Mechanistic Insights into Plant Chiral Growth
Abstract
1. Introduction
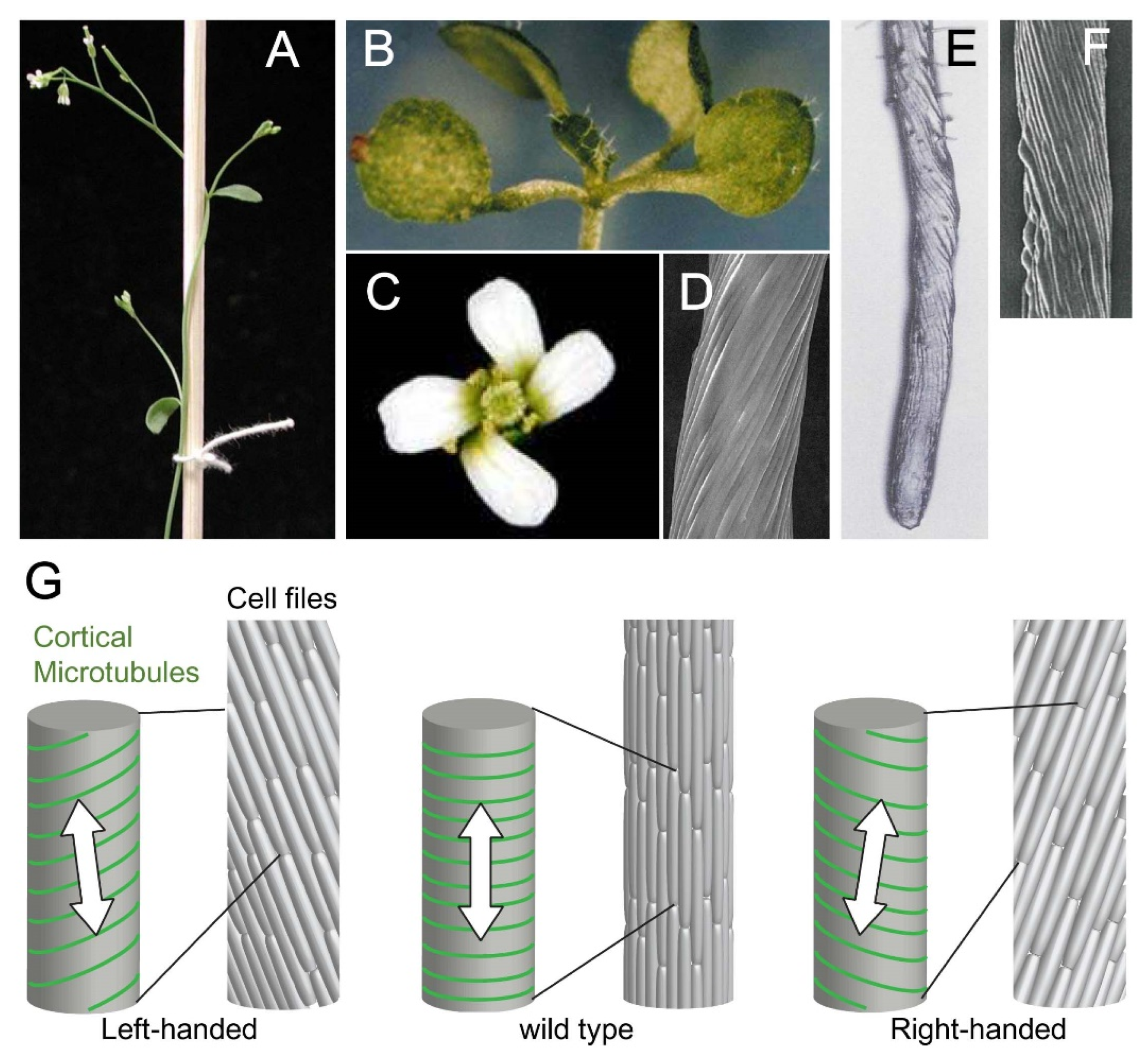
2. Cellular Basis of Organ Twisting
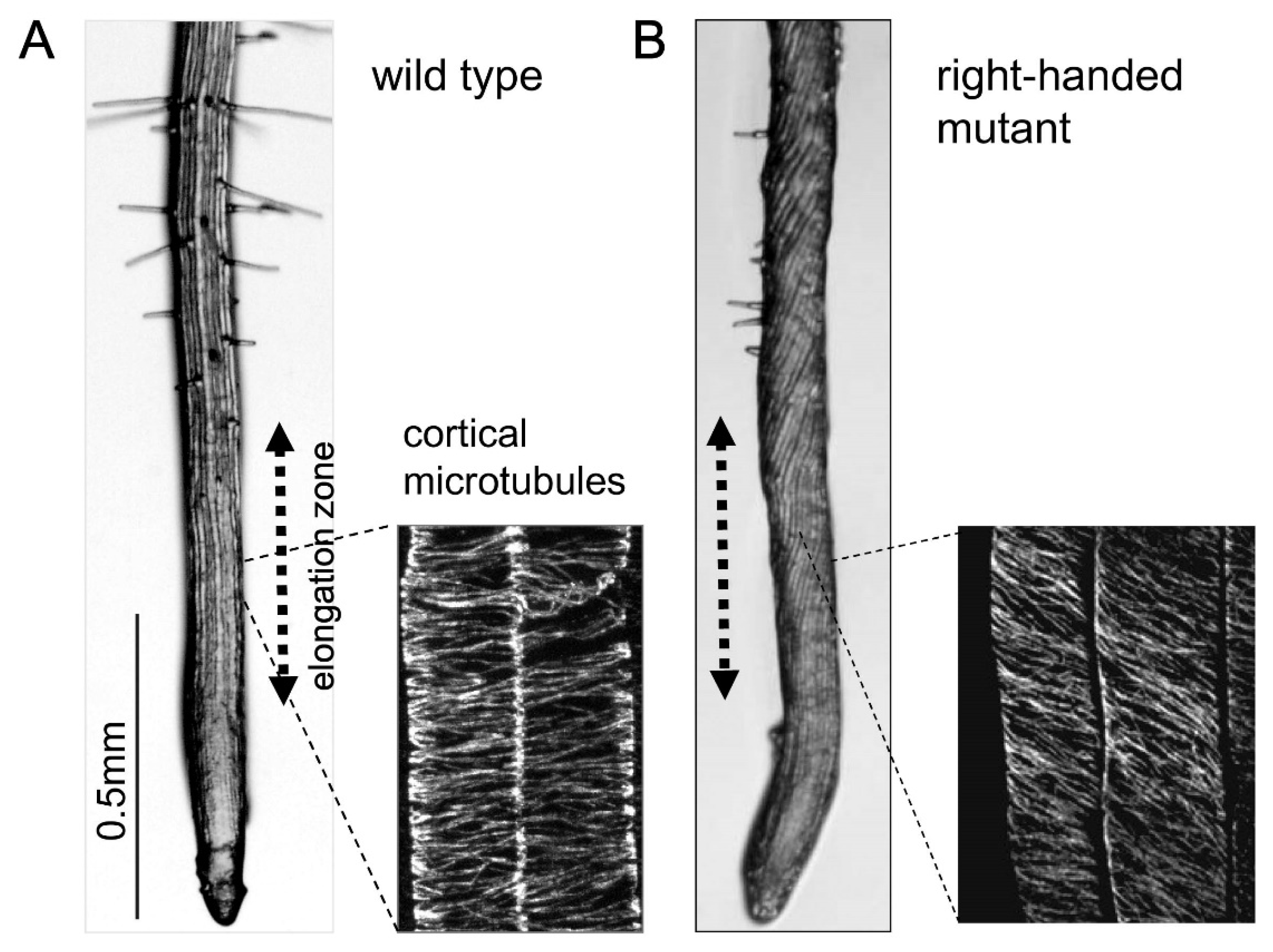
3. Microtubule Defects Cause Helical Growth
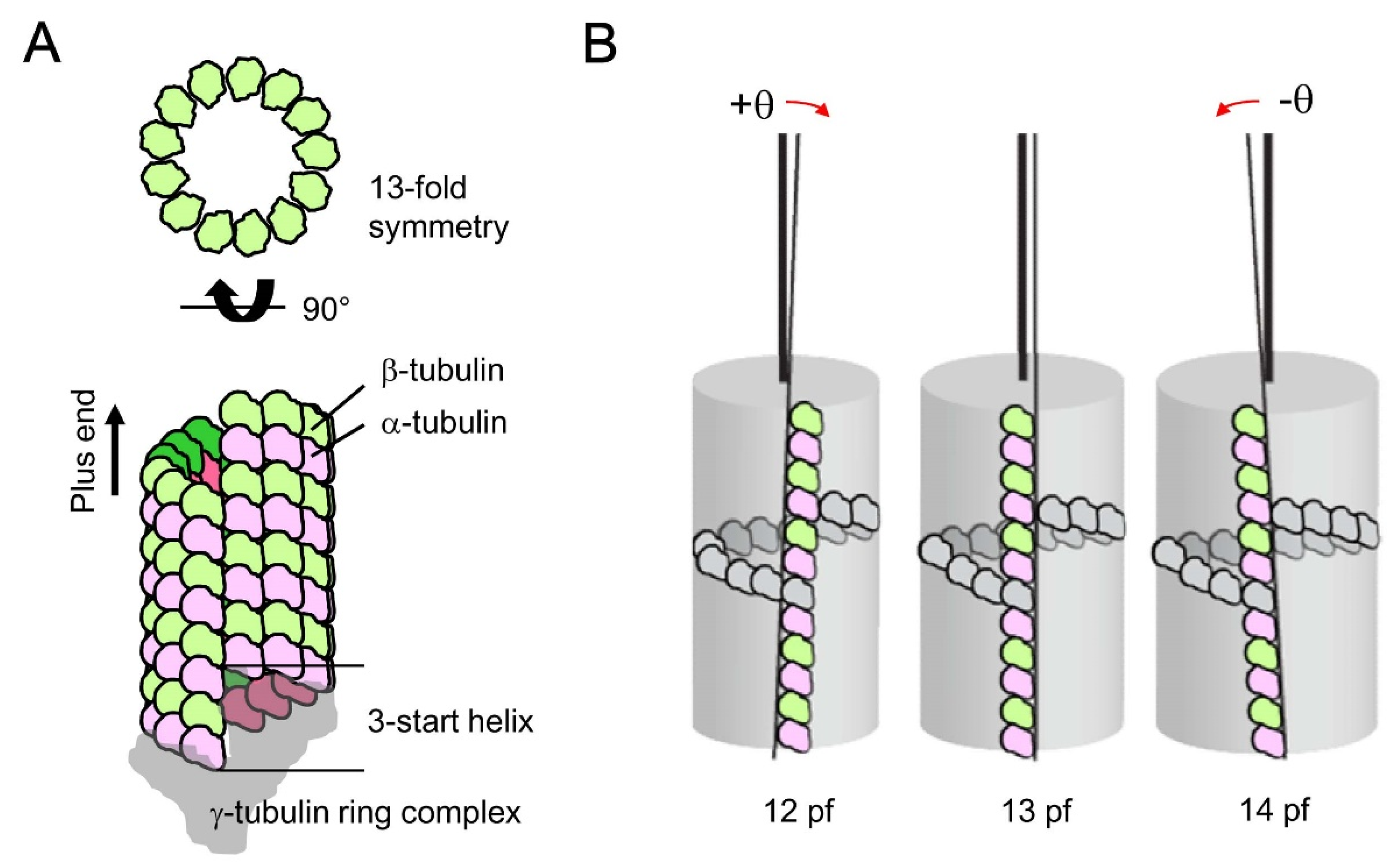
4. Possible Origin of Microtubule Chirality
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Darwin, C. On the movements and habits of climbing plants. Bot. J. Linn. Soc. 1865, 9, 1–118. [Google Scholar] [CrossRef]
- Darwin, C. The Power of Movement in Plants; John Murray: London, UK, 2017. [Google Scholar]
- Gianoli, E. The behavioural ecology of climbing plants. AoB Plants 2015, 7, plv013. [Google Scholar] [CrossRef]
- Isnard, S.; Silk, W.K. Moving with climbing plants from Charles Darwin’s time into the 21st century. Am. J. Bot. 2009, 96, 1205–1221. [Google Scholar] [CrossRef] [PubMed]
- Compton, J.A.; Lack, H.W. The discovery, naming and typification of Wisteria floribunda and W. brachybotrys (Fabaveae) with notes on associated names. Willdenowia 2012, 42, 219–240. [Google Scholar] [CrossRef]
- Edwards, W.; Moles, A.T.; Franks, P. The global trend in plant twining direction. Glob. Ecol. Biogeogr. 2007, 16, 795–800. [Google Scholar] [CrossRef]
- Stolarz, M. Circumnutation as a visible plant action and reaction. Plant Signal. Behav. 2009, 4, 380–387. [Google Scholar] [CrossRef]
- Hashimoto, T. Molecular genetic analysis of left-right handedness in plants. Philos. Trans. R. Soc. Biol. Sci. 2002, 357, 799–808. [Google Scholar] [CrossRef]
- Smyth, D.R. Helical growth in plant organs: Mechanisms and significance. Development 2016, 143, 3272–3282. [Google Scholar] [CrossRef]
- Furutani, I.; Watanabe, Y.; Prieto, R.; Masukawa, M.; Suzuki, K.; Naoi, S.; Thitamadee, T.; Shikanai, T. The SPIRAL genes are required for directional control of cell elongation in Arabidopsis thaliana. Development 2000, 127, 4443–4453. [Google Scholar] [PubMed]
- Thitamadee, S.; Tuchihara, K.; Hashimoto, T. Microtubule basis for left-handed helical growth in Arabidopsis. Nature 2002, 417, 193–196. [Google Scholar] [CrossRef]
- Gendreau, E.; Traas, J.; Desnos, T.; Grandjean, O.; Caboche, M.; Hofte, H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997, 114, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Weizbauer, R.; Peters, W.S.; Schulz, B. Geometric constraints and the anatomical interpretation of twisted plant organ phenotypes. Front. Plant Sci. 2011, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Verger, S.; Liu, M.; Hamant, O. Mechanical conflicts in twisting growth revealed by cell-cell adhesion defects. Front. Plant Sci. 2019, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kawamura, T.; Hashimoto, T. Role of the SPIRAL gene family in anisotropic growth of Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 513–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Savalde-Goldstein, S.; Chory, J. Growth cooridination and the shoot epidermis. Curr. Opin. Plant Biol. 2008, 11, 42–48. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; van der Straeten, D. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc. Natl. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef]
- Ishida, T.; Thitamadee, S.; Hashimoto, T. Twisted growth and organization of cortical microtubules. J. Plant Res. 2007, 120, 61–70. [Google Scholar] [CrossRef]
- Wu, G.; Otegui, M.S.; Spalding, E.P. The ER-localized TWD1 immunophilin is necessary for localization of multidrug resistance-like proteins required for polar auxin transport in Arabidopsis roots. Plant Cell 2010, 22, 3295–3304. [Google Scholar] [CrossRef]
- Hashimoto, T. Dissecting the cellular functions of plant microtubules using mutant tubulins. Cytoskeleton 2013, 70, 191–200. [Google Scholar] [CrossRef]
- Ishida, T.; Kaneko, Y.; Iwano, M.; Hashimoto, T. Helical microtubule arrays in a collection of tubulin mutants of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 8544–8549. [Google Scholar] [CrossRef]
- Nakajima, K.; Furutani, I.; Tachimoto, H.; Matsubara, K.; Hashimoto, T. SPIRAL1 encodes a novel plant-specific microtubule-localized protein required for directional cell control of rapidly expanding Arabidopsis cells. Plant Cell 2004, 16, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Sedbrook, J.C.; Ehrhardt, D.W.; Fisher, S.E.; Scheible, W.R.; Somerville, C.R. The Arabidopsis SKU6/SPIRAL1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. Plant Cell 2004, 16, 1506–1520. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Narita, N.N.; Hayashi, K.; Asada, J.; Hamada, T.; Sonobe, S.; Nakajima, K.; Hashimoto, T. Plant-specific microtubule-associated protein SPIRAL2 is required for anisotropic growth in Arabidopsis. Plant Physiol. 2004, 136, 3933–3944. [Google Scholar] [CrossRef] [PubMed]
- Bushmann, H.; Fabri, C.O.; Hauptmann, M.; Hutzler, P.; Laux, T.; Lloyd, C.W.; Schäffner, A.R. Helical growth of the Arabidopsis mutant tortifolia1 reveals a plant-specific microtubule-associated protein. Curr. Biol. 2004, 14, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wakamatsu, Y.; Itoh, T.J.; Shoji, T.; Hashimoto, T. Arabidopsis SPIRAL2 promotes uninterrupted microtubule growth by suppressing the pause state of microtubule dynamics. J. Cell Sci. 2008, 121, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Lideboom, J.J.; Saltini, M.; Mulder, B.M.; Ehrhardt, D.W. SPR2 protects minus ends to promote severing and reorientation of plant cortical microtubule arrays. J. Cell Biol. 2018, 217, 915–927. [Google Scholar] [CrossRef]
- Fan, Y.; Burkart, G.M.; Dixit, R. The Arabidopsis SPIRAL2 protein targets and stabilizes microtubule minus ends. Curr. Biol. 2018, 28, 987–994. [Google Scholar] [CrossRef]
- Nakamura, M.; Hashimoto, T. A mutation in the Arabidopsis γ-tubulin-containing complex subunit causes helical growth and abnormal microtubule branching. J. Cell Sci. 2009, 122, 2208–2217. [Google Scholar] [CrossRef]
- Naoi, K.; Hashimoto, T. A semi-dominant mutation in an Arabidopsis mitogen-activated protein kinase phosphatase-like gene compromises cortical microtubule organization. Plant Cell 2004, 16, 1841–1853. [Google Scholar] [CrossRef]
- Fujita, S.; Pytela, J.; Hotta, T.; Kato, T.; Hamada, T.; Akamatsu, R.; Ishida, Y.; Kutsuna, N.; Hasezawa, S.; Nomura, Y.; et al. An atypical tubulin kinase mediates stress-induced microtubule depolymerization in Arabidopsis. Curr. Biol. 2013, 23, 1969–1978. [Google Scholar] [CrossRef]
- Nakamura, M.; Naoi, K.; Shoji, T.; Hashimoto, T. Low concentrations of propyzamide and oryzalin alter microtubule dynamics in Arabidopsis epidermal cells. Plant Cell Physiol. 2004, 45, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Baskin, T.I. On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 2001, 215, 150–171. [Google Scholar] [CrossRef] [PubMed]
- Paredez, A.R.; Somerville, C.R.; Ehrhardt, D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006, 312, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, P.; Ho, R.; Zimmer, J. Architecture of a catalytically active homotrimeric plant cellulose synthase complex. Science 2020, 369, 1089–1094. [Google Scholar] [CrossRef]
- Green, P.B. Mechanism for plant cellular morphogenesis. Science 1962, 138, 1404–1405. [Google Scholar] [CrossRef]
- Hamant, O.; Inoue, D.; Bouchez, D.; Dumais, J.; Mjolsness, E. Are microtubule tension sensors? Nat. Commun. 2019, 10, 2360. [Google Scholar] [CrossRef]
- Buschmann, H.; Hauptmann, M.; Niessing, D.; Lloyd, C.W.; Schäffner, A.R. Helical growth of the Arabidopsis mutant tortifolia2 does not depend on cell division patterns but involves handed twisting of isolated cells. Plant Cell 2009, 21, 2090–2106. [Google Scholar] [CrossRef]
- Murata, T.; Sonobe, S.; Baskin, T.I.; Hyodo, S.; Hasezawa, S.; Nagata, T.; Horio, T.; Hasebe, M. Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat. Cell Biol. 2005, 7, 961–968. [Google Scholar] [CrossRef]
- Nakamura, M.; Ehrhardt, D.; Hashimoto, T. Microtubule and katanin dependent dynamics of microtubule nucleation complexes in the Arabidopsis cortical array. Nat. Cell Biol. 2010, 12, 1064–1070. [Google Scholar] [CrossRef]
- Shaw, S.; Kamyar, R.; Ehrhardt, D. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 2003, 300, 1715–1718. [Google Scholar] [CrossRef]
- Lindeboom, J.J.; Nakamura, M.; Hibbel, A.; Shundyak, K.; Guierrez, R.; Ketelaar, T.; Emons, A.M.C.; Mulder, B.M.; Kirik, V.; Ehrhardt, D.W. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 2013, 342, 1245533. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Cyr, R. Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior. Plant Cell 2004, 16, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Tindermans, S.H.; Deinum, E.E.; Lindeboom, J.J.; Mulder, B.M. Efficient event-driven simulations shed new light on microtubule organization in the plant cortical array. Front. Phys. 2014, 2, 19. [Google Scholar]
- Sedbrook, J.C.; Carroll, K.L.; Hung, K.F.; Masson, P.H.; Somerville, C.R. The Arabidopsis SKU5 gene encodes an extracellular glycosyl phosphatidylinositol-anchored glycoprotein involved in directional root growth. Plant Cell 2002, 14, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Saffer, A.M.; Carpita, N.C.; Irish, V.F. Rhamnose-containing cell wall polymers suppress helical plant growth independently of microtubule orientation. Curr. Biol. 2017, 27, 2248–2259. [Google Scholar] [CrossRef] [PubMed]
- Pyrpassopoulos, S.; Feeser, E.A.; Mazerik, J.N.; Tyska, M.J.; Ostap, E.M. Membrane-bound myo1c powers asymmetric motility of actin filaments. Curr. Biol. 2012, 22, 1688–1692. [Google Scholar] [CrossRef]
- Ali, M.Y.; Uemura, S.; Adachi, K.; Itoh, H.; Kionoshita, K.; Ishiwata, S. Myosin V is a left-handed spiral motor on the right-handed actin helix. Nat. Struct. Biol. 2002, 9, 464–467. [Google Scholar] [CrossRef]
- Pierson, G.B.; Burton, P.R.; Himes, R.H. Alterations in number of protofilaments in microtubules assembled in vitro. J. Cell Biol. 1978, 76, 223–228. [Google Scholar] [CrossRef]
- Chretien, D.; Wade, R.H. New data on the microtubule surface lattice. Biol. Cell 1991, 71, 161–174. [Google Scholar] [CrossRef]
- Sui, H.; Downing, K.H. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 2010, 18, 1022–1031. [Google Scholar] [CrossRef]
- Yagi, N.; Matsunaga, S.; Hashimoto, T. Insights into cortical microtubule nucleation and dynamics in Arabidopsis leaf cells. J. Cell Sci. 2018, 131, jcs203778. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T. Microtubule and cell shape determination. In The Plant Cytoskeleton; Liu, B., Ed.; Springer: New York, NY, USA, 2011; Chapter 11; pp. 245–257. [Google Scholar]
- Ledbetter, M.C.; Porter, K.R. Morphology of microtubules of plant cells. Science 1964, 144, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, S.; Brouhard, G.J. A microtubule bestiary: Structural diversity in tubulin polymers. Mol. Biol. Cell 2017, 28, 2924–2931. [Google Scholar] [CrossRef] [PubMed]
- Chalfie, M.; Thomson, J.N. Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. J. Cell Biol. 1982, 93, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, S.; Jariwala, S.; Hsu, C.T.; Redemann, S.; Kollman, J.M.; Müller-Reichert, T.; Sept, D.; Bui, K.H.; Brouhard, G.J. The structure and dynamics of C. elegans tubulin reveals the mechanistic basis of microtubule growth. Dev. Cell 2018, 47, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wurz, M.; Zupa, E.; Pfeffer, S.; Schiebel, E. Microtubule nucleation: The waltz between γ-tubulin ring complex and associated proteins. Curr. Opin. Cell Biol. 2020, 68, 124–131. [Google Scholar] [CrossRef]
- Chretien, D.; Fuller, S.D. Microtubule switch occasionally into unfavorable configurations during elongation. J. Mol. Biol. 2000, 298, 663–676. [Google Scholar] [CrossRef]
- Vitre, B.; Coquelle, F.M.; Heichette, C.; Garnier, C.; Chretien, D.; Arnal, I. EB1 regulates microtubule dynamics and tubulin sheet closure in vitro. Nat. Cell Biol. 2008, 10, 415–421. [Google Scholar] [CrossRef]
- Maurer, S.P.; Fourniol, F.J.; Bohner, G.; Moores, C.A.; Surrey, T. EBs recognize a nucleotide-dependent structural cap at growing microtubule ends. Cell 2012, 149, 371–382. [Google Scholar] [CrossRef]
- Moores, C.A.; Perderiset, M.; Francis, F.; Chelly, J.; Houdusse, A.; Milligan, R.A. Mechanism of microtubule stabilization by doublecortin. Mol. Cell 2004, 14, 833–839. [Google Scholar] [CrossRef]
- Hamada, T. Microtubule organization and microtubule-associated proteins in plants. In International Review of Cell and Molecular Biology; Academic Press: London, UK, 2014; Volume 312, pp. 1–52. [Google Scholar]
- Ti, S.C.; Alushin, G.M.; Kapoor, T.M. Human β-tubulin isotypes can regulate microtubule protofilament number and stability. Dev. Cell 2018, 47, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Sumino, Y.; Nagai, K.H.; Shitaka, Y.; Tanaka, D.; Yoshikawa, K.; Chaté, H.; Oiwa, K. Large-scale vortex lattice emerging from collectively moving microtubules. Nature 2012, 483, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Duclos, G.; Adkins, R.; Banerjee, D.; Peterson, M.S.E.; Varghese, M.; Kolvin, I.; Baskaran, A.; Pelcovits, R.A.; Powers, T.R.; Baskaran, A.; et al. Topological structure and dynamics of three-dimensional active nematics. Science 2020, 357, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Pokroy, B.; Kang, S.H.; Mahadevan, L.; Aizenberg, J. Self-organization of a mesoscale bristle into ordered, hierarchical helical assemblies. Science 2009, 323, 237–240. [Google Scholar] [CrossRef]
- Shen, H.; Fallas, J.A.; Lynch, E.; Sheffler, W.; Parry, B.; Jannetty, N.; Decarreau, J.; Wagenbach, M.; Vicente, J.J.; Chen, J.; et al. De novo design of self-assembling helical protein filaments. Science 2018, 362, 705–709. [Google Scholar] [CrossRef]
- Wang, S.; Furchtgott, L.; Huang, K.C.; Shaevitz, J.W. Helical insertion of peptidoglycan produces chiral ordering of the bacterial cell wall. Proc. Natl. Acad. Sci. USA 2012, 109, 3611. [Google Scholar] [CrossRef]
- Shi, H.; Quint, D.A.; Grason, G.M.; Gopinathan, A.; Huang, K.C. Chiral twisting in a bacterial cytoskeletal polymer affects filament size and orientation. Nat. Commun. 2020, 11, 1408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Hashimoto, T. Mechanistic Insights into Plant Chiral Growth. Symmetry 2020, 12, 2056. https://doi.org/10.3390/sym12122056
Nakamura M, Hashimoto T. Mechanistic Insights into Plant Chiral Growth. Symmetry. 2020; 12(12):2056. https://doi.org/10.3390/sym12122056
Chicago/Turabian StyleNakamura, Masayoshi, and Takashi Hashimoto. 2020. "Mechanistic Insights into Plant Chiral Growth" Symmetry 12, no. 12: 2056. https://doi.org/10.3390/sym12122056
APA StyleNakamura, M., & Hashimoto, T. (2020). Mechanistic Insights into Plant Chiral Growth. Symmetry, 12(12), 2056. https://doi.org/10.3390/sym12122056




