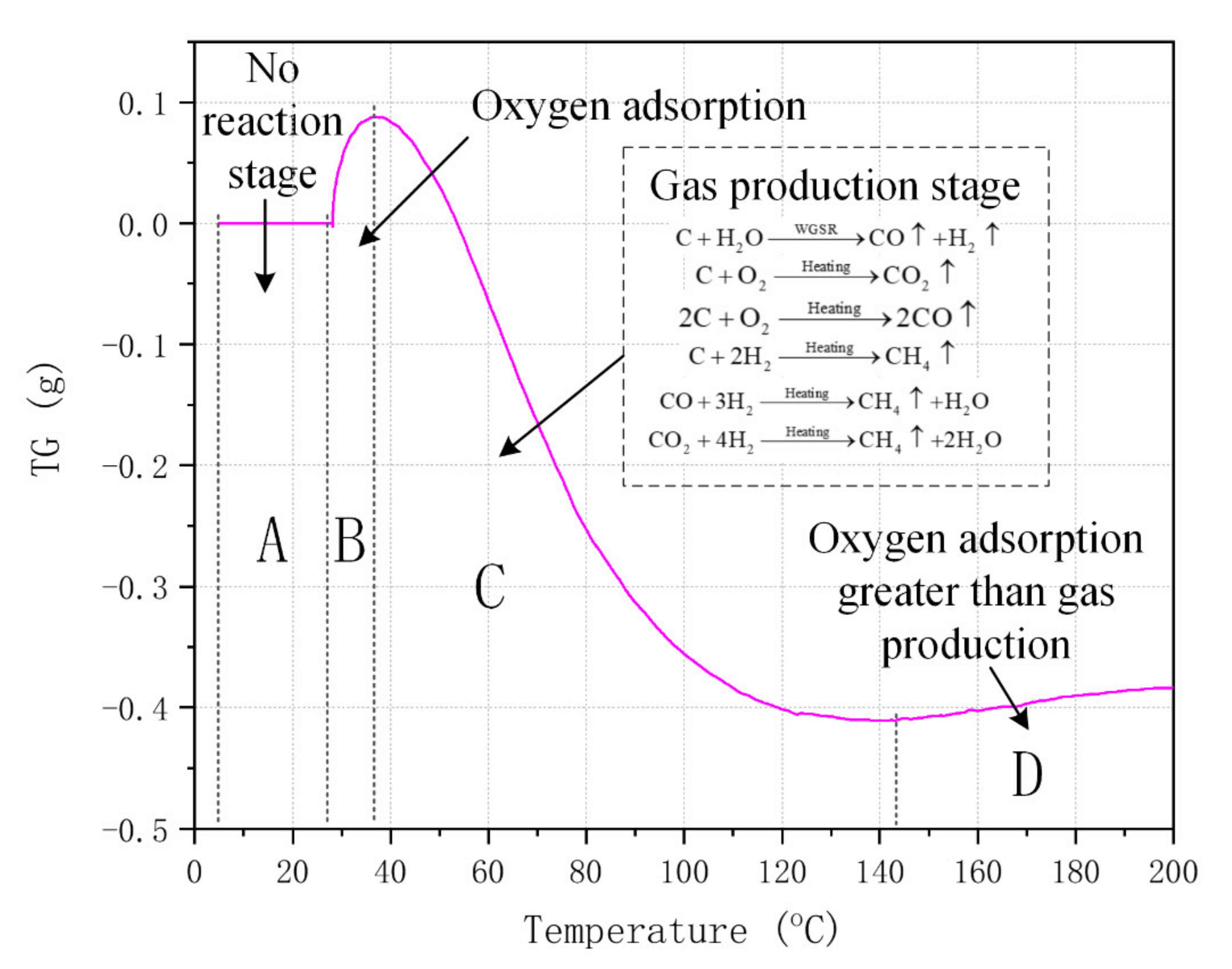
| Temperature (°C) | Collection Duration (min) | Heating Time (min) |
|---|---|---|
| 85 | 20 | 15 |
| 105 | 20 | 15 |
| 125 | 20 | 15 |
| 145 | 20 | 15 |
| 165 | 20 | 15 |
| 185 | 20 | 15 |
| Name of Parameters | Parameters |
|---|---|
| Reproducibility of retention time | <0.008% |
| Reproducibility of gas chromatography mass spectrometry | <1% RSD |
| Electronic pressure control accuracy | 0.001 psi |
| Maximum temperature | 400 °C |
| Mass range | 1.6–1050 amu |
| Scan rate | 12,500 u/s |
| Temperature (°C) | Average Flow Rate (mL/min) | Collection Duration (min) | Gas Collection Volume (mL) |
|---|---|---|---|
| 95 | 30 | 10 | 300 |
| 105 | 20 | 20 | 400 |
| 125 | 25 | 20 | 500 |
| 145 | 25 | 20 | 500 |
| 165 | 17 | 13 | 221 |
| 185 | 16 | 13 | 208 |
| Temperature/°C | Composition (vol.%) | |||
|---|---|---|---|---|
| Nitrogen | Oxygen | Hydrogen | Carbon Monoxide | |
| 95 | 76.0158 | 20.0321 | 0.0197 | 0 |
| 105 | 76.3142 | 20.7831 | 0 | 0 |
| 125 | 76.5528 | 20.8694 | 0.0446 | 0 |
| 145 | 76.6052 | 20.8847 | 0.0328 | 0.1002 |
| 165 | 76.3281 | 20.7723 | 0 | 0 |
| 185 | 77.3182 | 20.9654 | 0.0394 | 0 |
| Temperature (°C) | CAS Number | Composition Name | Peak Area (%) |
|---|---|---|---|
| 95 | 74-82-8 | Methane | 99.59 |
| 105 | 74-82-8 | Methane | 99.65 |
| 4076-39-5 | 1-Methylbenzo[c]phenanthrene | 0.14 | |
| 125 | 74-82-8 | Methane | 99.11 |
| 67-64-1 | Acetone | 0.11 | |
| 540-97-6 | Dodecamethylcyclohexasiloxane | 0.1 | |
| 145 | 74-82-8 | Methane | 98.3 |
| 540-97-6 | Dodecamethylcyclohexasiloxane | 0.54 | |
| 107-50-6 | Tetradecamethylcycloheptasiloxane | 0.4 | |
| 556-68-3 | Hexadecamethylcyclooctasiloxane | 0.26 | |
| 67-64-1 | Acetone | 0.21 | |
| 541-02-6 | Cyclopentasiloxane | 0.15 | |
| 165 | 74-82-8 | Methane | 99.47 |
| 540-97-6 | Dodecamethylcyclohexasiloxane | 0.17 | |
| 557-30-2 | Glyoxime | 0.05 | |
| 3555-47-3 | Tetrakis (trimethylsilyl) orthosilicate | 0.11 | |
| 185 | 74-82-8 | Methane | 99.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Shi, J.; Ji, H.; Li, J.; Fan, J. Investigating on the Iconic Gas Compositions Produced by Low-Temperature Heating Cotton. Symmetry 2020, 12, 883. https://doi.org/10.3390/sym12060883
Su H, Shi J, Ji H, Li J, Fan J. Investigating on the Iconic Gas Compositions Produced by Low-Temperature Heating Cotton. Symmetry. 2020; 12(6):883. https://doi.org/10.3390/sym12060883
Chicago/Turabian StyleSu, Hetao, Jingdong Shi, Huaijun Ji, Jiake Li, and Jingru Fan. 2020. "Investigating on the Iconic Gas Compositions Produced by Low-Temperature Heating Cotton" Symmetry 12, no. 6: 883. https://doi.org/10.3390/sym12060883
APA StyleSu, H., Shi, J., Ji, H., Li, J., & Fan, J. (2020). Investigating on the Iconic Gas Compositions Produced by Low-Temperature Heating Cotton. Symmetry, 12(6), 883. https://doi.org/10.3390/sym12060883






