One-Step Preparation of Z-Isomer-Rich β-Carotene Nanosuspensions Utilizing a Natural Catalyst, Allyl Isothiocyanate, via Supercritical CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
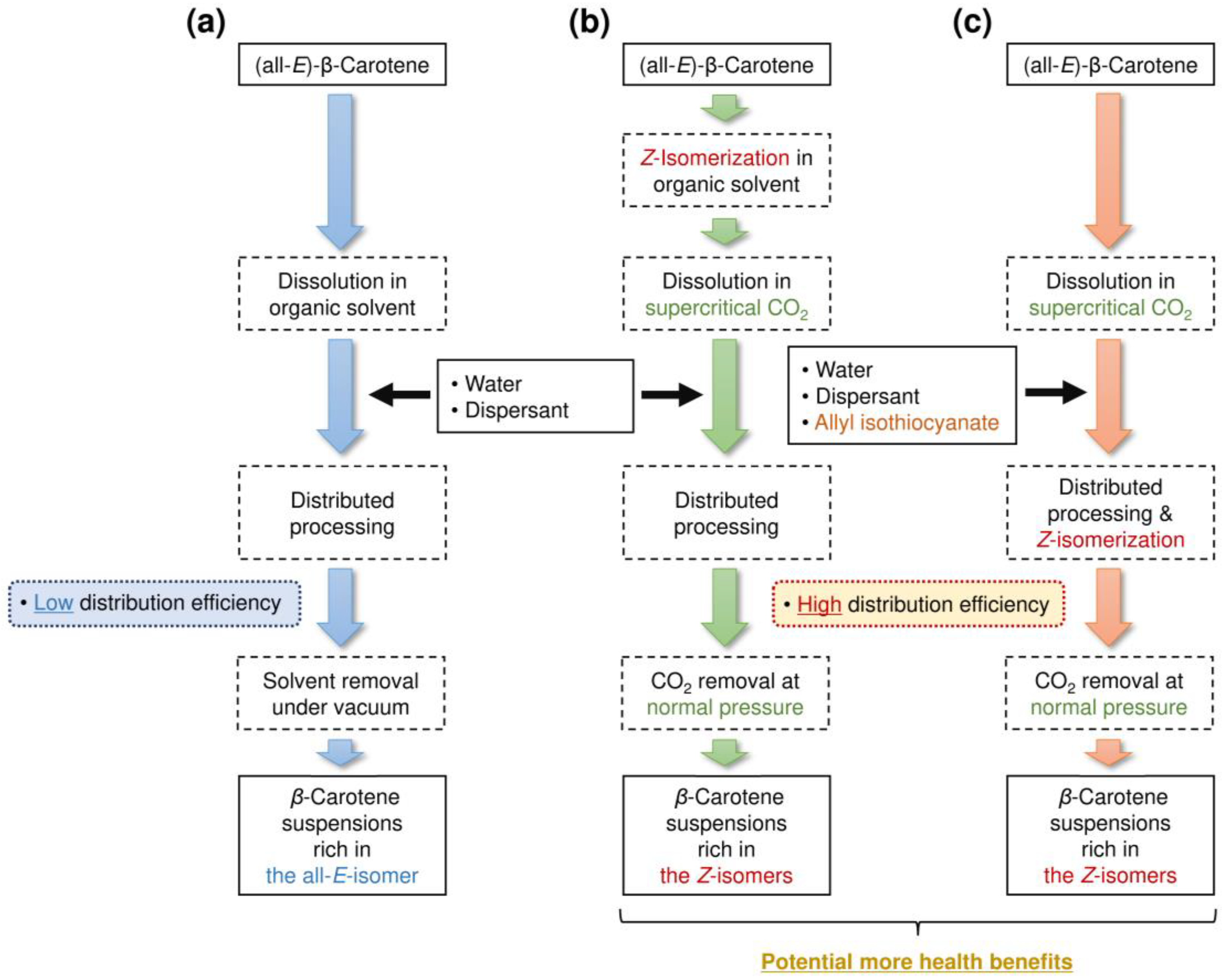
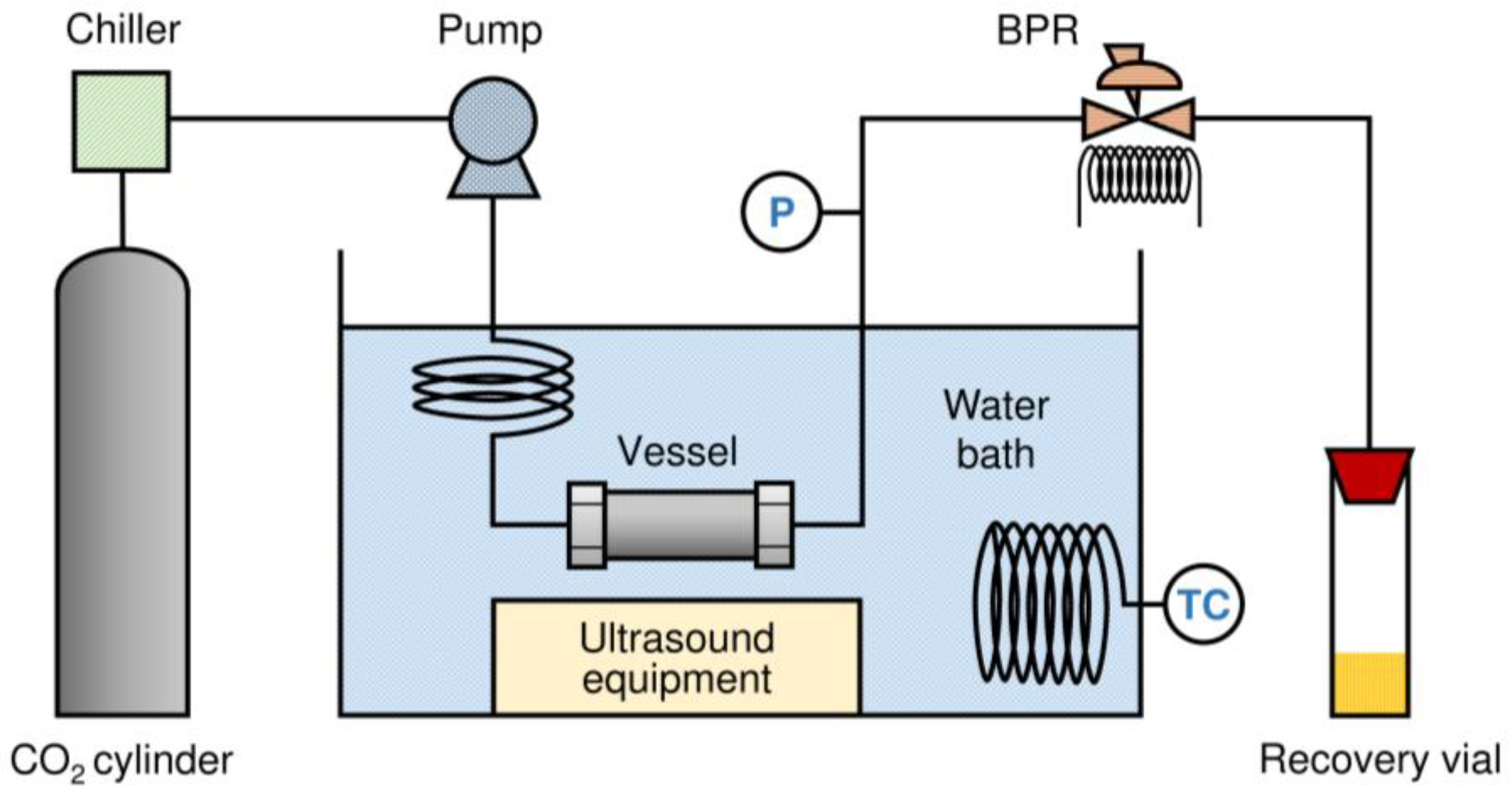
2.2. Isomerization and Distributed Processing of β-Carotene
2.3. Evaluation and Characterization of β-Carotene Suspensions
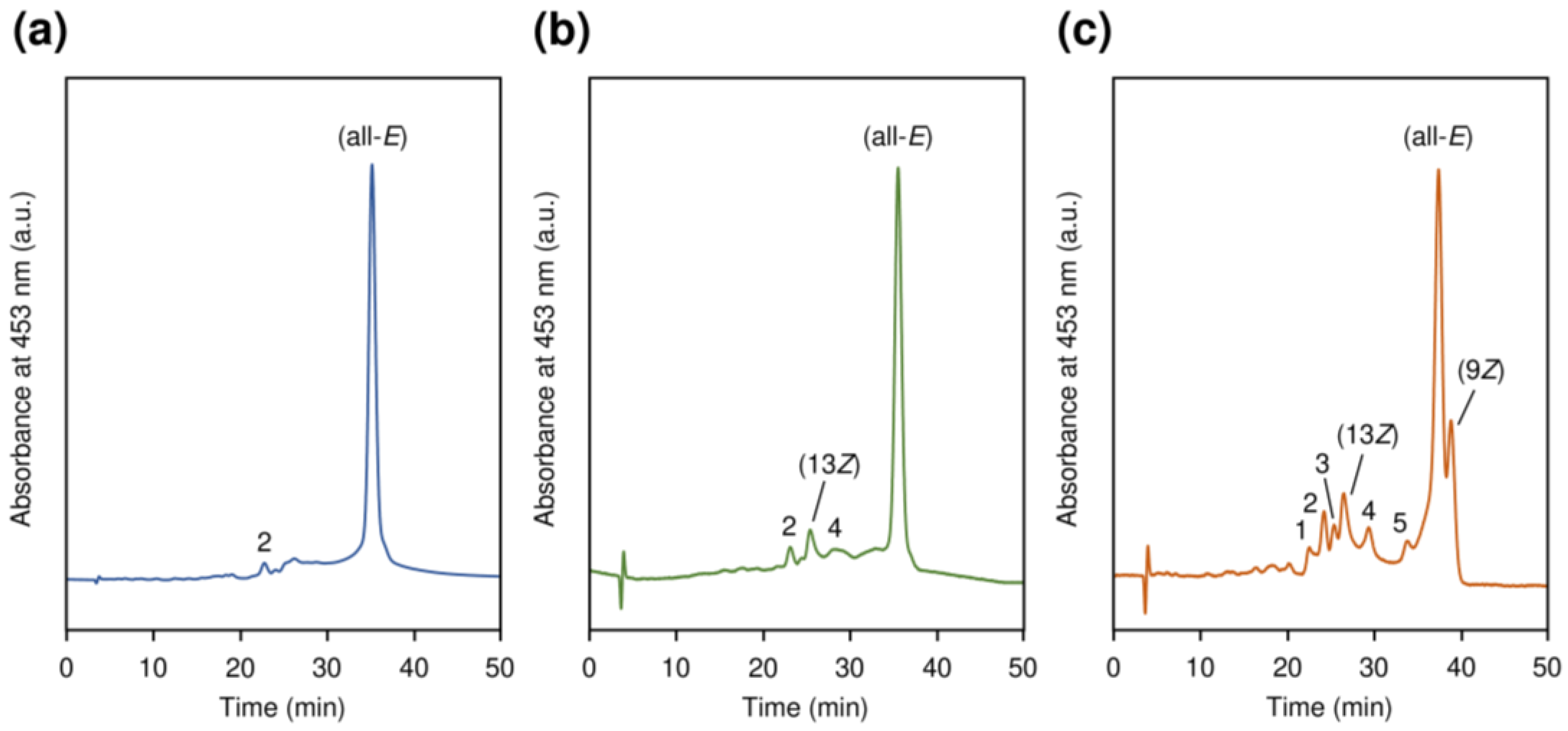
2.3.1. Z-Isomer Ratio of β-Carotene in Suspensions
2.3.2. Encapsulated β-Carotene Content
2.3.3. Absorption Spectra of β-Carotene Suspensions
2.3.4. Particle Size Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effects of AITC on Z-Isomer Ratio and β-Carotene Content in Nanosuspensions
3.2. Characterization of β-Carotene Suspensions
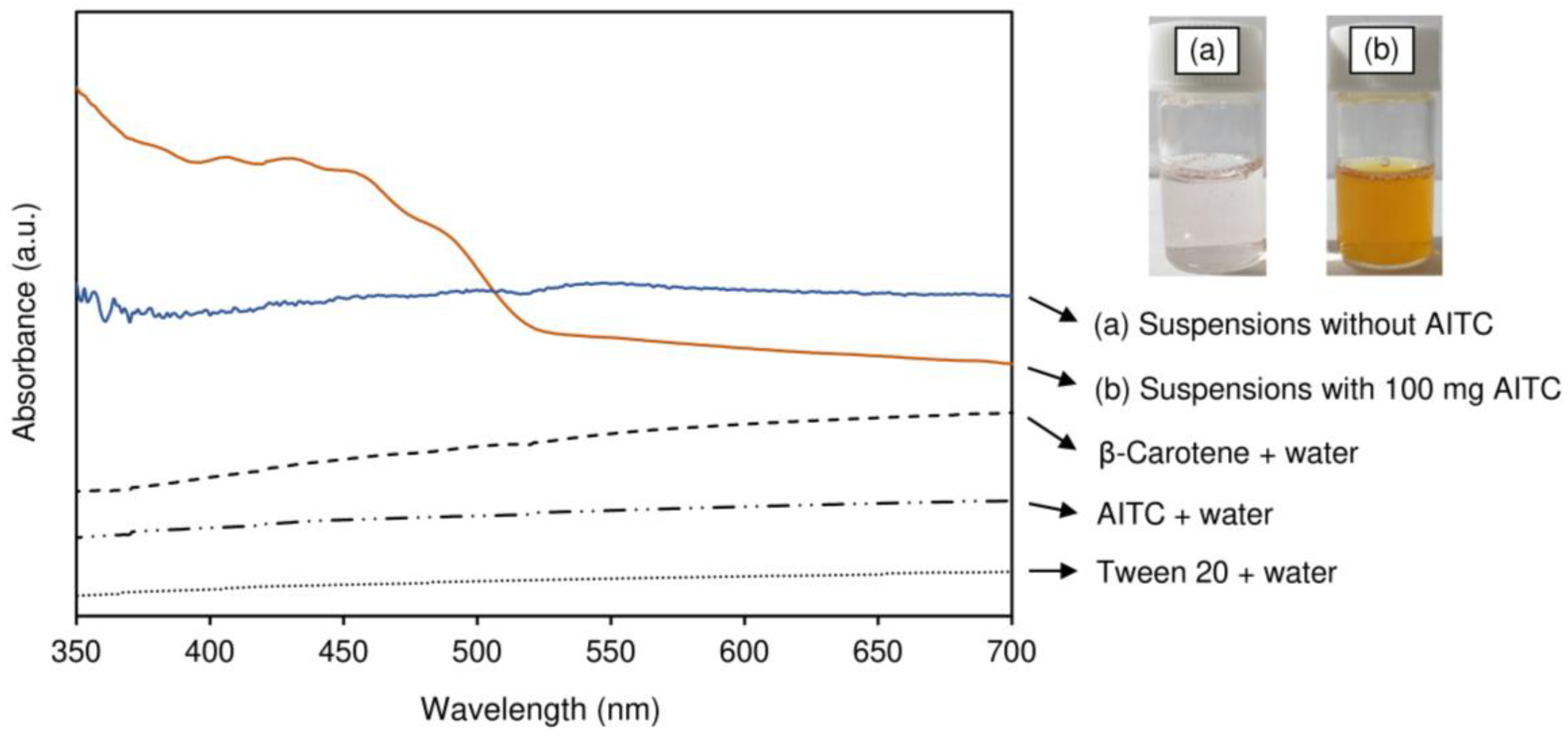
3.2.1. Color
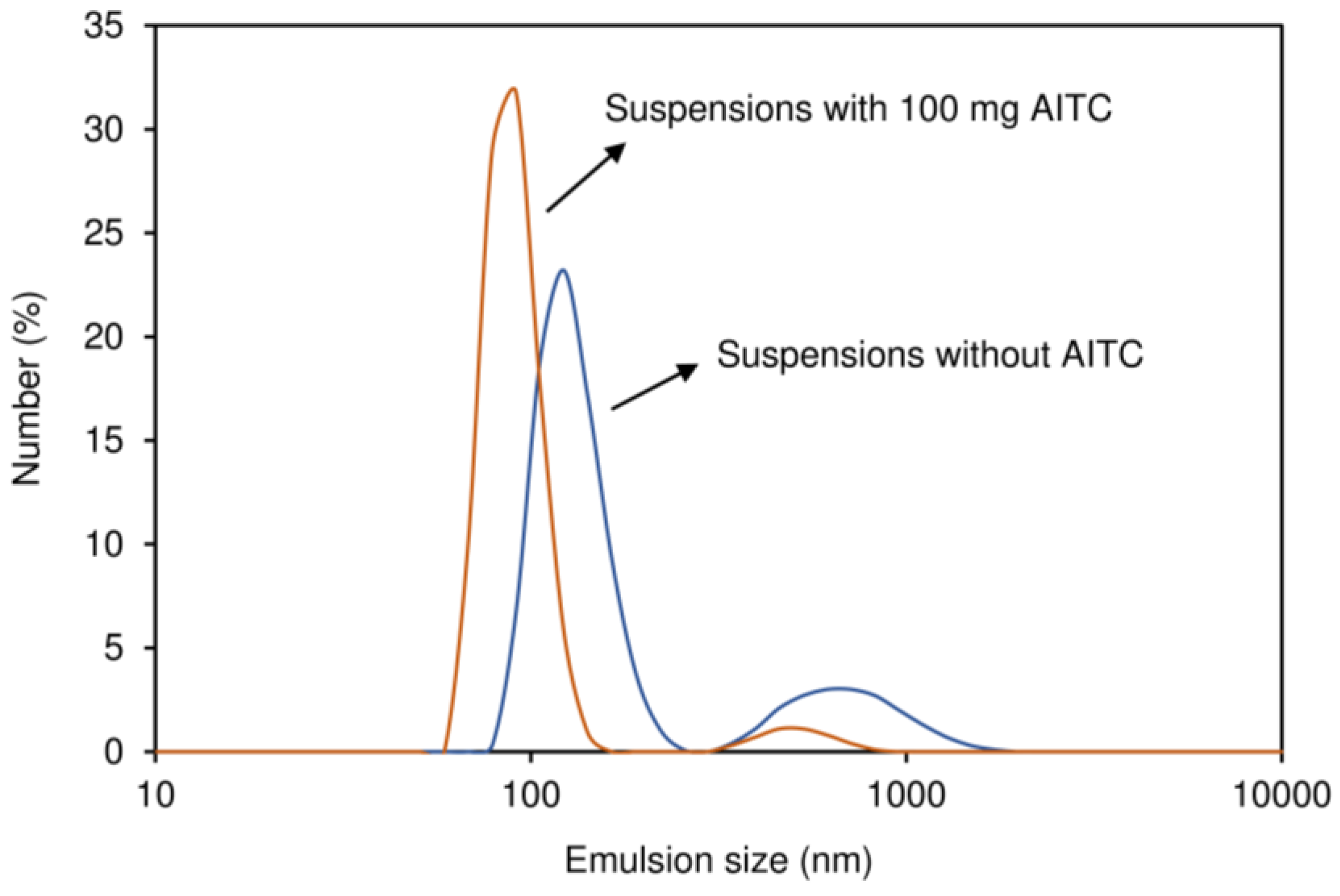
3.2.2. Size Distribution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 2011, 5, 7119–7131. [Google Scholar]
- Seo, J.S.; Burri, B.J.; Quan, Z.; Neidlinger, T.R. Extraction and chromatography of carotenoids from pumpkin. J. Chromatogr. A 2005, 1073, 371–375. [Google Scholar] [CrossRef] [PubMed]
- D’Odorico, A.; Martines, D.; Kiechl, S.; Egger, G.; Oberhollenzer, F.; Bonvicini, P.; Sturniolo, G.C.; Naccarato, R.; Willeit, J. High plasma levels of α-and β-carotene are associated with a lower risk of atherosclerosis: Results from the Bruneck study. Atherosclerosis 2000, 153, 231–239. [Google Scholar] [CrossRef]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, encapsulation, and health benefits of β-carotene—A review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Honda, M.; Kodama, T.; Kageyama, H.; Hibino, T.; Wahyudiono; Kanda, H.; Goto, M. Enhanced solubility and reduced crystallinity of carotenoids, β-carotene and astaxanthin, by Z-isomerization. Eur. J. Lipid Sci. Technol. 2018, 120, 1800191. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Zhang, Y.; Wahyudiono; Kanda, H.; Yamaguchi, R.; Takemura, R.; Fukaya, T.; Goto, M. Improved carotenoid processing with sustainable solvents utilizing Z-isomerization-induced alteration in physicochemical properties: A review and future directions. Molecules 2019, 24, 2149. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Cruz, R.C. Highly concentrated carotenoid-containing emulsions. Eng. Life Sci. 2005, 5, 84–88. [Google Scholar] [CrossRef]
- Tan, C.P.; Nakajima, M. β-Carotene nanodispersions: Preparation, characterization and stability evaluation. Food Chem. 2005, 92, 661–671. [Google Scholar] [CrossRef]
- De Paz, E.; Martín, Á.; Mateos, E.; Cocero, M.J. Production of water-soluble β-carotene micellar formulations by novel emulsion techniques. Chem. Eng. Proc. Proc. Int. 2013, 74, 90–96. [Google Scholar] [CrossRef]
- Ono, M.; Honda, M.; Wahyudiono; Yasuda, K.; Kanda, H.; Goto, M. Production of β-carotene nanosuspensions using supercritical CO2 and improvement of its efficiency by Z-isomerization pre-treatment. J. Supercrit. Fluids 2018, 138, 124–131. [Google Scholar] [CrossRef]
- Gamlieli-Bonshtein, I.; Korin, E.; Cohen, S. Selective separation of cis-trans geometrical isomers of β-carotene via CO2 supercritical fluid extraction. Biotechnol. Bioeng. 2002, 80, 169–174. [Google Scholar] [CrossRef]
- Murakami, K.; Honda, M.; Takemura, R.; Fukaya, T.; Kubota, M.; Wahyudiono; Kanda, H.; Goto, M. The thermal Z-isomerization-induced change in solubility and physical properties of (all-E)-lycopene. Biochem. Biophys. Res. Commun. 2017, 491, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Watanabe, Y.; Murakami, K.; Hoang, N.N.; Wahyudiono; Kanda, H.; Goto, M. Enhanced lycopene extraction from gac (Momordica cochinchinensis Spreng.) by the Z-isomerization induced with microwave irradiation pre-treatment. Eur. J. Lipid Sci. Technol. 2018, 120, 1700293. [Google Scholar] [CrossRef]
- Harari, A.; Harats, D.; Marko, D.; Cohen, H.; Barshack, I.; Kamari, Y.; Gonen, A.; Gerber, Y.; Ben-Amotz, A.; Shaish, A. A 9-cis β-carotene–enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice. J. Nutr. 2008, 138, 1923–1930. [Google Scholar] [CrossRef]
- Relevy, N.Z.; Rühl, R.; Harari, A.; Grosskopf, I.; Barshack, I.; Ben-Amotz, A.; Nir, U.; Gottlieb, H.; Kamari, Y.; Harats, D.; et al. 9-cis β-Carotene inhibits atherosclerosis development in female LDLR-/- mice. Funct. Foods Health Dis. 2015, 5, 67–79. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Ichihashi, K.; Takada, W.; Goto, M. Isomerization of commercially important carotenoids (lycopene, β-carotene, astaxanthin) by natural catalysts: Isothiocyanates and polysulfides. J. Agric. Food Chem. 2020, 68, 3228–3237. [Google Scholar] [CrossRef]
- Watanabe, Y.; Honda, M.; Higashiura, T.; Fukaya, T.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Rapid and selective concentration of lycopene Z-isomers from tomato pulp by supercritical CO2 with co-solvents. Solvent Extr. Res. Dev. 2018, 25, 47–57. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Takemura, R.; Goto, M.; Fukaya, T. Enhanced Z-isomerization of tomato lycopene through the optimal combination of food ingredients. Sci. Rep. 2019, 9, 7979. [Google Scholar] [CrossRef]
- Honda, M.; Sato, H.; Takehara, M.; Inoue, Y.; Kitamura, C.; Takemura, R.; Fukaya, T.; Wahyudiono; Kanda, H.; Goto, M. Microwave-accelerated Z-isomerization of (all-E)-lycopene in tomato oleoresin and enhancement of the conversion by vegetable oils containing disulfide compounds. Eur. J. Lipid Sci. Technol. 2018, 120, 180060. [Google Scholar] [CrossRef]
- Wagner, A.E.; Boesch-Saadatmandi, C.; Dose, J.; Schultheiss, G.; Rimbach, G. Anti-inflammatory potential of allyl-isothiocyanate–role of Nrf2, NF-κB and microRNA-155. J. Cell. Mol. Med. 2012, 16, 836–843. [Google Scholar] [CrossRef]
- Zhang, Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010, 54, 127–135. [Google Scholar] [CrossRef]
- Kamihira, M.; Asai, T.; Yamagata, Y.; Taniguchi, M.; Kobayashi, T. Formation of inclusion complexes between cyclodextrins and aromatic compounds under pressurized carbon dioxide. J. Ferm. Bioeng. 1990, 69, 350–353. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Souza, B.W.S.; Ribeiro, C.; Avides, M.C.; Quintas, M.A.C.; Coimbra, J.S.R.; Carneiro-da-Cunha, M.G.; Vicente, A.A. Nanoemulsions of β-carotene using a high-energy emulsification–evaporation technique. J. Food Eng. 2011, 102, 130–135. [Google Scholar] [CrossRef]
- Emenhiser, C.; Simunovic, N.; Sander, L.C.; Schwartz, S.J. Separation of geometrical carotenoid isomers in biological extracts using a polymeric C30 column in reversed-phase liquid chromatography. J. Agric. Food Chem. 1996, 44, 3887–3893. [Google Scholar] [CrossRef]
- Imsic, M.; Winkler, S.; Tomkins, B.; Jones, R. Effect of storage and cooking on β-carotene isomers in carrots (Daucus carota L. cv. ‘Stefano’). J. Agric. Food Chem. 2010, 58, 5109–5113. [Google Scholar] [CrossRef]
- Böhm, V.; Puspitasari-Nienaber, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene, and zeaxanthin. J. Agric. Food Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef]
- De Paz, E.; Martín, Á.; Estrella, A.; Rodríguez-Rojo, S.; Matias, A.A.; Duarte, C.M.M.; Cocero, M.J. Formulation of β-carotene by precipitation from pressurized ethyl acetate-on-water emulsions for application as natural colorant. Food Hydrocol. 2012, 26, 17–27. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Sowa, T.; Kawashima, Y. Efficient and environmentally friendly method for carotenoid extraction from Paracoccus carotinifaciens utilizing naturally occurring Z-isomerization-accelerating catalysts. Proc. Biochem. 2020, 89, 146–154. [Google Scholar] [CrossRef]
- Gao, G.; Wei, C.C.; Jeevarajan, A.S.; Kispert, L.D. Geometrical isomerization of carotenoids mediated by cation radical/dication formation. J. Phys. Chem. 1996, 100, 5362–5366. [Google Scholar] [CrossRef]
- Kaga, K.; Honda, M.; Adachi, T.; Honjo, M.; Kanda, H.; Goto, M. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): Effect of PVP and astaxanthin Z-isomer content. J. Supercrit. Fluids 2018, 136, 44–51. [Google Scholar] [CrossRef]
- Affandi, M.M.M.; Julianto, T.; Majeed, A.B.A. Enhanced oral bioavailability of astaxanthin with droplet size reduction. Food Sci. Technol. Res. 2012, 18, 549–554. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Wilson, T.A.; Nicolosi, R.J. Bioavailability of a nanoemulsion of lutein is greater than a lutein supplement. Nano Biomed. Eng. 2009, 1, 38–49. [Google Scholar] [CrossRef]

| λmax (nm) | % DB/DII | ||||
|---|---|---|---|---|---|
| Peak No. | β-Carotene Isomer a | In-Line | Reported a | In-Line | Reported a |
| 1 | UZ | 336,458,483,582 | – | 56.7 | – |
| 2 | UZ | 342,434,452,484 | – | 55.6 | – |
| 3 | UZ | 329,393,442,486 | – | 64.1 | – |
| (13Z) | 336,425,442,472 | 339,420,445,470 | 44.2 | 37.1 | |
| 4 | UZ | 332,412,442,484 | – | 70.1 | – |
| 5 | UZ | 338,442,462,473 | – | 61.7 | – |
| (all-E) | 423,450,473 | 426,452,478 | ND | ND | |
| (9Z) | 338,420,446,470 | 340,422,447,473 | 6.6 | 9.4 | |
| Amount of AITC Added (mg) | Z-Isomers Ratio (%) | β-Carotene Content (mg/L) |
|---|---|---|
| 0 | 15.1 ± 2.0 a | 29.6 ± 19.1 a |
| 50 | 31.8 ± 1.5 b | 66.4 ± 8.1 b |
| 100 | 37.4 ± 1.7 c | 102.5 ± 21.7 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Honda, M.; Fukaya, T.; Wahyudiono; Kanda, H.; Goto, M. One-Step Preparation of Z-Isomer-Rich β-Carotene Nanosuspensions Utilizing a Natural Catalyst, Allyl Isothiocyanate, via Supercritical CO2. Symmetry 2020, 12, 777. https://doi.org/10.3390/sym12050777
Zhang Y, Honda M, Fukaya T, Wahyudiono, Kanda H, Goto M. One-Step Preparation of Z-Isomer-Rich β-Carotene Nanosuspensions Utilizing a Natural Catalyst, Allyl Isothiocyanate, via Supercritical CO2. Symmetry. 2020; 12(5):777. https://doi.org/10.3390/sym12050777
Chicago/Turabian StyleZhang, Yelin, Masaki Honda, Tetsuya Fukaya, Wahyudiono, Hideki Kanda, and Motonobu Goto. 2020. "One-Step Preparation of Z-Isomer-Rich β-Carotene Nanosuspensions Utilizing a Natural Catalyst, Allyl Isothiocyanate, via Supercritical CO2" Symmetry 12, no. 5: 777. https://doi.org/10.3390/sym12050777
APA StyleZhang, Y., Honda, M., Fukaya, T., Wahyudiono, Kanda, H., & Goto, M. (2020). One-Step Preparation of Z-Isomer-Rich β-Carotene Nanosuspensions Utilizing a Natural Catalyst, Allyl Isothiocyanate, via Supercritical CO2. Symmetry, 12(5), 777. https://doi.org/10.3390/sym12050777







