Elastic Organic Crystals of π-Conjugated Molecules: New Concept for Materials Chemistry
Abstract
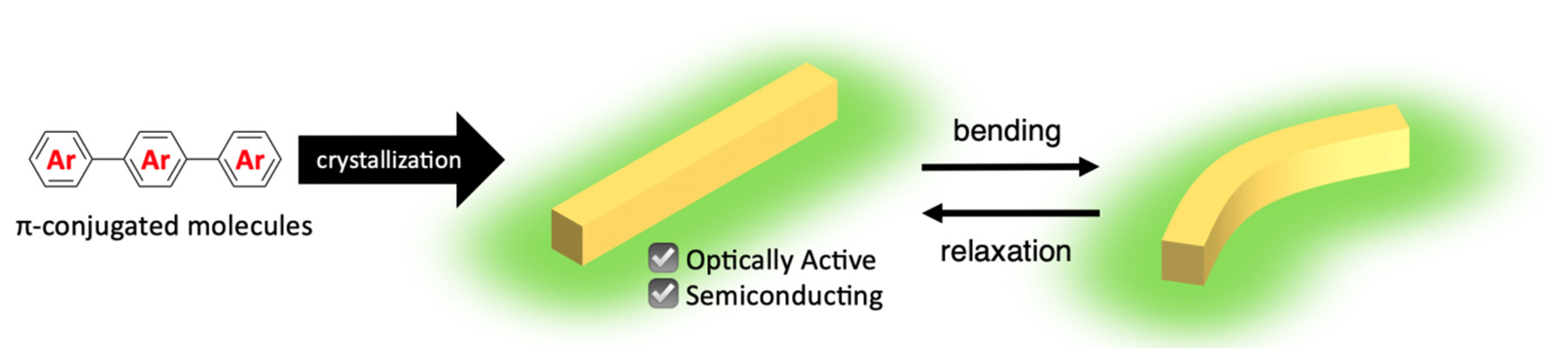
:1. Introduction
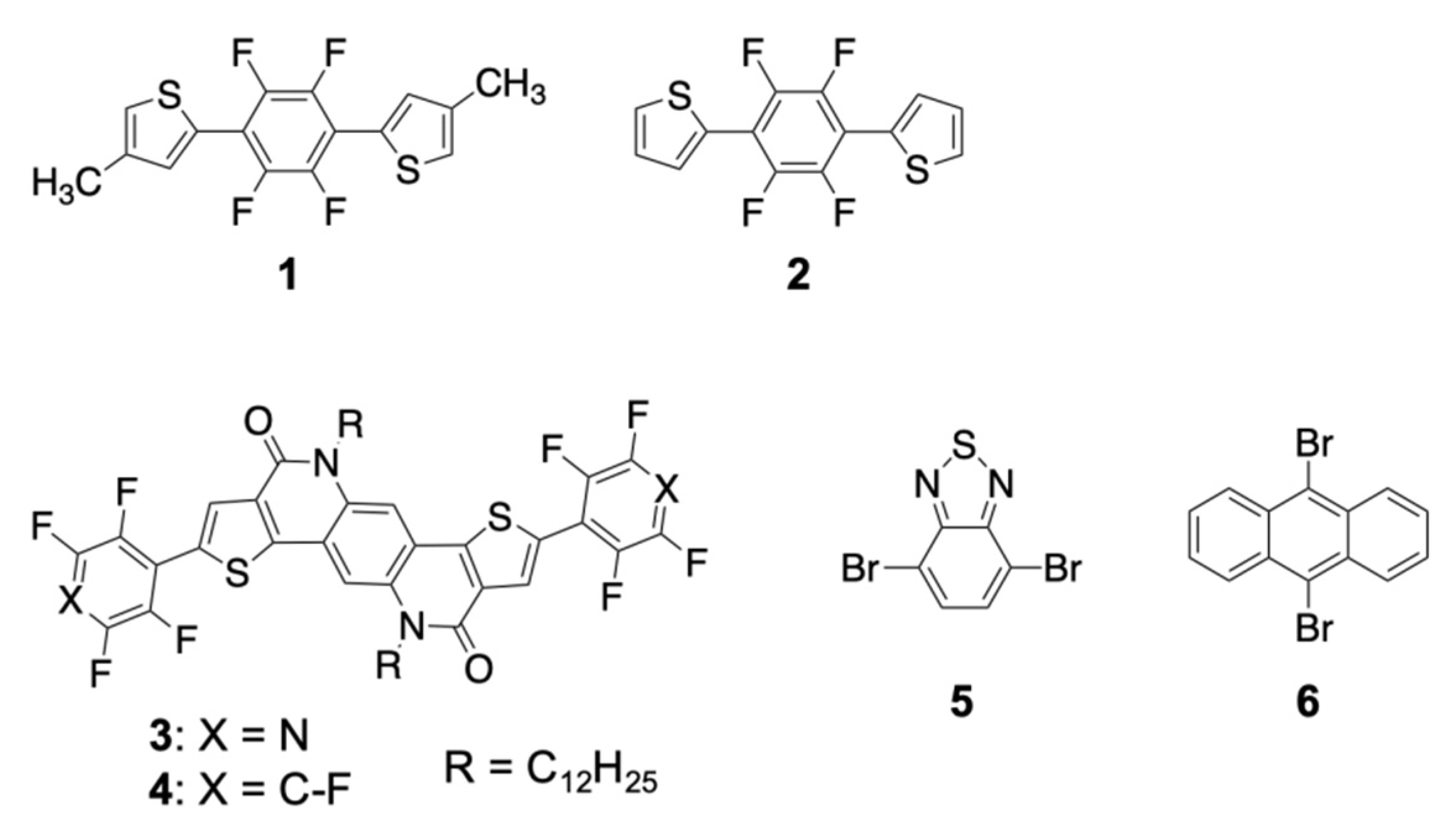
2. Design of Elastic Organic Crystals Composed of π-Conjugated Molecules
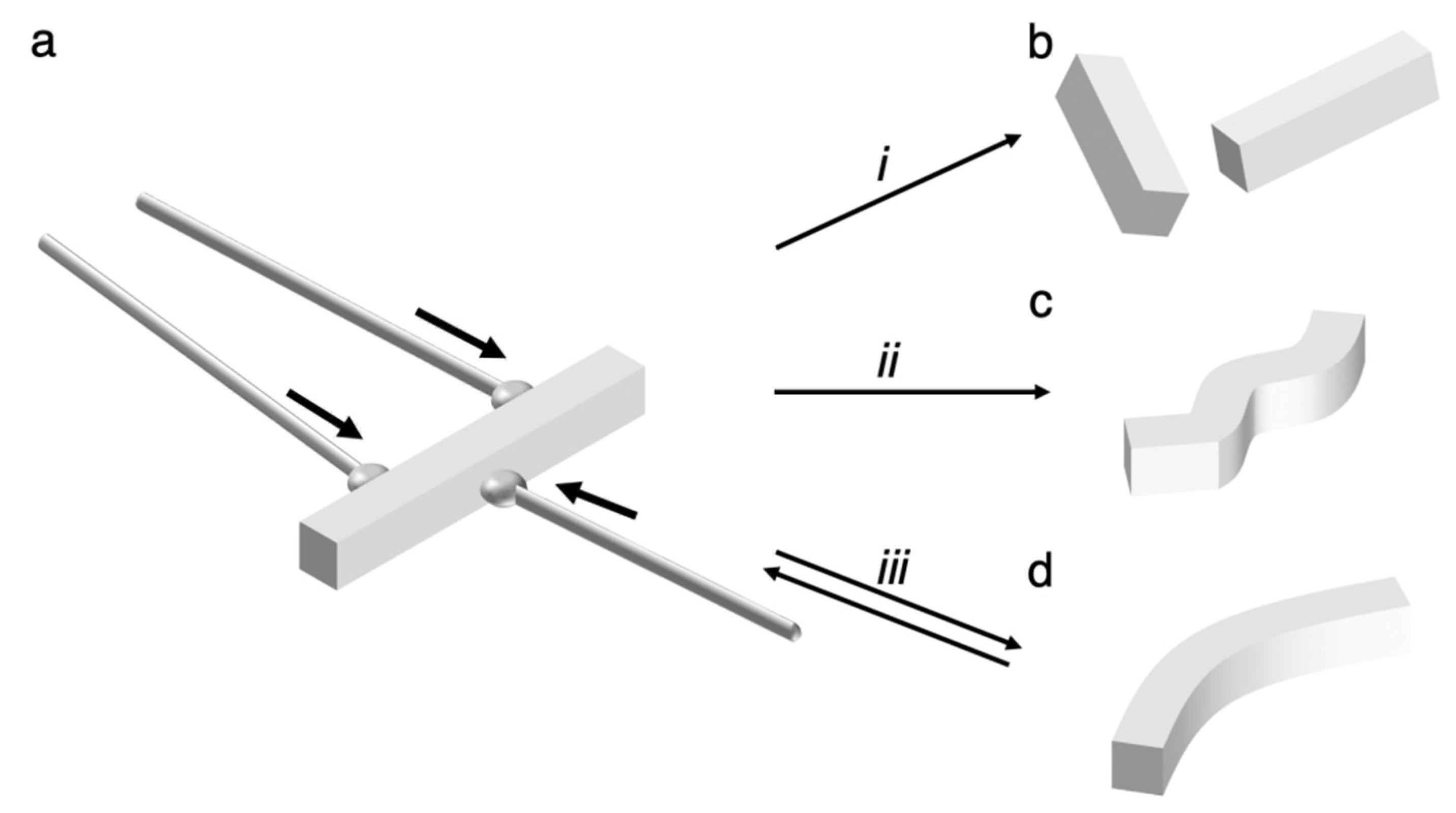
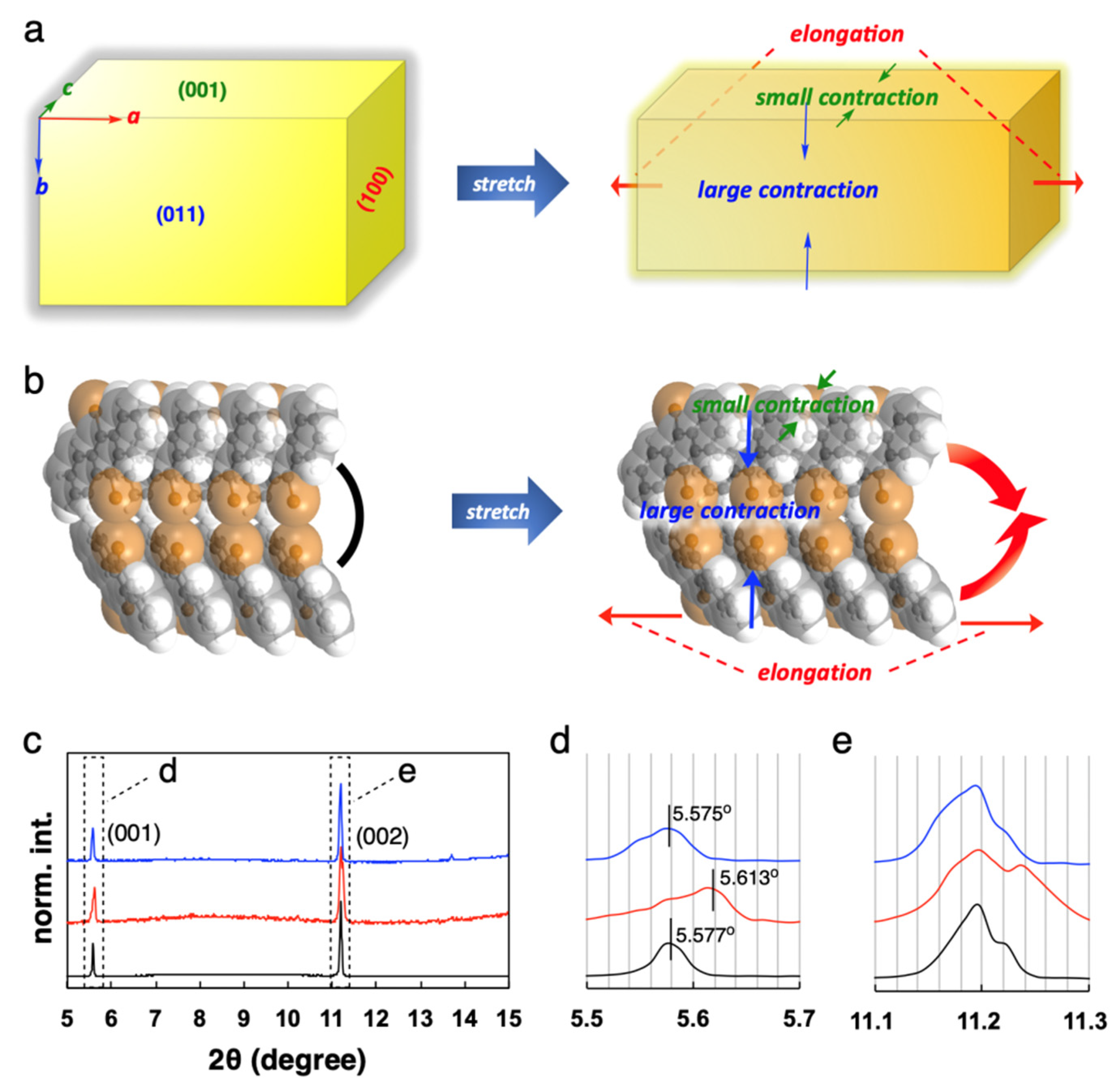
3. Potential Structures of Elastic Organic Crystals
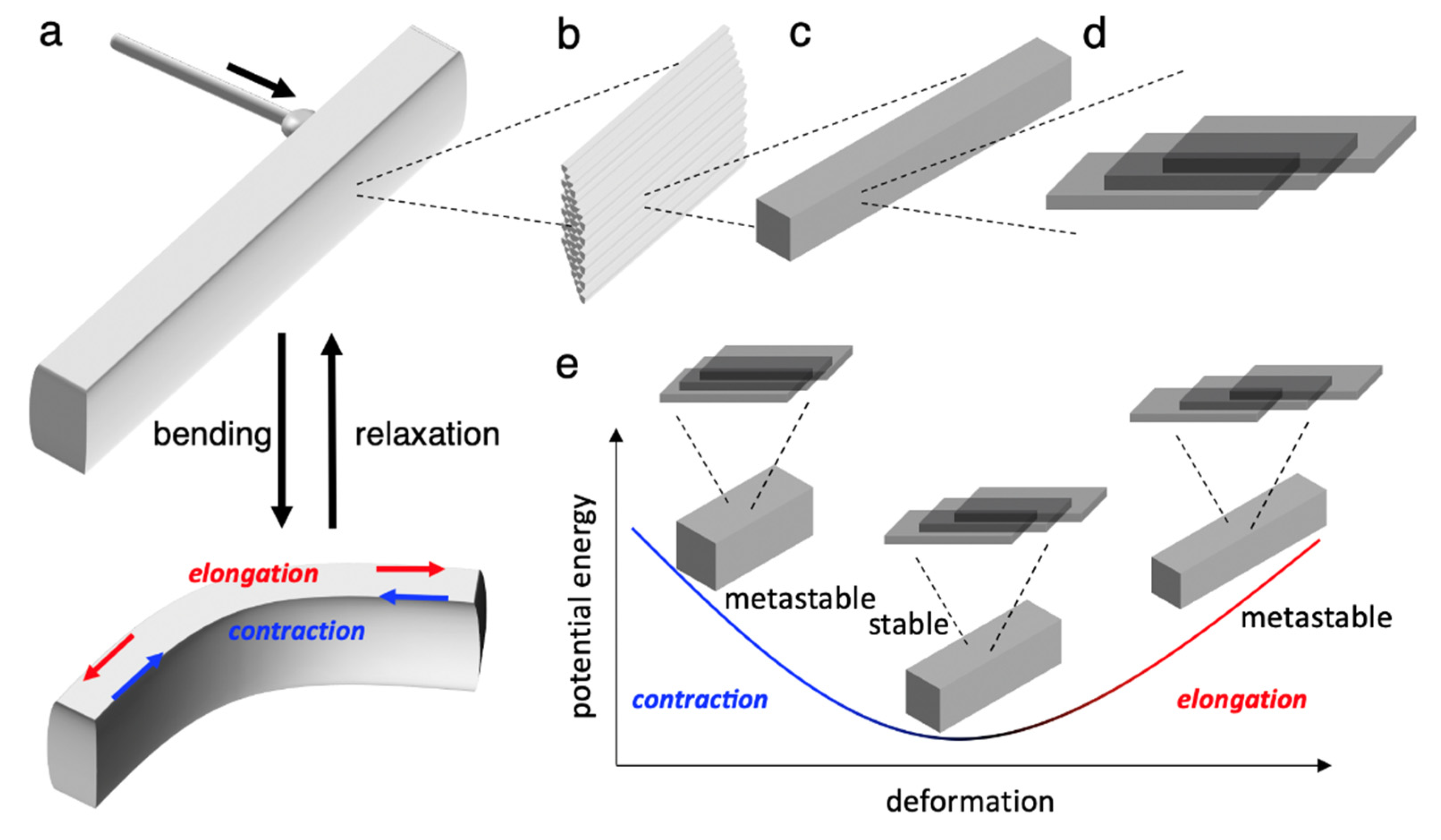
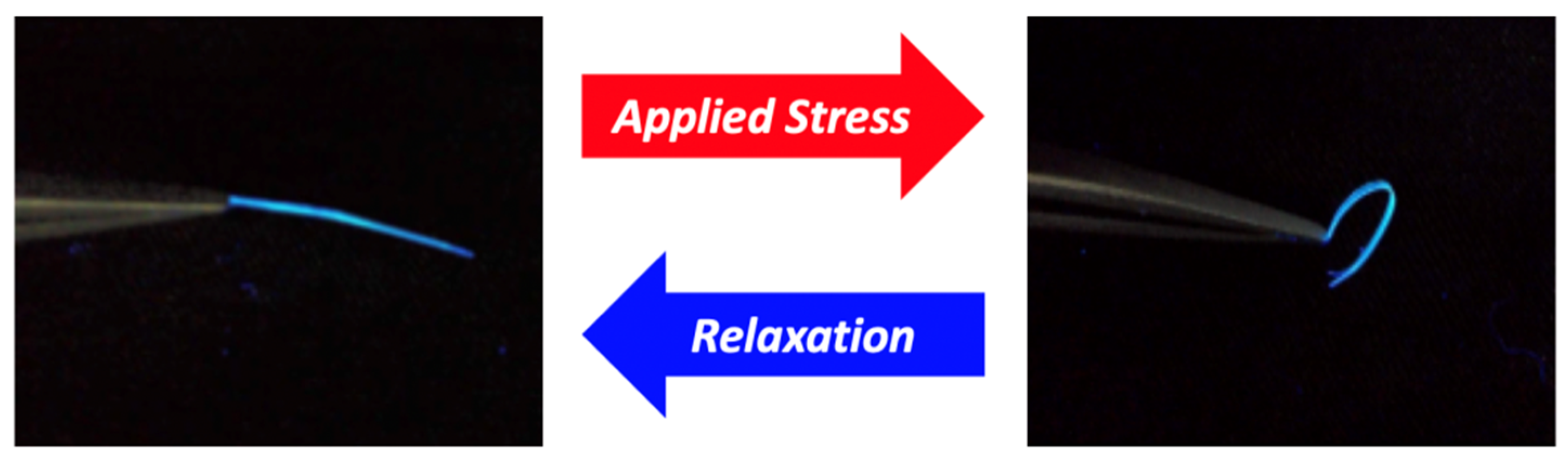
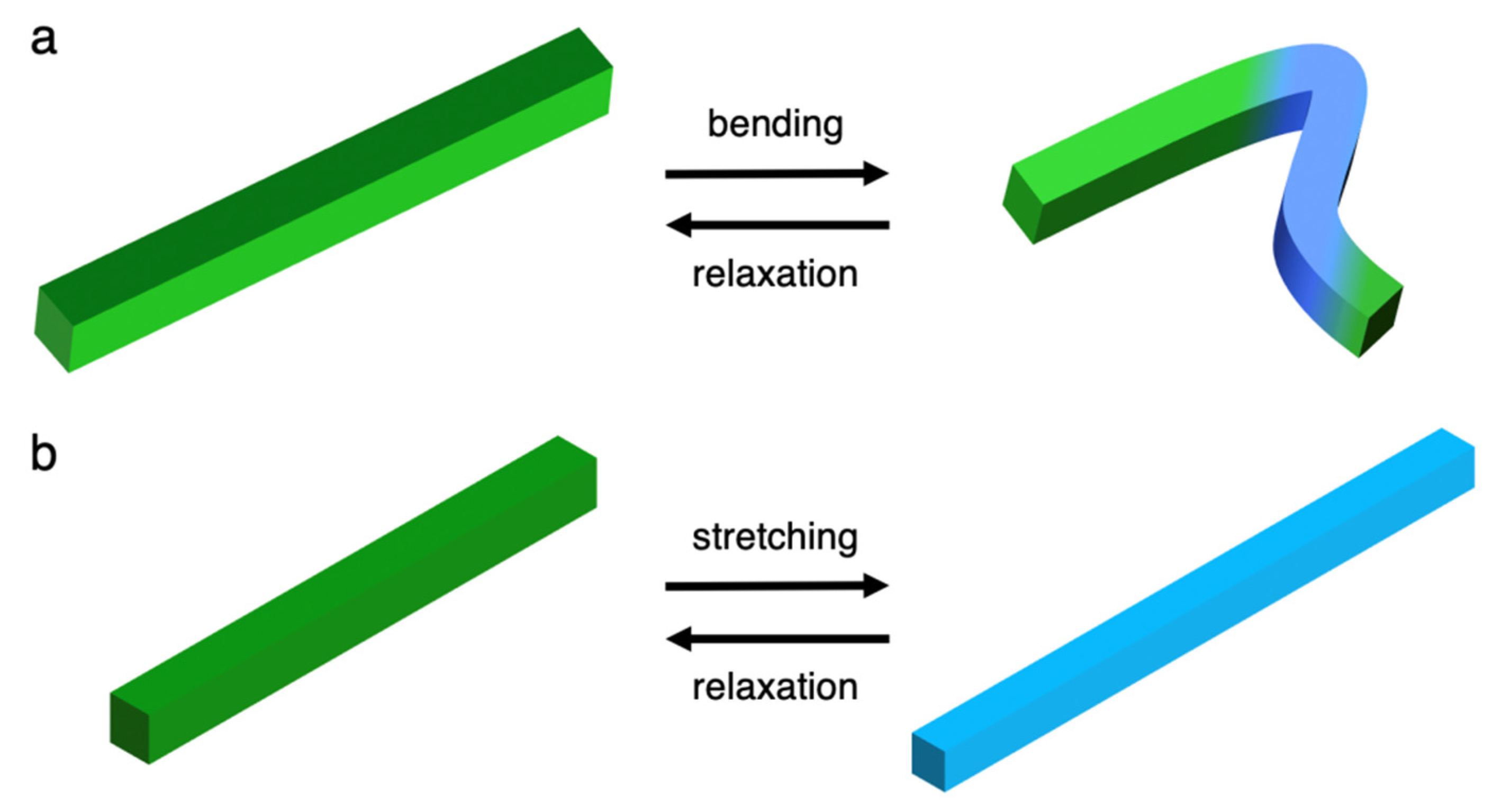
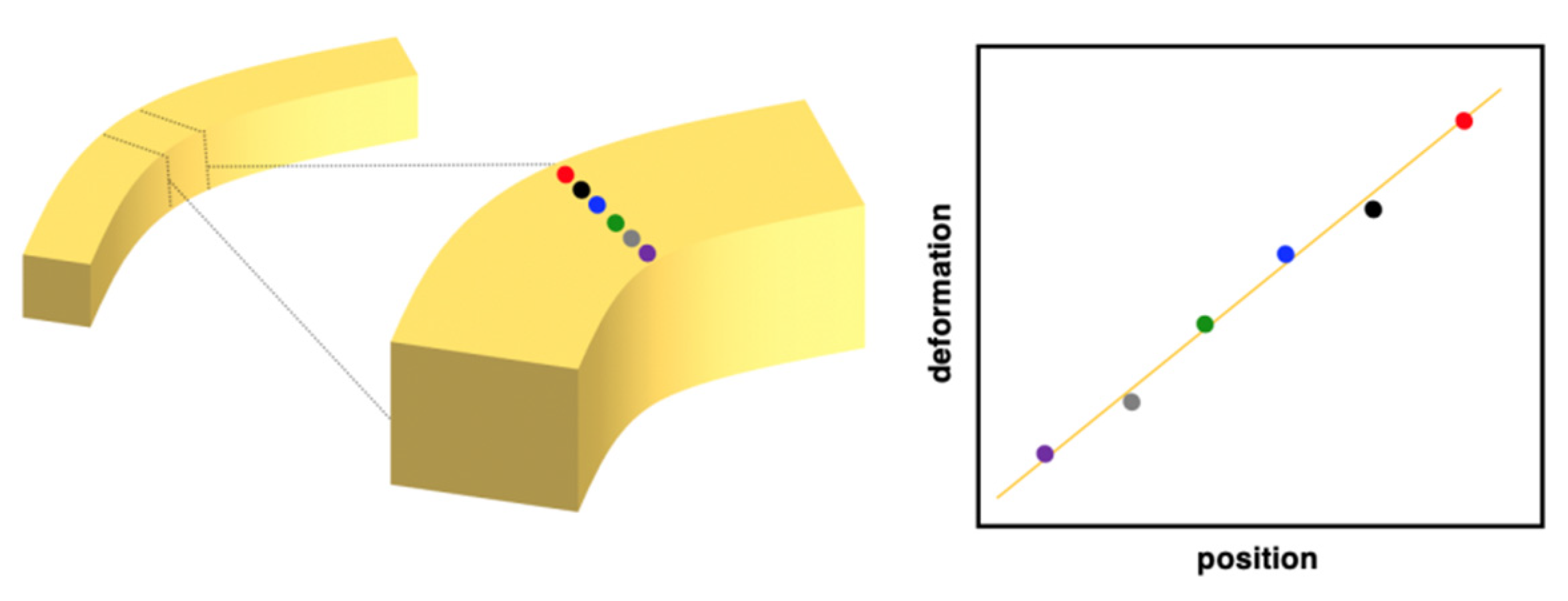
4. Detailed Deformation of Elastic Organic Crystals
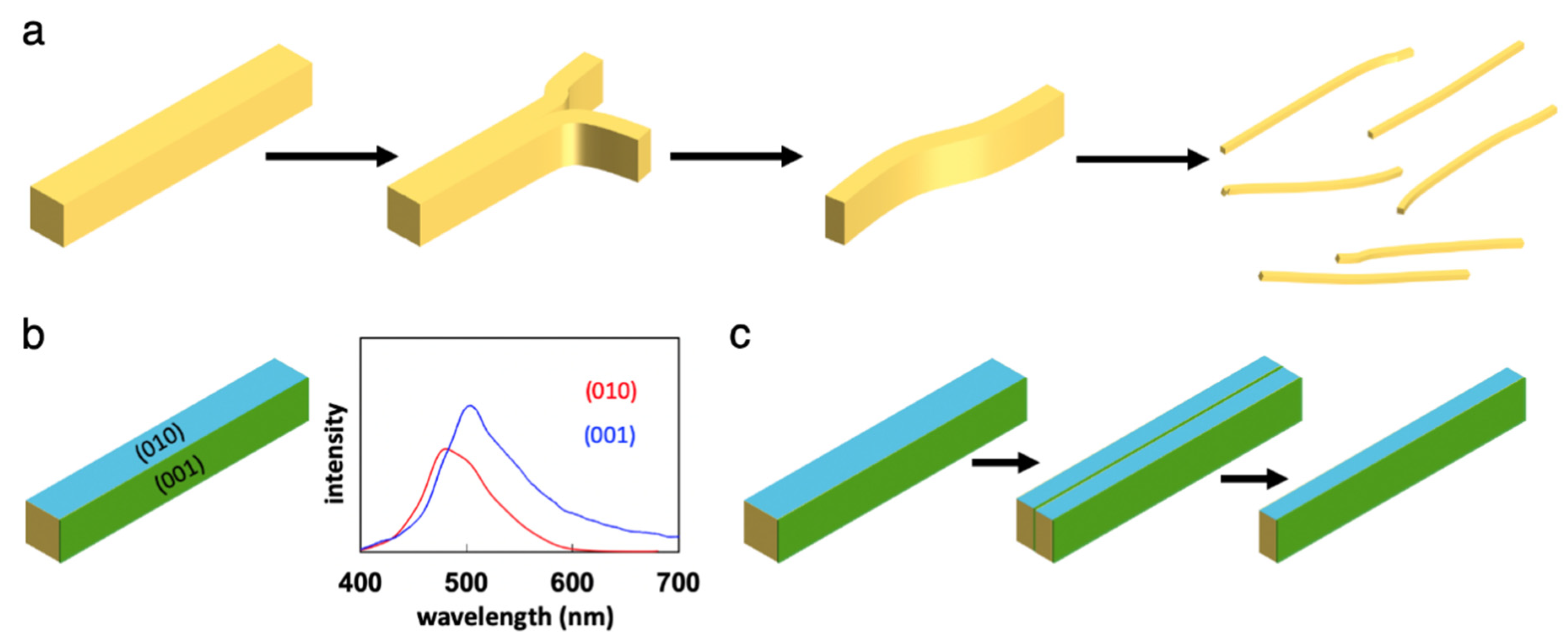
5. Unique Properties of Elastic Organic Crystals: Mechanically Induced Shaping into Perfect Crystal Fibers
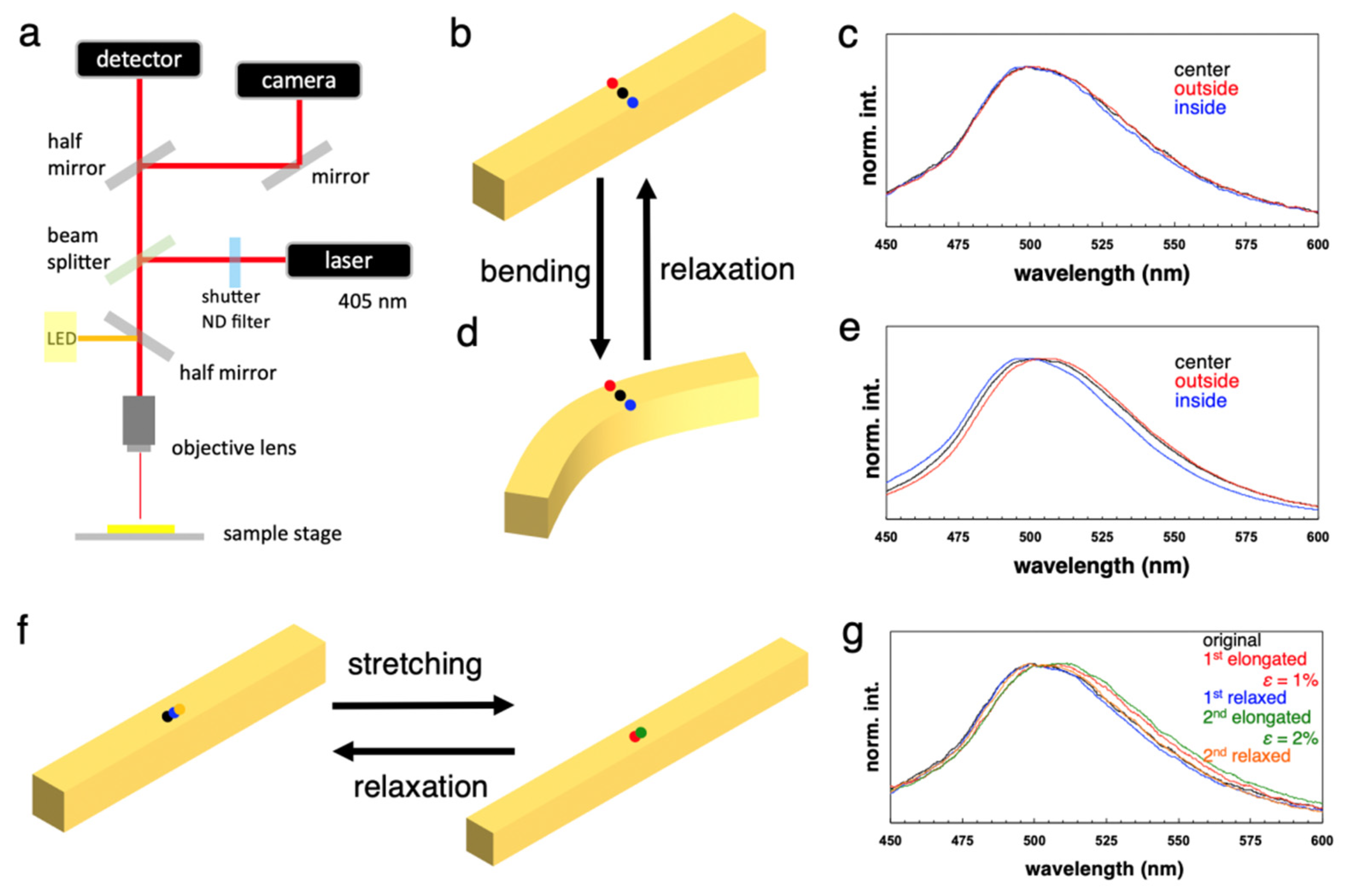
6. Unique Properties of Elastic Organic Crystals: Mechanically Induced Photoluminescence Change
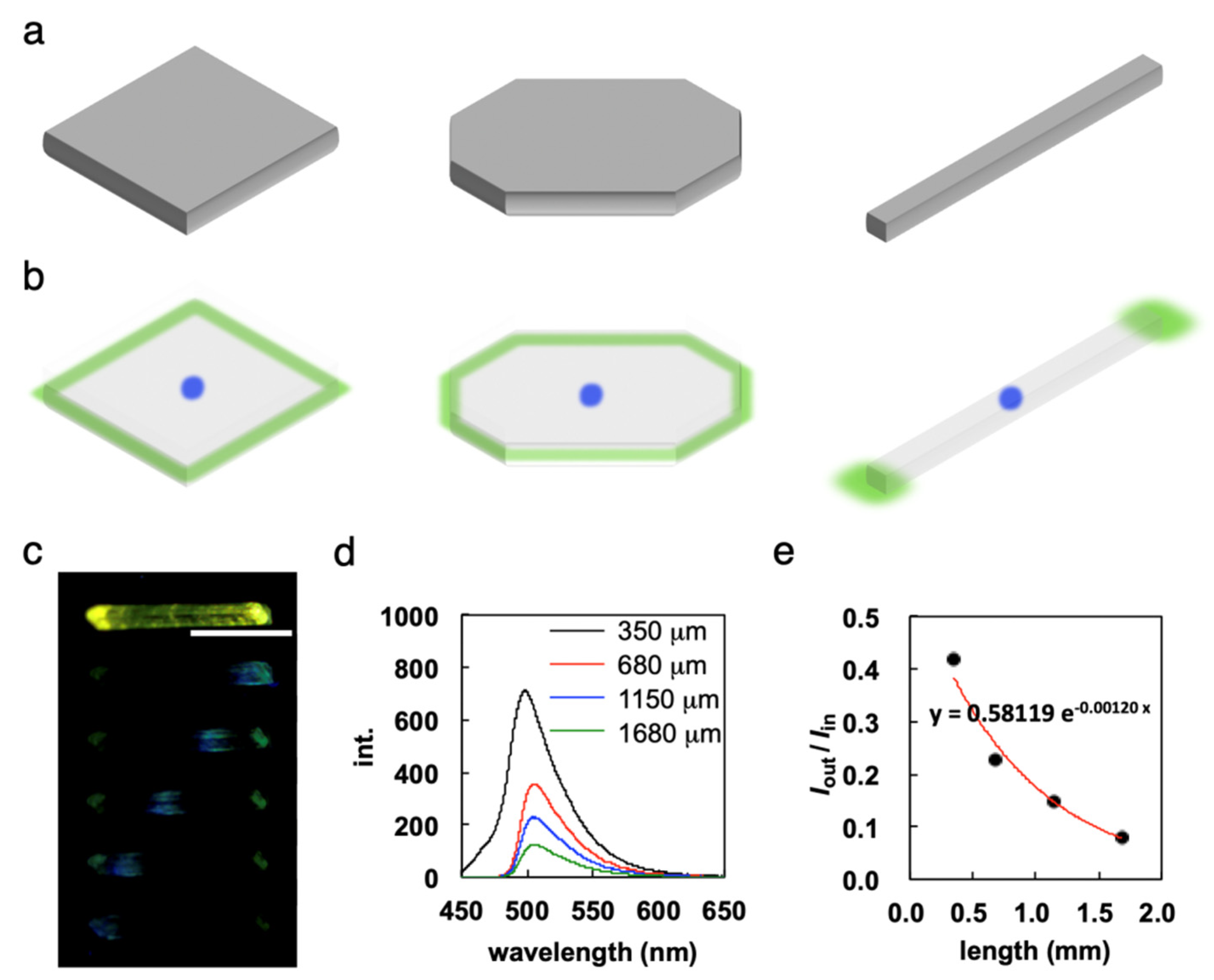
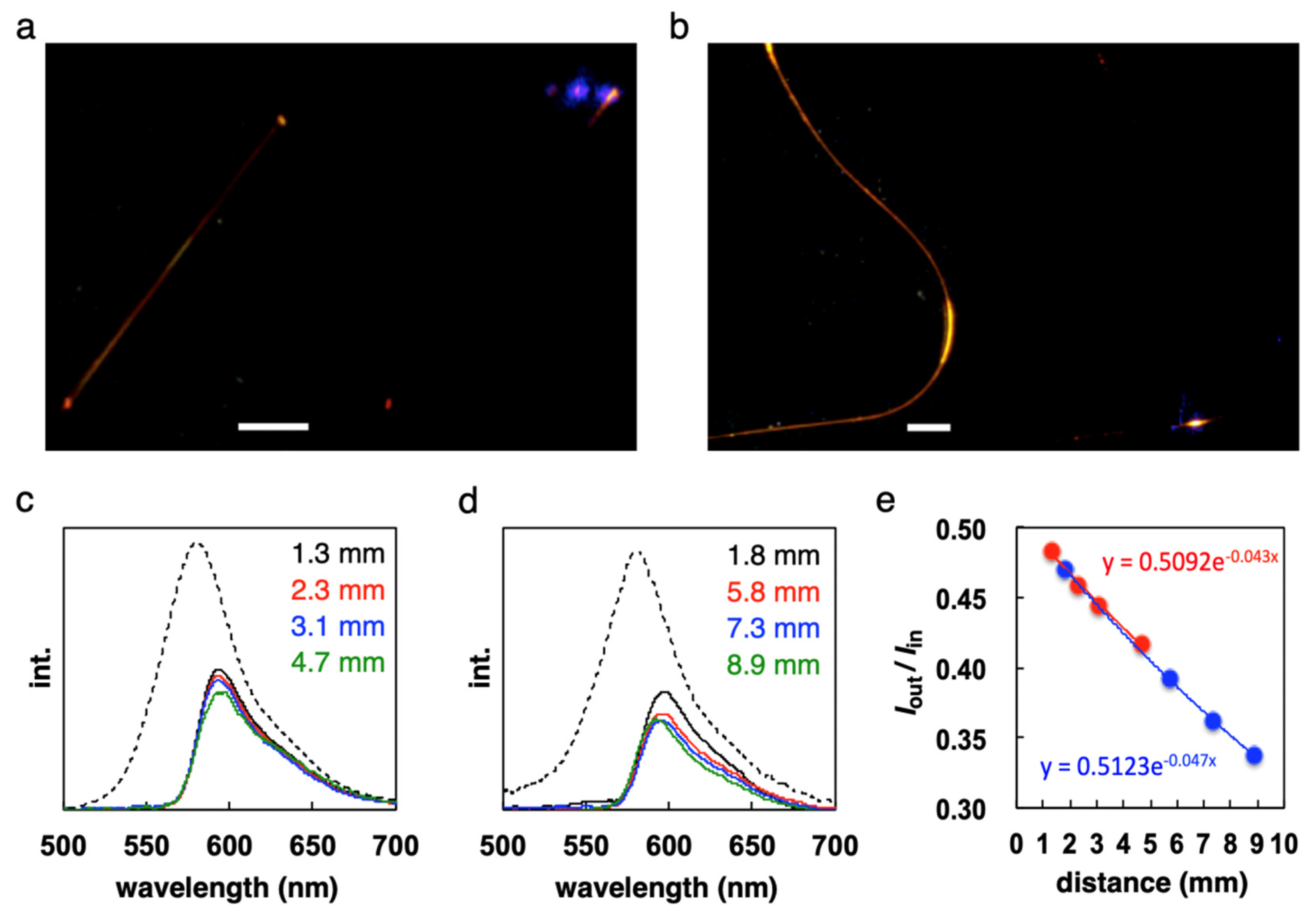
7. Unique Properties of Elastic Organic Crystals: Flexible Optical Waveguide
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Saha, S.; Mishra, M.K.; Reddy, C.M.; Desiraju, G.R. From molecules to interactions to crystal engineering: Mechanical properties of organic solids. Acc. Chem. Res. 2018, 51, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.M.; Kirchner, M.T.; Gundakaram, R.C.; Padmanabhan, K.A.; Desiraju, G.R. Isostructurality, polymorphism and mechanical properties of some hexahalogenated benzenes: The nature of halogen⋯halogen interactions. Chem. Eur. J. 2006, 12, 2222–2234. [Google Scholar] [CrossRef] [PubMed]
- Panda, M.K.; Ghosh, S.; Yasuda, N.; Moriwaki, T.; Mukherjee, G.D.; Reddy, C.M.; Naumov, P. Spatially resolved analysis of short-range structure perturbations in a plastically bent molecular crystal. Nat. Chem. 2015, 7, 65–72. [Google Scholar] [CrossRef]
- Reddy, C.M.; Gundakaram, R.C.; Basavoju, S.; Kirchner, M.T.; Padmanabhan, K.A.; Desiraju, G.R. Structural basis for bending of organic crystals. Chem. Commun. 2005, 31, 3945–3947. [Google Scholar] [CrossRef]
- Reddy, C.M.; Padmanabhan, K.A.; Desiraju, G.R. Structure-property correlations in bending and brittle organic crystals. Cryst. Growth Des. 2006, 6, 2720–2731. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Arkhipov, S.G.; Rychkov, D.A. Simple crystallographic model for anomalous plasticity of L-Leucinium hydrogen maleate crystals. Mater. Today Proc. 2020, 25, 412–415. [Google Scholar] [CrossRef]
- Takamizawa, S.; Takasaki, Y. Shape-Memory Effect in an Organosuperelastic Crystal. Chem. Sci. 2016, 7, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Takamizawa, S. Dynamic gas-inclusion in a single crystal. Angew. Chem. Int. Ed. 2015, 54, 7033–7036. [Google Scholar] [CrossRef]
- Takamizawa, S.; Miyamoto, Y. Superelastic organic crystals. Angew. Chem. Int. Ed. 2014, 53, 6970–6973. [Google Scholar] [CrossRef]
- DeRosa, C.; Auriemma, F. From entropic to enthalpic elasticity: Novel thermoplastic elastomers from syndiotactic propylene–ethylene copolymers. Adv. Mater. 2005, 17, 1503–1507. [Google Scholar] [CrossRef]
- DeRosa, C.; Auriemma, F. Structure and physical properties of syndiotactic polypropylene: A highly crystalline thermoplastic elastomer. Prog. Polym. Sci. 2006, 31, 1145–1237. [Google Scholar] [CrossRef]
- Ghosh, S.; Reddy, C.M. Elastic and bendable caffeine cocrystals: Implications for the design of flexible organic materials. Angew. Chem. Int. Ed. 2012, 51, 10319–10323. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mishra, M.K.; Kadambi, S.B.; Ramamurty, U.; Desiraju, G.R. Designing elastic organic crystals: Highly flexible polyhalogenated N-benzylideneanilines. Angew. Chem. Int. Ed. 2015, 54, 2674–2678. [Google Scholar] [CrossRef]
- Hayashi, S.; Koizumi, T. Elastic organic crystal of a fluorescent π-conjugated molecule. Angew. Chem. Int. Ed. 2016, 55, 2701–2704. [Google Scholar] [CrossRef]
- Hayashi, S.; Asano, A.; Kamiya, N.; Yokomori, Y.; Maeda, T.; Koizumi, T. Fluorescent organic single crystals with elastic bending flexibility: 1,4-bis(thien-2-yl)-2,3,5,6-tetrafluorobenzene derivatives. Sci. Rep. 2017, 7, 9453. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Koizumi, T. Mechanically induced shaping of organic single crystals: Facile fabrication of fluorescent and elastic crystal fibers. Chem. Eur. J. 2018, 24, 8507–8512. [Google Scholar] [CrossRef]
- Hayashi, S.; Koizumi, T. Direction-specific fluorescence of an engineered organic crystal and the appearance of a new face caused by mechanically induced shaping. CrystEngComm 2019, 21, 5990–5996. [Google Scholar] [CrossRef]
- Hayashi, S.; Koizumi, T.; Kamiya, N. Elastic bending flexibility of fluorescent organic single crystal: New aspects of commonly used building block “4,7-dibromo-2,1,3-benzothiadiazole”. Cryst. Growth Des. 2017, 17, 6158–6162. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamamoto, S.; Takeuchi, D.; Ie, Y.; Takagi, K. Creating elastic organic crystals of π-conjugated molecules with flexible optical waveguide and bending mechanofluorochromism. Angew. Chem. Int. Ed. 2018, 57, 17002–17008. [Google Scholar] [CrossRef]
- Hayashi, S.; Ishiwari, F.; Fukushima, T.; Mikage, S.; Imamura, Y.; Tashiro, M.; Katouda, M. Anisotropic Poisson’s effect and deformation-induced fluorescence change of elastic 9,10-dibromoanthracence single crystals. Angew. Chem. Int. Ed. 2020, 59, 16195–16201. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S. Elastic organic crystals of π-conjugated molecules: Anisotropic densely packed supramolecular 3D polymers exhibit mechanical flexibility and shape tunability. Polym. J. 2019, 51, 813–823. [Google Scholar] [CrossRef]
- Hayashi, S. Flexible and densely packed π-figuration system: Creating elastic organic crystals of π-conjugated molecules. J. Synth. Org. Chem. Jpn. 2020, 58, 962–970. [Google Scholar] [CrossRef]
- Hayashi, S. Elastic Organic Crystals. J. Synth. Org. Chem. Jpn. 2020, 58, 985. [Google Scholar]
- Saito, M.; Shinokubo, H.; Sakurai, H. Figuration of bowl-shaped π-conjugated molecules: Properties and function. Mater. Chem. Front. 2018, 2, 635–661. [Google Scholar] [CrossRef]
- Hayashi, S.; Inagi, S.; Fuchigami, T. Synthesis of 9-substituted fluorene copolymers via chemical and electrochemical polymer reaction and their optoelectronic properties. Macromolecules 2009, 42, 3755–3760. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Praveen, V.K. π-Organogels of self-assembled p-phenylenevinylenes: Soft materials with distinct size, shape, and functions. Acc. Chem. Res. 2007, 40, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, J.; Amsharov, K.Y.; Sharapa, D.I.; Reger, D.; Roschyna, K.; Lungerich, D.; Jux, N.; Hauke, F.; Clark, T.; Hirsch, A. Highly regioselective alkylation of hexabenzocoronenes: Fundamental insights into the covalent chemistry of graphene. Angew. Chem. Int. Ed. 2017, 56, 12184–12190. [Google Scholar] [CrossRef]
- Omachi, H.; Segawa, Y.; Itami, K. Synthesis of cyclophenylenes and related carbon nanorings: A step toward the controlled sysnthesis of carbon nanotubes. Acc. Chem. Res. 2012, 45, 1378–1389. [Google Scholar] [CrossRef]
- Hayashi, S.; Asano, A.; Koizumi, T. Modification of pyridine-based conjugated polymer films via Lewis acid: Halochromism, characterization and macroscopic gradation patterning. Polym. Chem. 2011, 2, 2764–2766. [Google Scholar] [CrossRef]
- Hayashi, S.; Koizumi, T. From benzodiazaborole-based compound to donor-acceptor polymer via electropolymerization. Polym. Chem. 2012, 3, 613–616. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamamoto, S.; Koizumi, T. Effects of molecular weight on the optical and electrochemical properties of EDOT-based π-conjugated polymers. Sci. Rep. 2017, 7, 1027. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Hirai, R.; Yamamoto, S.; Koizumi, T. A simple route to unsymmetric cyano-substituted oligo(p-phenylene-vinylene)s. Chem. Lett. 2018, 47, 1003–1005. [Google Scholar] [CrossRef]
- Hayashi, S.; Takigami, A.; Koizumi, T. Solvent control over supramolecular gel formation and fluorescence for a highly crystalline π-conjugated polymer. Chem. Asian J. 2018, 13, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Yamamoto, S.; Nishi, K.; Asano, A.; Koizumi, T. Synthesis of network polymer emitters: Tunable detection of chemicals by the geometric design. Polym. J. 2019, 51, 1055–1061. [Google Scholar] [CrossRef]
- Hayashi, S.; Sakamoto, M.; Ishiwari, F.; Fukushima, T.; Yamamoto, S.; Koizumi, T. A versatile scaffold for facile synthesis of fluorescent cyano-substituted stilbenes. Tetrahedron 2019, 75, 1079–1084. [Google Scholar] [CrossRef]
- Hayashi, S. Highly crystalline and efficient red-emissive π-conjugated polymer film: Tuning of macrostructure for light-emitting properties. Mater. Adv. 2020, 1, 632–638. [Google Scholar] [CrossRef]
- Hayashi, S.; Nishioka, N.; Nishiyama, H.; Koizumi, T. π-Conjugated alternating copolymer based on the 3,5-dinitro-9-fluorenone for electron-acceptor type materials. Synth. Met. 2012, 162, 1485–1489. [Google Scholar] [CrossRef]
- Facchetti, A.; Yoon, M.-H.; Stern, C.L.; Katz, H.E.; Marks, T.J. Building blocks for n-type organic electronics: Regiochemically modulated inversion of majority carrier sign in perfluoroarene-modified polythiophene semiconductors. Angew. Chem. Int. Ed. 2003, 42, 3900–3903. [Google Scholar] [CrossRef]
- Reese, C.; Bao, Z. Organic single crystals: Tools for the exploration of charge transport phenomena in organic materials. J. Mater. Chem. 2006, 16, 329–333. [Google Scholar] [CrossRef]
- Inada, Y.; Yamao, T.; Inada, M.; Itami, T.; Hotta, S. Giant organic single-crystals of a thiophene/phenylene co-oligomer toward device applications. Synth. Met. 2011, 161, 1869–1877. [Google Scholar] [CrossRef]
- Kim, T.-D.; Lee, K.-S. D-π-A conjugated molecules for optoelectronic applications. Macromol. Rapid. Commun. 2015, 36, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Supramolecular synthon in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Ghosh, S.; Mishra, M.K.; Ganguly, S.; Desiraju, G.R. Dual stress and thermally driven mechanical properties of the same organic crystal: 2,6-dichlorobenzylidene-4-fluoro-3-nitroaniline. J. Am. Chem. Soc. 2015, 137, 9912–9921. [Google Scholar] [CrossRef] [PubMed]
- Crouch, D.J.; Skabara, P.J.; Heeney, M.; McCulloch, I.; Coles, S.J.; Hursthouse, M.B. Hexyl-substituted oligothiophenes with a central tetrafluorophenylene unit: Crystal engineering of planar structures for p-type organic semiconductors. Chem. Commun. 2005, 1465–1467. [Google Scholar] [CrossRef]
- Crouch, D.J.; Skabara, P.J.; Lohr, J.E.; McDouall, J.J.W.; Heeney, M.; McCulloch, I.; Sparrowe, D.; Shkunov, M.; Coles, S.J.; Horton, P.N.; et al. Thiophene and selenophene copolymers incorporating fluorinated phenylene units in the main chain: Synthesis, characterization, and application in organic field-effect transistors. Chem. Mater. 2005, 17, 6567–6578. [Google Scholar] [CrossRef]
- Ahmed, E.; Karothu, D.P.; Naumov, P. Crystal adaptronics: Mechanically reconfigurable elastic and superelastic molecular crystals. Angew. Chem. Int. Ed. 2018, 57, 8837–8846. [Google Scholar] [CrossRef]
- Mishra, M.K.; Sun, C.C. Conformation directed interaction anisotropy leading to distinct bending behaviors of two ROY polymorphs. Cryst. Growth Des. 2020, 20, 4764–4769. [Google Scholar] [CrossRef]
- Wang, K.; Mishra, M.K.; Sun, C.C. Exceptionally elastic single-component pharmaceutical crystals. Chem. Mater. 2019, 31, 1794–1799. [Google Scholar] [CrossRef]
- Mishra, M.K.; Mishra, K.; Asif, S.A.S.; Manimunda, P. Structural analysis of elastically bent organic crystals using in situ indentation and micro-raman spectroscopy. Chem. Commun. 2017, 53, 13035–13038. [Google Scholar] [CrossRef]
- Horstman, E.M.; Keswani, R.K.; Frey, B.A.; Rzeczycki, P.M.; LaLone, V.; Bertke, J.A.; Kenis, P.J.A.; Rosania, G.R. Elasticity in macrophage-synthesized biocrystals. Angew. Chem. Int. Ed. 2017, 56, 1815–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Karothu, D.P.; Ahmed, E.; Naumov, P.; Nath, N.K. Thermally twistable, photobendable, elastically deformable, and self-healable soft crystals. Angew. Chem. Int. Ed. 2018, 57, 8498–8502. [Google Scholar] [CrossRef] [PubMed]
- Commins, P.; Karothu, D.P.; Naumov, P. Is a bent crystal still a single crystal? Angew. Chem. Int. Ed. 2019, 58, 10052–10060. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Sugimoto, M.; Hisaeda, Y. Multicomponent molecular puzzles for photofunction design: Emission color variation in Lewis acid–base pair crystals coupled with guest-to-host charge transfer excitation. J. Am. Chem. Soc. 2015, 137, 9519–9522. [Google Scholar] [CrossRef]
- Worthy, A.; Grosjean, A.; Pfrunder, M.C.; Xu, Y.; Yan, C.; Edwards, G.; Clegg, J.K.; McMurtrie, J.C. Atomic resolution of structural changes in elastic crystals of copper(II) acetylacetonate. Nat. Chem. 2018, 10, 65–69. [Google Scholar] [CrossRef]
- Hayashi, S. Facile investigation of reversible nanostructure changes in flexible crystals. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Kenny, E.P.; Jacko, A.C.; Powell, B.J. Mechanomagnetics in elastic crystals: Insights from [Cu(acac)2]. Angew. Chem. Int. Ed. 2019, 58, 15082–15088. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef]
- Gidron, O.; Bendikov, M. α-Oligofurans: An emerging class of conjugated oligomers for organic electronics. Angew. Chem. Int. Ed. 2014, 53, 2546–2555. [Google Scholar] [CrossRef]
- Kobaisi, M.A.; Bhosale, S.V.; Latham, K.; Raynor, A.M.; Bhosale, S.V. Functional naphthalene diimides: Synthesis, properties, and applications. Chem. Rev. 2016, 116, 11685–11796. [Google Scholar] [CrossRef]
- Uegaki, K.; Nakabayashi, K.; Yamamoto, S.; Koizumi, T.; Hayashi, S. Donor–acceptor random regioregular π-conjugated copolymers based on poly(3-hexylthiophene) with unsymmetrical monothienoisoindigo Units. RSC Adv. 2020, 10, 19034–19040. [Google Scholar] [CrossRef]
- Kakkar, S.; Bhattacharya, B.; Reddy, C.M.; Ghosh, S. Tuning mechanical behaviour by controlling the structure of a series of theophylline co-crystals. CrystEngComm 2018, 20, 1101–1109. [Google Scholar] [CrossRef]
- Seki, T.; Mashimo, T.; Ito, H. Anisotropic strain release in a thermosalient crystal: Correlation between the microscopic orientation of molecular rearrangements and the macroscopic mechanical motion. Chem. Sci. 2019, 10, 4185–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, N.K.; Panda, M.K.; Sahoo, S.C.; Naumov, P. Thermally induced and photoinduced mechanical effects in molecular single crystals—A revival. CrystEngComm 2014, 16, 1850–1858. [Google Scholar] [CrossRef]
- Sahoo, S.C.; Sinha, S.B.; Kiran, M.S.; Ramamurty, U.; Dericioglu, A.F.; Reddy, C.M.; Naumov, P. Kinematic and mechanical profile of the self-actuation of thermosalient crystal twins of 1,2,4,5-tetrabromobenzene: A molecular crystalline analogue of a bimetallic strip. J. Am. Chem. Soc. 2013, 135, 13843–13850. [Google Scholar] [CrossRef] [PubMed]
- Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. Distinct responses to mechanical grinding and hydrostatic pressure in luminescent chromism of tetrathiazolylthiophene. J. Am. Chem. Soc. 2013, 135, 10322–10325. [Google Scholar] [CrossRef]
- Ono, T.; Tsukiyama, Y.; Taema, A.; Sato, H.; Kiyooka, H.; Yamaguchi, Y.; Nagahashi, A.; Nishiyama, M.; Akahama, Y.; Ozawa, Y.; et al. Piezofluorochromism in charge-transfer inclusion crystals: The influence of high pressure versus mechanical grinding. ChemPhotoChem 2018, 2, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chi, Z.; Zhang, Y.; Mao, Z.; Yang, Z.; Ubba, E.; Chi, Z. Recent progress in the mechanofluorochromism of cyanoethylene derivatives with aggregation-induced emission. J. Mater. Chem. C 2018, 6, 6327–6353. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.S.; Yao, J. Optical waveguides at micro/nanoscale based on functional small organic molecules. Phys. Chem. Chem. Phys. 2011, 13, 9060–9073. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Camposeo, A.; Pagliara, S.; Mele, E.; Persano, L.; Stabile, R.; Cingolani, R.; Pisignano, D. Patterning of light-emitting conjugated polymer nanofibres. Nat. Nanotechnol. 2008, 3, 614. [Google Scholar] [CrossRef]
- Chen, X.; Sun, T.; Wang, F. Lanthanide-based luminescent materials for waveguide and lasing. Chem. Asian J. 2020, 15, 1–33. [Google Scholar] [CrossRef]
- Hayashi, S.; Koizumi, T.; Kamiya, N. 2,5-Dimethoxybenzene-1,4-dicarboxaldehyde: An emissive organic crystal and highly efficient fluorescent waveguide. ChemPlusChem 2019, 84, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Z.; Li, A.; Xu, W.; Wang, Y.; Jiang, H.; Wang, K.; Zhao, Y.; Jia, X. A single crystal with multiple functions of optical waveguide, aggregation-induced emission, and mechanochromism. ACS Appl. Mater. Interfaces 2017, 9, 8910–8918. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-H.; Lei, T.; Jin, Z.-X.; Wang, J.-Y.; Pei, J. T-Shaped donor–acceptor molecules for low-loss red-emission optical waveguide. Org. Lett. 2013, 15, 3530–3533. [Google Scholar] [CrossRef]
- Liu, B.; Di, Q.; Liu, W.; Wang, C.; Wang, Y.; Zhang, H. Red-emissive organic crystals of a single-benzene molecule: Elastically bendable and flexible optical waveguide. J. Phys. Chem. Lett. 2019, 10, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, C.; Wang, Y.; Zhang, H. Elastic self-doping organic single crystals exhibiting flexible optical waveguide and amplified spontaneous emission. Adv. Mater. 2018, 30, 1800814. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, Z.; Zhang, Z.; Wang, Y.; Zhang, H. Highly elastic organic crystals for flexible optical waveguide. Angew. Chem. Int. Ed. 2018, 57, 8448–8452. [Google Scholar] [CrossRef] [PubMed]
- Varughese, S.; Kiran, M.S.R.N.; Ramamurty, U.; Desiraju, G.R. Nanoindentation in crystal engineering: Quantifying mechanical properties of molecular crystals. Angew. Chem. Int. Ed. 2013, 52, 2701–2712. [Google Scholar] [CrossRef]
- Turner, M.J.; Thomas, S.P.; Shi, M.W.; Jayatilaka, D.; Spackman, M.A. Energy frameworks: Insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem. Commun. 2015, 51, 3735–3738. [Google Scholar] [CrossRef]





| Materials | Inorganic Crystals | Polymer Solids | Organic Crystals |
|---|---|---|---|
| Preparation methods | Vapor-phase deposition | Template polymerization, Electro-spinning | Vapor-phase deposition, Self-assembly |
| Crystallinity | Single crystal | Amorphous, Less-crystalline | Single crystal |
| Surface defects | Less of defects | Unavoidable defects | Less of defects |
| Interactions | Covalent bond, Ionic bond | Weak interactions 1 | Weak interactions 1 |
| Photo-stability | Relative stable | Decomposition | Bleaching |
| Optical properties | Excellent but not easy tunable | Tunable 2 | Tunable 3 |
| Refractive index, n | >2 | 1.5–2.0 | >1.5 |
| Flexibilities | Less flexible | Flexible (viscoelastic) or brittle | No flexibility |
| Waveguide types | Passive and active | Passive and active | Active |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, S. Elastic Organic Crystals of π-Conjugated Molecules: New Concept for Materials Chemistry. Symmetry 2020, 12, 2022. https://doi.org/10.3390/sym12122022
Hayashi S. Elastic Organic Crystals of π-Conjugated Molecules: New Concept for Materials Chemistry. Symmetry. 2020; 12(12):2022. https://doi.org/10.3390/sym12122022
Chicago/Turabian StyleHayashi, Shotaro. 2020. "Elastic Organic Crystals of π-Conjugated Molecules: New Concept for Materials Chemistry" Symmetry 12, no. 12: 2022. https://doi.org/10.3390/sym12122022





